Introduction
Propofol is an intravenous anesthetic extensively
used in clinical practice and is characterized by rapid induction
of anesthesia, as well as prompt recovery from its effects.
However, propofol has an obvious side effect of inducing
hypotension, particularly in patients with high blood pressure or
in elderly patients. Furthermore, patients with vena cava collapse
are prone to develop significant hypotension when treated with
propofol, which may be a direct result of vascular relaxation
(1–4). Certain studies have indicated that
propofol elicits vascular relaxation via the following mechanisms:
i) Activation of large-conductance calcium voltage-activated
potassium channels (BKCa) (2); ii) activation of ATP-sensitive
K+ channels (KATP) (5); iii) inhibition of voltage-operated
calcium channels and receptor-operated calcium channels (6); and iv) increases in the availability of
nitric oxide (7,8). Propofol may cause vasodilation via four
different pathways, however, to the best of our knowledge, no
experimental study has assessed the mechanism of vasodilation
induced by propofol on the rat mesenteric artery. The differences
between these results may thus be due to different subjects, while
propofol has no significant effect on the BKCa channel
of mesenteric arteries in rats.
BKCa channels are abundant on vascular
smooth muscle cells (VSMCs) and have a dominant role in the
regulation of vascular tone. Peripheral resistance exerts a
significant function in regulating blood pressure and blood flow
distribution in tissues and organs. Peripheral vascular resistance
is the basic condition for the generation of blood pressure, and
the formation of peripheral resistance is mainly due to the
myogenic tone of MSCs. The BKCa channel mediates 70–80%
of the outward current of VSMCs, implying a close association
between the BKCa channel and the myogenic tone of VSMCs
(9). Of note, the BKCa
channel is able to regulate the contraction and relaxation of the
blood vessels by regulating the myogenic tone of VSMCs (9). Furthermore, the activation of
BKCa channels leads to K+ efflux,
contributing to membrane hyperpolarization. This membrane potential
change leads to the closure of L-type voltage-gated Ca2+
channels, which in turn reduces [Ca2+] and induces
vasorelaxation (10). A previous
study indicated that inhibition of BKCa channels
resulted in vasoconstriction (11).
Therefore, BKCa channels have a pivotal role in the
regulation of vascular tone and blood pressure (12,13).
Blood vessels are mainly composed of endothelial
cells (ECs) and VSMCs, and numerous gap junctions exist among ECs,
among VSMCs and between the layers of these two cell types
(14). Gap junctions, which directly
link the cytoplasm, are essential for coordinating tissue
homeostasis and regulating vascular responses, which allows for
conduction of intercellular signals between adjacent cells
(15). This behavior enables the
vasculature, which consists of numerous cell types, to behave as an
integrated system (16). Therefore,
gap junctions are of great importance to ensure the synchronization
and coordination of vasomotor activity, and to maintain the
stability and consistency of the physiological function of the
vessel (17–19).
The present study aimed to observe the relaxation of
propofol and to further clarify the roles of BKCa
channels and gap junctions in the vasodilation effect of
propofol.
Materials and methods
Animals
The present study was approved by the Animal
Experimental Ethical Inspection Committee of the First Affiliated
Hospital Shihezi University (Shihezi, China). A total of 80 Sprague
Dawley (SD) rats (age, 8–12 weeks; weight, 250–300 g), both males
and females, were obtained from the Center for Disease Control and
Prevention of Xinjiang Uygur Autonomous Region [Urumchi, China;
animal certificate of conformity no. SCXK (Xin) 2003–0001]. The
rats were housed in separate cages in a specific pathogen-free
environment (temperature, 24±3°C; humidity of ~49%) under a 12-h
light-dark cycle, and were provided food and water ad
libitum. All protocols were approved by the Animal Experimental
Ethical Inspection of First Affiliated Hospital, Shihezi University
School of Medicine (Shihezi, China) and were in accordance with the
Guidelines for the Care and Use of Laboratory Animals published by
the US National Institutes of Health. The SD rats were euthanized
under deep general anesthesia by intraperitoneal injection of 350
mg/kg 10% chloral hydrate. No rats exhibited peritonitis at this
dosage. Rats were then sacrificed via exsanguination and the MA and
its branches were harvested from the upper ileum mesentery.
Reagents
Propofol was purchased from AstraZeneca (Cambridge,
UK; production lot no. GF357). EDTA, ethylene
glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA),
tetraethylammonium (TEA), collagenase A, papain, bovine serum
albumin (BSA), dithiothreitol (DTT), Na-hydroxyethyl piperazine
ethanesulfonic acid (HEPES), 2-aminoethoxydiphenyl borate (2-APB),
18β-glycyrrhetinic acid (18β-GA), TritonX-100, propidium iodide
(PI) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Connexin (Cx)40 antibody was purchased from Abcam
(Cambridge, MA, USA; cat. no. ab213688), Cx43 antibody was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA;
cat. no. 3512) and Cx45 antibody was purchased from Abcam
(Cambridge, MA, USA; cat. no. ab78408). KCl and all other reagents
were acquired locally. All solutions used in the pressure myograph
system and whole-cell patch-clamp technique were prepared using
physiological saline solution (PSS). Extracellular solution was a
stock sample prepared prior to further dilution with external
solution to achieve the final concentration. The formulations of
PSS/saline solution with high kalium and the external solution were
in accordance with the literature (17).
Instruments
The following instruments were used in the present
study: Pressure myograph system (110P; Danish Myo Technology A/S,
Aarhus, Denmark), MyoVIEW software (Danish Myo Technology A/S,
version 2.0), Axon MultiClamp 700B patch-clamp amplifier (Axon;
Molecular Devices LLC, Sunnyvale, CA, USA), micromanipulator
(PCS5001; Siskiyou Design, Grants Pass, OR, USA), P-97
microelectrode pullers (Sutter Instrument Co., Novato, CA, USA),
heated water bath (HSS-1B; Chengdu Science Instrument Factory,
Chengdu, China), multiple perfusion administration system (Huazhong
University of Science and Technology, Wuhan, China) and a laser
scanning confocal microscope (Zeiss LSA 510 META; Zeiss AG,
Oberkochen, Germany).
Pressure myograph measurement
The MA was freed from surrounding fat and connective
tissues, and placed in normal PSS supplemented with the following
(in mM): NaCl, 118.9; KCl, 4.69; MgSO4x7H2O,
1.17; KH2PO4, 1.18; CaCl2, 2.5;
NaHCO3, 25; EDTA, 0.026; and glucose, 5.5 (pH 7.4;
osmolarity, 300 mOsm/l). A short segment of the vessel (with the
diameter of 2–3 mm) was attached to a bath glass microelectrode.
Nylon wire (10–0) was used to fix both ends of blood vessels to
prevent air leakage and was connected to the experimental device,
and maintained at a constant saturation of 95% O2 and a
constant temperature of 37°C, and the vascular cavity pressure was
maintained at 60 mmHg. After the sample had been equilibrated in
PSS for 30 min, the experiment was started. After addition of 60
mmol/l KCl, the vascular contraction reached a steady state, and
subsequently, portions of propofol were added to reach
concentrations of 1×10−7−3×10−4 mol/l. When
the maximum relaxation effect was reached at a high concentration
of propofol, the changes in blood vessel diameter were observed and
recorded. The diameter was continuously determined and recorded via
a video dimension analyzer and the DMT Vessel Acquisition Suite
comprising a Pressure myograph system (110P; Danish Myo Technology
A/S, Aarhus, Denmark) and MyoVIEW software version 2.0 (Danish Myo
Technology A/S). Diameter changes between contraction and
relaxation (D; in µm) were calculated via the formula D=Dx-Dp,
where Dx is the value of the vasodilation stability after propofol
administration and Dp is the diameter of the vessel in the KCl
solution. The MyoVIEW software (Danish Myo Technology A/S) was used
to control the blood vessel pressure and record the experimental
data.
Whole-cell patch-clamp recording
The arterioles were detached in a buffer solution
[NaCl, 142 nM; KCl, 5.0 nM; CaCl2, 0.05 nM;
MgCl2, 1.0 nM; Na-HEPES, 4.0 nM; HEPES, 5.0 nM; and
glucose, 7.5 nM (pH 7.3)] containing 1.0 mg/ml BSA, 0.5 mg/ml
collagenase A, 1.0 mg/ml papain and 1 mg/ml DTT for 10 min at 37°C.
After replacing the supernatant with normal extracellular solution
[in mM: NaCl, 142; KCl, 5.0; CaCl2, 0.05;
MgCl2, 1.0; Na-HEPES, 4.0; HEPES, 5.0; and glucose, 7.5
(pH 7.3)], the single MA SMCs were obtained, and the cells were
transferred to a Petri dish containing poly-L-lysine-coated
coverslips at the bottom. Samples were then incubated for 10 min at
37°C. Once the dispersed cells were attached to the surface of the
cover slips, they were mounted on an inverted microscope and
perfused with normal extracellular solution for whole-cell
recording. The specimen was continuously superfused in normal
external solution (0.2 ml/min) at room temperature (22–25°C).
Conventional whole-cell recordings were performed using an Axon
700B amplifier (Axon; Molecular Devices LLC). Recording pipettes
were fabricated from borosilicate glass capillaries and filament
with a P-97 microelectrode puller. Typically, the pipette had a tip
with an outer diameter of 1 µm and a resistance of 6–9 MΩ after
being filled with normal internal solution (NIS), which was
composed of the following (in mM): K-gluconate, 130; NaCl, 10;
CaCl2, 2.0; MgCl2, 1.2; HEPES, 10; EGTA, 5;
and glucose, 7.5; adjusted to pH 7.2 and an osmolarity of 290
mOsm/l. The pipette capacitance was well-compensated when a GΩ seal
with the cell was achieved. The membrane current or voltage signal
was low-pass filtered at 5 kHz (−3 dB). Data were recorded on a
personal computer equipped with a Digidata 1440A AD-interface and
pClamp 10.2 software (Axon; Molecular Devices LLC). A Minidigi
digitizer and Axoscope 10.2 software (Axon; Molecular Devices LLC)
were used to perform a gap-free recording at a sampling interval of
50 msec throughout the experiment.
Immunofluorescence technique
For the purpose of identifying the expression of
Cx40, Cx43 and Cx45 on the MA, immunostaining was performed. The MA
samples were randomly assigned to two groups, namely the
experimental and control groups. After harvesting, the MAs were
placed in 4% paraformaldehyde for 2 h at room temperature. After
rinsing thrice with PBS, the MAs were incubated in immunostaining
blocking liquid (5% BSA) for 1 h. Subsequently, the MA was rinsed
thrice with PBS and incubated with 5% BSA for 1 h at room
temperature. Each sample was then rinsed with PBS and treated with
1:200 dilutions of anti-Cx40, anti-Cx43 and anti-Cx45 and incubated
for 1 h at room temperature, separately. Finally, each sample was
placed in a wet box at 4°C for 12 h for maintenance. Subsequently,
the samples were brought to 37°C over 1 h. The MAs were transferred
to Eppendorf tubes and rinsed thrice in PBS, and any remaining
liquid was absorbed with filter paper strips. The samples were
treated with the secondary antibody (fluorescein isothiocyanate
conjugated goat anti-rabbit immunoglobulin G; 1:200 dilution;
OriGene Technologies, Inc., Rockville, MD, USA; cat. no. ZF-0311)
and then placed in a wet box for 1 h of incubation. Each sample was
rinsed for 20 sec prior to staining with 1:200 PI and incubated for
20 sec at room temperature. Finally, each MA was rinsed thrice with
PBS again, and was then transferred onto a microscope slide. The
extra PBS was absorbed with filter papers before the slide was
sealed with 85% glycerinum for fluorescence quenching. The
fluorescence was observed and recorded with a laser scanning
confocal microscope. The MA in the control group underwent the same
treatment, apart from the anti-Cx40, anti-Cx43 and anti-Cx45
antibody being replaced by PBS.
Statistical analysis
Values are expressed as the mean ± standard error.
Statistical analysis was performed using the SPSS statistical
software package, version 17.0 (SPSS Inc., Chicago, IL, USA). A
homogeneity test for variance was performed, followed by one-way
analysis of variance, and comparisons between two groups were
assessed using the paired t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
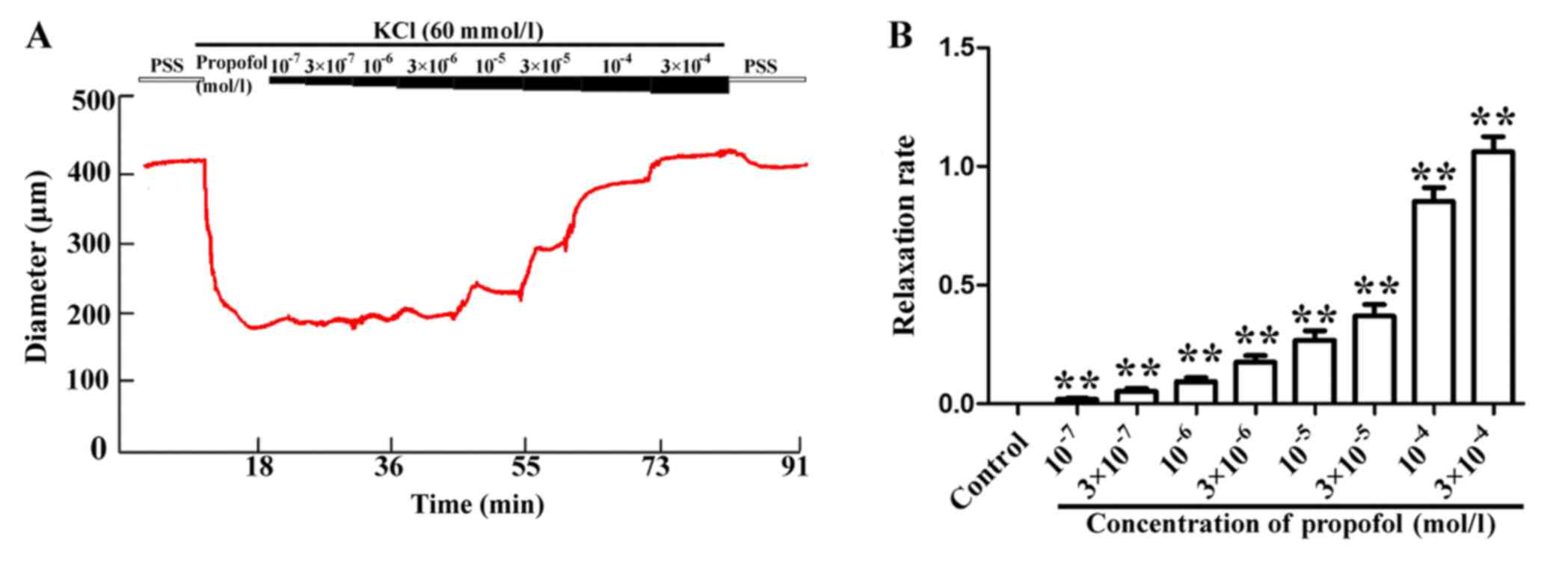
Propofol relaxes rat MAs in a
concentration-dependent manner
After the diameter of the MAs was stable in the
presence of 60 mmol/l KCl, the isolated vasculature exhibited a
concentration-induced response, namely a gradual increase of the
diameter when propofol was added to reach final concentrations of
1×10−7, 3×10−7, 1×10−6,
3×10−6, 1×10−5, 3×10−5,
1×10−4 and 3×10−4 mol/l (Fig. 1A). The respective increase in MA
diameter was by 4.01±0.10, 10.06±2.45, 17.81±3.06, 32.67±4.79,
49.43±6.93, 75.71±8.24, 161.24±11.43 and 195.88±11.04 µm. The
relaxation rate of the MAs is presented in Fig. 1B, which also indicated that propofol
caused concentration-dependent increases in the relaxation of MAs.
All of the results indicated that propofol increased the vascular
diameter in a concentration-dependent manner.
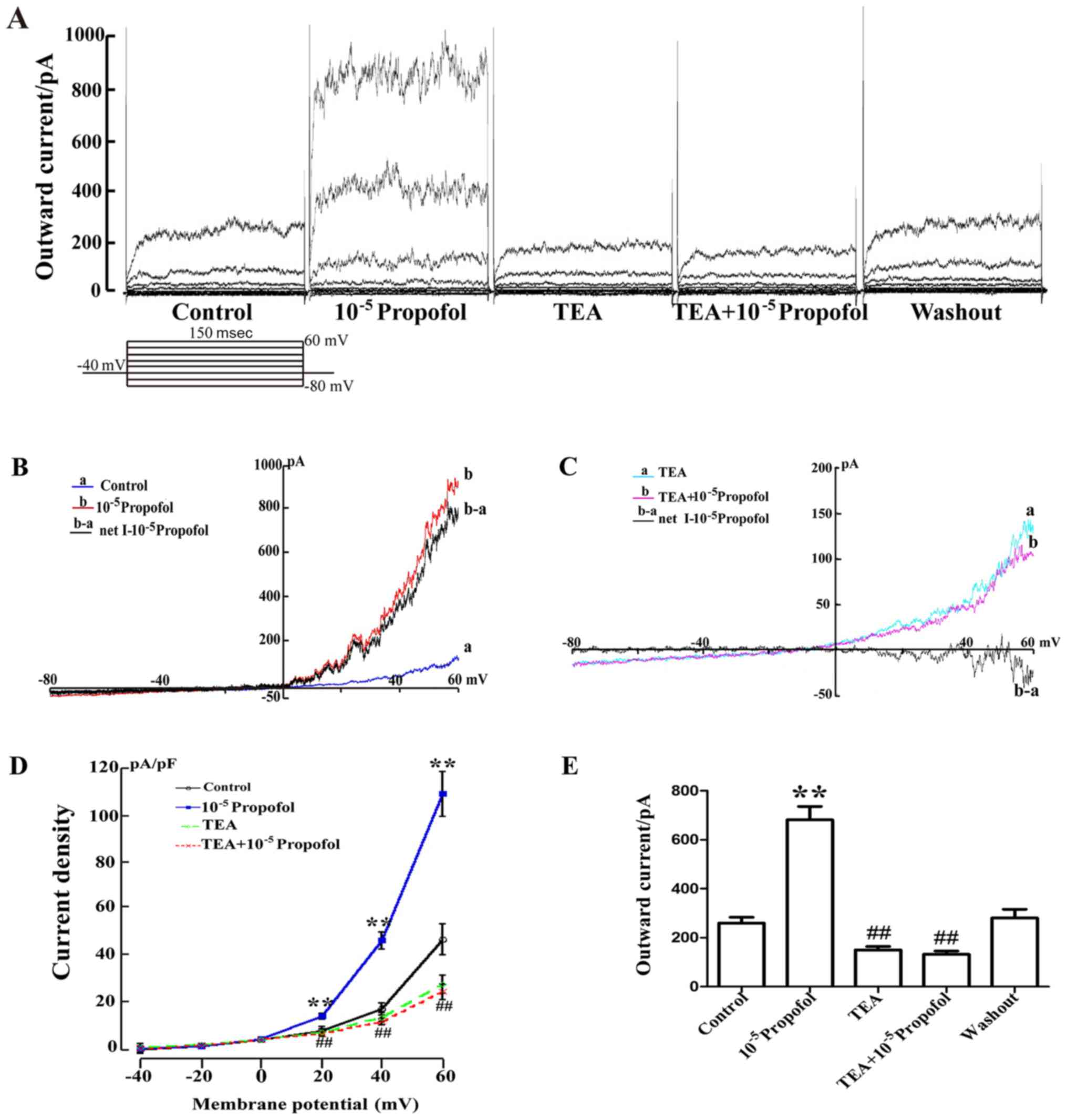
Propofol enhances the outward current
of VSMCs from MAs in a concentration-dependent manner
Whole-cell voltage-clamp experiments were performed
on dispersed VSMCs obtained from MAs. Application of different
concentrations of propofol increased the outward current induced by
voltage steps from the holding potential (HP) of −40 mV in the
VSMCs (Fig. 2A). The results in
Fig. 2B indicated that the
whole-cell current/voltage (I/V) curve slope, following stimulus
(−80 to 60 mV), was increased in the entire voltage range, and the
3×10−5 mol/l propofol-induced net current exhibited a
significant enhancement. The cells displayed a
concentration-dependent response after treatment with
1×10−7, 3×10−7, 1×10−6,
3×10−6, 1×10−5, 3×10−5,
1×10−4 and 3×10−4 mol/l propofol. The outward
current was increased from 185.33±33.32 pA (when cells were stable)
to 247.72±37.54, 325.76±35.12, 454.90±29.23, 628.28±64.68,
975.39±56.06 and 1,451.9±31.67 pA, respectively (Fig. 2C). These data suggest that the
outward current was enhanced by propofol.
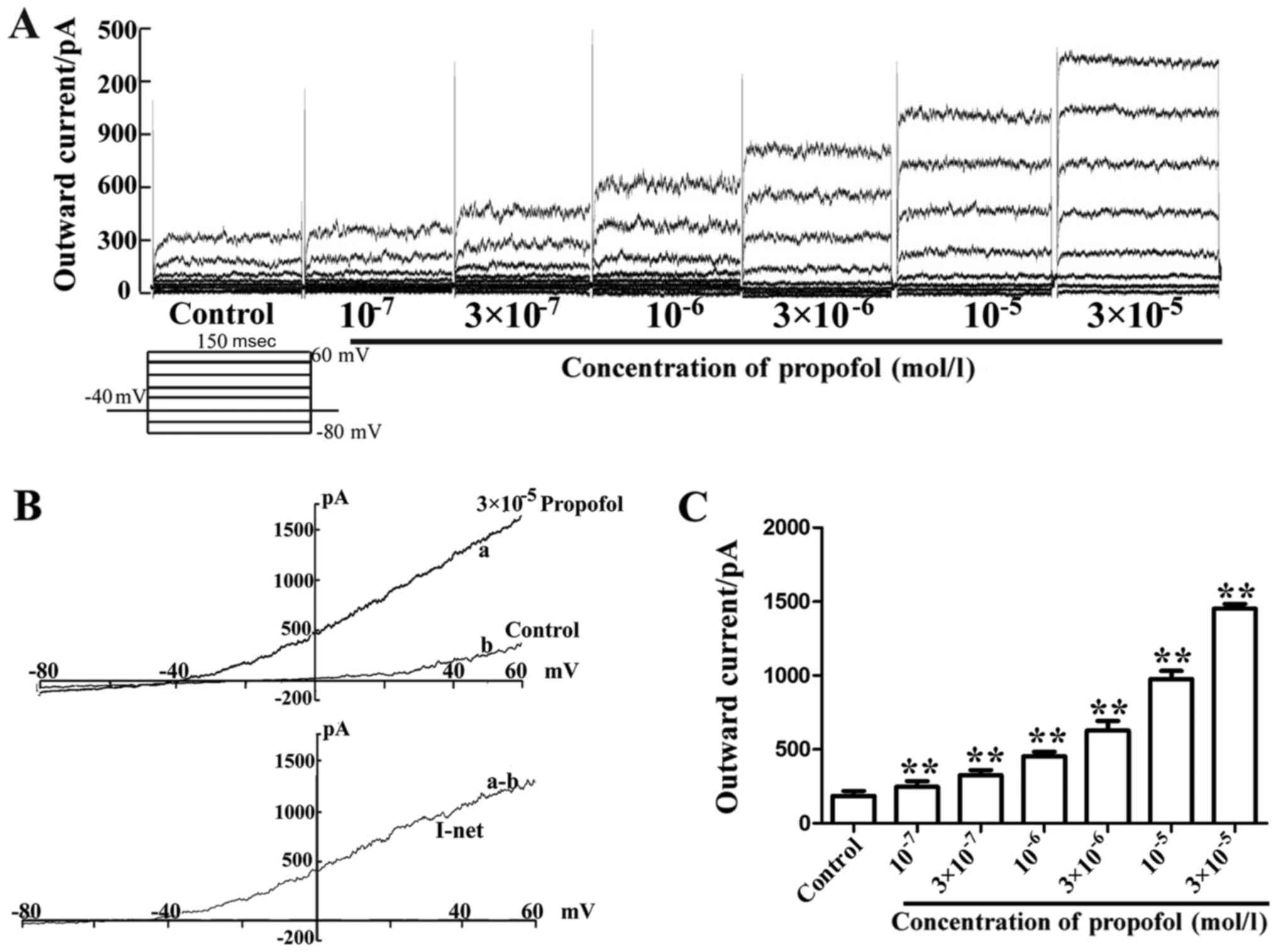 | Figure 2.Propofol enhances the outward current
of vascular smooth muscle cells in MAs in a concentration-dependent
manner. (A) Whole-cell current traces induced by voltage steps
prior to and during application of 1×10−7,
3×10−7, 1×10−6, 3×10−6,
1×10−5 and 3×10−5 mol/l propofol. (B)
Whole-cell I/V curves in the absence and presence of
3×10−5 mol/l propofol in cells of MA (top panel) and the
respective propofol-sensitive net current I/V curves (lower
panels). Note that propofol caused an increase of the slope
conductance of single cells. (a) Application of 3×10−5
mol/l propofol; (b) ramp trace in the absence of 3×10−5
mol/l propofol; (a-b) net current induced by 3×10−5
mol/l propofol. (C) Mean outward current induced by
1×10−7, 3×10−7, 1×10−6,
3×10−6, 1×10−5 and 3×10−5 mol/l
propofol in comparison to the control. **P<0.01 vs. control
(n=6). MA, mesenteric arteriole; I/V, current/voltage. |
Propofol-induced increases in the
outward current are blocked by TEA
The cells were maintained at −40 mV and then
subjected to a series of test potentials ranging from −80 to +60 mV
(At a holding potential of −40 mV, the stimulation voltage was
increased from −80 to 60 mV with a 20 mV a ladder; Fig. 3). Taking the data obtained at +60 mV
as an example, the outward current was initially 259.89±24.11 pA,
and after addition of 1×10-5 mol/l propofol, the outward current
was enhanced to 727.11±39.95 pA. However, supplementation with 1
mmol/l TEA (BKCa channel blocker) significantly reduced
the outward current to 150.14±14.43 pA, while the inhibition was
recovered to 280.78±35.86 pA after washing with drug-free normal
external solution (Fig. 3A and E).
The data obtained from 6 similar experiments focusing on the effect
of TEA on the mean current density are summarized in Fig. 3D. The results demonstrated that TEA
reduced the current density from 109.23±9.65 to 25.66±3.91. In
addition, the whole-cell I/V curve slope exhibited an increasing
trend in the entire voltage range and the 1×10−5 mol/l
propofol-induced net current was significantly enhanced (Fig. 3B). Furthermore, as displayed in
Fig. 3C, it was indicated that after
perfusion with 1 mmol/l TEA, no significant increase in the current
was obtained following addition of 1×10−5 mol/l
propofol. All of the results indicated that propofol enhanced the
outward current, which was mediated via the BKCa
channel.
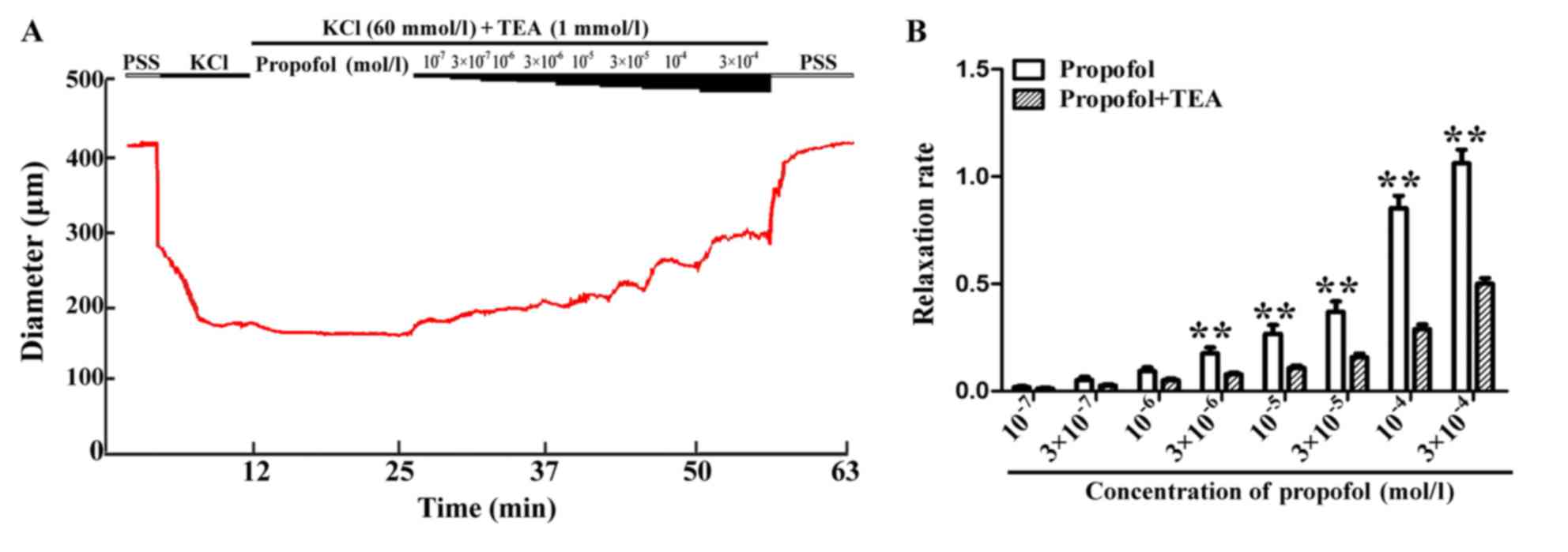
Inhibitory effect of TEA on the
relaxation elicited by propofol
The vessel diameter was in a stable, constricted
state in the presence of 60 mmol/l KCl, and the increases in
diameter obtained by addition of propofol (1×10−7,
3×10−7, 1×10−6, 3×10−6,
1×10−5, 3×10−5, 1×10−4 and
3×10−4 mol/l) were significantly inhibited by
pre-treatment with 1 mmol/l TEA (a BKCa inhibitor) for
20 min, resulting in increases in diameter by 2.45±0.90, 5.28±1.17,
10.46±1.46, 15.95±1.55, 21.93±1.96, 31.98±3.10, 59.17±4.45 and
102.85±5.91 µm, respectively. Furthermore, the vasodilator effect
of propofol attenuated significantly in the presence of TEA
(Fig. 4B). As indicated in Fig. 4B, the propofol-induced increase in
diameter/vessel relaxation was inhibited in the presence of TEA. In
addition, in the absence and presence of the BKCa
inhibitor TEA, a significant difference was obtained with different
concentrations of propofol, indicating a regulatory role for
BKCa in vascular vasodilation induced by propofol.
However, propofol induced vasodilatation is not entirely mediated
by BKca.
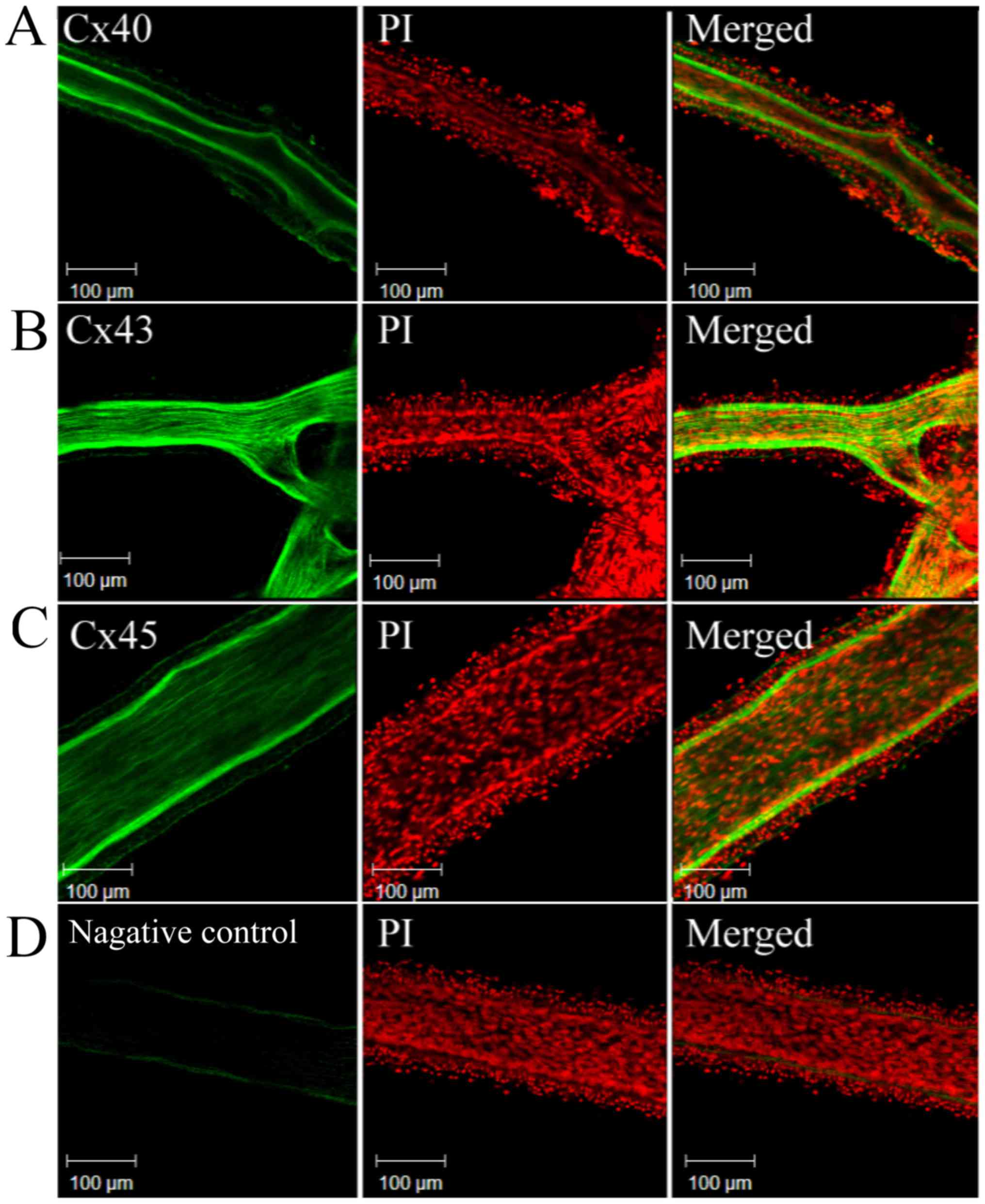
MAs possess gap junctions
Immunofluorescence microscopy was used to observe
the expression of Cx in the MAs. The Mas were labelled with
polyclonal antibody to Cx40, Cx43 or Cx45, and the nuclei were
stained with PI. The results indicated that the MAs contained Cx40,
Cx43 and Cx45 on their inner surface, indicating the presence of
gap junctions (Fig. 5).
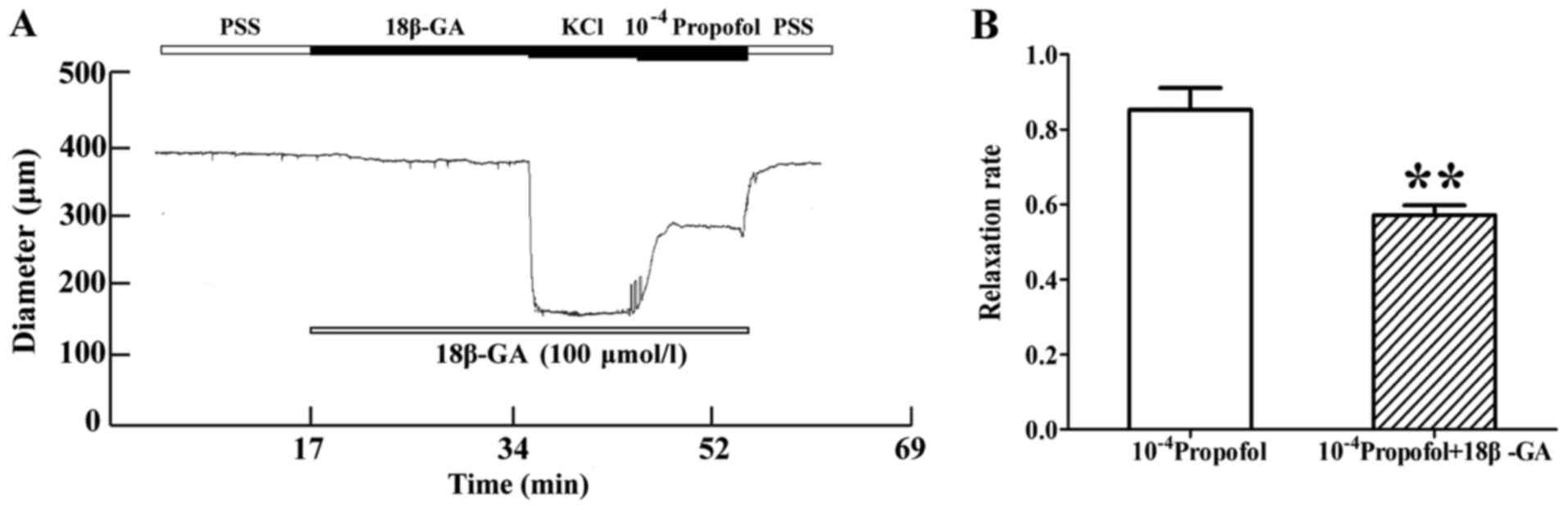
Inhibitory effect of 18β-GA on the
vasorelaxation induced by propofol
The MA was pre-treated with gap junction inhibitor
18β-GA (100 µmmol/l) for 20 min, and 1×10−4 mol/l
propofol was added after stable constriction of the MA with 60
mmol/l KCl. In the presence of 18β-GA, the vasodilation effect of
propofol was decreased. The increase in diameter induced by
1×10−4 mol/l propofol was 161.24±11.43 and 130.63±8.80
µm in the absence and presence of 18β-GA, respectively. 18β-GA
reduced the diameter increment induced by 1×10−4 mol/l
propofol by 34.03±3.43 µm, and its inhibition rate was 26.19±3.61%
(P<0.01, n=8; Fig. 6A and B). The
diastolic diameter was not marked in the picture; however, the
relaxation rate of blood vessels was measured in Figs. 6B and 7B. From the results of relaxation rate, it
was determined that vasodilatation was weakened.
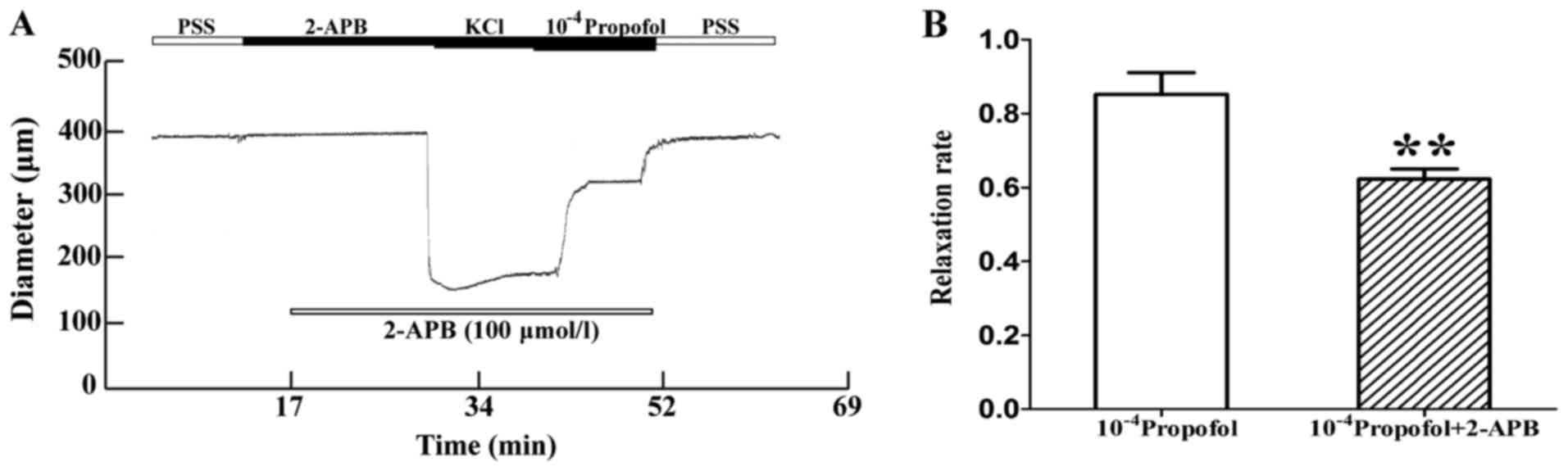
Inhibitory effect of 2-APB on the
relaxation induced by propofol
In another experiment, the MAs were pre-treated with
the gap junction inhibitor 2-APB (100 µmmol/l) for 20 min, and
after stable vasoconstriction was achieved with 60 mmol/l KCl,
1×10−4 mol/l propofol was added. The vasodilation effect
of propofol was decreased in the presence of 2-APB. The increase in
diameter induced by 1×10−4 mol/l propofol was
161.24±11.43 and 143.15±4.69 µm, respectively, in the absence and
presence of 2-APB. 2-APB reduced the diameter increment induced by
1×10−4 mol/l propofol by 19.16±3.67 µm, and its
inhibition rate was 20.52±4.54% (P<0.01, n=8; Fig. 7A and B).
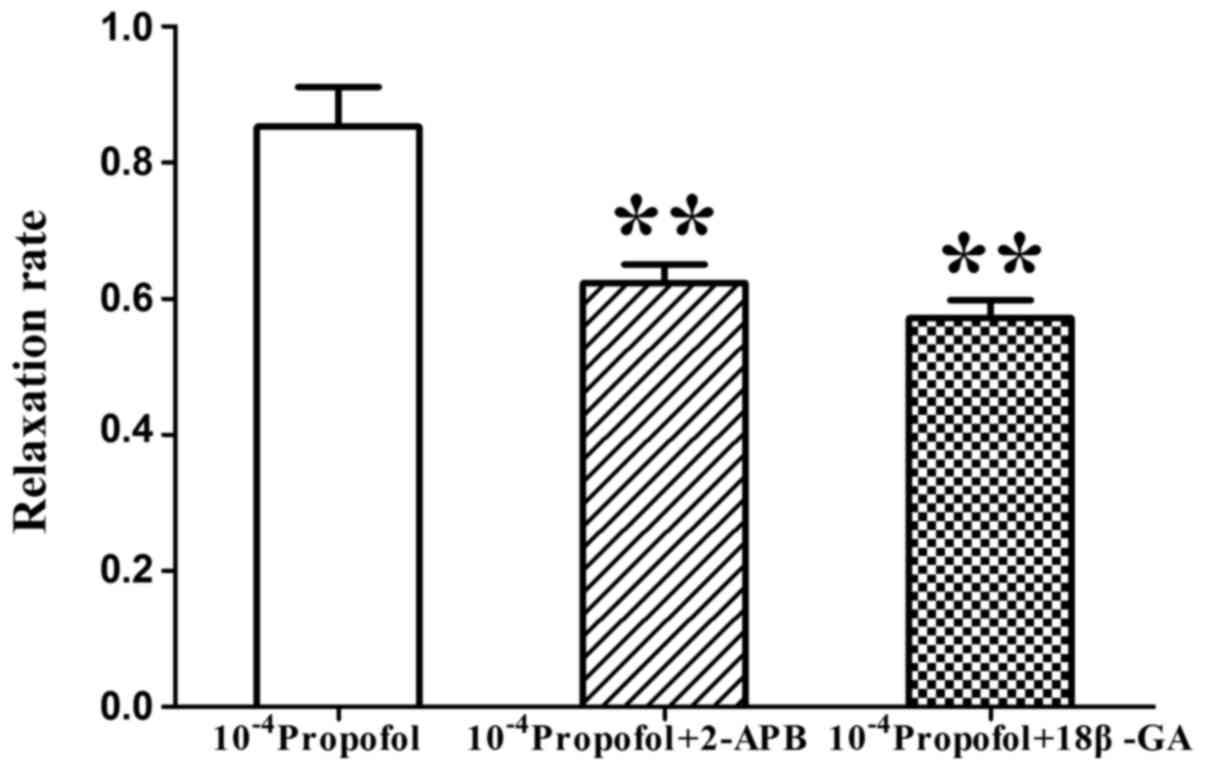
Comparison between the inhibitory
effects of 18β-GA and 2-APB on the relaxation induced by
propofol
Next, the vasodilation response to 1×10−4
mol/l propofol in the presence of 18β-GA or 2-APB was compared. The
relaxation rate of MAs induced by 1×10−4 mol/l propofol
was 85.26±5.83%, but the relaxation rate was reduced to 57.73±2.69
and 62.27±2.73%, respectively, in the presence of 18β-GA and 2-APB.
The relaxation induced by propofol was inhibited by 18β-GA and
2-APB, but no significant difference between the inhibitory effect
of 18β-GA and 2-APB was identified (P<0.01, n=8; Fig. 8).
Discussion
Propofol is widely applied in the clinic as an
intravenous anesthetic; however, it frequently causes hypotension
at the time of induction of anesthesia (20), particularly in elderly and
hypertension patients, whose cardiac cycle fluctuations are more
obvious, and which may contribute to various conditions of the
cardiovascular system, including arrhythmias, myocardial ischemia
and myocardial infarction (21). The
blood pressure in the circulatory system is dependent on blood
volume, cardiac ejection and peripheral resistance. The peripheral
resistance of the circulatory system is a prerequisite for the
generation of blood pressure, and this resistance refers to
micro/small arterial resistance to blood flow. It has been
suggested that the increased peripheral resistance often increases
the diastolic and systolic blood pressure (22). The blood flow and vascular resistance
are inversely proportional to the fourth power of the radius
(23), which mainly depends on the
caliber of blood vessels that may be modified by VSM contraction.
Therefore, the present study aimed to investigate these types of
blood vessel based on an experiment in separated 2–3 mm segments
from MAs, which may be categorized as small arteries, are
resistance vessels and also have a role in blood pressure
regulation.
The principal findings of the present study may be
summarized as follows: i) Propofol relaxes the MA in a
concentration-dependent manner; ii) propofol enhances the outward
current of VSMCs; iii) propofol enhances the outward current
mediated via the BKCa channel, as the propofol-induced
increases in the outward current and relaxation of MAs were blocked
by the BKCa channel inhibitor TEA; iv) MAs contain gap
junctions; v) propofol relaxes the vasculature via gap junctions,
as the as the propofol-induced relaxation of MAs were blocked by
two different gap junction inhibitors. These results suggest that
the relaxation effect of propofol on MAs may be mediated via
BKCa channels and gap junctions.
The experimental design of the present study was
divided into two parts. In the first part, the MAs of experimental
SD rats were used as the experimental models. The pressure myograph
technique was applied to examine the effect of different
concentrations of propofol on the relaxation of blood vessels, and
the whole-cell patch clamp technique was employed to observe the
outward currents of VSMCs induced by different concentrations of
propofol. The experimental results obtained from the pressure
myograph technique revealed that propofol was capable of relaxing
MAs, and the whole-cell patch clamp assay indicated that propofol
enhanced the outward current of VSMCs. To further investigate the
mechanisms of propofol-induced vasodilation and the increased
outward current and identify the channels involved, various channel
inhibitors were applied. The effect of propofol on the relaxation
of blood vessels was decreased after treatment with TEA (a
BKCa channel blocker), as indicated in the pressure
myograph experiment, and the whole-cell patch clamp assay also
demonstrated that pre-treatment with TEA inhibits the increase of
the outward current induced by propofol. According to these
experimental results, it may be concluded that the relaxation
effect of propofol on the MA may exerted via enhancement of the
BKCa current. The whole-cell patch clamp assay is a
technique that is only performed on single SMCs, but in theory, the
propofol-induced activation of the BKCa channel may lead
to transfer of the hyperpolarization information, which further
results in the fast and synchronized relaxation of the MA.
Due to the long artery span and alterations in blood
flow intensity, regulation of the blood pressure by the
microcirculation is required along the full length of the blood
vessels. In order to achieve this equilibration, the cells in the
blood vessels form a coordinated response (24), i.e. they constitute a network of
coupled cells to facilitate a coordinated response. Gap junctions
provide a pathway for the formation of intercellular junctions,
which have a key role in cell communication and conduction of
vasodilation (19).
The possible implication of gap junctions in the
vasodilation effect of propofol was then investigated. In this
second part of the present study, the expression of Cx40, Cx43 and
Cx45 was verified by immunofluorescence microscopy, which was
consistent with the results of previous studies (25,26), and
indicated that gap junctions were present on MAs. In addition, it
was demonstrated that pre-treatment with gap junction blockers,
namely 18β-GA and 2-APB, dampened the relaxation effect of
propofol, indicating that gap junction communication has a role in
propofol-induced vasodilation.
Klockgether-Radke et al (27) suggested that activation of the
BKCa channel may contribute to the vasodilating effect
of propofol on coronary arteries, and Sinha et al (28) indicated that propofol-induced
vasodilation is mediated by transient receptor potential A1 ion
channels and includes the activation BKCa channels.
These studies provide compelling evidence that BKCa
channels are important effectors in mediating VSM hyperpolarization
and relaxation of numerous vessel types. Hyperpolarization is a
highly efficient means of synchronizing cells, as it may exert an
electric strain along a variety of cells that are coupled to each
other. In addition, hyperpolarization has an important role in
coordinating the behavior of the entire vasculature. The activation
of BKCa and K+ efflux leads to cell membrane
hyperpolarization, which contributes to the closure of
voltage-dependent Ca2+ channels to block the influx of
extracellular Ca2+ and thereby induce vasorelaxation
(29,30). The membrane potential is one of the
major factors that regulate the contractile activity of SMCs. Since
the coordination of contraction or dilatation of SMCs is required
to exert full control over the local circulation, synchronous
changes in membrane potential in regions of neighboring SMCs are
indispensable (24). Due to the low
impedance of gap junctions and the high electrical conductivity,
cells tend to transform into syncytium. The gap junction provides a
good platform for the rapid conduction of hyperpolarization along
the blood vessels. Furthermore, the hyperpolarization mediated by
gap junctions is able to ensure the synchronous change in membrane
potential. The flow of K+ may result in the
hyperpolarization of the membrane. Activation of the
BKCa channel may cause membrane hyperpolarization, which
leads to a corresponding hyperpolarization of the cell membrane
potential due to the electrical communication between the gap
junctions (31). Therefore,
propofol-induced activation of the BKCa channel causes
hyperpolarization, which may further affect the SMC potential via
gap junction communication, and it is well recognized as a
potential mechanism of vascular relaxation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81560175 and
81260159) and the High Level Talent Research Project of Shihezi
University (grant no. RCSX201705).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HJW participated in designing and performing the
experiments, analyzed the data, and wrote and revised the article.
YW assisted in the experimental process, designed the
immunofluorescence experiment, and contributed in data analysis and
writing and revising the article. JQS participated in the
conceptual design of the experiments and provided funding for
research projects. LL participated in the study and design
experimental design, assisted in performing the experiments, and
provided funding for research projects.
Ethical approval and consent to
participate
The use of animals was approved by the Ethical
Inspection of the First Affiliated Hospital, Shihezi University
School of Medicine (Shihexi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gragasin FS and Davidge ST: The effects of
propofol on vascular function in mesenteric arteries of the aging
rat. Am J Physiol Heart Circ Physiol. 297:H466–H474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu XR, Tan XQ, Yang Y, Zeng XR and Tang
XL: Propofol increases the Ca2+ sensitivity of BKCa in the cerebral
arterial smooth muscle cells of mice. Acta Pharmacol Sin. 33:19–26.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Au AK and Matthew Fields J: Ultrasound
measurement of inferior vena cava collapse predicts propofol
induced hypotension. Am J Emerg Med. 35:508–509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakai Y, Kawahito S, Takaishi K, Mita N,
Kinoshita H, Hatakeyama N, Azma T, Nakaya Y and Kitahata H:
Propofol-induced relaxation of rat aorta is altered by aging. J Med
Invest. 61:278–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lam CF, Chang PJ, Chen YA, Yeh CY and Tsai
YC: Inhibition of ATP-sensitive potassium channels attenuates
propofol-induced vasorelaxation. Crit Care Resusc. 12:186–190.
2010.PubMed/NCBI
|
|
6
|
Zhang G, Cui J, Chen Y and Ma J: The
relaxant effect of propofol on isolated rat intrapulmonary
arteries. Korean J Physiol Pharmacol. 18:377–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folino T and Parks L: Propofol.
StatPearls. StatPearls Publishing StatPearls Publishing LLC,
Treasure Island (FL). 2017.
|
|
8
|
Wang Y, Zhou H, Wu B, Zhou Q, Cui D and
Wang L: Protein Kinase C isoforms distinctly regulate
Propofol-induced Endothelium-dependent and Endothelium-independent
vasodilation. J Cardiovasc Pharmacol. 66:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hill MA, Yang Y, Ella SR, Davis MJ and
Braun AP: Large conductance, Ca2+-activated K+ channels (BKCa) and
arteriolar myogenic signaling. FEBS Lett. 584:2033–2042. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan
XQ, Liu ZF and Zeng XR: Function of BKCa channels is reduced in
human vascular smooth muscle cells from Han Chinese patients with
hypertension. Hypertension. 61:519–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brayden JE and Nelson MT: Regulation of
arterial tone by activation of calcium-dependent potassium
channels. Science. 256:532–535. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kohler R, Kaistha BP and Wulff H: Vascular
KCa-channels as therapeutic targets in hypertension and restenosis
disease. Expert Opin Ther Targets. 14:143–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tykocki NR, Boerman EM and Jackson WF:
Smooth muscle ion channels and regulation of vascular tone in
resistance arteries and arterioles. Compr Physiol. 7:485–581. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tran CH, Vigmond EJ, Goldman D, Plane F
and Welsh DG: Electrical communication in branching arterial
networks. Am J Physiol Heart Circ Physiol. 303:H680–H692. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ampey BC, Morschauser TJ, Lampe PD and
Magness RR: Gap junction regulation of vascular tone: Implications
of modulatory intercellular communication during gestation. Adv Exp
Med Biol. 814:117–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bou Saab J, Losa D, Chanson M and Ruez R:
Connexins in respiratory and gastrointestinal mucosal immunity.
FEBS Lett. 588:1288–1296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma KT, Guan BC, Yang YQ, Nuttall AL and
Jiang ZG: 2-Aminoethoxydiphenyl borate blocks electrical coupling
and inhibits voltage-gated K+ channels in guinea pig arteriole
cells. Am J Physiol Heart Circ Physiol. 300:H335–H346. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Figueroa XF, Lillo MA, Gaete PS, Riquelme
MA and Sáez JC: Diffusion of nitric oxide across cell membranes of
the vascular wall requires specific connexin-based channels.
Neuropharmacology. 75:471–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Triggle CR, Samuel SM, Ravishankar S,
Marei I, Arunachalam G and Ding H: The endothelium: Influencing
vascular smooth muscle in many ways. Can J Physiol Pharmacol.
90:713–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ilyas M, Butt MFU, Bilal M, Mahmood K,
Khaqan A and Ali Riaz R: A review of modern control strategies for
clinical evaluation of propofol anesthesia administration employing
hypnosis level regulation. Biomed Res Int. 2017:74323102017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Kong AL, Chen R, Qian C, Liu SW,
Sun BG, Wang LX, Song LS and Hong J: Propofol and arrhythmias: Two
sides of the coin. Acta Pharmacol Sin. 32:817–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall JE and Guyton AC: The
microcirculation and the lymphatic system: Capillary fluid
exchange, interstitial fluid, and lymph flowGuyton and Hall
Textbook of Medical Physiology. 13th. Elsevier; Philadelphia, PA:
pp. 182–185. 2015
|
|
23
|
Hall JE and Guyton AC: Vascular
distensibility and functions of the arterial and venous
systemsGuyton and Hall Textbook of Medical Physiology. 13th.
Elsevier; Philadelphia, PA: pp. 171–175. 2015
|
|
24
|
Yamamoto Y, Klemm MF, Edwards FR and
Suzuki H: Intercellular electrical communication among smooth
muscle and endothelial cells in guinea-pig mesenteric arterioles. J
Physiol. 535:181–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brink PR, Ricotta J and Christ GJ:
Biophysical characteristics of gap junctions in vascular wall
cells: Implications for vascular biology and disease. Braz J Med
Biol Res. 33:415–422. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haefliger JA, Nicod P and Meda P:
Contribution of connexins to the function of the vascular wall.
Cardiovasc Res. 62:345–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klockgether-Radke AP, Schulze H, Neumann P
and Hellige G: Activation of the K+ channel BK(Ca) is involved in
the relaxing effect of propofol on coronary arteries. Eur J
Anaesthesiol. 21:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sinha S, Sinharoy P, Bratz IN and Damron
DS: Propofol causes vasodilation in vivo via TRPA1 ion channels:
Role of nitric oxide and BKCa channels. PLoS One. 10:e01221892015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ledoux J, Werner ME, Brayden JE and Nelson
MT: Calcium-activated potassium channels and the regulation of
vascular tone. Physiology (Bethesda). 21:69–78. 2006.PubMed/NCBI
|
|
30
|
Hu XQ and Zhang L: Function and regulation
of large conductance Ca(2+)-activated K+ channel in vascular smooth
muscle cells. Drug Discov Today. 17:974–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allen T, Iftinca M, Cole WC and Plane F:
Smooth muscle membrane potential modulates endothelium-dependent
relaxation of rat basilar artery via myo-endothelial gap junctions.
J Physiol. 545:975–986. 2002. View Article : Google Scholar : PubMed/NCBI
|