Introduction
At present, the neurotoxicity of local anesthetics
is receiving an increasing amount of attention (1), and therefore, it is particularly
important to identify safe methods of prevention and treatment. One
pharmacological strategy against the neurotoxicity of local
anesthetics is the use of drugs to induce autophagy to treat the
associated diseases (2,3). Studies have shown that certain drugs
can directly or indirectly activate autophagy to prevent cell death
(4,5). Based on these observations, it has been
hypothesized that the regulation of autophagy could serve as the
basis for the development of candidate neuroprotective agents
against neurotoxicity caused by local anesthetics. However, the
components of the autophagy signaling pathway remains at an initial
stage (6), therefore, the major aims
of the current study were to explore drugs for the prevention and
treatment of nerve injury caused by local anesthetics via the
autophagy signaling pathway.
In previous years, polysaccharides have been
investigated in numerous research studies and serve roles in
controlling cell division and differentiation, regulate cell growth
and aging, and aid in maintaining the normal metabolism of an
organism (7–9). Numerous studies have indicated that
polysaccharides can activate autophagy via a
non-serine/threonine-protein kinase mTOR pathway and serve a
protective role in glomerular lesions and neurodegenerative
diseases (10,11). It was also thought that
polysaccharides, as natural products, were more safe. Therefore,
polysaccharides have attracted increasing research attention
(12). Aphanizomenon
flos-aquae, a filamentous, heterocystous and dominant
cyanobacterium, is common in nutrient-rich freshwater
cyanobacterial blooms (13). During
the process of growth until death, A. flos-aquae
continuously secretes extracellular polymeric substances (EPS) into
the surrounding environment (13).
EPS are high molecular weight polysaccharides consisting of three
or four different monosaccharides arranged in groups of £10 to form
repeating units (14). Numerous
studies have indicated that EPS exhibit numerous bioactivities,
including antitumor, immune-adjusting, antithrombotic, ameliorating
endothelial cell injury, antioxidant and antiviral (14,15).
Although several studies on the biological activities of EPS have
been performed, with a particular focus on their anti-apoptotic
activity (16–18), little is known about their
neuroprotective effects in vivo. Therefore, in the present
study, rats received intraperitoneal injections of EPS from A.
flos-aquae (EPS-A), and apoptosis and autophagy were observed
in spinal cord neurons. The aim was to investigate the protective
effects of EPS-A on neuronal injury and the possible underlying
mechanisms.
Materials and methods
EPS-A
A strain of A.flos-aquae, isolated from Lake
Dianchi in China, was obtained from the Freshwater Algae Culture
Collection of the Institute of Hydrobiology, Chinese Academy of
Sciences in Wuhan city of China. The culture of A.
flos-aquae was performed according to the methods of Zhang
et al (19), and extraction
and purification of EPS-A were performed as previously described by
Hu et al (20).
Animal selection and housing
The present in vivo study was approved by the
Medical Ethics Committee of the First Hospital of Lanzhou
University (Lanzhou, China). A total of 18 healthy adult male
Sprague-Dawley rats (age, 5–7 weeks; weight, 180–220 g), were
supplied by the Animal Breeding and Research Center of Lanzhou
University (Lanzhou City, China). Rats were housed in separate
cages with food and water freely available until the time of
testing and kept in temperature-controlled rooms (20–24°C; relative
humidity, 50–60%) with a 12-h light/dark cycle (6:00 a.m.-6:00
p.m.).
Groups and treatment
A total of 18 rats were randomly divided into groups
A, B and C (n=6/group). In groups A and B, rats received
intraperitoneal injections of 1 ml 0.9% NaCl, whole group C
received an intraperitoneal injection of EPS-A (100 mg/kg), once a
day for 3 days. Subsequently, the group A rats were subjected to 2%
isoflurane inhalation and intrathecal injection of 0.9% NaCl 50 µl.
Groups B and C received 2% isoflurane inhalation and intrathecal
injection of 1% bupivacaine 50 µl (2.5 mg/kg animal body
weight).
Bupivacaine in lumbar anesthesia
When optimal flexion of the rat lumbar spine was
achieved in a prone position, a 27-gauge needle attached to a 100
µl syringe (model, KL-34; Hamilton Medical, Inc., Reno, NV, USA)
was inserted into the midline of the lumbar 4–5 (L4-5)
intervertebral space and 50 µl of the respective drug was injected.
While a tail-flick indicated entrance into the intrathecal space,
rats were subsequently observed for paralysis of the hind limbs,
indicative of a spinal blockade. During the selection process, rats
were excluded if they lacked a healthy appearance or required more
than one spinal puncture. A total of 6 rats were included in each
group following this.
Spinal cord section specimen
Rats in each group were sacrificed at 6 h following
the aforementioned anaesthesia and the spinal cord was rapidly
collected, then sections were transported in fixative (10% neutral
buffered formalin) at room temperature for 36–48 h. A section of
tissue was frozen in liquid nitrogen and stored at −70°C until
further use.
Hematoxylin & eosin (H&E)
staining
H&E staining is a standard method used for
detecting morphological alterations. First, the spinal cord
sections were sliced into 5 µm sections using a microtomer,
deparaffinized at 40°C in a water bath and rehydrated. Samples were
then washed with distilled water and dried. Hematoxylin was added
for 5 min and rinsed with water. Subsequently 1% HCl ethanol
solution (1 ml HCl added to 99 ml 70% ethanol) was added for 10 sec
in triplicate to remove excess haematoxylin. Following this,
sections were washed using distilled water for 25 min, 0.5% eosin
was added for 2 min and slices were dehydrated with 95 and 100%
ethanol. Dimethylbenzene (Absin Bioscience, Inc., Shanghai, China)
was added for 5 min twice and incubated at 37°C for 24 h. Finally,
sections were kept in a special slide container at room temperature
and observed under a light microscope.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL)
TUNEL staining was performed to examine apoptosis.
The spinal cord samples were fixed in 10% neutralized formalin at
room temperature for 20 min and embedded in paraffin. Sections were
deparaffinized, rehydrated and incubated for 15 min at 37°C with
proteinase K working solution (Shanghai Xiangsheng biotechnology
Co., Ltd., Shanghai, China). Sections were rinsed twice with PBS
(pH 7.4), blocked with hydrogen peroxide for 15 min, and 50 µl
TUNEL reaction mixture was added and incubated for 60 min at 37°C
in a humidified atmosphere in the dark. Following rinsing with PBS
(pH 7.4) three times, sections were incubated for 30 min at 37°C
with 50 µl converter peroxidase (Shanghai Xiangsheng biotechnology
Co., Ltd.). A total of 50 µl diaminobenzidine [5 µl 20×DAB, 1 µl
30% H2O2, 94 µl PBS (pH 7.4)] was added and
incubated for 10 min at 25°C. Sections were rinsed with PBS (pH
7.4) three times and cover slips were mounted onto 50% glycerol
treated slides. Samples were analyzed under a light microscope
(magnification, ×400). Slices were photographed continuously and
six visual fields were randomly selected under an optical
microscope. Pictures were numbered and random fields were obtained
using random numbers generated by a computer. The final value of a
slice was obtained by a mean value from random high-power fields.
The optical density was quantified using Image-Pro Plus (version
7.0; Media Cybernetics, Inc., Rockville, MD, USA).
Immunohistochemical (IHC)
staining
IHC is widely used in research to understand the
distribution and localization of biomarkers and differentially
expressed proteins in different parts of cells or tissues. In the
present study, spinal cord samples were replaced with ethanol in
decreasing concentrations (100, 90 and 70%), washed with PBS (pH
7.4) three times for 5 min each and heated in a water bath
(95–98°C) in 0.01 M trisodium citrate (pH 6.0) for 15 min for
antigen retrieval. Subsequently, the sections were incubated with a
blocking reagent (3% milk and 5% fetal bovine serum; Absin
Bioscience, Inc.) for 1 h at room temperature and further incubated
with anti-caspase-3 (cat. no. ab20816; dilution 1:500; Abcam,
Cambridge, UK), anti-microtubule-associated protein 1A light chain
3 (LC3) polyclonal rabbit antibody (cat. no. L8918; dilution 1:200;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or anti-beclin1
(cat. no. SAB2103299; dilution 1:500; Sigma-Aldrich; Merck KGaA) at
4°C overnight. Following washing with PBS (pH 7.4), the tissue
samples were exposed to biotinylated anti-rabbit immunoglobulin G
(cat. no. A0239; dilution 1:1,000; Beyotime Institute of
Biotechnology, Fuzhou, China) and horseradish peroxidase conjugated
streptavidin (Vector Laboratories, Inc., Burlingame, CA, USA).
After the slides were sealed, the sections were imaged with a
confocal microscope. The optical density was quantified using
Image-Pro Plus version 7.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Western blot analysis
Western blotting is used for the detection and
quantification of specific proteins in a sample of tissue. Protein
levels of two autophagy markers, LC3 and beclin1, were detected. To
investigate autophagy activation, the ratio of LC3-I to LC3-II, a
marker of autophagic vacuole formation, was measured. The spinal
cord samples were cut into small pieces (2 mm long; 2 mm thick) and
homogenized in lysis buffer [50 mM Tris HCl (pH 7.6), 20 mM
MgCl2, 150 mM NaCl, 0.5% Triton-X, 5
units.ml−1 aprotinin, 5 µg.ml-1 leupeptin, 5 µg.ml-1
pepstatin, 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride].
Lysate protein levels were determined using a BCA protein assay kit
(Shanghai Qcbio Science&Technologies Co., Ltd., Shanghai,
China). Equal amounts of proteins (~30 µg) were loaded per lane and
subjected to 10% SDS-PAGE, and subsequently transferred to a
nitrocellulose membrane. Membranes were blocked with 5% nonfat
dried milk in Tris-buffered saline with Tween 20 (TBST; 137 mM
sodium chloride, 20 mM Tris, 0.1% Tween 20; Absin Bioscience, Inc.)
for 1 h at room temperature. Primary antibodies specific to LC3
(cat. no. bsm-33309M; dilution 1:1,000; Biosynthesis Biotechnology
Co., Ltd., Beijing, China), beclin-l (cat. no. bs-1353R; dilution
1:200; Biosynthesis Biotechnology Co., Ltd., Beijing, China),
caspase-3 (cat. no. ab20816; dilution 1:500; Abcam) and β-actin
(cat. no. bsm-33139M; 1:1,000; Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, China) were diluted in TBST overnight at 4°C.
Membranes were incubated with Goat-anti-mouse immunoglobulin G
secondary antibodies conjugated to alkaline phosphatase (cat. no.
A32723; dilution 1:1,000; Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 1 h at room temperature. Reactive bands
were detected by incubating with nitro blue tetrazolium and
5-bromo-4-chloro-3-indolyl phosphate (Sigma-Aldrich; Merck KGaA)
for 5 min. Band densities were detected with an imaging
densitometer (GS-800; Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and the optical density was quantified using Image-Pro Plus
(version 7.0; Media Cybernetics, Inc.), which was calibrated
against β-actin.
Statistical analysis
Statistical analysis was performed using SPSS
software (verison 20.0; IBM Corp., Armonk, NY, USA). Data were
presented as the mean ± standard deviation. All experiments were
performed in triplicate. The differences between groups were
evaluated by one-way analysis of variance and multiple group
comparisons were performed by using Dunnett's tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pathological alterations
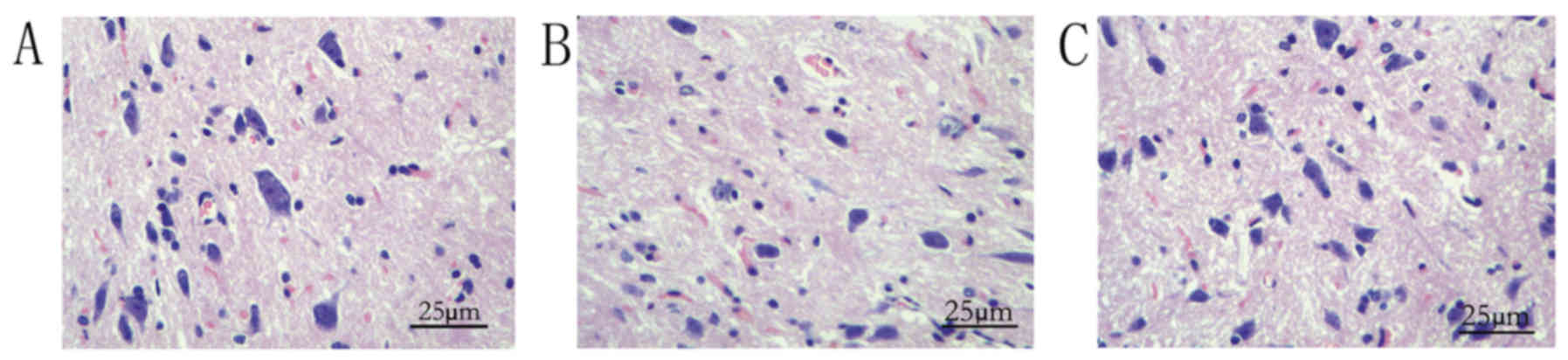
As demonstrated by light microscopy, in group A,
spinal cord neurons in the rats were uniformly distributed, the
morphology was normal, nissl bodies were clear, cell membranes were
intact, the neural fibers of the white matter were arranged in an
orderly manner and the intercellular matrix was uniform (Fig. 1A). However, following bupivacaine
injection, the number of spinal cord neurons was reduced, neurons
were shrunken and darkly stained and the nuclei were condensed
(Fig. 1B). However, following
administration of EPS-A, injury was ameliorated markedly (Fig. 1C). Therefore, the rat models were
successfully established.
Alterations in apoptosis
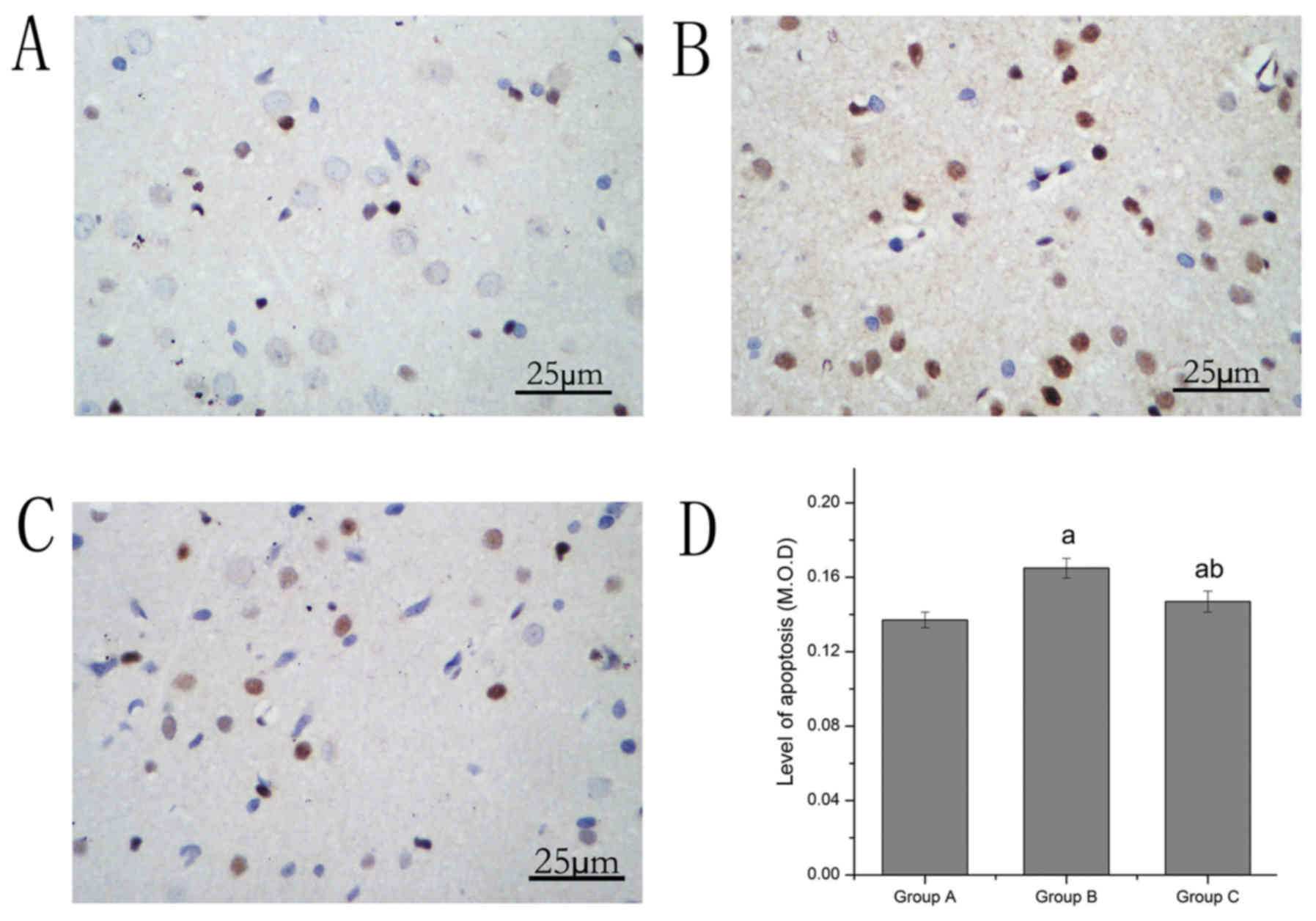
TUNEL staining was used to observe neuronal
apoptosis under a light microscope. The results indicated that
neuronal apoptosis in the spinal cord was altered. The apoptosis
level in group B increased compared with group A. Compared with
group B, the level of apoptosis in group C decreased significantly
(Table I; Fig. 2).
 | Table I.Comparison of apoptosis and autophagy
of spinal cord neurons in rats treated with bupivacaine and
extracellular polymeric substances from Aphanizomenon
flos-aquae as determined by terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling and
immunohistochemistry. |
Table I.
Comparison of apoptosis and autophagy
of spinal cord neurons in rats treated with bupivacaine and
extracellular polymeric substances from Aphanizomenon
flos-aquae as determined by terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling and
immunohistochemistry.
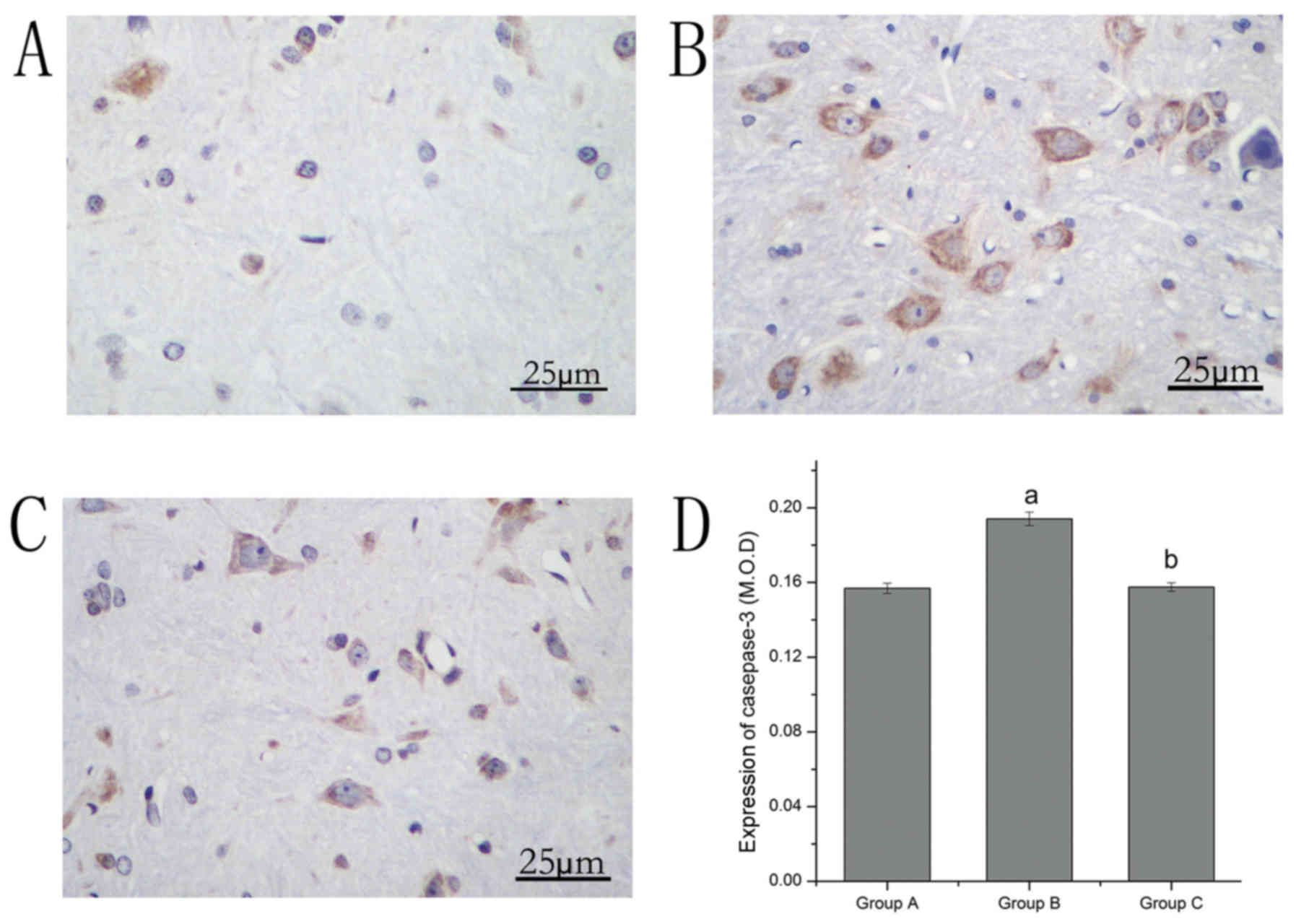
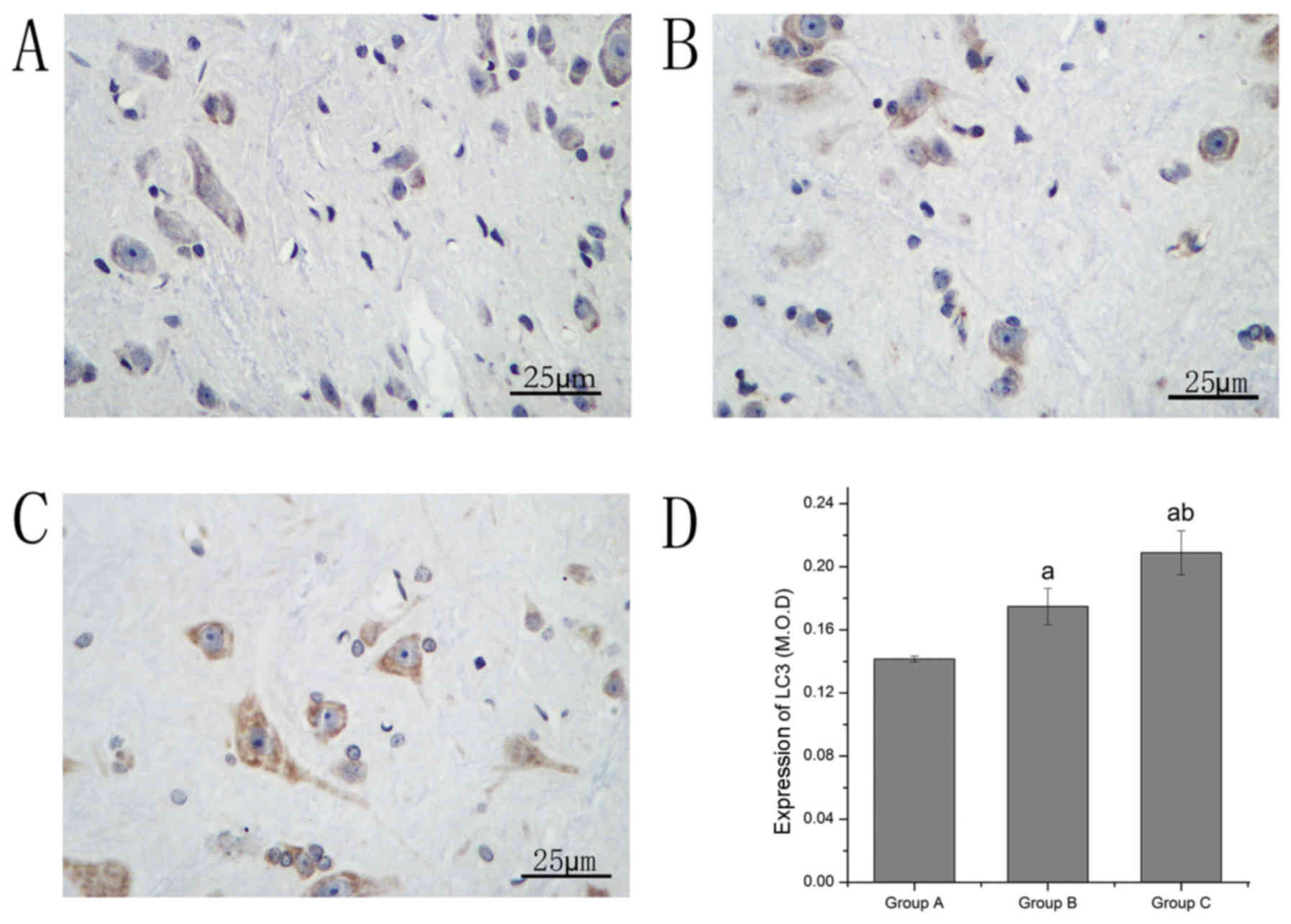
| Group | Apoptosis | Casepase-3 | Beclin1 | LC3 |
|---|
| A | 0.137±0.004 | 0.157±0.002 | 0.182±0.006 | 0.141±0.008 |
| B |
0.164±0.005a |
0.194±0.004a |
0.199±0.003a |
0.178±0.010a |
| C |
0.146±0.006a,b |
0.157±0.002b |
0.238±0.003a,b |
0.208±0.012a,b |
| Sum | 0.149±0.012 | 0.169±0.018 | 0.206±0.025 | 0.175±0.030 |
| F | 41.06 | 314.47 | 260.87 | 68.27 |
| P | 0.000 | 0.000 | 0.000 | 0.000 |
Alterations in the expression levels
of caspase-3
The expression of casepase-3 was observed by
immunohistochemical staining. Caspase-3-positive neurons were
stained brown. The expression of caspase-3 in group B increased
compared with group A. Compared with group B, the expression of
caspase-3 in group C significantly decreased (Table I; Fig.
3).
Alterations in the expression levels
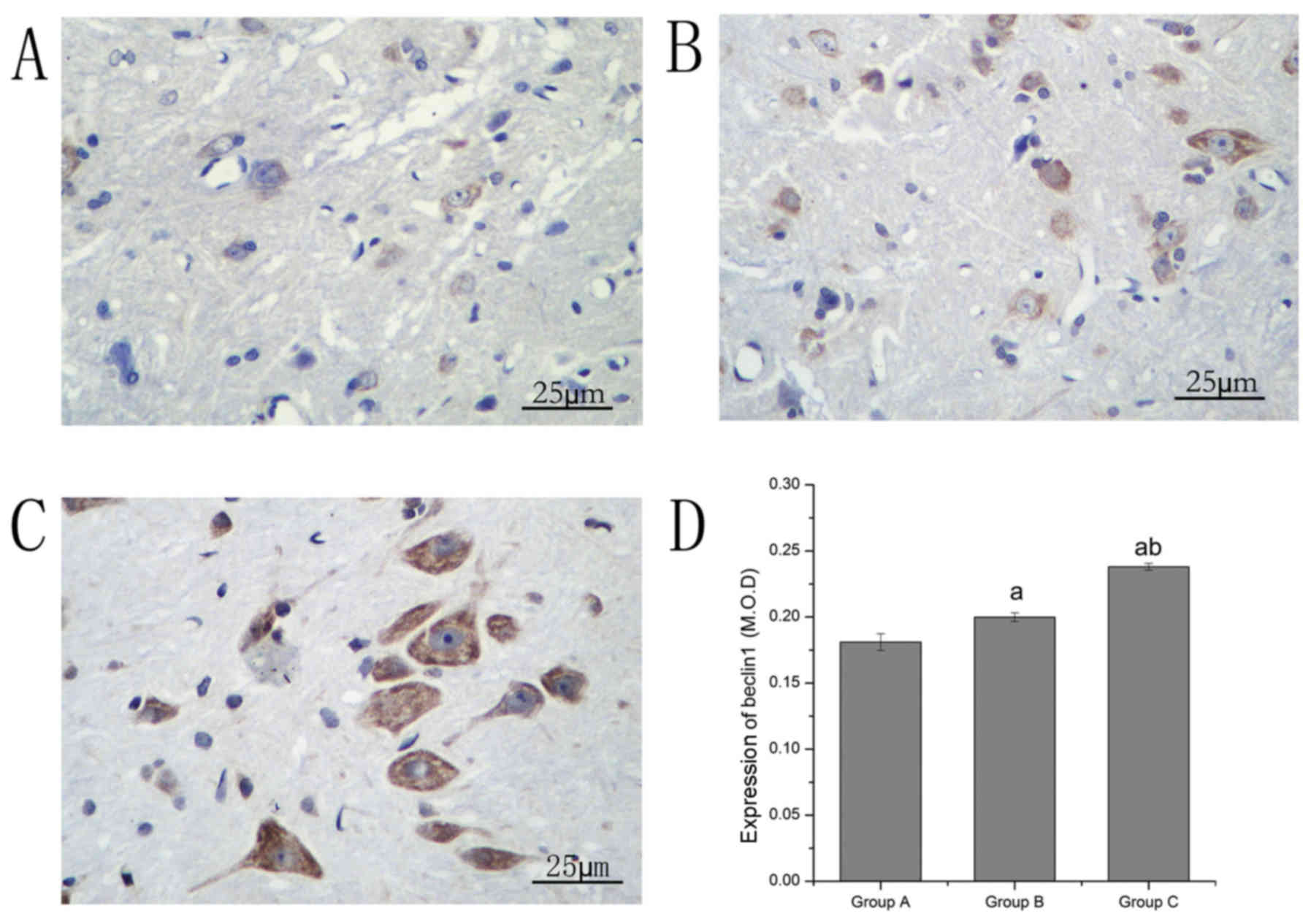
of LC3 and beclin1
Immunohistochemical staining was performed to
investigate alterations in the expression levels of LC3 and
beclin1. The LC3- and beclin1-positive neurons were stained brown.
LC3 and beclin1 levels in group B increased compared with group A.
Compared with group B, the LC3 and beclin1 levels in group C
significantly increased (Table I;
Figs. 4 and 5).
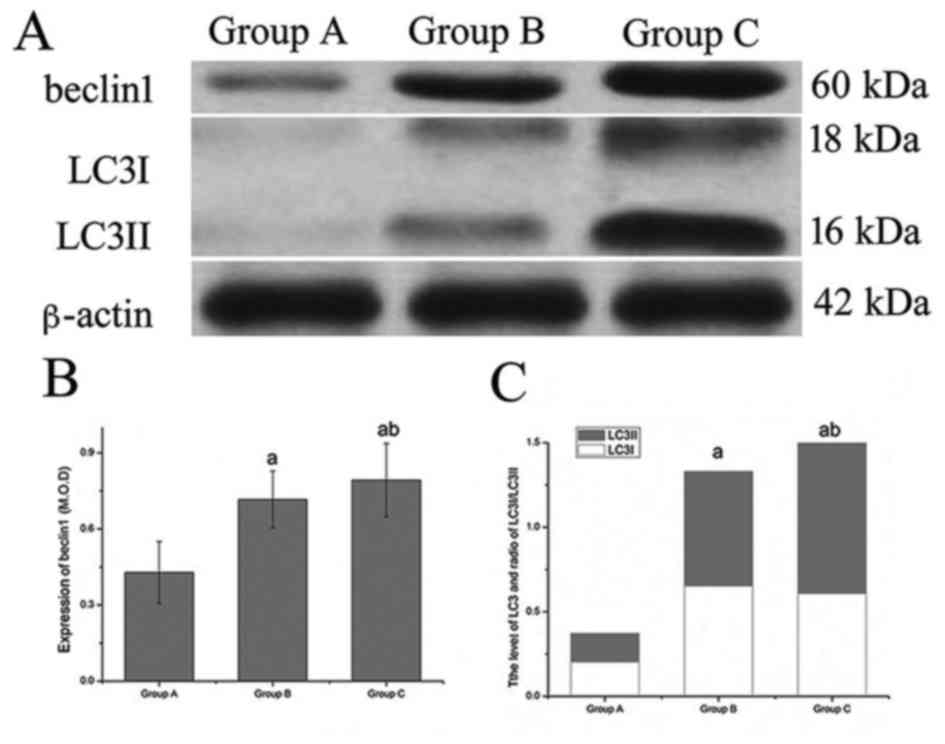
Western blotting
In order to further determine the level of
autophagy, western blot analysis was performed to observe the
expression levels of LC3 and beclin1. The results of the present
study indicated that the expression level of beclin1 and the ratio
of LC3-II/LC3-I differed between the groups. Compared with group A,
the level of beclin1 and the ratio of LC3-II/LC3-I increased
significantly in group B. Compared with group B, the level of
beclin1 and the ratio of LC3-II/LC3-I increased even further in
group C, and these results were consistent with those of the
immunohistochemical staining (Table
II; Fig. 6).
 | Table II.Comparison of the expression levels
of beclin1 and LC3 of spinal cord neurons in rats treated with
bupivacaine and extracellular polymeric substances from
Aphanizomenon flos-aquae as determined by western
blotting. |
Table II.
Comparison of the expression levels
of beclin1 and LC3 of spinal cord neurons in rats treated with
bupivacaine and extracellular polymeric substances from
Aphanizomenon flos-aquae as determined by western
blotting.
| Group | Beclin1 | LC3 |
|---|
| A | 0.428±0.099 | 0.141±0.008 |
| B |
0.712±0.064a |
0.178±0.010a |
| C |
0.849±0.129a,b |
0.208±0.012a,b |
| Sum | 0.663±0.203 | 0.175±0.030 |
| F | 27.112 | 1005.334 |
| P | 0.000 | 0.000 |
Discussion
Local anesthetics are widely used in clinical
anesthesia and pain treatment (21).
When their effects end, in general, neurological function can be
restored; however, their neurotoxicity has attracted an increasing
amount of attention from clinicians (22,23).
Identification of novel drugs which serve a protective role in
neuronal injury may provide theoretical basis for further research
to protect damaged neurons. Previously, studies have shown that
polysaccharides can reduce the swelling and shrinking of neurons,
promote nervous regeneration, reduce neurological defects and exert
protective effects on damaged neurons (24–26).
Therefore, the present study aimed to evaluate the beneficial
effects of EPS-A on neurotoxicity induced by local anesthetics in
an intraperitoneal injection bupivacaine rat model.
Morphological alteration is the primary way to
detect injury. H&E is one of principal histological stains and
the most widely used staining method in medical diagnosis (27). The results of the present study
indicated that following injection of bupivacaine, the number of
spinal cord neurons in rats was reduced. However, upon
administration of EPS-A, injury was ameliorated markedly. The
results suggested that EPS-A markedly attenuated neuronal injury
compared with bupivacaine induced neuronal injury. However, EPS-A
was able to attenuate these pathological alterations and,
therefore, the effect of EPS-A on the level of pathological
alterations may be involved in the reduction of neuronal injury in
rats treated with bupivacaine.
Apoptosis leads to the loss of a large number of
neurons, which is an important mechanism of secondary spinal cord
injury (28,29). It was also hypothesized to be one
mechanism of neurotoxicity caused by local anesthetics (30). A previous study suggested that
apoptosis is more common in the neurotoxicity of local anesthesia
as induced by local anesthetics (31). In the present study, the results
indicated that the apoptosis rate in spinal cord neurons increased
following intrathecal injection of bupivacaine, however, the
pre-administration of EPS-A resulted in a significant decrease in
apoptosis. Furthermore, the results indicated that EPS-A could
ameliorate the increase of apoptosis rate, thus the neuroprotective
effects of EPS-A may be mediated by its capacity to reduce neuronal
apoptosis.
The expression of caspase-3 is closely associated
with the level of neuronal apoptosis following spinal cord injury
(32). The expression of caspase-3
may also reflect the degree of neuronal damage to some extent
(33). In the present study, the
results indicated that the injection of bupivacaine significantly
increased the expression of caspase-3. However, following treatment
with EPS-A, the expression of caspase-3 significantly decreased.
The results suggested that the neurotoxic effects caused by
bupivacaine were associated with casepase3-dependent apoptosis.
EPS-A pre-administration was able to alleviate the neurotoxic
effects caused by bupivacaine and significantly reduced
casepase3-dependent apoptosis, which was consistent with the
results of the TUNEL staining. Therefore, the role of EPS-A in the
expression of caspase-3 may be involved in the attenuation of
apoptosis in rats treated with bupivacaine. The results implied
that EPS-A may affect the level of apoptosis, and this may aid in
understanding the beneficial neuroprotective effects of EPS-A
against local anesthetics.
Autophagy serves an important role in death and
survival of neuronal cells, which may influence several
neurodegenerative disorders (34,35).
Basal autophagy acts as one of the cytoprotective mechanisms and
participates in maintaining homeostasis (36). Additionally, in disease conditions of
myocardial ischemia-reperfusion and neuronal ischemic anoxia,
autophagy enhancement serves a role as a protective mechanism
(37). Recent studies (38,39) have
shown that drug-induced autophagy is characterized by the altered
expression of autophagy-associated proteins LC3 and beclin1. LC3
and beclin1 are two pacemakers in the autophagic cascade. LC3,
exists in cytosolic (LC3-I) and membrane bound forms (LC3-II)
(40). The ratio of conversion from
LC3-I to LC3-II is closely associated with the extent of
autophagosome formation (41).
Beclin1 is essential for the recruitment of other autophagic
proteins during the expansion of the pre-autophagosomal membrane
(42,43). The results of the present study
indicated that bupivacaine increased the protein expression levels
of beclin1 and LC3; however, with pre-administration of EPS-A, the
LC3 and beclin1 protein expression levels improved further. The
present study also suggested that the induction of autophagy was
associated with the alteration of the LC3II/LC3I ratio. In
addition, further data confirming the protective effects of EPS-A
were obtained from the western blot analysis of beclin1 and LC3.
The results demonstrated that bupivacaine increased the ratio of
LC3II/LC3I and the expression levels of beclin1; however, following
pre-administration of EPS-A, the ratio of LC3II/LC3I and the
beclin1 expression level increased further. The results implied
that EPS-A may affect the autophagy level, and this may aid in
understanding the beneficial neuroprotective effects of EPS-A
against local anesthetics by inducing the expression of
autophagy.
Autophagy is associated with apoptosis in a number
of ways, which are as complex as the roles of autophagy in cell
survival and death (44). It has
been suggested that autophagy may be a trigger for apoptotic cell
death (45), while others have
argued that autophagy protects against apoptosis and inflammation;
apoptosis and autophagy may cooperate, coexist or antagonize each
other to balance death and survival signaling (46). In the present study, the results
suggested that autophagy and apoptosis exhibited cross-inhibitory
interactions; the activation of autophagy could inhibit apoptosis
and autophagy could promote cell survival against apoptosis, which
was consistent with a previous report (47).
In conclusion, EPS-A exhibited neuroprotective
effects against bupivacaine-induced neurotoxicity in an
intraperitoneal injection bupivacaine rat model. The mechanisms may
be attributed to inhibiting apoptosis, inducing autophagy and
improving cell survival. These results suggested that EPS-A may be
a candidate neuroprotective agent against the neurotoxicity of
local anesthetics. The promotion and utilization of autophagy
induced by drugs has attracted an increasing amount of attention in
the field of medicine. However, further details on the beneficial
mechanisms of EPS-A in apoptosis and autophagy in an
intraperitoneal injection model are required, and this remains to
be evaluated in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Gansu
Province Health Industry Scientific Research Plan (grant no.
GSWSKY2017-18).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX wrote the manuscript. XX, YLv and YLe conceived
and designed the present study. XX and YaZ performed the majority
of the experimental procedures. YLv analyzed the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the First Hospital of Lanzhou University in (Lanzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Verlinde M, Hollmann MW, Stevens MF,
Henning H, Werdehausen R and Lirk P: Local anesthetic-induced
neurotoxicity. Int J Mol Sci. 17:3392016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HC, Fong TH, Hsu PW and Chiu WT:
Multifaceted effects of rapamycin on functional recovery after
spinal cord injury in rats through autophagy promotion,
anti-inflammation, and neuroprotection. J Surg Res. 179:e203–e210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiong J, Kong Q, Dai L, Ma H, Cao X, Li L
and Ding Z: Autophagy activated by tuberin/mTOR/p70S6K suppression
is a protective mechanism against local anaesthetics neurotoxicity.
J Cell Mol Med. 21:579–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang M, Cui SW, Cheung PCK and Wang Q:
Antitumor polysaccharides from mushrooms: A review on their
isolation process, structural characteristics and antitumor
activity. Trends Food Sci Tech. 18:4–19. 2007. View Article : Google Scholar
|
|
9
|
Jaehrig SC, Rohn S, Kroh LW, Wildenauer
FX, Lisdat F, Fleischer LG and Kurz T: Antioxidative activity of
(1->3), (1->6)-beta-D-glucan from Saccharomyces cerevisiae
grown on different media. LWT-Food Sci Tech. 41:868–877. 2008.
View Article : Google Scholar
|
|
10
|
Kang YL, Saleem MA, Chan KW, Yung BY and
Law HK: Trehalose, an mTOR independent autophagy inducer,
alleviates human podocyte injury after puromycin aminonucleoside
treatment. PLoS One. 9:e1135202014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emanuele E: Can trehalose prevent
neurodegeneration? Insights from experimental studies. Curr Drug
Targets. 15:551–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao F, Zhao J, Song L, Zhang YQ, Guo Z
and Yang KH: The induction of apoptosis and autophagy in human
hepatoma SMMC-7721 cells by combined treatment with vitamin C and
polysaccharides extracted from Grifola frondosa. Apoptosis.
22:1461–1472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv Y, Xue X, Tao L, Zhang D, Hu C, Liu Y
and Ren JF: Effect of extracellular polymeric substances from
aphanizomenon flos-aquae (EPS-A) on human epidermoid carcinoma
(A431 cells). Fresen Environ Bull. 24:3307–3314. 2015.
|
|
14
|
Jiao G, Yu G, Zhang J and Ewart HS:
Chemical structures and bioactivities of sulfated polysaccharides
from marine algae. Mar Drugs. 9:196–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei W, Wang SX and Guan HS: The antiviral
activities and mechanisms of marine polysaccharides: An overview.
Mar Drugs. 10:2795–2816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue X, Lv Y, Liu Q, Zhang X, Zhao Y, Zhang
L and Xu S: Extracellular polymeric substance from Aphanizomenon
flos-aquae induces apoptosis via the mitochondrial pathway in A431
human epidermoid carcinoma cells. Exp Ther Med. 10:927–932. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imatani T, Kato T, Okuda K and Yamashita
Y: Histatin 5 inhibits apoptosis in human gingival fibroblasts
induced by porphyromonas gingivalis cell-surface polysaccharide.
Eur J Med Res. 9:528–532. 2004.PubMed/NCBI
|
|
18
|
Zhang CX and Huang KX: Mechanism of
apoptosis induced by a polysaccharide, from the loach Misgurnus
anguillicaudatus (MAP) in human hepatocellular carcinoma cells.
Toxicol Appl Pharmacol. 210:236–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang D, Hu C, Wang G, Li D, Li G and Liu
Y: Zebrafish neurotoxicity from aphantoxins-cyanobacterial
paralytic shellfish poisons (PSPs) from Aphanizomenon flos-aquae
DC-1. Environ Toxicol. 28:239–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu C, Liu Y, Paulsen BS, Petersen D and
Klaveness D: Extracellular carbohydrate polymers from five desert
soil algae with different cohesion in the stabilization of fine
sand grain. Carbohyd Polym. 54:33–42. 2003. View Article : Google Scholar
|
|
21
|
Sigirci A: Pain management in total knee
arthroplasty by intraoperative local anesthetic application and
one-shot femoral block. Indian J Orthop. 51:280–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rigler ML, Drasner K, Krejcie TC, Yelich
SJ, Scholnick FT, DeFontes J and Bohner D: Cauda equina syndrome
after continuous spinal anesthesia. Anesth Analg. 72:275–281. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Z, Liu H, Guo Q, Xu X, Zhang Z and
Wang N: In vivo and in vitro evidence of the neurotoxic effects of
ropivacaine: the role of the Akt signaling pathway. Mol Med Rep.
6:1455–1459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, Li C, Yin J, Shen J, Wang H, Wu Y
and Jin H: Fucoidan, a sulfated polysaccharide from brown algae,
improves cognitive impairment induced by infusion of Aβ peptide in
rats. Environ Toxicol Pharmacol. 33:304–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao J, Liong EC, Ching YP, Chang RC, Fung
ML, Xu AM, So KF and Tipoe GL: Lycium barbarum polysaccharides
protect rat liver from non-alcoholic steatohepatitis-induced
injury. Nutr Diabetes. 3:e812013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect human
lens epithelial cells against oxidative stress-induced apoptosis
and senescence. PLoS One. 9:e1102752014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laine L, Lewin DN, Naritoku W and Cohen H:
Prospective comparison of H&E, Giemsa, and Genta stains for the
diagnosis of Helicobacter pylori. Gastrointest Endosc. 45:463–467.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ,
Kim YC, Markelonis GJ and Oh TH: Systemic administration of
17beta-estradiol reduces apoptotic cell death and improves
functional recovery following traumatic spinal cord injury in rats.
J Neurotrauma. 21:293–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Whalley K, O'Neill P and Ferretti P:
Changes in response to spinal cord injury with development:
Vascularization, hemorrhage and apoptosis. Neuroscience.
137:821–832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park CJ, Park SA, Yoon TG, Lee SJ, Yum KW
and Kim HJ: Bupivacaine induces apoptosis via ROS in the Schwann
cell line. J Dent Res. 84:852–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Werdehausen R, Fazeli S, Braun S, Hermanns
H, Essmann F, Hollmann MW, Bauer I and Stevens MF: Apoptosis
induction by different local anaesthetics in a neuroblastoma cell
line. Brit J Anaesth. 103:711–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boselli E, Duflo F, Debon R, Allaouchiche
B, Chassard D, Thomas L and Portoukalian J: The induction of
apoptosis by local anesthetics: A comparison between lidocaine and
ropivacaine. Anesth Analg. 96:755–756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ananth C, Dheen Thameem S,
Gopalakrishnakone P and Kaur C: Domoic acid-induced neuronal damage
in the rat hippocampus: Changes in apoptosis related genes (bcl-2,
bax, caspase-3) and microglial response. J Neurosci Res.
66:177–190. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nixon RA: The role of autophagy in
neurodegenerative disease. Nat Med. 19:983–997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang ZY, Lin JH, Muharram A and Liu WG:
Beclin-1-mediated autophagy protects spinal cord neurons against
mechanical injury-induced apoptosis. Apoptosis. 19:933–945. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HJ, Kim J, Kang KS, Lee KT and Yang
HO: Neuroprotective effect of chebulagic acid via autophagy
induction in SH-SY5Y cells. Biomol Ther (Seoul). 22:275–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamurcu Z, Delibaşı N, Geçene S, Şener EF,
Dönmez-Altuntaş H, Özkul Y, Canatan H and Ozpolat B: Targeting LC3
and Beclin-1 autophagy genes suppresses proliferation, survival,
migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin
β1/Src signaling in triple negative breast cancer cells. J Cancer
Res Clin Oncol. 144:415–430. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niu Y, Zhang Y, Li K, Xing C and Sun C:
miR-124-3p and miR-140-3p.2 act as negative regulators of Beclin1
and LC3 expression in the liver of rat model with hepatic impact
injury. Bio Res. 29:2018.(ISSN: 0970-938X).
|
|
40
|
Liu C, Xu P, Chen D, Fan X, Xu Y, Li M,
Yang X and Wang C: Roles of autophagy-related genes Beclin-1 and
LC3 in the development and progression of prostate cancer and
benign prostatic hyperplasia. Biomed Rep. 1:855–860. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miracco C, Cevenini G, Franchi A, Luzi P,
Cosci E, Mourmouras V, Monciatti I, Mannucci S, Biagioli M, Toscano
M, et al: Beclin 1 and LC3 autophagic gene expression in cutaneous
melanocytic lesions. Hum Pathol. 41:503–512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao L, Jiang T, Guo J, Liu Y, Cui G, Gu L,
Su L and Zhang Y: Inhibition of autophagy contributes to ischemic
postconditioning-induced neuroprotection against focal cerebral
ischemia in rats. PLoS One. 7:e460922012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu C, Xu P, Chen D, Fan X, Xu Y, Li M,
Yang X and Wang C: Roles of autophagy-related genes Beclin-1 and
LC3 in the development and progression of prostate cancer and
benign prostatic hyperplasia. Biomed Rep. 1:855–860. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao M, Li L, Li C, Zhang P, Hu Q, Ma L
and Zhang H: Role of autophagy and apoptosis in wound tissue of
deep second-degree burn in rats. Acad Emerg Med. 21:383–391. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shrivastava A, Kuzontkoski PM, Groopman JE
and Prasad A: Cannabidiol induces programmed cell death in breast
cancer cells by coordinating the cross-talk between apoptosis and
autophagy. Mol Cancer Ther. 10:1161–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zeng R, He J, Peng J, Chen Y, Yi S, Zhao F
and Cui G: The time-dependent autophagy protects against apoptosis
with possible involvement of Sirt1 protein in multiple myeloma
under nutrient depletion. Ann Hematol. 91:407–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|