Introduction
Areca catechu is a species of palm widely
cultivated in India, Indonesia, Sri Lanka, the Philippines and
other South and Southeast Asian countries (1). A total of four alkaloids are presented
in the betel nut, including arecoline, arecaidine, guavacoline and
guavacine (2). Arecoline is the
major alkaloid, soluble in water or any concentration of ethanol
(3). In Southeast Asia, South
Pacific Islands and the surrounding areas, people chew the betel
nut frequently for its comforting and pleasant effects (4). Betel nut is the fourth most frequently
consumed recreational drug globally, after nicotine, ethanol and
caffeine. As a common clinical drug, arecoline can be used in the
treatment of tapeworm infection due to its insecticidal properties
(5) and for protection of vascular
endothelial cells to prevent atherosclerosis (6). However, due to its cytotoxicity and
immunotoxicity, long-term administration of arecoline can lead to
cellular DNA damage and promotion of apoptosis (7). Arecoline can cause submucosal fibrosis
and oral cancer (8), and promote
apoptosis and damage of liver cells, leading to hepatic cirrhosis
and hepatic cancer (9–11). Several researchers have reported that
arecoline-induced liver injury involves not only apoptosis but also
autophagy (9,12,13),
however, the exact role of autophagy remains to be elucidated.
Autophagy is a highly conserved, self-protective
behavior of eukaryotic cells when subjected to adverse external
stimuli, including hypoxia, sudden pressure elevation and drug
stimulation (14,15). A variety of autophagy-related (ATG)
proteins are involved in the autophagy process, including the
ATG12-ATG5 conjugation and beclin 1 (16,17).
Under different conditions, the level of autophagy is strictly
regulated by various signal transduction pathways (18). Mammalian target of rapamycin (mTOR),
a factor involved in the regulation of autophagy, serves a role of
a ‘gatekeeper’ (19). Microtubule
associated protein 1 light chain 3 (MAP1LC3), as a specific
substrate of autophagy, is essential for autophagosome formation
and is recognized as a marker of autophagic activity (20–22).
Therefore, the present study detected cellular autophagic activity
by measuring the content of MAP1LC3B.
L-glutathione is commonly used in the clinical
treatment of liver disease and drug-induced hepatic injury, and
serves a role in the protection and repair of hepatocytes by
scavenging free radicals and superoxide ions (23). A previous study by the authors of the
present study has found that MAP1LC3B expression increased in the
injured and L-glutathione treated hepatic cells (24), however, the mechanism remains to be
elucidated. In the present study, the function and regulation of
autophagy were further investigated by detecting the expression of
mTOR, beclin 1 and MAP1LC3B, and the results may provide an insight
into the protection or treatment for drug-induced liver damage.
Materials and methods
Chemicals and mice
Arecoline hydrobromide (98%) and L-glutathione were
purchased from J&K Scientific Ltd. (Beijing, China). Mouse
aspartate aminotransferase enzyme-linked immunosorbent assay kit
(AST ELISA kit; cat. no. BPE20184) and mouse alanine
aminotransferase (ALT) ELISA kit (cat. no. BPE20168) were purchased
from Shanghai Lengton Bioscience Co., Ltd. (Shanghai, China). The
following reagents were used for immunohistochemistry and western
blotting: Rabbit anti-mTOR (cat. no. ab32028), anti-beclin 1 (cat.
no. ab55878), anti-MAP1LC3B (cat. no. ab63817) polyclonal
antibodies purchased from Abcam (Cambridge, UK); and anti-caspase-3
(cat. no. ab90437) polyclonal antibodies purchased from Abcam
(Cambridge, UK) for western blotting; and the PV-6000
immunohistochemical detection kit (cat. no. K145213B) from OriGene
Technologies, Inc. (Beijing, China). A total of 80 Male ICR mice
(age, 6–8 weeks; weight, 20±2 g) were provided by the Laboratory
Animal Center of Binzhou Medical University (Yantai, China). All
animal experiments were performed in accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health, the animal use
protocol was reviewed and approved by the Ethics Committee of
Binzhou Medical University (Yantai, China).
Animal treatments
All experimental mice were housed in plastic cages
in a pathogen-free environment, with controlled temperature
(23–25°C) and humidity (50–70% relative humidity), with ad
libitum access to food and water, and a 12-h light/dark cycle.
These animals were randomly divided into four groups, with 20 mice
in each group (n=20). Arecoline hydrobromide was dissolved in
physiological saline (0.9% NaCl). The control group mice were given
physiological saline gavage (4 ml/kg/day) and the model group mice
were given arecoline hydrobromide (20 mg/kg/day). The low-dose
glutathione group mice were given a mixture of L-glutathione (1
mg/kg/day) and arecoline hydrobromide (20 mg/kg/day), and high-dose
glutathione group mice were given a mixture of L-glutathione (2
mg/kg/day) and arecoline hydrobromide (20 mg/kg/d). During the
experiment, physiological saline, arecoline hydrobromide or a
mixture of L-glutathione and arecoline hydrobromide were
administered to corresponding group of mice by gavage twice daily
for 15 or 30 days. The dosage of arecoline hydrobromide was
determined according to a previous study (25).
Behavior and bodyweights of the
experimental mice
During the experiment, the behavior of mice was
observed using an open field test (26) and the mental states were detected
through mechanical stimulus method (27). The bodyweights were recorded on days
15 and 30.
Serum collection and liver function
measurement
Blood samples were collected from the orbit sinus
after ether anesthesia and spinal cord dislocation on days 15 or 30
(10 mice/group). During ether anesthesia, the respiratory rate of
mice was monitored by the biological signal analytical system
(BL-420F; Chengdu Techman Software Co., Ltd. Chengdu, China) and
controlled within 90–120 beats/min. Marker enzymes of liver
function (ALT and AST) in the serum were assessed with the
respective ELISA kits following the manufacturer's protocol in an
automatic biochemical analyzer.
Liver morphology
The morphological alterations of the liver were
observed. Liver samples were dissected, partly placed in liquid
nitrogen immediately for western blotting, and partly in 10%
formalin solution, fixed for 48 h at room temperature and
progressively dehydrated prior to embedding in paraffin. The
paraffin blocks were cut into 4-µm-thick sections, deparaffinized,
rehydrated and stained with hematoxylin for 15 min and eosin for 5
min at room temperature. Histological examination was performed
under a light microscope (magnification, ×400; Olympus BX43;
Olympus Corporation, Tokyo, Japan).
Immunohistochemistry
After deparaffinization and rehydration in a
descending alcohol series, each section was incubated with 0.01
mol/l citrate buffer (pH 6.0) in a microwave at 98°C for 10 min for
antigen retrieval. Endogenous peroxidase was blocked with 3%
H2O2 for 20 min at room temperature. Each
sample was pretreated with normal goat serum (OriGene Technologies,
Inc.) for 30 min at room temperature to block nonspecific binding
and incubated with rabbit anti-mTOR (diluted 1:800), rabbit
anti-beclin 1 (diluted 1:120) or rabbit anti-MAP1LC3B (diluted
1:60) antibodies overnight at 4°C. Human colon carcinoma tissue
obtained from a 50-year-old male patient (Collected in January 2017
from the Pathology Department, of Binzhou Medical University
Hospital, Binzhou, China) was used as the positive control. The
participant provided written informed consent. An ethical approval
for the use of human samples was obtained from the Ethics Committee
of the Binzhou Medical University. For the negative control, the
primary antibody was replaced with phosphate-buffered saline. A
biotinylated goat anti-rabbit immunoglobulin G antibody (cat. no.
ZB-2305; OriGene Technologies, Inc.; 1:400) was used as the
secondary antibody and incubated with each section at 37°C for 15
min. These sections were subsequently exposed to
streptavidin-horseradish peroxidase conjugate (Beijing Bioneeds,
Technologies Co Ltd. Beijing, China; cat. no. CJ30H; 1:500) at 37°C
for 10 min. The peroxidase reactivity was visualized by the
application of 3,3′-diaminobenzidine solution for 5 min at room
temperature. These sections were counterstained with hematoxylin
for 10 min and mounted. Two sections were selected from the right
and left liver lobes separately in each mouse, and five
non-repeating fields of view were observed for each section. The
percentage of positive immunoreactivity cell area for mTOR, beclin
1 or MAP1LC3B and the reactive intensity were determined using a
light microscope at a magnification of ×400 (Olympus BX43; Olympus
Corporation). The proportions of liver tissues that stained
positively in the cytoplasm and/or cytomembrane were scored
according the following criteria: i) 0, <1% cell staining; ii)
1, staining in 1–10% cells; iii) 2, staining in 11–50% cells; and
iv) 3, staining in >50% cells. The intensity of staining was
also recorded as: i) 0, negative (no staining); ii) 1, weak (faint
yellow); iii) 2, moderate (yellow); and iv) 3, strong (brown)
(28). The total score was the
product of the proportion and strength of staining, ranging from 0
to 9. The total score ≤3 was recorded as negative (−) and >3 was
recorded as positive (+). The positive expression rate was
calculated.
Western blotting
The proteins of liver samples were prepared with
radioimmunoprecipitation assay lysis buffer and
phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was detected
with a BCA Protein Assay kit (Beyotime Institute of Biotechnology)
with a loading mass of 20 µg/well. The samples were separated by
12% SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Membranes were blocked with 5% nonfat milk in PBST buffer (PBS
+0.1% Tween 20) for 2 h at room temperature, then incubated with
primary antibodies overnight at 4°C and secondary antibodies
(Beijing ZhongShan Golden Bridge Biotechnology Co., Ltd Beijing,
China; 1:5,000) at 37°C for 0.5 h. The following primary antibodies
were used: mTOR (1:1,000), beclin1 (1:1,000), MAP1LC3B (1:1,000),
caspase-3 (1:1,000) and GAPDH (cat. no. SC-32233; 1:10,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). Protein bands were
visualized by Enhanced Chemiluminescence kit (Beyotime Institute of
Biotechnology). Integral optical density (IOD) value measured with
Image-Pro Plus (version 6.0; Media Cybernetics, Inc., Rockville,
MD, USA) was used to calculate the relative expression of the
target protein (ratio) using the following equation: Ratio=IOD
target protein/IOD GAPDH.
Statistical analysis
All experiments were repeated three times
independently. Data were analyzed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA) and presented as the mean ± standard
deviation. Multigroup comparisons were carried out by one-way
analysis of variance with Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
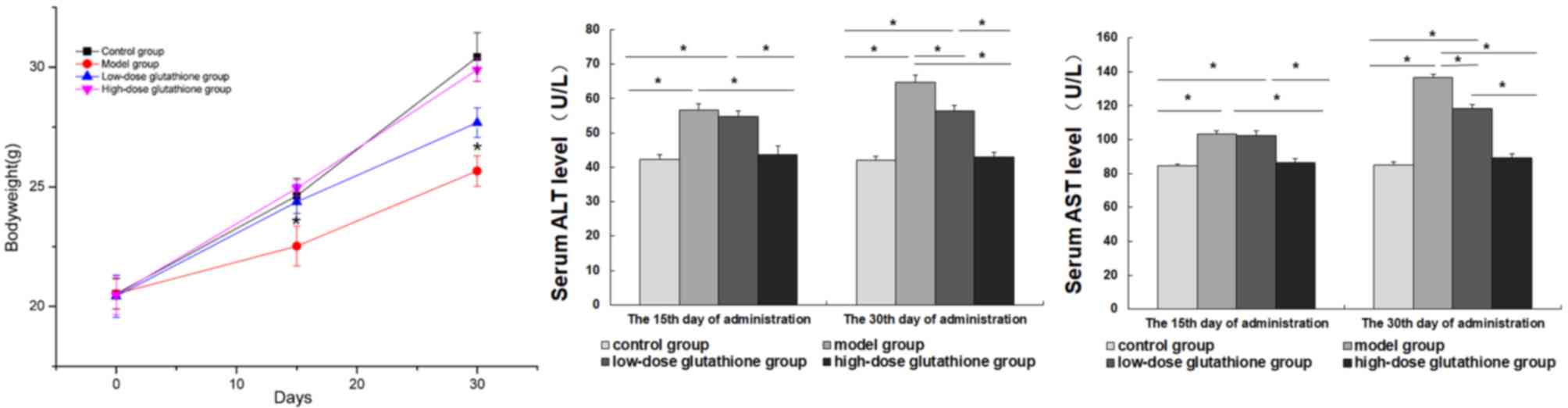
Alterations in bodyweights and serum
markers of liver function in the experimental mice
In the model group, the mice exhibited hair loss,
thinning and increased irritability. The activity of mice was
decreased (data not shown), and weight losses were significantly
more severe in the model mice compared with the control group
(P<0.05; Fig 1). Glutathione
administration groups exhibited minor alterations in behavior or
bodyweight, especially in the high-dose glutathione group, when
compared with control and model groups. The serum levels of ALT and
AST were measured on days 15 and 30 of the experiment. Serum levels
of ALT and AST in the model group significantly increased compared
with the control group and high-dose glutathione group (all
P<0.05). On day 15, there was no significant difference in serum
levels of ALT and AST between the model group and the low-dose
group, whereas the serum levels in the low-dose glutathione group
were significantly lower compared with the model group on day 30
(P<0.05; Fig. 1).
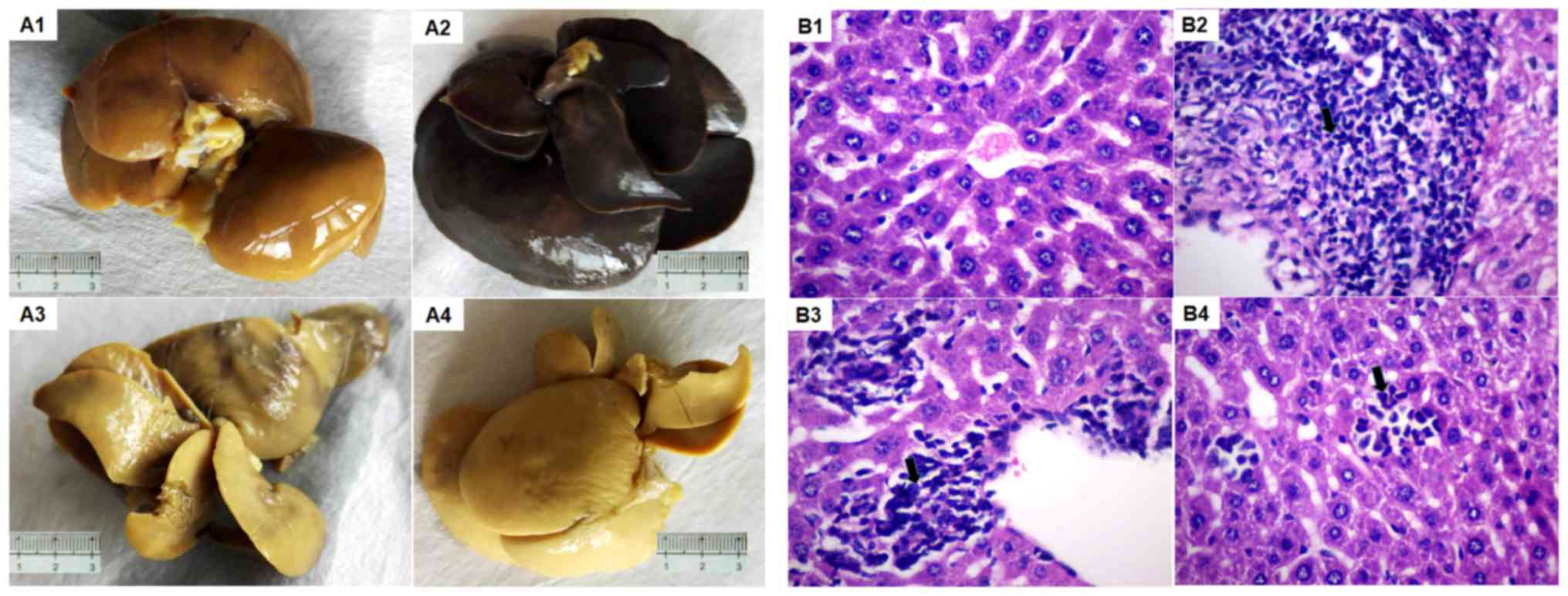
Liver morphology of the mice
In the control group, it was observed that the liver
capsule was smooth, the sections were gray and red, and the texture
was uniform and soft. Under the microscope, the structures of the
hepatic lobule and portal area were clear, without obvious signs of
the presence of fibrous tissue; the liver cells were aligned and
the cytoplasm was eosinophilic uniformly. In the model group, the
livers were clearly congested, swollen and rough. Hepatocellular
edema, a large number of hepatocytes undergoing hepatic steatosis
and mild necrosis could be observed on day 15 in the model group.
On day 30 in model group, the liver cells exhibited severe edema
and multifocal dotted necrosis; fibrosis and hyperplasia were
visible around the portal vein, with inflammatory cell
infiltration. In the low-dose glutathione group, cell morphology
was similar to that of the model group, with occasional hepatic
cell degeneration. In the high-dose glutathione group, the livers
exhibited slight congestion, mild hepatocyte edema, slight dotted
necrosis and no significant fatty degeneration, however, obvious
hepatic cell degeneration was observed (Fig. 2).
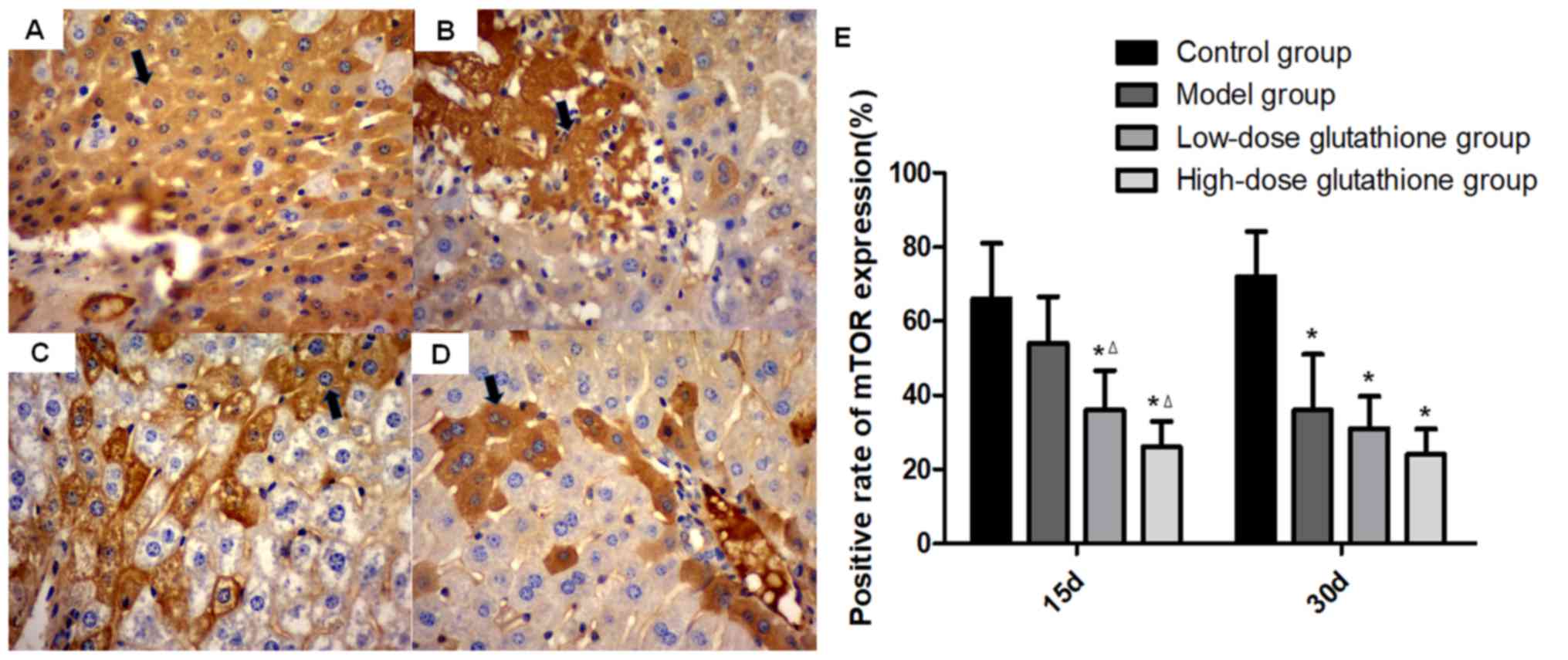
Expression of autophagy-associated
proteins mTOR, beclin 1 and MAP1LC3B in mouse livers
High levels of mTOR expression were observed in the
cytoplasm in the control group, however these levels were reduced
in the model group. On day 15 of the experiment, mTOR expression
was significantly lower in the glutathione treatment groups
compared with the control group and model group (both P<0.05),
especially in the high-dose glutathione group. On day 30, compared
with the control group, mTOR expression decreased significantly in
all other groups (including the model group and glutathione
treatment groups; all P<0.05; Fig.
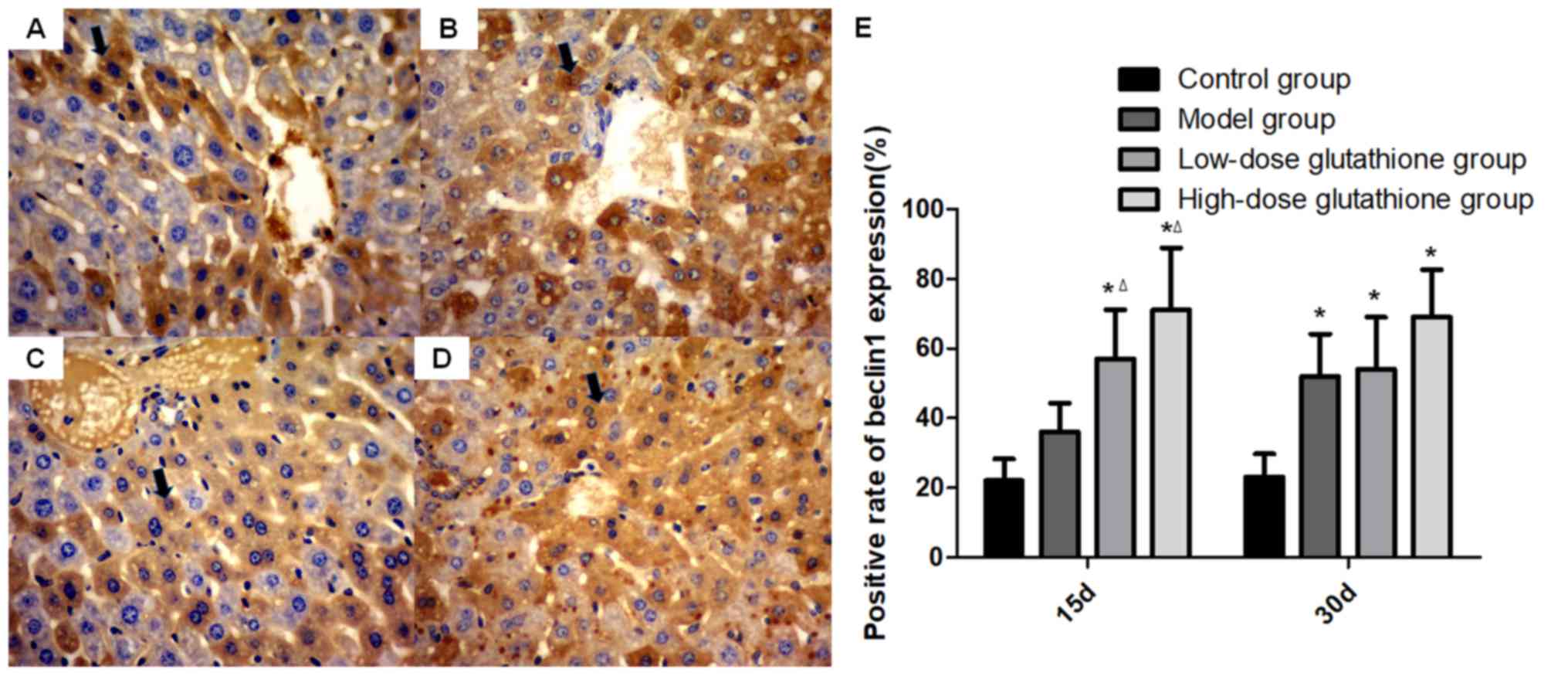
3). The trend of beclin 1 expression was different compared
with that of mTOR expression. On day 15 of the experiment,
expression levels of beclin 1 increased significantly in the
glutathione treatment groups compared with the control group and
model group (both P<0.05). On day 30, beclin 1 expression was
significantly higher in all groups compared with the control group
(all P<0.05; Fig. 4). The
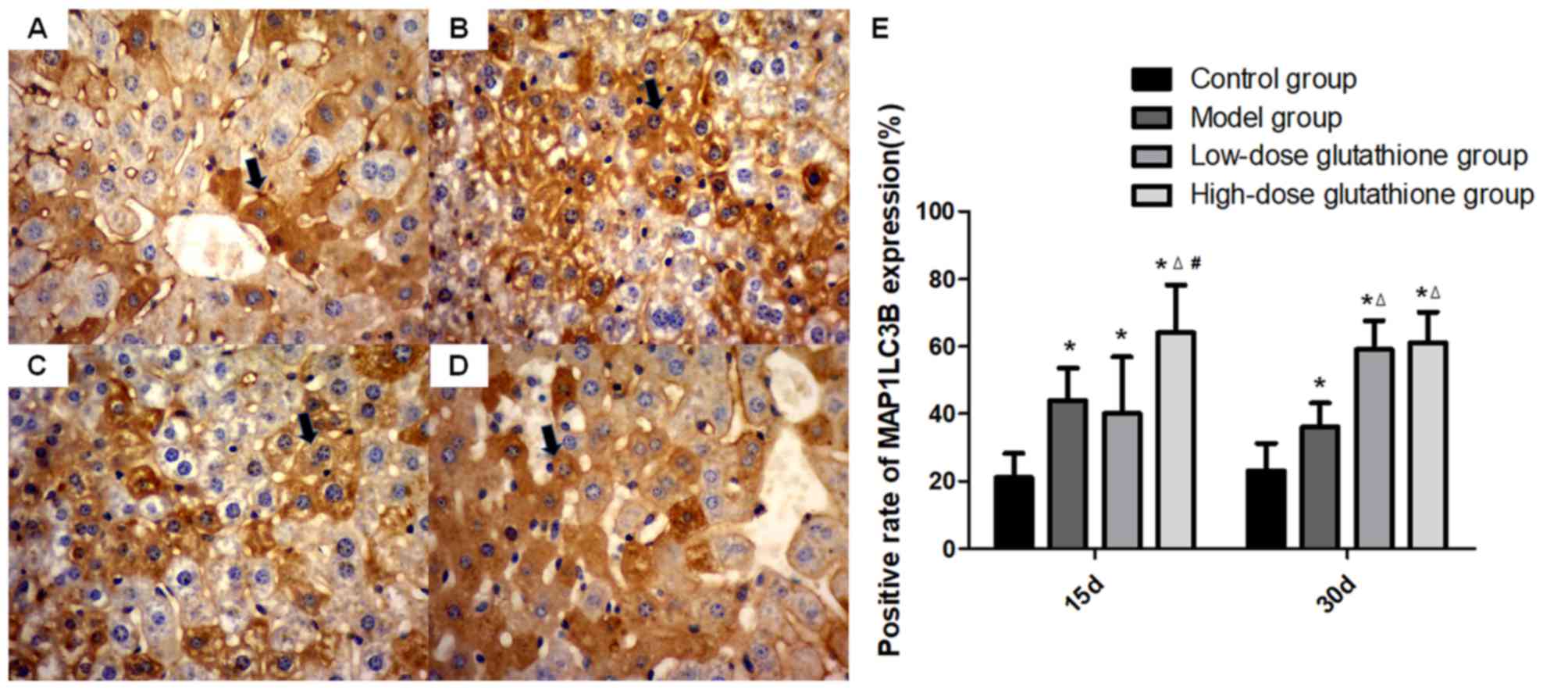
expression of MAP1LC3B was similar to that of beclin 1. On day 15,
the expression of MAP1LC3B in all drug-administrated groups was
significantly higher than the control group (P<0.05) and
MAP1LC3B expression in the high-dose glutathione group was also
higher than the model and low-dose glutathione group. On day 30,
MAP1LC3B expression in all drug-administrated groups was higher
than the control group and glutathione-administrated groups were
also higher when compared with the model groups (all P<0.05;
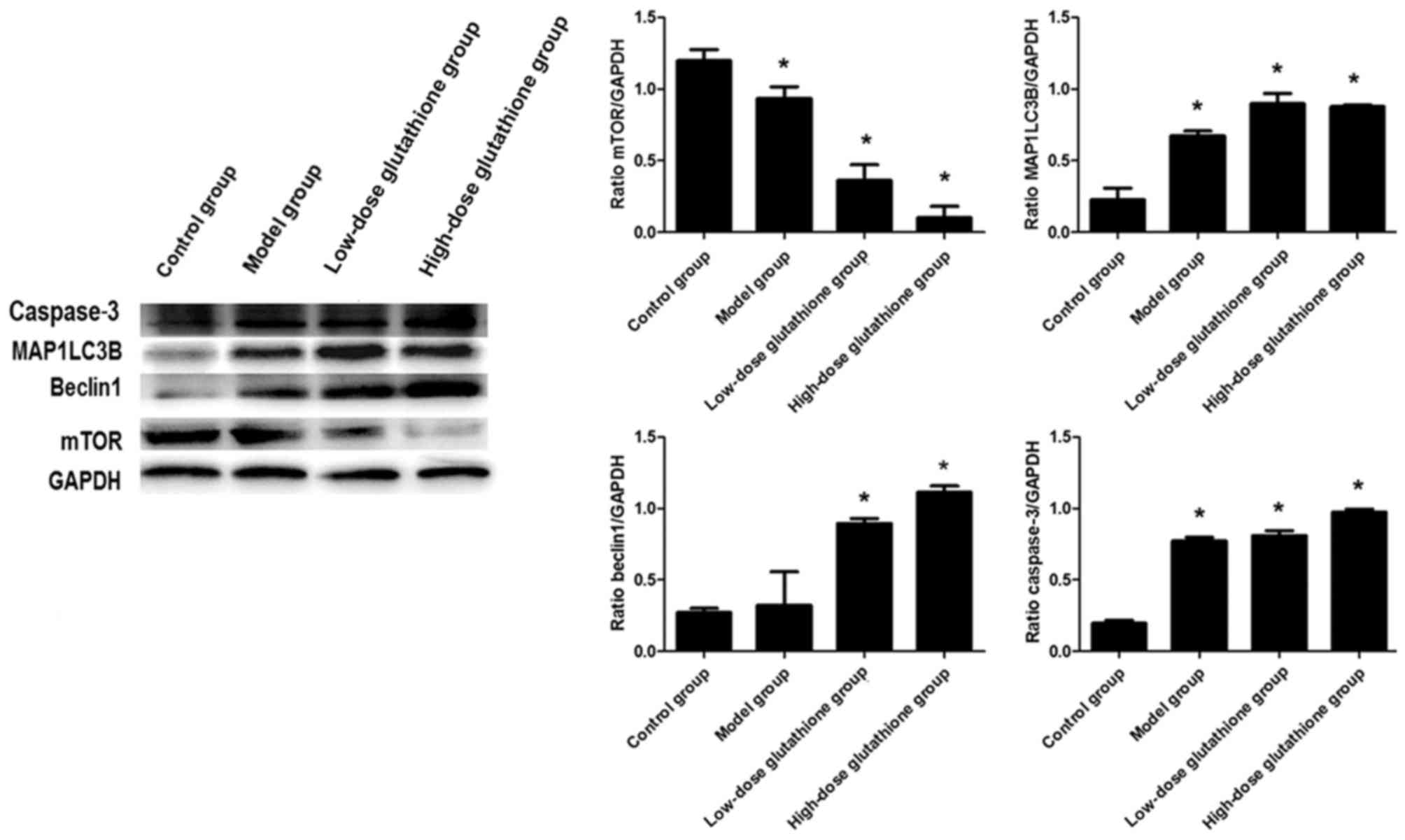
Fig. 5). The results of western
blotting indicated that mTOR expression decreased obviously in all
groups compared with the control group. The expression of beclin1
increased in both glutathione treatment groups compared with the
control group, which was consistent with the results for MAP1LC3B
and caspase-3 expression (all P<0.05; Fig. 6).
Discussion
Hepatic injury caused by the long-term
administration of particular drugs, including acetaminophen,
amoxicillin-clavulanate and antiepileptic drugs are common clinical
phenomenons (29,30). The degree of hepatocyte damage
depends on the accumulation of toxicity and the protection afforded
by physical or exogenous factors (31,32).
Among these cell protection mechanisms, autophagy is currently
widely investigated (13).
Under normal physiological conditions, the autophagy
level of cells is low, designated as ‘basic autophagy’, which
primarily maintains the stable state of cells (15). However, autophagy is induced by
pathological factors, including starvation, ischemia/hypoxia and
injury (15,33). To maintain homeostasis and ensure
cell survival, energy is generated by the degradation of damaged
organelles and protein aggregates (15,33,34).
Several studies have demonstrated that autophagy may serve dual
roles in alcoholic liver disease, hepatic ischemia/reperfusion
injury and bile duct ligation or carbon tetrachloride cirrhosis
models (35–37). As a cellular self-protective
mechanism, autophagy significantly increases in hepatic injury
caused by various factors, including drug use, alcohol consumption
and chronic hepatitis B and C (38,39).
Furthermore, autophagy may attenuate fibrosis and reduce hepatic
injury by inhibiting inflammatory reaction and decreasing oxidative
stress response (40). Therefore,
autophagy may be a new therapeutic target for liver injury and
fibrosis (40). However, reports on
autophagy in the context of arecoline-induced hepatic injury are
scarce.
Arecoline is a commonly used Chinese medicine
extracted from the betel nut with numerous properties, including
parasite expulsion and antiatherosclerotic properties (5,6).
However, due to its cytotoxicity and immunotoxicity, long-term use
of arecoline can damage cellular DNA and promote cell apoptosis
(7). Long-term chewing of the betel
nut can lead to oral submucosal fibrosis and oral cancer (4). Several studies have shown that the
long-term administration of arecoline accelerates apoptosis,
degeneration and necrosis of hepatic cells, leading to liver
cirrhosis and liver cancer (9–11). The
betel nut has been identified as a first-class carcinogen by the
International Agency for Research on Cancer (41,42). In
the present study, intragastric administration of arecoline
resulted in alterations in mice behavior and bodyweight, increased
levels of AST and ALT, and induction of histological hepatocyte
edema and spotty necrosis, and these alterations aggravated with
the progress of administration. The above results demonstrated that
the excessive use of arecoline may damage liver cells. Thangjam and
Kondaiah (43) reported that
arecoline-induced cytotoxicity could promote the generation of
reactive oxygen species (ROS) and activate inflammatory cells in
stress. Furthermore, in the presence of ROS, arecoline may inhibit
adenosine monophosphate-activated protein kinase (AMPK)
phosphorylation and induce autophagy (44). However, the exact associations
between ROS, AMPK and autophagy in arecoline-induced hepatic injury
remain unclear, and further investigation is required.
In clinical practice, L-glutathione is commonly used
to protect undamaged liver cells and repair damaged cells by
scavenging free radicals and superoxide ions (23). In the present study, L-glutathione
was used to treat the hepatic injury induced by arecoline. Compared
with the model group, the behavior and serum levels of AST and ALT
altered only marginally in the low-dose glutathione group. In the
high dose glutathione group, the serum levels of AST and ALT were
restored to normal, and histological examinations indicated cell
regeneration with minimal necrosis, suggesting that glutathione
could protect liver function and promote the regeneration of
hepatocytes.
MAP1LC3 is required for autophagosome formation and
the level of MAP1LC3 is proportional to the number of autophagic
vacuoles present in cells, and, therefore, the autophagic activity
can be assessed by measuring the expression of MAP1LC3 (20–22). The
phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB)/mTOR
signaling pathway is involved in the inhibition of autophagy
(45). Under nutrient-rich
conditions, the mTOR signal is activated and autophagy is inhibited
(19). In the case of nutrient
deficiency, mTOR is suppressed, and autophagy is activated by the
negative feedback of the PI3K/PKB/mTOR signaling pathway (46). The PI3K-VPS34/beclin 1 pathway is
involved in the positive regulation of autophagy (47). Beclin 1, as a ‘platform’ for
molecular reactions, promotes the localization of
autophagy-associated proteins to the phagosome and regulates the
formation and maturation of autophagosome by binding with VPS34
(48). Cursio et al (49) investigated the role of autophagy in
hepatic ischemia/reperfusion injury. The authors found that hypoxia
and nutrient deficiency activated autophagy, which reduced the
extent of hepatic reperfusion injury and restored the function of
the damaged mitochondria (49). In
another study, the researchers found that knock out of
autophagy-associated protein cysteine protease ATG4B aggravated
hepatic damage in mice due to increased sensitivity to
ischemia/reperfusion injury (50).
Studies on the association between autophagy and alcoholic and
foodborne liver damage have suggested that autophagy protects liver
cells by selectively removing harmful substances and repairing
damaged mitochondria (51,52). Betsuyaku et al (51) reported that, to maintain immune
homeostasis, the levels of both apoptosis and autophagy were
increased in rats with acute ethanol-induced damage to the thymus.
Regarding the association between autophagy and drug-induced liver
damage, Ni et al (52)
reported that the excessive application of acetaminophen could
cause mitochondrial damage and liver cells necrosis. Inhibition of
autophagy by chloroquine could aggravate the necrosis of liver
cells, whereas induction of autophagy with rapamycin could reduce
or even reverse liver damage (53).
However, a previous study indicated that ATG5-defective mice
exhibited high tolerance to acetaminophen and increased resistance
to pathological stimuli by reducing autophagy (54).
In the present study, expression levels of MAP1LC3B
and beclin 1 were low in the control group, and mTOR was highly
expressed; MAP1LC3B expression indicated autophagy level in these
cells. In the model group on day 30, following administrated of
arecoline, the expression of MAP1LC3B and beclin 1 in hepatocytes
increased significantly compared with the control group, and the
expression of mTOR decreased. One study has indicated that
carboxylesterase, the main metabolic enzyme of arecoline, is highly
active in liver mitochondria (55).
When the accumulation of arecoline in the body exceeds the
metabolic capacity, it can damage mitochondria and activate
autophagy to ameliorate damage and ensure the survival of liver
cells (32). Increased expression of
MAP1LC3B and beclin 1 can indicate the increased autophagic
activity (22). Jung et al
(56) reported that mTOR complex 1
localized near mitochondria and was inhibited by oxidative stress
and mitochondrial dysfunction. Furthermore, the expression of mTOR
primarily in necrotic cells may imply that severely damaged cells
cannot survive by activating autophagy (57). Therefore, the authors of the present
study hypothesized that autophagy may be induced by an interaction
between mTOR and beclin 1 (PI3K-VPS34/beclin 1), and via the
destruction of mitochondria (56).
Previous studies suggested that the phosphorylation levels of
autophagy regulatory genes were closely associated with autophagy
activity (58,59). Wang et al (58) indicated that PKB-mediated
phosphorylation of beclin 1 served a role in autophagy inhibition.
The phosphorylation level of 4E-binding protein 1, the first
downstream substrate of mTOR, can directly reflect the activated
state of mTOR (59). Therefore, the
following study should evaluate the phosphorylation levels of
autophagy-associated proteins.
Furthermore, the present study demonstrated that the
expression of caspase-3, a key executor of apoptosis, was
upregulated in the process of liver damage. Zhu et al
(60) reported that beclin 1 was a
substrate of caspase-3 and the cleavage of beclin 1 may contribute
to inactivation of autophagy leading to augmentation of apoptosis.
Beclin1 protein includes a bcl-2-homology-3 domain, which can
combine with bcl-2/bcl-xl and abrogate anti-apoptotic effects
(51,61). In the present study, administration
of L-glutathione decreased the necrosis of hepatocytes. Expression
of MAP1LC3B in the high-dose glutathione group increased
significantly compared with the low-dose and model groups. The
expression of MAP1LC3B may be associated with the concentration of
glutathione. When mitochondria undergo damage by arecoline, ROS
accumulation may accelerate apoptosis and lead to necrosis
(32). However, L-glutathione, a
major component of the endogenous antioxidant defense system, may
accelerate autophagy to generate energy, decrease ROS production
and reduce liver damage (62). In
addition, the reactive sulfur atoms of glutathione can protect
hepatocytes by binding to a variety of chemicals and metabolites,
and increase the membrane stability (63). However, the exact association between
L-glutathione, apoptosis and liver injury remains to be further
elucidated in the future.
In conclusion, L-glutathione can effectively protect
against the hepatic injury caused by arecoline. Autophagy may be an
important mechanism in the procession of hepatic injury and
autophagic activity may be promoted by targeting
autophagy-associated genes. The results of the present study may
thus aid the treatment of drug-induced liver injury.
Acknowledgements
The authors would like to acknowledge postgraduate
Mr Wei Liu, undergraduates Mr Kai Sun, Miss Changyan Xiao and Miss
Shengnan Li of Binzhou Medical University (Yantai, China) for their
assistance. The authors would also like to thank the teachers of
the Medical Research Centre of Binzhou Medical University (Yantai,
China).
Funding
The present study was supported by the Medical and
Health Technology Development Program of Shandong Province (grant
no. 2016WS0055) and the Natural Science Fund of Shandong Province
(grant no. ZR2018MH034) grants for Associate Professor Peiyuan
Wang.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PW and XW designed the experiments; XW, XS and YS
performed the experiments; JX and BW analyzed the data; PW and XW
drafted the manuscript. All authors have read and approved the
final version for publification.
Ethics approval and consent to
participate
All experimental protocols involving animals and
human tissue samples were approved by the Ethics Committee of
Binzhou Medical University (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gupta PC and Warnakulasuriya S: Global
epidemiology of areca nut usage. Addict Biol. 7:77–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu NS: Effects of betel chewing on the
central and autonomic nervous system. J Biomed Sci. 8:229–236.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niu X, Li W, Xu H, Liu X and Qi L:
Simultaneous quantification of 11 isoquinoline alkaloids in
Corydalis impatiens (Pall.) Fisch by HPLC. J Sep Sci. 36:2090–2095.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warnakulasuriya S, Trivedy C and Peters
TJ: Areca nut use: An independent risk factor for oral cancer. BMJ.
324:799–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green PW, Simmonds MS and Blaney WM:
Toxicity and behavioural effects of diet-borne alkaloids on larvae
of the black blowfly, Phormia regina. Med Vet Entomol. 16:157–160.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shan LM and Wang H: Pharmacological
characteristics of the endothelial target for acetylcholine induced
vascular relaxation. Life Sci. 70:1285–1298. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellinger-Ziegelbauer H, Stuart B, Wahle B,
Bomann W and Ahr HJ: Characteristic expression profiles induced by
genotoxic carcinogens in rat liver. Toxicol Sci. 77:19–34. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin KH, Lin CY, Liu CC, Chou MY and Lin
JK: Arecoline N-oxide: Its mutagenicity and possible role as
ultimate carcinogen in areca oral carcinogenesis. J Agric Food
Chem. 59:3420–3428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou WW, Gun JY, Tsai JF, Hwang CC, Chen
HC, Huang JS, Yang YL, Hung WC and Chuang LY: Arecoline-induced
growth arrest and p21WAF1 expression are dependent on p53 in rat
hepatocytes Toxicology. 243:1–10. 2008.PubMed/NCBI
|
|
10
|
Zhou J, Sun Q, Yang Z and Zhang J: The
hepatotoxicity and testicular toxicity induced by arecoline in mice
and protective effects of vitamins C and E. Korean J Physiol
Pharmacol. 18:143–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasgupta R, Saha I, Pal S, Bhattacharyya
A, Sa G, Nag TC, Das T and Maiti BR: Immunosuppression,
hepatotoxicity and depression of antioxidant status by arecoline in
albino mice. Toxicology. 227:94–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng HL, Su SJ, Huang LW, Hsieh BS, Hu
YC, Hung TC and Chang KL: Arecoline induces HA22T/VGH hepatoma
cells to undergo anoikis-involvement of STAT3 and RhoA activation.
Mol Cancer. 9:1262010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang SW, Haydar G, Taniane C, Farrell G,
Arias IM, Lippincott-Schwartz J and Fu D: AMPK activation prevents
and reverses drug-induced mitochondrial and hepatocyte injury by
promoting mitochondrial fusion and function. PLoS One.
11:e01656382016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshimori T: Autophagy: A regulated bulk
degradation process inside cells. Biochem Biophys Res Commun.
313:453–458. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lozy F and Karantza V: Autophagy and
cancer cell metabolism. Semin Cell Dev Biol. 23:395–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong R, Xu H, Chen G, Zhao G, Gao Y, Liu
X, Ma S and Dong L: The role of hypoxia-inducible factor-1α in
radiation-induced autophagic cell death in breast cancer cells.
Tumor Biol. 36:7077–7083. 2015. View Article : Google Scholar
|
|
17
|
Weng J, Wang C, Wang Y, Tang H, Liang J,
Liu X, Huang H and Hou J: Beclin1 inhibits proliferation, migration
and invasion in tongue squamous cell carcinoma cell lines. Oral
Oncol. 50:983–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang C and Avery L: Death-associated
protein kinase (DAPK) and signal transduction: Fine-tuning of
autophagy in Caenorhabditis elegans homeostasis. FEBS J. 277:66–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicklin P, Bergman P, Zhang B,
Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson
C, et al: Bidirectional transport of amino acids regulates mTOR and
autophagy. Cell. 136:521–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Wang PY, Zhu YH and Li S:
Correlation between autophagy related genes expression and clinical
features in carcinogenesis of oral squamous cell carcinoma. Int J
Clin Exp Pathol. 9:6307–6316. 2016.
|
|
22
|
Yoshioka A, Miyata H, Doki Y, Yamasaki M,
Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y and Monden M:
LC3, an autophagosome marker, is highly expressed in
gastrointestinal cancers. Int J Oncol. 33:461–468. 2008.PubMed/NCBI
|
|
23
|
Dunning S, Rehman Ur A, Tiebosch MH,
Hannivoort RA, Haijer FW, Woudenberg J, van den Heuvel FA,
Buist-Homan M, Faber KN and Moshage H: Glutathione and antioxidant
enzymes serve complementary roles in protecting activated hepatic
stellate cells against hydrogen peroxide-induced cell death.
Biochim Biophys Acta. 1832:2027–2034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Sun K, Xiao CY, Li SN, Tian YP and
Wang CY: Effect of autophagy on glutathione protecting against
arecoline-induced hepatic injury. Chin J Clin Exp Pathol.
6:660–664. 2016.(In Chinese).
|
|
25
|
Saha I, Chatterjee A, Mondal A, Maiti BR
and Chatterji U: Arecoline augments cellular proliferation in the
prostate gland of male Wistar rats. Toxicol Appl Pharmacol.
255:160–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu XS, Shi H, Cao XL, Dong XF, Li T, Jiao
JJ, Qi JS and Wu MN: Effects of adiponectin on the anxiety and
memory impairment in triple transgenic Alzheimer's disease model
mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 33:405–409. 2017.(In
Chinese). PubMed/NCBI
|
|
27
|
Honoré PH, Basnet A, Eljaja L, Kristensen
P, Andersen LM, Neustrup S, Møllgaard P and Bjerrum OJ: Neuropathic
pain models in the development of analgesic drugs. Scand J Pain.
2:172–177. 2017. View Article : Google Scholar
|
|
28
|
Kimura H, Weisz A, Kurashima Y, Hashimoto
K, Ogura T, D'Acquisto F, Addeo R, Makuuchi M and Esumi H: Hypoxia
response element of the human vascular endothelial growth factor
gene mediates transcriptional regulation by nitric oxide: Control
of hypoxia-inducible factor-1 activity by nitric oxide. Blood.
95:189–197. 2000.PubMed/NCBI
|
|
29
|
Giordano C, Rivas J and Zervos X: An
update on treatment of drug-induced liver injury. J Clin Transl
Hepatol. 2:74–79. 2014.PubMed/NCBI
|
|
30
|
Björnsson ES, Bergmann OM, Björnsson HK,
Kvaran RB and Olafsson S: Incidence, presentation, and outcomes in
patients with drug-induced liver injury in the general population
of Iceland. Gastroenterology. 144:1419–1425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Au JS and Pockro PJ: Drug-induced liver
injury from antiepileptic drugs. Clin Liver Dis. 17:687–697. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshikawa Y, Miyashita T, Higuchi S,
Tsuneyama K, Endo S, Tsukui T, Toyoda Y, Fukami T, Nakajima M and
Yokoi T: Mechanisms of the hepatoprotective effects of tamoxifen
against drug-induced and chemical-induced acute liver injuries.
Toxicol Appl Pharmacol. 264:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eskelinen EL: The dual role of autophagy
in cancer. Curr Opin Pharmacol. 11:294–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen N and Karantza-Wadsworth V: Role and
regulation of autophagy in cancer. Biochim Biophys Acta.
1793:1516–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao
W, Lu B, Stolz DB, Clemens DL and Yin XM: Autophagy reduces acute
ethanol-induced hepatotoxicity and steatosis in mice.
Gastroenterology. 139:1740–1752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kan C, Liu A, Fang H, Dirsch O, Dahmen U
and Boettcher M: Induction of autophagy reduces
ischemia/reperfusion injury in steatotic rat livers. J Surg Res.
216:207–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu G, Yang Q, Yu Y, Lin S, Feng Y, Lv Q,
Yang J and Hu J: taurine inhibits kupffer cells activation induced
by lipopolysaccharide in alcoholic liver damaged rats. Adv Exp Med
Biol. 975:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Albanis E and Friedman SL: Antifibrotic
agents for liver disease. Am J Transplant. 6:12–19. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen J, Yu Y, Li S, Liu Y, Zhou S, Cao S,
Yin J and Li G: MicroRNA-30a ameliorates hepatic fibrosis by
inhibiting Beclin1-mediated autophagy. J Cell Mol Med.
21:3679–3692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mao YQ and Fan XM: Autophagy: A new
therapeutic target for liver fibrosis. World J Hepatol.
7:1982–1986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chaturvedi P, Vaishampayan SS, Nair S,
Nair D, Agarwal JP, Kane SV, Pawar P and Datta S: Oral squamous
cell carcinoma arising in background of oral submucous fibrosis: A
clinicopathologically distinct disease. Head Neck. 35:1404–1409.
2013.PubMed/NCBI
|
|
42
|
Mehrotra D and Kumar S, Agarwal GG,
Asthana A and Kumar S: Odds ratio of risk factors for oral
submucous fibrosis in a case control model. Br J Oral Maxillofac
Surg. 51:e169–e173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thangjam GS and Kondaiah P: Regulation of
oxidative-stress responsive genes by arecoline in human
keratinocytes. J Periodontal Res. 44:673–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yen CY, Lin MH, Liu SY, Chiang WF, Hsieh
WF, Cheng YC, Hsu KC and Liu YC: Arecoline-mediated inhibition of
AMP-activated protein kinase through reactive oxygen species is
required for apoptosis induction. Oral Oncol. 47:345–351. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McAuliffe PF, Meric-Bernstam F, Mills GB
and Gonzalez-Angulo AM: Deciphering the role of PI3K/Akt/mTOR
pathway in breast cancer biology and pathogenesis. Clin Breast
Cancer. 10 Suppl 3:S59–S65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang RC, Wei Y, An Z, Zou Z, Xiao G,
Bhagat G, White M, Reichelt J and Levine B: Akt-mediated regulation
of autophagy and tumorigenesis through Beclin 1 phosphorylation.
Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Menon S, Dibble CC, Talbott G, Hoxhaj G,
Valvezan AJ, Takahashi H, Cantley LC and Manning BD: Spatial
control of the TSC complex integrates insulin and nutrient
regulation of mTORC1 at the lysosome. Cell. 156:771–785. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cursio R, Colosetti P and Gugenheim J:
Autophagy and liver ischemia-reperfusion injury. Biomed Res Int.
2015:4175902015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang JH, Ahn IS, Fischer TD, Byeon JI,
Dunn WA Jr, Behrns KE, Leeuwenburgh C and Kim JS: Autophagy
suppresses age-dependent ischemia and reperfusion injury in livers
of mice. Gastroenterology. 141(2188–2199): e62011.PubMed/NCBI
|
|
51
|
Betsuyaku T, Eid N, Ito Y, Tanaka Y,
Otsuki Y and Kondo Y: Ethanol enhances thymocyte apoptosis and
autophagy in macrophages of rat thymi. Histol Histopathol.
32:963–975. 2017.PubMed/NCBI
|
|
52
|
Ni HM, Boggess N, McGill MR, Lebofsky M,
Borude P, Apte U, Jaeschke H and Ding WX: Liver-specific loss of
Atg5 causes persistent activation of Nrf2 and protects against
acetaminophen-induced liver injury. Toxicol Sci. 127:438–450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fang H, Liu A, Dahmen U and Dirsch O: Dual
role of chloroquine in liver ischemia reperfusion injury: Reduction
of liver damage in early phase, but aggravation in late phase. Cell
Death Dis. 4:e6942013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ni HM, Bockus A, Boggess N, Jaeschke H and
Ding WX: Activation of autophagy protects against
acetaminophen-induced hepatotoxicity. Hepatology. 55:222–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Patterson TA and Kosh JW: Elucidation of
the rapid in vivo metabolism of arecoline. Gen Pharmacol.
24:641–647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Codogno P, Mehrpour M and Proikas-Cezanne
T: Canonical and non-canonical autophagy: Variations on a common
theme of self-eating? Nat Rev Mol Cell Biol. 13:7–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang RC, Wei Y, An Z, Zou Z, Xiao G,
Bhagat G, White M, Reichelt J and Levine B: Akt-mediated regulation
of autophagy and tumorigenesis through Beclin 1 phosphorylation.
Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fingar DC, Salama S, Tsou C, Harlow E and
Blenis J: Mammalian cell size is controlled by mTOR and its
downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16:1472–1487.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhu Y, Zhao L, Liu L, Gao P, Tian W, Wang
X, Jin H, Xu H and Chen Q: Beclin 1 cleavage by caspase-3
inactivates autophagy and promotes apoptosis. Protein Cell.
1:468–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang W, Choi W, Hu W, Mi N, Guo Q, Ma M,
Liu M, Tian Y, Lu P, Wang FL, et al: Crystal structure and
biochemical analyses reveal Beclin 1 as a novel membrane binding
protein. Cell Res. 22:473–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
de Barboza Diaz G, Guizzardi S, Moine L
and de Talamoni Tolosa N: Oxidative stress, antioxidants and
intestinal calcium absorption. World J Gastroenterol. 23:2841–2853.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Circu ML and Aw TY: Glutathione and
modulation of cell apoptosis. Biochim Biophys Acta. 1823:1767–1677.
2012. View Article : Google Scholar : PubMed/NCBI
|