Introduction
Heroin addiction is a global problem with widespread
social and public health implications. Methadone maintenance
treatment (MMT) has been widely used to treat patients addicted to
heroin. However, it remains controversial whether methadone is
effective or not.
Behavioral studies (1–4) have
demonstrated that patients dependent on heroin or other opioids
have a significantly lower inhibitory control function than normal
volunteers. Animal experiments in mice have revealed that methadone
treatment (2.5–10 mg/kg) once daily for three weeks with repeated
withdrawals on Saturdays and Sundays has a negative impact on
cognitive function, regardless of whether methadone is detectable
in brain tissues (5). In addition,
neural apoptotic damage caused by chronic methadone administration
occurs in mouse brain white matter (6) and correlates with memory dysfunction
and depression in former heroin users receiving MMT (7).
Certain studies provided behavioral evidence that
MMT improves inhibitory control. Using a reaction time test based
on a stop-signal task (SST), one study (8) revealed that prior to MMT, the reaction
time in opioid-dependent individuals was significantly longer than
that in healthy controls. However, this difference disappeared
after these dependent individuals had been opioid-abstinent for two
months with MMT. Another study used the digit symbol substitution
task and continuous performance task to assess psychomotor speed,
and selective attention/impulsivity assessment revealed that
methadone-maintained individuals performed worse than controls
after <1 year of MMT. However, their performance approached that
of controls after longer treatment (1). A study that used the Stroop Color-Word
test to examine verbal learning, visuospatial memory and
psychomotor speed suggested that opioid-dependent subjects exhibit
significant improvement in cognitive function after two months of
MMT (9).
The disagreement between studies regarding whether
MMT affects inhibitory control function likely reflects the
inadequate consideration of numerous factors that may affect it,
including social support, personality and cognitive as well as
neural factors (10). For instance,
depression is ubiquitous among patients receiving MMT, although
different studies report a wide variety of prevalence rates
(11) and depressive disorders are
associated with interference control (12). A voxel-based morphometric study
revealed that MMT patients have structural deficits in the emotion
control circuit and cerebellum, which are associated with
depression, anxiety and cognitive dysfunction (13). Using diffusion tensor imaging, MMT
patients were found to have more memory and emotional deficits than
healthy subjects (7). Furthermore,
significant differences were also found in the white matter content
of the reward circuit and in depression- and memory-associated
regions. Individuals with high impulsivity and low inhibitory
control are more likely to become dependent on drugs (14). In addition, few event-associated
functional magnetic resonance imaging (fMRI) studies have directly
examined how therapy curbs the addictive thoughts and behaviors of
patients. Another noteworthy phenomenon is that most former studies
on MMT have been cross-sectional, preventing investigators from
adequately controlling for possible effects of previous addictive
drug use. Understanding whether and how MMT affects inhibitory
control function is essential for improving efficacy, which is
particularly important, as therapy is associated with a relatively
high rate of relapse (15).
In order to address these gaps in the literature,
the present study combined behavioral experiments with fMRI and
examined whether MMT alters particular neuronal circuits associated
with inhibitory control. This technique has been demonstrated to
provide sufficient temporal resolution to capture changes in
inhibitory control (16,17). Previous fMRI studies have indicated
that significant activation of inhibitory control primarily occurs
in the bilateral medial prefrontal cortex, anterior cingulate
cortex and inferior frontal gyrus (IFG) in healthy controls
(18–20). Experiments using fMRI have already
revealed that fewer brain regions and smaller overall brain areas
are activated under conditions of inhibitory control in
heroin-dependent individuals compared with those in healthy
controls (18).
The objective of the present study was to determine
whether MMT in heroin-dependent patients affects inhibitory
control, whether any MMT-induced changes correlate with the
methadone dose and MMT duration, and whether these changes depend
on the psychological characteristics of patients such as
depression, anxiety and impulsivity.
Materials and methods
Design
In the present study, performance in the classical
GO/NO-GO task at baseline and after one year of MMT was assessed.
Similar to SST, this is often used to assess the inhibitory control
of the motor response (21–24). These tests were complemented with
several standard behavioral assessments of inhibitory function,
including the Hamilton anxiety scale (HAMA) (25), the Beck Depression Inventory-II
(BDI-II) (26), the Protracted
Withdrawal Symptoms Scale (PWSS) (27) and the Barratt Impulsivity Scale 11
(BIS-11) (28).
Participants
The study protocol was approved by the Tangdu
Hospital Review Board (the Fourth Military Medical University,
Xi'an, China). MMT subjects were recruited from outpatients
receiving standard treatment at one of the Baqiao methadone
substitution treatment centers in Xi'an (China). All participants
provided written informed consent prior to entering into the study.
These patients underwent behavioral tests and fMRI scans between
October and December 2012, and these were taken again one year
later.
Inclusion criteria were as follows: i)
Right-handedness, normal vision and an age of 18–50 years; ii)
heroin dependence according to the Diagnostic and Statistical
Manual of Mental Disorders, Fourth Edition, Text Revision
(DSM-IV-TR) criteria (29); iii) a
score of >90 points on Raven's intelligence test, with no
clinically significant deficits in intelligence or language
communication; iv) a history of heroin use followed by complete
detoxification; v) intake of a stable dose of methadone for at
least three months prior to enrollment into the study.
Subjects were excluded from the study if they
presented any of the following: i) history of using cocaine or
other drugs of abuse besides heroin; ii) current alcohol intake of
>15 drinks (210 g alcohol; 1.5 oz of liquor or 12 oz of beer)
per week; iii) cerebral organic disease; iv) positivity for human
immunodeficiency virus; v) neurological signs and/or history of
neurological disease in the patient or first-degree relatives; vi)
decreased hepatic, renal, or cardiac function, and history of
long-term use of associated drugs; vii) claustrophobia or other MRI
contraindications; viii) a history of cardiovascular or endocrine
disease; ix) a current medical illness or recent medicine use; x)
fMRI data showing >1.5 mm of displacement and/or >1.5°
rotation in any of the axes during any of the task repetitions; or
xi) a combined accuracy rate <90% on the GO and NO-GO tasks and
an error rate of >50% on the NO-GO task.
Procedure
The test took 50 min to complete. In the first step,
clinico-demographic and psychological scale data were collected
(Tables I and II). In the second step, fMRI scans and the
GO/NO-go task were simultaneously performed. Once fMRI data were
acquired, participants were paid and verbally debriefed.
 | Table I.Demographic characteristics of
heroin-dependent Han Chinese patients undergoing methadone
maintenance treatment. |
Table I.
Demographic characteristics of
heroin-dependent Han Chinese patients undergoing methadone
maintenance treatment.
| Characteristic | Value | Range |
|---|
| Age, years | 35.8±8.0 | 22–48 |
| Education,
years |
9.3±2.1 |
6–12 |
| Cigarette use |
|
|
| Overall
duration, years | 17.2±8.2 |
2–30 |
| Daily
amount, n | 19.0±8.2 |
5–45 |
|
Lifetime amount, n |
123,839.2±77,947.6 | 3,650–306,600 |
| Heroin use |
|
|
| Overall
duration, years |
7.3±6.5 | 0.5–19 |
| Daily
dose, g/day |
0.5±0.4 | 0.1–2.0 |
|
Lifetime dose, g |
1,105.2±1,525.1 | 78–6,852 |
| Methadone use |
|
|
| Overall
duration, years |
2.3±1.4 | 0.3–4.7 |
| Daily
dose, mg |
42.2±16.7 | 12–80 |
|
Lifetime dose, mg |
50,994.7±35,460.4 | 9,480–162,425 |
| Total
dose during the 1-year study period, mg |
15,188.6±6,000.9 | 4,320–28,440 |
 | Table II.Personality and psychological
characteristics of heroin-dependent Han Chinese patients prior to
and after 1-year methadone maintenance treatment. |
Table II.
Personality and psychological
characteristics of heroin-dependent Han Chinese patients prior to
and after 1-year methadone maintenance treatment.
| Survey | Baseline value | Value after 1-year
therapy | t-value | P-value |
|---|
| BDI | 9.3±8.1 | 7.7±8.3 | 1.151 | 0.263 |
| HAMA | 6.4±7.6 | 7.9±6.3 | −1.104 | 0.283 |
| PWSS | 12.3±14.4 | 9.8±11.0 | 0.826 | 0.419 |
| BIS |
|
|
|
|
|
Total | 62.1±9.9 | 62.6±7.1 | −0.195 | 0.847 |
| AI | 14.0±3.0 | 14.3±2.8 | −0.255 | 0.801 |
| MI | 28.9±3.5 | 19.4±3.3 | 0.850 | 0.406 |
|
NPI | 27.9±5.5 | 28.9±3.5 | −0.708 | 0.487 |
Assessments
Clinico-demographic data collection.
Clinico-demographic characteristics of patients, as well as their
history of heroin use and methadone treatment prior to the start of
the one-year MMT period, were recorded. Patients were also assessed
at baseline for severity of psychological characteristics using
HAMA, BDI, PWSS and BIS-11. Patients were assessed again one year
later using the same instruments.
Relapse detection
To detect relapse events, participants were
interviewed monthly and their urine was tested for heroin.
GO/NO-GO test
The GO/NO-GO tasks were performed in two runs
(18,24,30),
which consisted of 316 GO blocks and 20 NO-GO blocks, which lasted
for 346 sec. The tasks were performed during each testing session.
Participants lay quietly in the MRI scanner during testing. They
were presented with a text description of the task for 10 sec prior
to commencement. During the GO trials, participants were presented
with the letters ‘X’ or ‘Y’ alternately. Letters appeared in white
on a black background at a resolution of 640×640; the letter
appeared for 900 msec, followed by a black screen that lasted for
100 msec (stimulus interval). Participants were instructed to press
button ‘1’ on their keypad as soon as they saw the letter ‘X’, and
press button ‘2’ when they saw the letter ‘Y’. If the alternate
rule was broken (as in the NO-GO trials), they were instructed not
to press any button. NO-GO trials were pseudo-randomly interspersed
throughout the GO trials. Performance measures on the GO/NO-GO
task, including reaction time, accuracy rate and error rate, were
collected and analyzed using E-prime version 2.0 (Psychology
Software Tools, Pittsburgh, PA, USA).
Participants were instructed to stop taking
methadone 20 h prior to the GO/NO-GO task, which was performed
between 8:00 a.m. and 12:00 p.m.
MRI data acquisition
fMRI data were acquired from participants during the
performance of the GO/NO-GO tasks. All imaging data were acquired
on a 3.0T MRI scanner (GE Signa Excite HD; GE Healthcare, Little
Chalfont, UK) equipped with an 8-channel head coil. After localizer
and conventional anatomical scans, GO/NO-GO blood oxygenation
level-dependent (BOLD) responses were measured as a function of
time using a T2*-weighted, single-shot, echo-planar imaging
sequence to acquire T2*-weighted image volumes with approximate
AC-PC alignment [repetition time (TR), 2,000 msec; echo time (TE),
30 msec, flip angle, 90°]. Each brain volume consisted of 32
transverse slices with the following characteristics: Matrix, 64
tri; field of view (FOV), 256×256 mm2; thickness, 4 mm;
no gap; spatial resolution, 4×4×4 mm3. Each patient
performed two GO/NO-GO task run, 168 echoplanar volumes were
collected in each run, therefore a total of 336 volumes were
collected for each patient. A high-resolution T1-weighted
structural image was acquired using a 166-slice, high-resolution,
fast-spoiled, gradient-echo 3-dimensional T1-weighted imaging
sequence with the following characteristics: TR, 7.8 msec; TE, 3.0
msec; matrix, 256×256 mm2; FOV, 256×256 mm2;
spatial resolution, 1×1×1 mm3.
MRI data processing
Structural data were first checked for abnormalities
independently by two experienced radiologists (Yarong Wang and Wei
Wang). Discrepancies were resolved through consultation. Data were
then analyzed using Statistical Parametric Mapping 8 software
(www.fil.ion.ucl.ac.uk/spm). A
6-parameter rigid-body transformation involving three rotations and
three translations was used to register images and correct for head
motion. Re-aligned datasets were spatially normalized to the
standard stereotactic space of the Montreal Neurological Institute
(MNI) using a 12-parameter affine transformation and a voxel size
of 3×3×3 mm3. Subjects that had >1.5 mm of head
translation or >1.5° head rotation were excluded from the
analysis. Data were spatially smoothed using an 8-mm
full-width-half-maximum Gaussian kernel.
The smoothed data were then analyzed using a General
Linear Model (17). Individual
contrast images captured under NO-GO conditions were compared with
individual images taken under the GO condition. In the second-level
analysis, these images were compared between the two groups using a
paired sample t-test. Subtraction results were presented by
overlaying the statistical maps onto the standardized MNI
anatomical image, with a threshold of P<0.05
(AlphaSim-corrected) and a cluster size of ≥12 voxels. For further
analysis, regions of interest (ROIs) were defined by positioning
spheres with a 2-mm radius at the local maxima in the statistical
map for each cluster.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc, Chicago, IL, USA), and the threshold of significance
was P<0.05. Normally distributed data were expressed as the mean
± standard deviation and assessed for significant differences
between baseline and after one year of MMT using the paired sample
t-test. Bivariate Pearson correlation analysis was used to examine
possible associations between the percentual change in ROI signal
intensity over the 1-year MMT period, as well as the
clinico-demographic characteristics and behavioral indicators of
the inhibitory control.
Results
Participants
Recruitment information was distributed to 139
heroin addicts undergoing regular MMT at Baqiao methadone
substitution treatment centers in Xi'an (China). A total of 78
subjects were initially recruited, of which 73 were males. However,
37 subjects failed to show up for baseline testing. Among the 41
subjects who performed baseline testing, 15 were lost to follow-up.
The remaining 26 subjects underwent testing at baseline as well as
one year later. However, five subjects had to be excluded due to
excessive motion during fMRI scanning or because their performance
on the GO/NO-GO task fell within the exclusion criteria. In the
end, the data of 21 Han Chinese men (mean age, 35.8 years; range,
22–48 years) were included in the final analysis (Table I).
During the one-year study period, monthly urine
testing detected 12 individuals who relapsed for an average of
three times. The average relapse dose was 0.4 g based on follow-up
interviews.
Depression and anxiety
characteristics
The BDI, HAMA, PWSS and BIS-11 scores of the
participants prior to and after the 1-year MMT period were similar
(Table II).
Inhibitory control behavior in the
GO/NO-GO task
Participants revealed no differences between
baseline and at 12 months in terms of reaction time, accuracy rate
under GO conditions, or false alarm rate under NO-GO conditions
(P<0.05; Table III).
 | Table III.Characteristics of inhibitory control
behavior of patients during execution of the GO/NO-GO task at
baseline and after 1 year of methadone maintenance treatment. |
Table III.
Characteristics of inhibitory control
behavior of patients during execution of the GO/NO-GO task at
baseline and after 1 year of methadone maintenance treatment.
| Items | Baseline value | Value after 1-year
therapy | t-value | P-value |
|---|
| Average reaction
time, msec | 407.33±71.99 | 400.30±77.65 | 0.337 | 0.710 |
| Average accuracy
rate on GO, % | 0.98±0.03 | 0.98±0.03 | −0.296 | 0.770 |
| False alarm rate on
NO-GO, % | 0.32±0.12 | 0.28±0.14 | 0.924 | 0.366 |
Inhibitory control-associated neural
activity during the GO/NO-GO task
Baseline fMRI scans taken prior to the one-year MMT
revealed numerous regions of increased activation during the
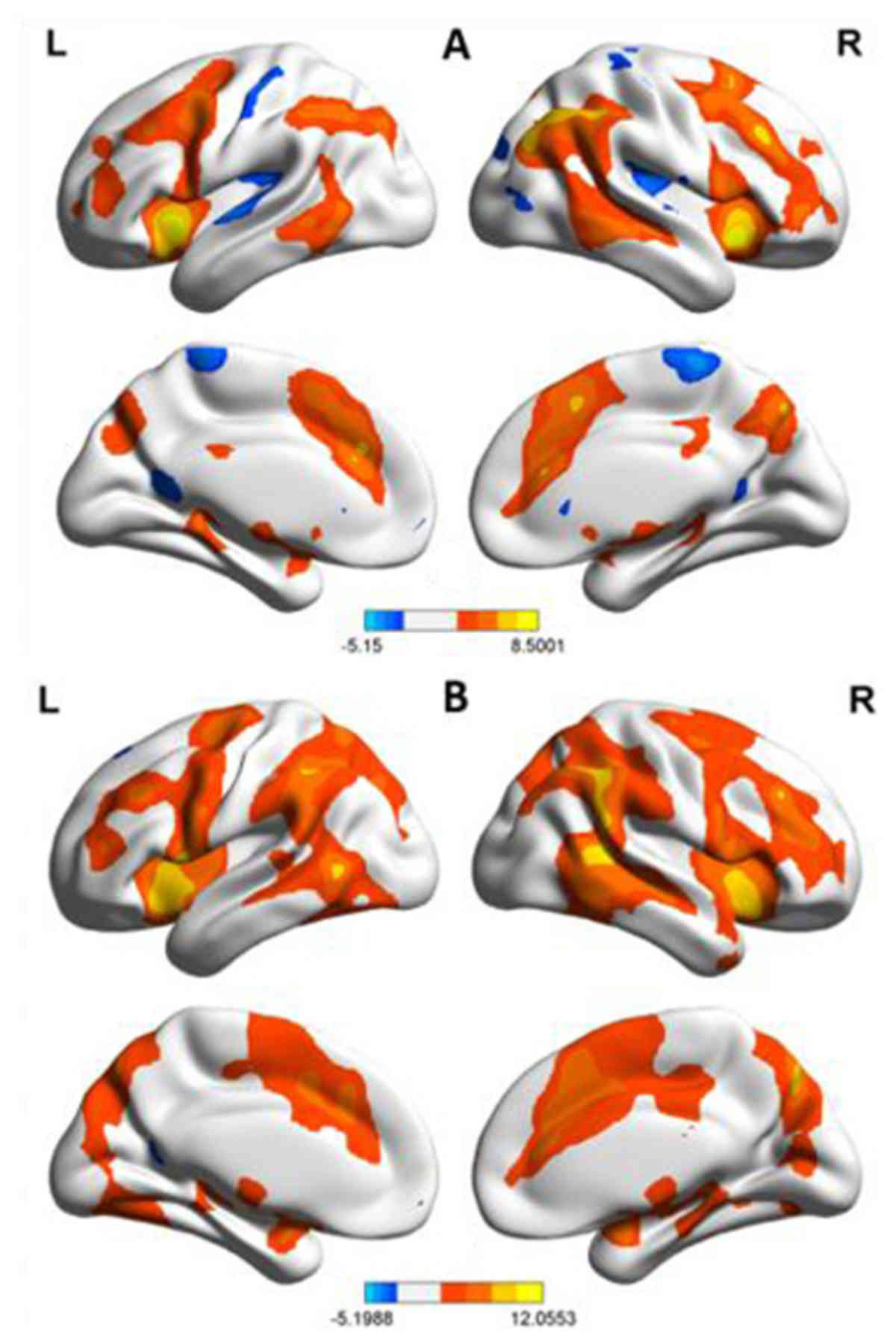
GO/NO-GO task (Fig. 1A). These
included the bilateral occipital lobe (cuneus and superior
occipital lobe), bilateral parietal lobe (superior parietal lobe,
inferior parietal lobule and precuneus), bilateral frontal lobule
(IFG, superior frontal gyrus, middle frontal gyrus and precentral
gyrus), limbic system (anterior cingulated cortex, insula, back of
the hippocampus and left parahippocampal gyrus), thalamencephalon
(caudate nucleus, lentiform nucleus, claustrum and red nucleus),
and cerebellum. Several regions of decreased activation were also
observed. These included left cuneus, bilateral paracentral lobule,
left precentral gyrus and left superior temporal gyrus.
On repeated fMRI scanning using the same protocol
after one year of MMT, the levels of activation were found to be
similar to those at baseline, although the overall area of
activation was greater (Fig. 1B). By
contrast, this decreased activation was observed in only two areas:
Left postcentral gyrus and right precuneus.
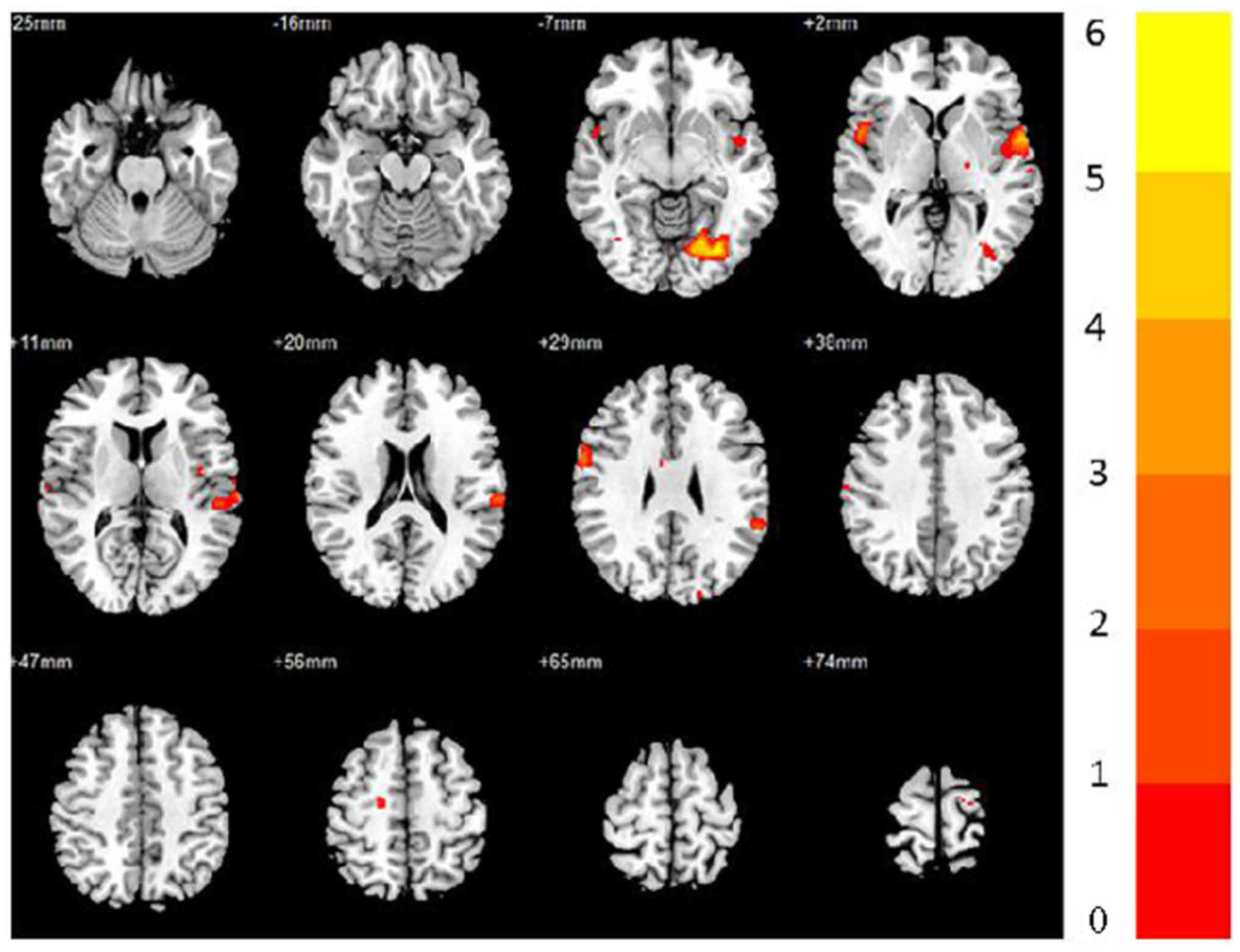
Differential analysis revealed that the following
regions had significantly higher activation levels at one year than
at baseline (AlphaSim-corrected P<0.005): Bilateral superior
temporal gyrus, precentral gyrus and insula; left postcentral
gyrus, cuneus, supramarginal gyrus, inferior parietal lobule and
lingual; right IFG and middle cingulate gyrus (Fig. 2; Table
IV). No brain areas were found to exhibit decreased activation
after the one-year MMT.
 | Table IV.Clusters of significant differences
in activation detected by functional magnetic resonance imaging in
patients during execution of the GO/NO-GO task at baseline and
after 1 year of methadone maintenance treatment (AlphaSim-corrected
P<0.005). |
Table IV.
Clusters of significant differences
in activation detected by functional magnetic resonance imaging in
patients during execution of the GO/NO-GO task at baseline and
after 1 year of methadone maintenance treatment (AlphaSim-corrected
P<0.005).
| Cluster | L/R | Brain region | x | y | z | Voxel | BA |
t-valuea |
|---|
| 1 | L | Superior temporal
gyrus | −60 | 4 | 2 | 165 | 22,41,42 | 4.89 |
|
| L | Insula | −44 | −6 | 6 | 34 | 13 | 3.68 |
| 2 | L | Lingual | −22 | −72 | −8 | 103 | 18,19 | 5.24 |
|
| L | Cuneus | −19 | −90 | 34 | 30 | 19 | 4.13 |
| 3 | L | Postcentral
gyrus | −64 | −26 | 20 | 45 | 40 | 4.08 |
|
| L | Inferior parietal
lobule | −60 | −42 | 28 | 28 | 40 | 3.94 |
|
| L | Supramarginal
gyrus | −60 | −44 | 30 | 17 | 40 | 3.98 |
| 4 | R | Superior temporal
gyrus | 54 | 6 | 2 | 45 | 22 | 4.22 |
|
| R | Inferior frontal
gyrus | 60 | 6 | 30 | 21 | 48 | 4.04 |
|
| R | Insula | 40 | −20 | 12 | 16 | 13 | 3.49 |
| 5 | L | Precentral
gyrus | −58 | 0 | 7 | 40 | 6 | 3.98 |
| 6 | R | Precentral
gyrus | 62 | 2 | 29 | 48 | 4,6 | 4.21 |
| 7 | R | Middle cingulate
gyrus | 4 | −2 | 45 | 20 | 24 | 3.62 |
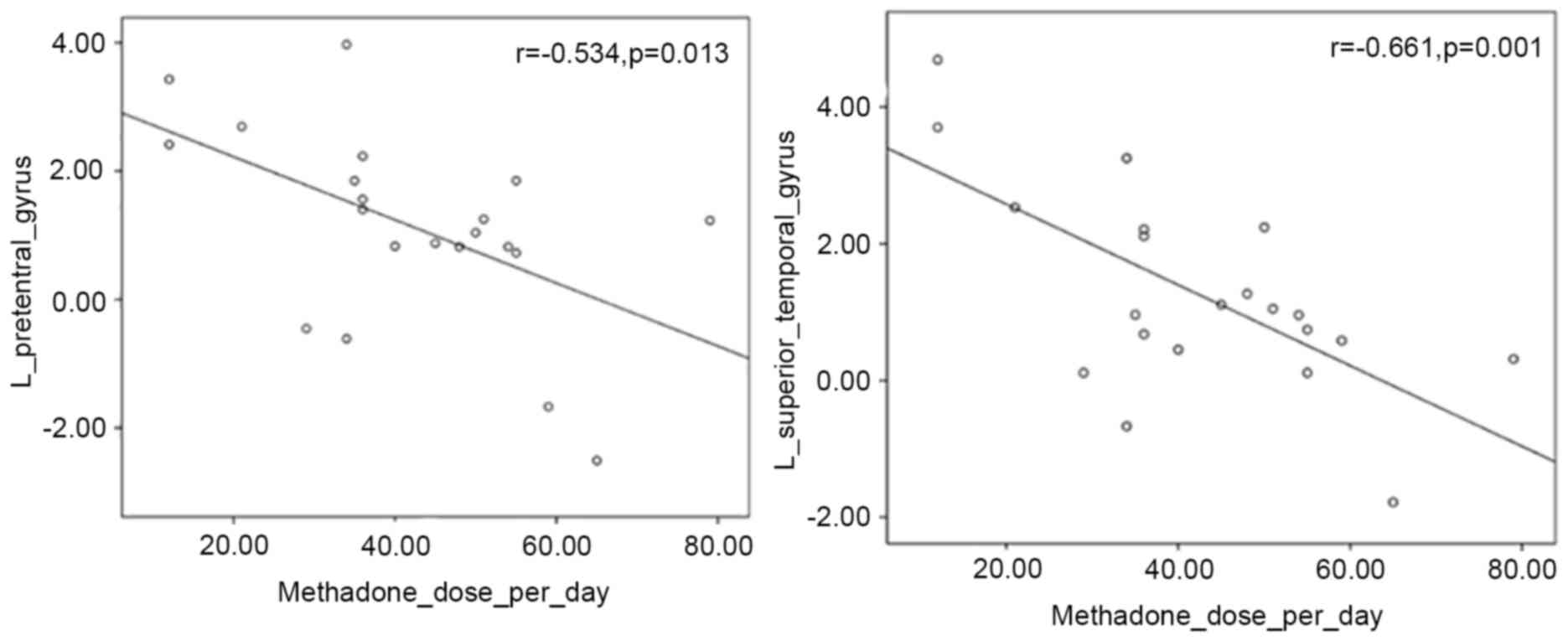
Correlations
Changes in fMRI signal intensities between baseline
and at one year were found to significantly correlate with one of
the clinico-demographic characteristics (Fig. 3). These were negatively correlated
with the total methadone dose during the one-year study period and
with the methadone dose per day in the left precentral gyrus
(P=0.013, r=−0.534) and in the left superior temporal gyrus
(P=0.001, r=−0.661).
At the same time, intensity changes in brain
activation did not display any significant associations with the
duration of heroin use, lifetime heroin dose or inhibitory control
behavior (data not shown).
Discussion
The present study aimed to clarify the controversial
question of whether MMT significantly affects inhibitory control
function in heroin-dependent patients, which was assessed in the
present study using a classical GO/NO-GO task. This behavioral task
was coupled with fMRI to capture dynamic changes in neural circuit
activation, which are potentially associated with inhibitory
control. In order to avoid certain limitations of previous studies,
the present study performed behavioral and fMRI measurements at
baseline and after one year of MMT. This did not compare patients
with healthy participants, thereby helping to isolate the effects
of methadone from those of heroin.
The present results suggested that one year of MMT
did not significantly change the personality inventory scores or
behavioral indicators of the inhibitory control function in
heroin-dependent patients. This is inconsistent with findings of
previous studies (3,8–10). This
apparent discrepancy may reflect the fact that these previous
studies were cross-sectional, whereas the present study was
longitudinal, which provided a potentially more reliable assessment
of the effects of MMT. In addition, previous studies compared their
patients with healthy controls, which meant that the outcomes may
have been attributed to the effects of heroin use, MMT or both. The
present study included only patients with a history of heroin use,
who had been undergoing MMT. Hence, the key difference between
measurements at baseline and at one year may only be MMT.
As far as we are aware, the effects of methadone on
inhibitory control in humans have not been previously reported,
although studies in animals suggested that the drug reduces
inhibitory control (4). The
observation of no significant change in inhibitory control behavior
in the GO/NO-GO task prior to or after one year of MMT may mean
that MMT actually augments inhibitory control in the long term. If
MMT initially induced such a decrease in the heroin-dependent
subjects of the present study, this would imply that there was a
prompt recovery to offset the damage. The event-associated fMRI
results further confirmed this hypothesis.
The fMRI scanning results of the present study
demonstrated greater activation in regions involved in inhibitory
control function after one year of MMT than at baseline. This is
consistent with the idea that one year of MMT increased inhibitory
control processing at the level of neural circuits, which may
reflect the increase in inhibitory control ability to a certain
extent. However, methadone is an opioid, and numerous studies on
the effects of MMT have reported that it may decrease certain
cognitive functions in the long term (1–4). These
two inconsistent findings may be explained by dual functions, the
negative methadone effect and the recovery of function after
withdrawal of heroin. Functional recovery after withdrawal of
opioids has also been confirmed by numerous studies (31–34).
This hypothesis was also supported by the correlation analysis
results between the change in signal intensity of brain activation
and the methadone dose found in the present study.
Increased BOLD intensity in the left precentral
gyrus and superior temporal gyrus was negatively correlated with
the methadone dose per day (mg/day) and the total methadone dose
during the one-year study period (mg), suggesting that methadone
negatively affected the activity in these brain regions. The
increase in signal may be explained by the functional recovery
after the withdrawal of heroin. In addition, the negative effects
of methadone appear to be outweighed by overall functional
recovery. Studies have demonstrated that heroin abstainers
gradually recover their inhibitory control function with increasing
abstinence time during MMT (1,8).
Consistent with these behavioral studies, an fMRI study revealed
that certain brain regions linked to inhibitory control gradually
became active with increasing abstinence time (31). The recovery of inhibitory control in
heroin-abstinent individuals independent of MMT may be assessed by
including an appropriate control arm in future studies. However,
whether this would comply with international ethical standards
remains controversial.
It was surprising that one-year MMT increased the
activation of brain regions associated with inhibitory control
without affecting inhibitory control behavior in the GO/NO-GO task.
A possible explanation may be that one year of MMT exerts effects
on brain regions associated with inhibitory control earlier than on
inhibitory control behavior. In 1995, Harnishfeger (35) proposed that inhibition occurs in two
stages: Cognitive inhibition and behavioral inhibition; and this
distinction continues to drive the field (36). Although two contrasting loop theories
have been proposed to explain inhibitory control (37,38),
each of the two viewpoints that inhibitory control is a top-down
process and moves from the back to the front of the brain have been
confirmed (39). The interaction of
the supplementary motor area with the subcortical brain and brain
stem nuclei then completes the inhibitory control process (39). The forehead-striatal loop, which
participates in motion control and response inhibition, is of
particular importance (40–44). The GO/NO-GO task is expected to
trigger cognitive as well as behavioral inhibition. All areas that
display higher activation after MMT than at baseline are associated
with the cognitive processes, including those occurring in the
superior frontal gyrus, which is responsible for receiving and
processing information (20,21,30,40,45,46).
However, no significant activation changes were observed in the
forehead-striatal loop, particularly in the basal ganglia domain
(47,48). This may explain why MMT patients
demonstrated no significant improvement in the behavioral test of
inhibitory control. The possible interpretation of these findings
is that MMT, for as short as one year, triggers improvement in the
cognitive inhibitory control processes. However, this time may be
too short to strengthen behavioral pathways. Future studies should
examine the two types of inhibitory control in greater detail over
a longer MMT.
The present study lays the groundwork for follow-up
studies to flesh out the effects of MMT at neural and behavioral
levels, but its results should be interpreted with caution. One
major limitation is the small sample size and heterogeneity in
patient characteristics, which may explain why the present study
failed to observe correlations of brain activity with either the
duration of heroin use, which ranged widely from 0.5 to 19 years,
or the lifetime heroin dose, which ranged from 78 to 6,852 g. Low
statistical power may also explain why the present study failed to
observe significant changes in behavioral inhibitory controls. All
study participants were male, highlighting the requirement to
perform similar studies in females. Relapses were detected based on
self-report and monthly urine testing, which may underestimate
actual relapse events. A large sample would also allow subgroup
analysis based on those who had relapsed and those who did not,
thereby providing further insight into the effects of MMT. Finally,
the present study assessed patients in a 12-month window, which may
be insufficient for assessing the therapeutic potential of MMT.
Despite these limitations, the present study
provided evidence that MMT increases inhibitory control function in
heroin-dependent patients and suggested that the therapy may
initially work by strengthening cognition-based inhibitory control
processes, with behavior-based processes modified in the longer
term. This may have implications for determining the minimal
effective MMT period. MMT should be beneficial for maintaining
heroin withdrawal and inhibitory control function recovery after
withdrawal. The present study also suggested that the psychological
and emotional state of patients may affect the efficacy of MMT.
However, the data did not reveal how the duration and the dose of
heroin or methadone affect MMT efficacy. The findings highlight the
requirement for more a detailed study with larger samples for
periods longer than one year.
Acknowledgements
The authors would like to thank the Baqiao methadone
substitution treatment centers in Xi'an (China) for providing
invaluable assistance. The authors are grateful to Dr Wan Yi
(Department of Statistics, The Fourth Military Medical University,
Xi'an, China) for his statistical assistance, and to Dr Wang Defeng
and Dr Liu Kai of the Department of Imaging and Interventional
Radiology, Chinese University of Hong Kong (Hong Kong, China) for
their editorial contribution to the manuscript. The authors also
thank Dr Zheng Dandan and Dr Zhou Zhenyu of GE Healthcare MR
Research (Beijing, China) for their support and assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81371532,
81201081, 81471648 and 81401393) and the Clinical Research
Foundation of Tangdu Hospital (grant no. 2013LCYJ003).
Authors' contributions
JJY, WL, DSZ, QL, WW and YRW conceived and designed
the experiments. JJY, JZ, JJC, YBL, XJY, JRL and XW performed the
experiments. JJY, DSZ and QL analyzed the data. JJY wrote the
manuscript.
Availability of data and materials
The datasets from our database are not publicly
available, but can be made available from the corresponding author
on reasonable request.
Ethics approval and consent to
participate
The study protocol was approved by the Tangdu
Hospital Review Board (the Fourth Military Medical University,
Xi'an, China). All participants provided written informed consent
prior to their inclusion in the study.
Consent for publication
All participants provided written informed consent
prior to entering into the study. In the informed consent, they
agreed to use the data for scientific research without revealing
their privacy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bracken BK, Trksak GH, Penetar DM,
Tartarini WL, Maywalt MA, Dorsey CM and Lukas SE: Response
inhibition and psychomotor speed during methadone maintenance:
Impact of treatment duration, dose, and sleep deprivation. Drug
Alcohol Depend. 125:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mintzer MZ, Copersino ML and Stitzer ML:
Opioid abuse and cognitive performance. Drug Alcohol Depend.
78:225–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Constantinou N, Morgan CJ, Battistella S,
O'Ryan D, Davis P and Curran HV: Attentional bias, inhibitory
control and acute stress in current and former opiate addicts. Drug
Alcohol Depend. 109:220–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verdejo A, Toribio I, Orozco C, Puente KL
and Pérez-García M: Neuropsychological functioning in methadone
maintenance patients versus abstinent heroin abusers. Drug Alcohol
Depend. 78:283–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersen JM, Olaussen CF, Ripel A and
Mørland J: Long-term methadone treatment impairs novelty preference
in rats both when present and absent in brain tissue. Pharmacol
Biochem Behavior. 98:412–416. 2011. View Article : Google Scholar
|
|
6
|
Tramullas M, Martínez-Cué C and Hurlé MA:
Chronic methadone treatment and repeated withdrawal impair
cognition and increase the expression of apoptosis-related proteins
in mouse brain. Psychopharmacology (Berl). 193:107–120. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin WC, Chou KH, Chen CC, Huang CC, Chen
HL, Lu CH, Li SH, Wang YL, Cheng YF and Lin CP: White matter
abnormalities correlating with memory and depression in heroin
users under methadone maintenance treatment. Plos One.
7:e338092012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao DL, Huang CY, Hu S, Fang SC, Wu CS,
Chen WT, Lee TS, Chen PC and Li CS: Cognitive control in opioid
dependence and methadone maintenance treatment. PloS One.
9:e945892014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gruber SA, Tzilos GK, Silveri MM, Pollack
M, Renshaw PF, Kaufman MJ and Yurgelun-Todd DA: Methadone
maintenance improves cognitive performance after two months of
treatment. Exp Clin Psychopharmacol. 14:157–164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nigg JT: On inhibition/disinhibition in
developmental psychopathology: Views from cognitive and personality
psychology and a working inhibition taxonomy. Psychol Bull.
126:220–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peles E, Schreiber S, Naumovsky Y and
Adelson M: Depression in methadone maintenance treatment patients:
Rate and risk factors. J Affect Disord. 99:213–220. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kavanaugh BC: The role of inhibitory
control in the hospitalization of children with severe psychiatric
disorders. Clin Neuropsychol. 30:369–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin WC, Chou KH, Chen HL, Huang CC, Lu CH,
Li SH, Wang YL, Cheng YF, Lin CP and Chen CC: Structural deficits
in the emotion circuit and cerebellum are associated with
depression, anxiety and cognitive dysfunction in methadone
maintenance patients: A voxel-based morphometric study. Psychiatry
Res. 201:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verdejo-Garcia A, Lawrence AJ and Clark L:
Impulsivity as a vulnerability marker for substance-use disorders:
Review of findings from high-risk research, problem gamblers and
genetic association studies. Neurosci Biobehav Rev. 32:777–810.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Camchong J, Stenger A and Fein G:
Resting-state synchrony during early alcohol abstinence can predict
subsequent relapse. Cereb Cortex. 23:2086–2099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mechelli A, Henson RN, Price CJ and
Friston KJ: Comparing event-related and epoch analysis in blocked
design fMRI. Neuroimage. 18:806–810. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friston KJ, Fletcher P, Josephs O, Holmes
A, Rugg MD and Turner R: Event-related fMRI: Characterizing
differential responses. Neuroimage. 7:30–40. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L,
Ming-Fan and Yang Z: Impaired response inhibition function in
abstinent heroin dependents: An fMRI study. Neurosci Lett.
438:322–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garavan H, Ross TJ, Murphy K, Roche RA and
Stein EA: Dissociable executive functions in the dynamic control of
behavior: Inhibition, error detection, and correction. Neuroimage.
17:1820–1829. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menon V, Adleman NE, White CD, Glover GH
and Reiss AL: Error-related brain activation during a Go/NoGo
response inhibition task. Hum Brain Mapp. 12:131–143. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swick D, Ashley V and Turken U: Are the
neural correlates of stopping and not going identical? Quantitative
meta-analysis of two response inhibition tasks. Neuroimage.
56:1655–1665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eagle DM, Bari A and Robbins TW: The
neuropsychopharmacology of action inhibition: Cross-species
translation of the stop-signal and go/no-go tasks.
Psychopharmacology (Berl). 199:439–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufman JN, Ross TJ, Stein EA and Garavan
H: Cingulate hypoactivity in cocaine users during a GO-NOGO task as
revealed by event-related functional magnetic resonance imaging. J
Neurosci. 23:7839–7843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simmonds DJ, Pekar JJ and Mostofsky SH:
Meta-analysis of Go/No-go tasks demonstrating that fMRI activation
associated with response inhibition is task-dependent.
Neuropsychologia. 46:224–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamilton M: The assessment of anxiety
states by rating. Br J Med Psychol. 32:50–55. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clinical practice guideline: Diagnosis and
evaluation of the child with attention-deficit/hyperactivity
disorder. American Academy of Pediatrics. Pediatrics.
105:1158–1170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
ZW H: The preliminary establishment of
opioid protracted withdrawal symptoms self evaluation scale. Chin
Mental Health J. 17:2942003.(In Chinese).
|
|
28
|
Patton JH, Stanford MS and Barratt ES:
Factor structure of the Barratt impulsiveness scale. J Clin
Psychol. 51:768–774. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Association AP: Diagnostic and statistical
manual of mental disorders. 4th ed. text rev.Washington, DC:
2002
|
|
30
|
Rubia K, Russell T, Overmeyer S, Brammer
MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V,
Andrew CM and Taylor E: Mapping motor inhibition: Conjunctive brain
activations across different versions of go/no-go and stop tasks.
Neuroimage. 13:250–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Li Q, Wang Y, Tian J, Yang W, Li
W, Qin W, Yuan K and Liu J: Brain fMRI and craving response to
heroin-related cues in patients on methadone maintenance treatment.
Am J Drug Alcohol Abuse. 37:123–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salo R, Nordahl TE, Galloway GP, Moore CD,
Waters C and Leamon MH: Drug abstinence and cognitive control in
methamphetamine-dependent individuals. J Subst Abuse Treat.
37:292–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schweinsburg AD, Schweinsburg BC, Medina
KL, McQueeny T, Brown SA and Tapert SF: The influence of recency of
use on fMRI response during spatial working memory in adolescent
marijuana users. J Psychoact Drugs. 42:401–412. 2010. View Article : Google Scholar
|
|
34
|
Li CS, Luo X, Sinha R, Rounsaville BJ,
Carroll KM, Malison RT, Ding YS, Zhang S and Ide JS: Increased
error-related thalamic activity during early compared to late
cocaine abstinence. Drug Alcohol Depend. 109:181–189. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harnishfeger KK: The development of
cognitive inhibitionInterferenee and Inhibition in Cognition.
Dempster FN and Brainerd CJ: Academic Press; San Diego, CA: 1995,
View Article : Google Scholar
|
|
36
|
Bari A and Robbins TW: Inhibition and
impulsivity: Behavioral and neural basis of response control. Prog
Neurobiol. 108:44–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nambu A, Tokuno H and Takada M: Functional
significance of the cortico-subthalamo-pallidal ‘hyperdirect’
pathway. Neurosci Res. 43:111–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aron AR and Poldrack RA: Cortical and
subcortical contributions to Stop signal response inhibition: Role
of the subthalamic nucleus. J Neurosci. 26:2424–2433. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Greenhouse I, Gould S, Houser M, Hicks G,
Gross J and Aron AR: Stimulation at dorsal and ventral electrode
contacts targeted at the subthalamic nucleus has different effects
on motor and emotion functions in Parkinson's disease.
Neuropsychologia. 49:528–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boehler CN, Appelbaum LG, Krebs RM, Hopf
JM and Woldorff MG: Pinning down response inhibition in the
brain-conjunction analyses of the Stop-signal task. NeuroImage.
52:1621–1632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alexander GE, Crutcher MD and DeLong MR:
Basal ganglia-thalamocortical circuits: Parallel substrates for
motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. Prog Brain
Res. 85:119–146. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghahremani DG, Lee B, Robertson CL,
Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron
AR, Mandelkern MA, et al: Striatal dopamine D(2)/D(3) receptors
mediate response inhibition and related activity in frontostriatal
neural circuitry in humans. J Neurosci. 32:7316–7324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jahfari S, Waldorp L, van den Wildenberg
WP, Scholte HS, Ridderinkhof KR and Forstmann BU: Effective
connectivity reveals important roles for both the hyperdirect
(fronto-subthalamic) and the indirect (fronto-striatal-pallidal)
fronto-basal ganglia pathways during response inhibition. J
Neurosci. 31:6891–6899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li CS, Yan P, Sinha R and Lee TW:
Subcortical processes of motor response inhibition during a stop
signal task. Neuroimage. 41:1352–1363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Swick D, Ashley V and Turken AU: Left
inferior frontal gyrus is critical for response inhibition. BMC
Neurosci. 9:1022008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dosenbach NU, Visscher KM, Palmer ED,
Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL
and Petersen SE: A core system for the implementation of task sets.
Neuron. 50:799–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brunia CH: Waiting in readiness: Gating in
attention and motor preparation. Psychophysiology. 30:327–339.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aron AR, Behrens TE, Smith S, Frank MJ and
Poldrack RA: Triangulating a cognitive control network using
diffusion-weighted magnetic resonance imaging (MRI) and functional
MRI. J Neurosci. 27:3743–3752. 2007. View Article : Google Scholar : PubMed/NCBI
|