Introduction
A number of factors contribute to nerve
injury-associated diseases (1–3). Among
these, spinal cord injury (SCI)-induced nerve damage is a serious
threat to health worldwide (4).
Following SCI, patients experience continuous and often diverse
neurological deficits and disability (5,6).
According to the location and severity of SCI, symptoms may vary
widely from pain or numbness to paralysis and incontinence
(7–10). SCIs may be primary or secondary.
Primary SCI involves the mechanical damage of neural elements,
including transection or distraction. This damage usually occurs in
cases of spinal fracture and/or dislocation, but may also occur in
its absence. Penetrating injury of the spinal cord, such as that
caused by bullets or weapons or the displacement of bone fragments,
may also lead to primary SCI. In addition, in oncology, spinal cord
compression may occur as a consequence of metastatic disease.
Secondary SCI is mainly caused by arterial rupture, arterial
thrombosis and insufficient perfusion (11,12).
Previous studies have indicated that reasonable and
effective exercise is able to promote recovery from nerve injury
(9,11). Aerobic exercise is regarded as an
effective means to reduce fat and weight, and enables patients with
obesity and diabetes to quickly improve their health (13,14). In
previous studies, researchers have focused on identifying the types
of exercise that are the most effective in helping patients who
have various diseases, such as diabetes (15,16). To
the best of our knowledge, there have been no studies concerning
the specific molecular mechanisms underlying the beneficial effects
of exercise, and it remains unclear whether exercise ameliorates
nerve injury (17,18). Thus, the aim of the present study was
to determine whether a defined amount of aerobic exercise is able
to ameliorate SCI and the pathogenesis of nerve injury, and
investigate the detailed molecular mechanisms by which aerobic
exercise promotes recovery from SCI-induced nerve injury.
Materials and methods
Animals
A total of 24 male Sprague-Dawley rats (6 weeks old;
weight, 150–200 g) were purchased from the Shanghai Experimental
Animal Center (Shanghai, China) and housed in a temperature and
humidity-controlled environment (25±2°C, 50±10% humidity) with a
standard 12-h light/dark cycle. Water and food were available ad
libitum in their housing. The study was approved by the ethics
committee of Sichuan Academy of Medical Sciences and Sichuan
Provincial People's Hospital (Chengdu, China). All protocols were
conducted in accordance with the guidelines of the Institutional
Animal Care and Use Committee of Sichuan Academy of Medical
Sciences.
The rats were randomly divided into four groups, six
in each group: i) Control (CT group); ii) SCI (model group); iii)
model with 15 min/day exercise (15 min/day group); iv) model with
30 min/day exercise (30 min/day group). A clinically relevant model
of SCI mimicking the impact injury that occurs following traffic
and other accidents was established. The rats were anesthetized
with 80 mg/kg ketamine (Ketaset; Pfizer, Inc., New York, NY, USA)
and 10 mg/kg xylazine (AnaSed; Lloyd Laboratories, Inc. Shenadoah,
IA, USA) by intraperitoneal administration, and a laminectomy was
performed at T10. Following immobilization of the spine with a
spinal stereotactic device, SCI was induced by the method of Perot,
which involved dropping a 5-g weight from a height of 8 cm onto an
impounder (0.3 cm in diameter) gently placed on the spinal cord
(19). Buprenorphine (0.01–0.05
mg/kg) was administered for the treatment of post-operative pain.
Following the surgery, the rats were helped to urinate via massage
of the bladder twice per day until the micturition reflex was
recovered. An intraperitoneal injection of penicillin (200,000
units) was administered twice per day to protect against infection
during the first week after surgery. Penicillin was also provided
in cases of infection occurrence.
The Basso Beattie Bresnahan (BBB) scoring system
(20) was employed to assess the
neurological function of the rats using a double-blind method. This
scoring system ranges from 0 to 21, where complete paraplegia is
scored 0 and normal is scored 21. The scores were determined in a
blinded manner by research assistants who were familiar with the
BBB scoring system. The rats were allowed to move freely on a wide
and flat surface for 2 min. The average score was obtained for each
assessment and three assessments for each rat were conducted. The
rats in the two treatment groups underwent passive walking
rehabilitation training in the roller training machine, which the
authors created, with a speed of 5 revolutions/min for 15 or 30
min/day, respectively, with the first training session occurring at
24 h post-injury. Following each 1-min period of movement during
the training, the rats were allowed to rest for 2 min. The duration
of the training period was 30 days. On day 30, following anesthesia
with 5% chloral hydrate (50 mg/kg intraperitoneally; rat body
weight at sacrifice, ~200 g), the rats were fixed in the supine
position and sacrificed by decapitation. A 1-cm section of spinal
cord centered on the lesion site, a 1-cm rostral section (starting
0.5 cm rostral from the impact site) and a caudal penumbra section
(starting 0.5 cm caudal to the impact site) were taken for all
groups for quantitative analysis.
Histological examination
Spinal cord sections of rats from the four groups
were subjected to hematoxylin and eosin (H&E) staining and
examined to detect tissue injury and inflammatory response using
light microscopy. Immunohistochemical (IHC) examination was
conducted to investigate astrocyte activation using glial
fibrillary acidic protein (GFAP) antibody. Immunofluorescence
examination of the tissues was also conducted to investigate
SCI-induced nerve injury via the detection of proteins associated
with nuclear factor (NF)-κB signaling, including TLR4, MyD88, GFAP
and p-IκBα. Briefly, 8-µm-thick tissues were fixed with 2.5%
glutaraldehyde in 0.1 mol/l phosphate buffer (pH 7.4) at room
temperature for 15 min. The sections were wash twice (4 min each
wash) with TBS supplemented by 0.1% saponin (Merck KGaA, Darmstadt,
Germany). Endogenous peroxidase activity was blocked by incubating
the sections with TBS supplemented by 0.3%
H2O2, 0.1% saponin and 0.02% NaN3
for 30 min at 4°C. The sections were wash three times (3 min each
wash) with TBS supplemented by 0.1% saponin. Next, non-specific
binding sites were blocked diluted goat serum (1:100; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in TBS/saponin for 20
min at 37°C. They were then incubated overnight at 4°C, with the
following primary antibodies: Rabbit anti-human TLR4 (1:500; cat.
no. ab13556), mouse anti-human MyD88 antibody (1:1,000; cat. no.
ab107585), rabbit anti-human GFAP polyclonal antibody (1:500; cat.
no. ab7260) and rabbit anti-human p-IκBα polyclonal antibody
(1:500; cat. no. ab92700; all Abcam, Cambridge, UK). The sections
were wash four times (3 min each wash) with TBS supplemented by
0.1% saponin, then incubated with the following secondary
antibodies for 30 min at 37°C: Horseradish peroxidase-conjugated
goat anti-mouse immunoglobulin (Ig)G (1:200; cat. no. ab6789) and
rabbit anti-mouse IgG (1:200; cat. no. ab6728; both Abcam). The
resulting peroxidase activity were revealed by incubating the
slides with a 0.5 mg/ml DAB (Beijing Zhongshan Golden Bridge
Biotechnology; OriGene Technologies, Inc., Beijing, China) +
H2O2 prepared in distilled water. The
sections were wash four times in TBS (5 min/wash) and
counterstained for 1 min at room temperature with hematoxylin.
Next, they were dehydrated with sequential washes (1 min each wash)
with 75, 80 and 100% ethanol. The sections were analyzed by optical
microscopy and Image-Pro Plus (version 5.0) was used to quantify
the data.
ELISA measurements
On day 30, following anesthesia with 1.0%
pentobarbital sodium (45 mg/kg intraperitoneally), blood was
extracted from the eyeball. The inflammatory cytokines interleukin
(IL)-1β (cat. no. MLB00C), IL-6 (cat. no. D6050), IL-18 (cat. no.
DBP180) and tumor necrosis factor (TNF)-α (cat. no. MTA00B) in the
serum were tested using ELISA kits in accordance with the
manufacturer's instructions (R&D Systems, Inc., Minneapolis,
MN, USA).
Western blot analysis
For western blotting, 100 mg spinal cord tissues
from rats in the different groups were lysed using
radioimmunoprecipitation assay lysis buffer [150 mM NaCl, 0.1%
Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate
and 50 mM Tris-HCl, pH 8.0] to yield a homogenate. The final
supernatants were then obtained by centrifugation at 4°C and 12,000
× g for 15 min. The protein concentration was calculated using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.) with bovine serum albumin (Gibco; Thermo Fisher Scientific,
Inc.) as a standard. The total protein extract was used for western
blot analysis. In this analysis, 40 µg total protein was loaded per
lane and proteins were separated using 10% SDS-PAGE and
electrophoretically transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
then blocked with 5% non-fat dry milk in Tris-buffered saline (20
mM Tris, pH 7.6, 137 mM NaCl) with 0.1% Tween-20 for 60 min at room
temperature, washed and then incubated with primary antibody
overnight at 4°C. The membranes were washed again, incubated with
secondary antibody at 37°C for 1 h and scanned following the use of
the ECL Advanced Western Blotting kit (GE Healthcare Life Sciences,
Little Chalfont, UK). The relative expression of protein was
analyzed by Quantity One 1-D software (version 4.6.9; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) following internal reference
calibration. The primary antibodies were as follows (1:1,000):
Rabbit anti-NF-κB (cat. no. ab16502), transforming growth factor
β1-activated kinase 1 (TAK1; cat. no. ab109526), TLR4 (cat. no.
ab22048), IκBα (cat. no. ab32518), MyD88 (cat. no. ab2064), p-NF-κB
(cat. no. ab86299), p-IκBα (cat. no. ab133462), p-IKKβ (cat. no.
ab59195), inhibitor of NF-κB kinase subunit β (IKKβ; cat. no.
ab178870; all Abcam) and GAPDH (cat. no. 14C10; Cell Signaling
Technology, Inc., Danvers, MA, USA). The secondary antibodies were
as follows: Horseradish peroxidase-conjugated horse anti-mouse
secondary antibodies (cat. no. 7076; 1:1,000; Cell Signaling
Technology, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was carried out to calculate RNA expression
levels. Total RNA of tissue samples was extracted, frozen and
pulverized using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and cDNA was synthesized from the RNA with oligo
(dT) primers using the Advantage RT-for-PCR kit (Clontech
Laboratories, Inc., Mountainview, CA, USA). qPCR was performed
using SYBR-Green I (Invitrogen; Thermo Fisher Scientific, Inc.) and
normalized to GAPDH gene expression. All primer sequences (Table I) used for qPCR were produced by
Invitrogen (Thermo Fisher Scientific, Inc.). The cycling conditions
were as follows: Pre-denaturation at 95°C for 5 min, and 40 cycles
of denaturation at 95°C for 10 sec, annealing at 58°C for 15 sec
and extension at 72°C for 20 sec. The copy number was calculated
according to the 2−ΔΔCq method described in a previous
study (21).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Protein | Direction | Sequence
(5′-3′) |
|---|
| TLR4 | Forward |
TGATGTCTGCCTCGCGCCTG |
|
| Reverse |
TAGGAACCACCTCCACGCAGGG |
| MyD88 | Forward |
GCATGGAACCAGTGGCTGTGAG |
|
| Reverse |
GAGGAAGTGGAATGGGCGGTGT |
| IκBα | Forward |
GCAAAATCCTGACCTGGTGT |
|
| Reverse |
GCTCGTCCTCTGTGAACTCC |
| TAK1 | Forward |
CAACTACAGCCTCTAGCAC |
|
| Reverse |
CTTATCATGTCTGCTCGAAG |
| IKKβ | Forward |
CCGTGACTGTTGACTACTG |
|
| Reverse |
GTCCACTTCGCTCTTCTG |
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The treated tissues and corresponding controls were
compared using GraphPad Prism version 6.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) by one-way analysis of variance with Dunn's
least significant difference test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Exercise improves the functional
outcomes of the rats
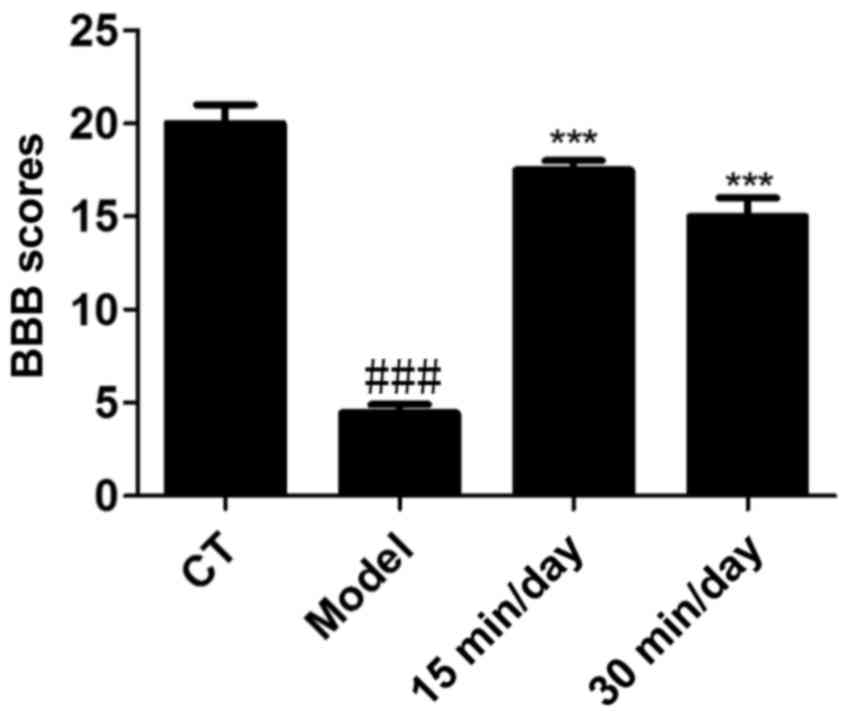
The BBB scoring system was employed to assess the
neurological functions of the rats in each group. Prior to
training, the BBB score of the SCI model rats was 1. As shown in
Fig. 1, the BBB scores of the rats
that received 15 and 30 min exercise training each day were
significantly higher compared with those of the model group. This
indicates that effective and moderate exercise helped to improve
the functional outcomes of the rats.
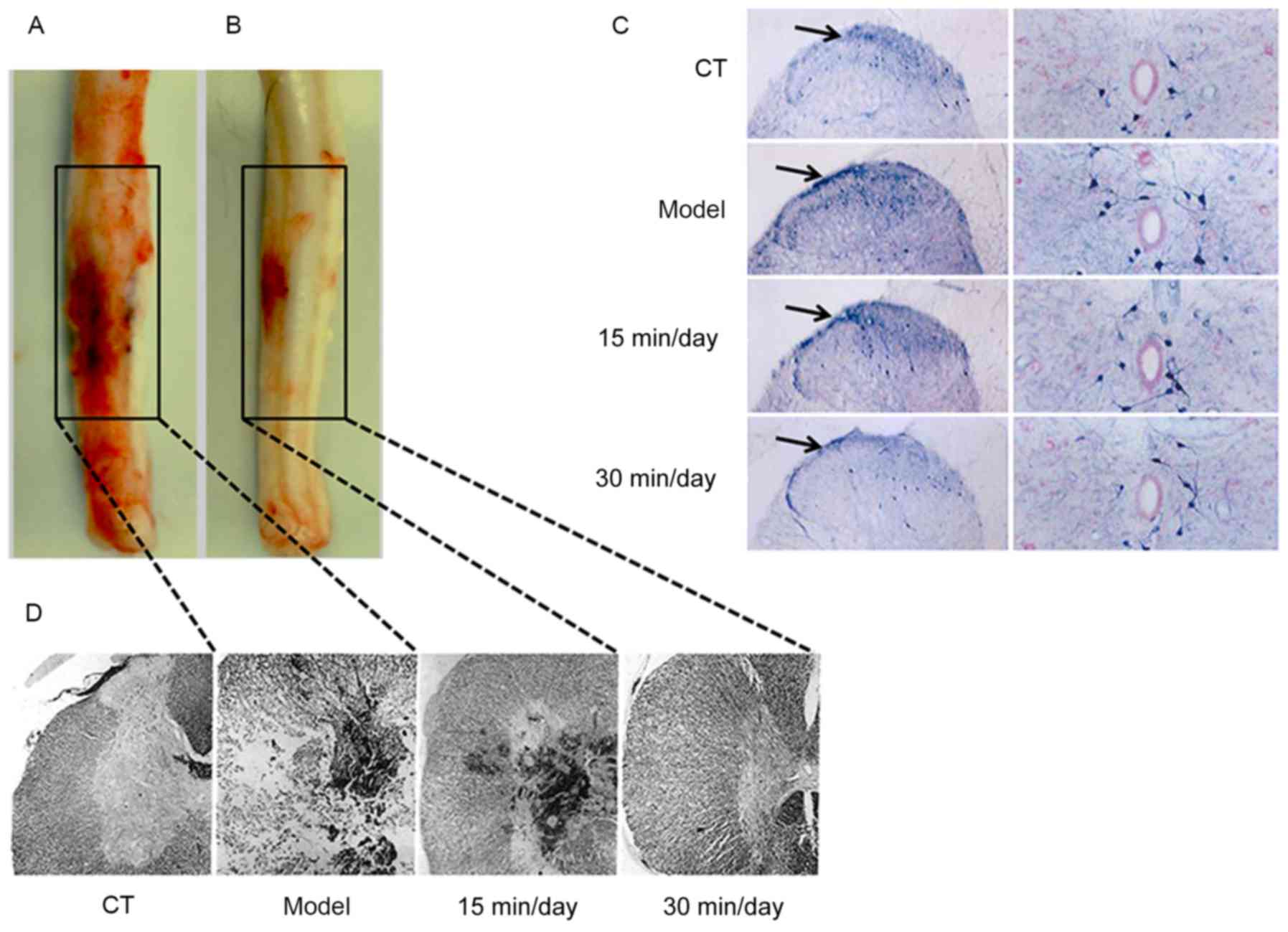
Exercise suppresses SCI-stimulated
tissue damage
Rats were treated with training exercise for 15 or
30 min/day for 30 days, after which the SCI and tissue inflammatory
responses were evaluated. As shown in Fig. 2A and B, the spinal cords of the model
and 30 min/day exercise treatment groups appeared clearly
different. Also, GFAP was markedly activated in the model group
compared with the CT and exercise treatment groups (Fig. 2C). These results indicate that
effective and moderate exercise helps to ameliorate SCI and
promotes the resolution of inflammation. It also appears that the
degree of improvement may be proportional to the duration of
exercise. The H&E staining images presented in Fig. 2D further demonstrate that SCI caused
tissue damage and induced an inflammatory response, and that
exercise was able to suppress the upregulated inflammatory
response.
Exercise ameliorates the inflammatory
response in the serum and spinal cord
Inflammation-associated cytokines serve an important
role in SCI-induced nerve injury and neuroinflammation. Studies
have demonstrated that a series of cytokines and
inflammation-related signaling pathways may be activated during the
occurrence of SCI (22). The ELISA
results in Fig. 3A-D illustrate the
changes of pro-inflammatory cytokines that occurred in the serum of
the rats following SCI. These results demonstrate that SCI
significantly increased the serum levels of pro-inflammatory
cytokines and promoted their release. However, the 30-min exercise
treatment significantly suppressed the pro-inflammatory response
compared with that in the model group. Fig. 3E-H shows the corresponding mRNA
expression levels of these cytokines in the spinal cord of the
different groups. These results indicate that moderate exercise
attenuated the SCI-induced changes in RNA levels. The results are
consistent with those for the respective proteins. Fig. 3I shows the relative mRNA expression
levels of other inflammatory cytokines (interferon-γ, IL-17, IL-10,
IL-2, IL-4 and IL-1) in the four groups. The mRNA expression levels
of these major cytokines were also significantly downregulated
following exercise training compared with those in the model
group.
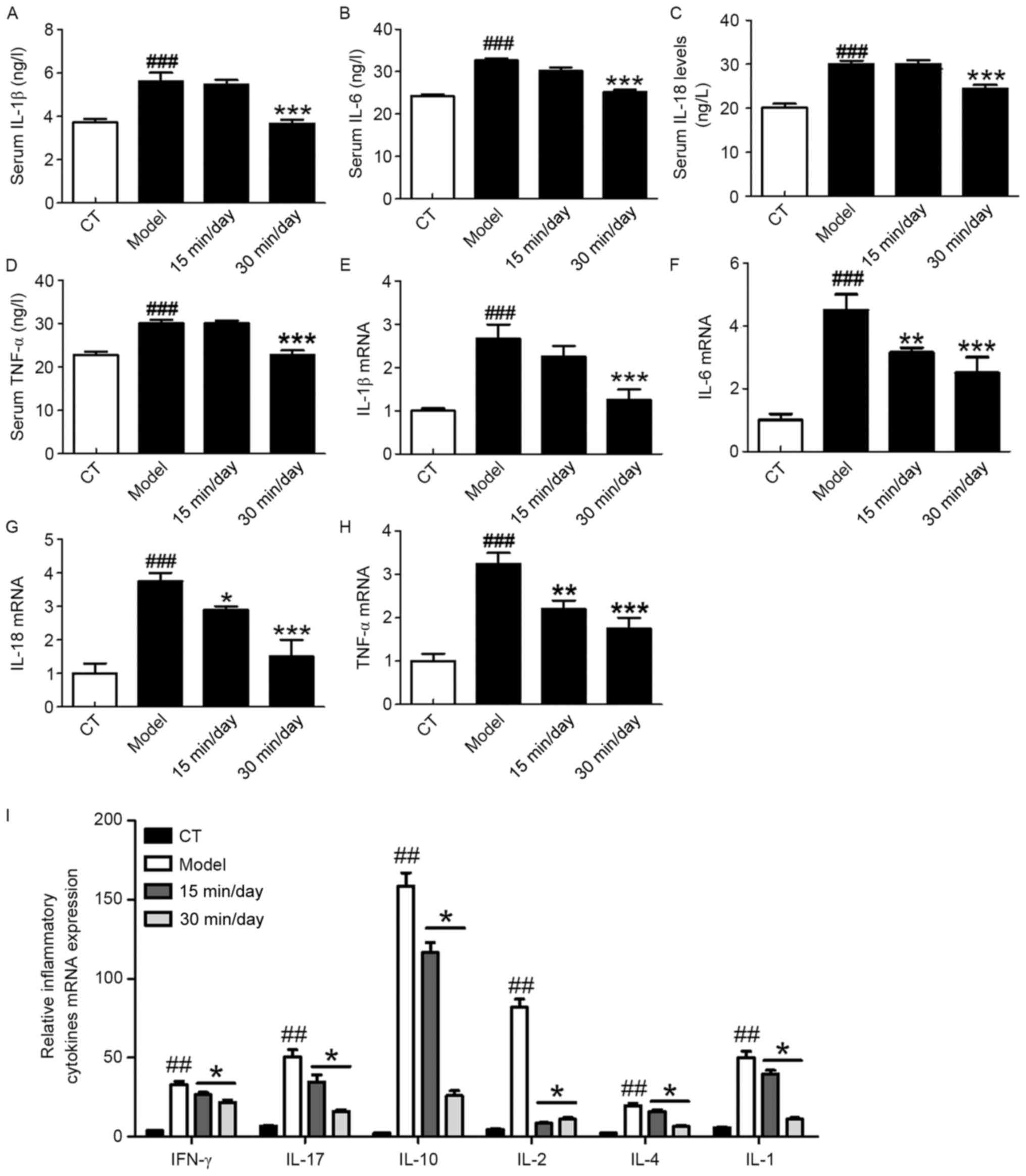 | Figure 3.Exercise attenuates the inflammatory
responses of peripheral tissues and spinal cord of rats following
SCI. ELISA analysis demonstrating the changes in the protein levels
of the pro-inflammatory cytokines (A) IL-1β, (B) IL-6, (C) IL-18
and (D) TNF-α in the serum. RT-qPCR analysis showing the changes in
the mRNA expression of (E) IL-1β, (F) IL-6, (G) IL-18 and (H) TNF-α
in the spinal cord. (I) RT-qPCR analysis of the mRNA expression of
further inflammatory cytokines in the SCI model. Significance,
using one-way analysis of variance with Dunn's least significant
difference tests, is indicated. ##P<0.01 and
###P<0.001 vs. the CT group; *P<0.05, **P<0.01
and ***P<0.001 vs. the model group. SCI, spinal cord injury; IL,
interleukin; TNF, tumor necrosis factor; IFN, interferon; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
Exercise inhibits SCI-induced glial
activation and nerve inflammation
Whether the NF-κB pathway is involved in the
inflammatory process was then investigated using immunofluorescence
(Fig. 4). The results in Fig. 4A-C and E show that components of the
NF-κB pathway were significantly upregulated in the model group
compared with the CT group. The immunofluorescence results in
Fig. 4D indicate that GFAP positive
cells, which serve as a marker of nerve injury (23), were also induced in the SCI model.
These results demonstrate that SCI significantly increased GFAP
cell activation, and promoted neuroinflammation and nerve injury.
However, the training exercise helped to attenuate the damage.
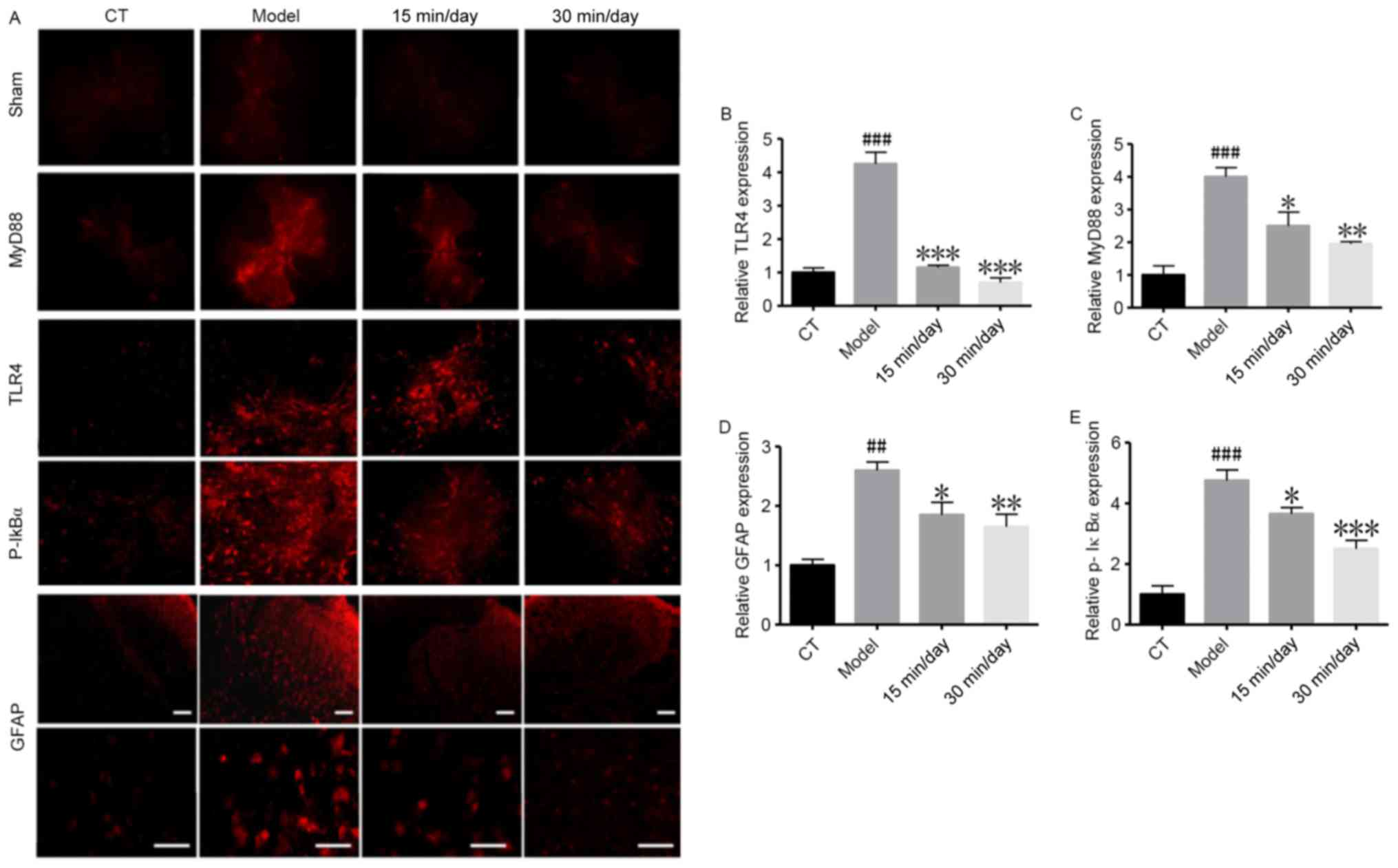 | Figure 4.Exercise inhibits SCI-induced glial
activation and neural inflammation. Immunofluorescence assays were
used to analyse GFAP activation in glial cells and nuclear
factor-κB-associated indicators following SCI. (A) Representative
immunofluorescence staining images. Magnification, ×400. Quantified
results showing the relative (B) TLR4, (C) MyD88, (D) GFAP and (E)
p-IκBα protein expression. Data of all groups are normalized to the
control. Significance, using one-way analysis of variance with
Dunn's least significant difference tests, is indicated.
##P<0.01 and ###P<0.001 vs. the CT
group; *P<0.05; **P<0.01 and ***P<0.001 vs. the model
group. SCI, spinal cord injury; GFAP, glial fibrillary acidic
protein; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation
primary response gene 88; p-IκBα, phosphorylated inhibitor of κB;
CT, control. Scale bar, 100 µm. |
Exercise regulates SCI-induced
activation of the NF-κB inflammatory signaling pathway
Western blot analysis further indicated that SCI
significantly induced activation of the NF-κB-related inflammatory
signaling pathway. As shown in Fig.
5, in the model group, the protein expression of TLR4, MyD88,
TAK1, p-IκBα, p-IKKβ and p-NF-κB was significantly upregulated
compared with that in the control group. Also, the treatment groups
exhibited significantly lower expression levels of these proteins
compared with the model group, indicating that reasonable exercise
has the ability to attenuate SCI. RT-qPCR analysis was also used to
investigate these NF-κB-associated indicators, and the demonstrated
that the mRNA levels of TLR4, MyD88, IκBα, TAK1 and IKKβ were
significantly increased in the animals with SCI compared with the
CT group (Fig. 6). The results were
consistent with the results of western blotting.
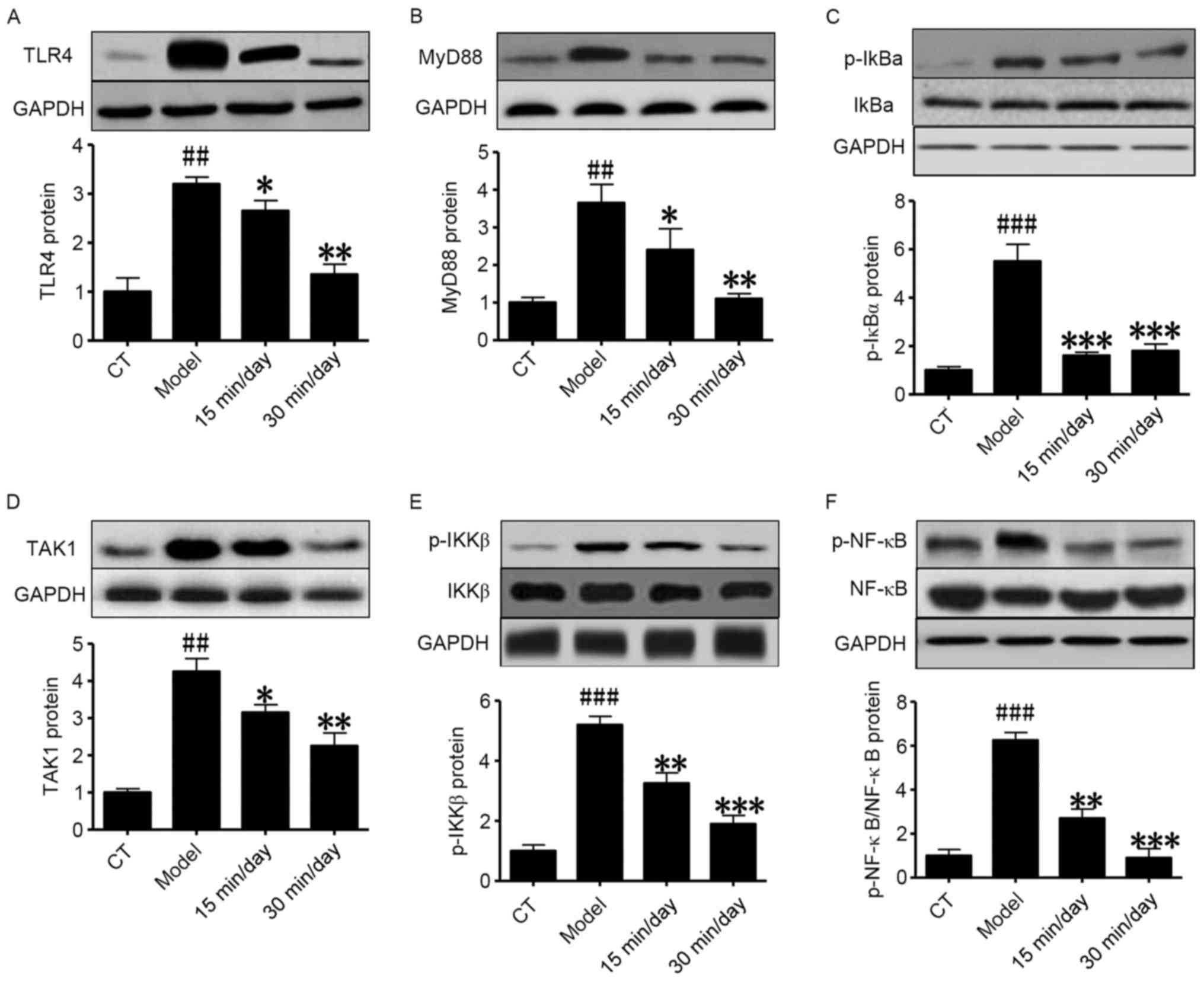 | Figure 5.Exercise inhibits SCI via the
inhibition of NF-κB pathways. Western blot analysis of the
expression of NF-κB pathway-associated proteins in a rat model of
SCI. Relative protein expression of (A) TLR4, (B) MyD88, (C)
p-IκBα, (D) TAK1, (E) p-IKKβ and (F) p-NF-κB. Significance, using
one-way analysis of variance with Dunn's least significant
difference tests, is indicated. ##P<0.01 and
###P<0.001 vs. the CT group; *P<0.05; **P<0.01
and ***P<0.001 vs. the model group. SCI, spinal cord injury;
NF-κB, nuclear factor-κB; TLR4, Toll-like receptor 4; MyD88,
myeloid differentiation primary response gene 88; p-IκBα,
phosphorylated inhibitor of κB; TAK1, transforming growth factor
β1-activated kinase 1; p-IKKβ, phosphorylated inhibitor of NF-κB
kinase subunit β; CT, control. |
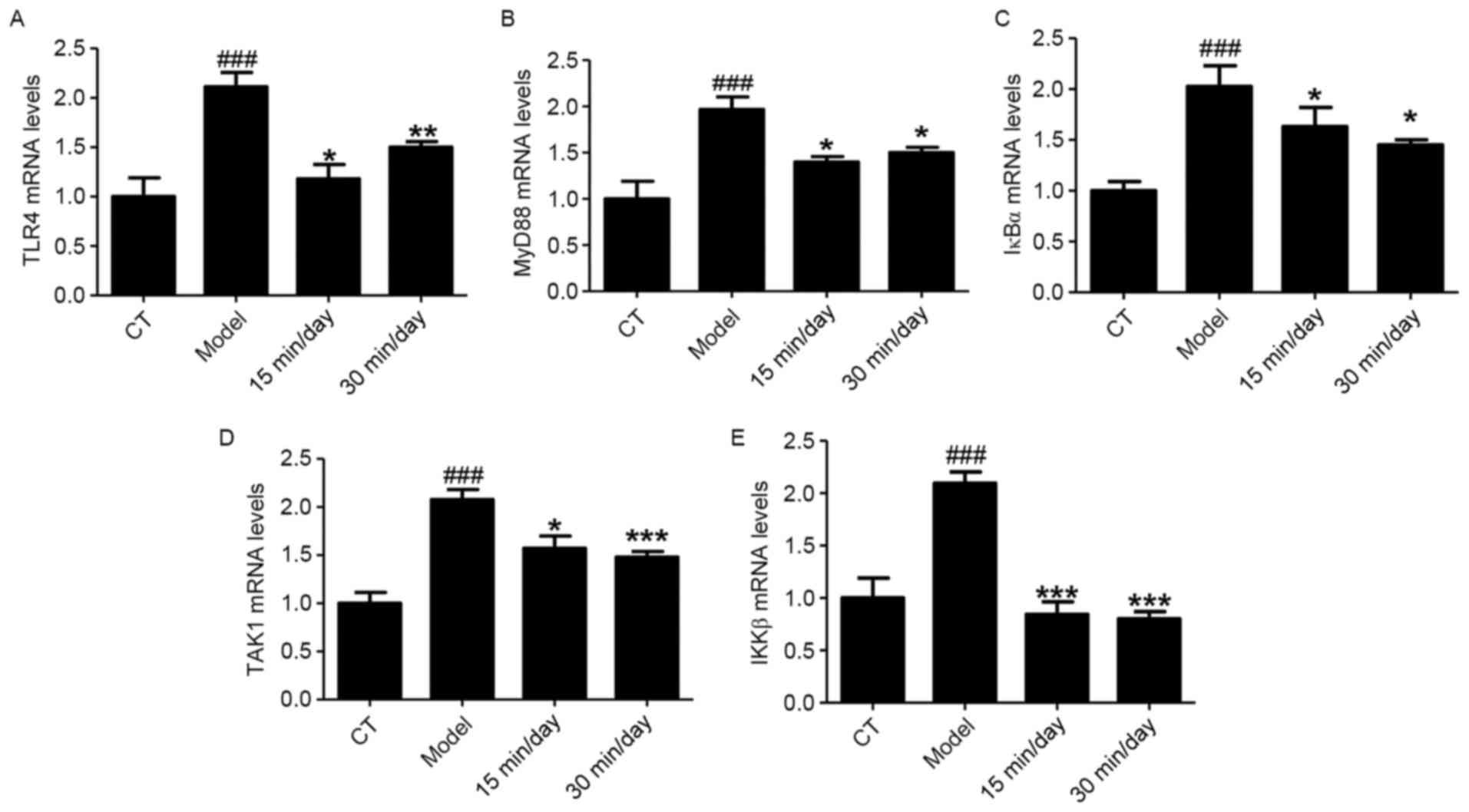 | Figure 6.Exercise suppresses SCI by regulating
NF-κB pathways at the mRNA level. Reverse
transcription-quantitative polymerase chain reaction analysis was
used to test the mRNA levels of NF-κB related-indicators in a rat
model of SCI. Relative mRNA expression of (A) TLR4, (B) MyD88, (C)
IκBα, (D) TAK1 mRNA and (E) IKKβ. Data of all groups are normalized
to the control. Significance, using one-way ANOVA with Dunn's least
significant difference tests, is indicated.
###P<0.001 vs. the CT group; *P<0.05, **P<0.01
and ***P<0.001 vs. the model group. SCI, spinal cord injury;
NF-κB, nuclear factor-κB; TLR4, Toll-like receptor 4; MyD88,
myeloid differentiation primary response gene 88; IκBα, inhibitor
of κB; TAK1, transforming growth factor β1-activated kinase 1;
IKKβ, inhibitor of NF-κB kinase subunit β; CT, control. |
Discussion
SCI-induced nerve damage is a serious threat to the
health of people worldwide (24–26). SCI
is associated with the secretion of pro-inflammatory cytokines,
including IL-1β, IL-6 and TNF-α (27). While an inflammatory response is
necessary to clear debris from the site of injury, if it becomes
out of control, an enlargement of the initial lesion results, with
additional axonal damage, contusion, compression or stretch injury,
and demyelination with concomitant exacerbation of neurological
function loss (28). Congenital
lesions and tumor compression may also cause SCI, disc herniation
and ischemia (29–32). The initial physical injury of SCI
causes progressive damage, which spreads from the epicenter of
injury. The initial physical injury causes tissue necrosis,
neuronal disruption and vascular damage, and progressive damage may
ensue, including oxidative stress, necrosis, inflammation, ischemia
and apoptotic response (12,24). However, pharmacotherapies for the
amelioration of neuronal injury and enhancement of regeneration are
limited.
Individuals who survive SCI are likely to suffer
from medical complications such as chronic pain, bladder and bowel
dysfunction, and increased susceptibility to respiratory and heart
problems, and their successful recovery is dependent upon the daily
treatment of these chronic diseases (33). Numerous researchers consider that
reasonable exercise may help patients with SCI to recover, by
improving their health and restoring normal physiological functions
(34,35). Therefore, the current study aimed to
investigate whether exercise is able to ameliorate SCI.
The results of the present study indicated that SCI
caused nerve damage and systemic inflammation, and that moderate
exercise had the ability to ameliorate SCI through the regulation
of NF-κB signaling pathways and inhibition of nerve injury. The IHC
and western blot analyses demonstrated that inflammation of the
spinal cord was induced by SCI, and exercise was able to suppress
this. The stimulation of peripheral inflammation by SCI was also
examined in the present study. The increased serum cytokine levels
detected by ELISA indicated that SCI caused a peripheral
inflammatory response, this was also suppressed by exercise. NF-κB
regulates the expression of genes associated with apoptosis, viral
replication, tumorigenesis, inflammation and autoimmune disease
(36). NF-κB activation is
considered to be part of the stress response, as it is induced by a
variety of stimuli, including growth factors, cytokines,
lymphokines, ultraviolet, pharmacological agents and stress
(37). The TLR4/NF-κB pathway is one
of the most important signal transduction pathways in the
initiation and development of inflammation (37). The aforementioned stimuli also
activate the phosphorylation, ubiquitination and degradation of
IκB, which leads to the translocation of NF-κB to the nucleus where
it binds to the consensus sequence of target genes and activates
their transcription (38). The
phosphorylation of IκB is mediated by an IκB kinase complex
comprising IKK1/IKKα, IKK2/IKKβ and IKK3/IKKγ (39). In the present study, the expression
of these TLR4/NF-κB pathway components in the spinal cord tissues
of the rats was investigated using western blotting and RT-qPCR.
The analysis indicated that SCI increased inflammatory gene
transcription and protein expression, and significantly activated
the TLR4/NF-κB signaling pathway. By contrast, exercise training
suppressed protein phosphorylation in this pathway, and attenuated
the SCI-induced inflammatory response.
In summary, the results of the present study
indicate that SCI stimulates inflammatory responses by increasing
the activation of glial cells and TLR4/NF-κB signaling pathways.
However, exercise training may alleviate SCI-induced nerve injury
by suppressing the expression of inflammatory mediators via
TLR4/NF-κB signaling, and thus be beneficial to recovery following
SCI.
Acknowledgements
The authors would like to thank Mr. Yaqiong Song and
Miss Jiaojiao Chen (Emergency Department, Sichuan Academy of
Medical Sciences and Sichuan Provincial People's Hospital) for
assisting in the current study.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FT conceived the study. YS and JQL conducted the
experiments. FT, YS and JQL analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sichuan Academy of Medical Sciences and Sichuan Provincial People's
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siriphorn A, Dunham KA, Chompoopong S and
Floyd CL: Postinjury administration of 17β-estradiol induces
protection in the gray and white matter with associated functional
recovery after cervical spinal cord injury in male rats. J Comp
Neurol. 520:2630–2646. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrinovic MM, Hourez R, Aloy EM, Dewarrat
G, Gall D, Weinmann O, Gaudias J, Bachmann LC, Schiffmann SN, Vogt
KE and Schwab ME: Neuronal Nogo-A negatively regulates dendritic
morphology and synaptic transmission in the cerebellum. Proc Natl
Acad Sci USA. 110:1083–1088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang F, Xing S, He M, Hou Q, Chen S, Zou
X, Pei Z and Zeng J: Nogo-A is associated with secondary
degeneration of substantia nigra in hypertensive rats with focal
cortical infarction. Brain Res. 1469:153–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu WW, Ma XL, Guo AL, Zhao HY and Luo HH:
Neuroprotective effects of NEP1-40 and fasudil on Nogo-A expression
in neonatal rats with hypoxic-ischemic brain damage. Genet Mol Res.
10:2987–2995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JH, Choi KH, Jang YJ, Bae SS, Shin BC,
Choi BT and Shin HK: Electroacupuncture acutely improves cerebral
blood flow and attenuates moderate ischemic injury via an
endothelial mechanism in mice. PLoS One. 8:e567362013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MW, Chung YC, Jung HC, Park MS, Han
YM, Chung YA, Maeng LS, Park SI, Lim J, Im WS, et al:
Electroacupuncture enhances motor recovery performance with
brain-derived neurotrophic factor expression in rats with cerebral
infarction. Acupunct Med. 30:222–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan F, Wan S, Wu H, Huo Q, Wang J, Chen W,
Fang M, Liu X, Wang X and Sun J: Expression of neurocan mRNA and
ultrastructure of brain tissue after cerebral ischemia and
reperfusion in stroke-prone renovascular hypertensive rats treated
by electroacupuncture. Neural Regen Res. 6:2834–2838. 2011.
|
|
8
|
Hwang L, Choi IY, Kim SE, Ko IG, Shin MS,
Kim CJ, Kim SH, Jin JJ, Chung JY and Yi JW: Dexmedetomidine
ameliorates intracerebral hemorrhage-induced memory impairment by
inhibiting apoptosis and enhancing brain-derived neurotrophic
factor expression in the rat hippocampus. Int J Mol Med.
31:1047–1056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung YH, Kim SC, Hong HP, Park CY, Shin
MS, Kim CJ, Seo JH, Kim DY, Kim DJ and Cho HJ: Treadmill exercise
ameliorates dopaminergic neuronal loss through suppressing
microglial activation in Parkinson's disease mice. Life Sci.
91:1309–1316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donnelly DJ and Popovich PG: Inflammation
and its role in neuroprotection, axonal regeneration and functional
recovery after spinal cord injury. Exp Neurol. 209:378–388. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anwar MA, Al Shehabi TS and Eid AH:
Inflammogenesis of secondary spinal cord injury. Front Cell
Neurosci. 10:982016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dominguez E, Mauborgne A, Mallet J,
Desclaux M and Pohl M: SOCS3-mediated blockade of JAK/STAT3
signaling pathway reveals its major contribution to spinal cord
neuroinflammation and mechanical allodynia after peripheral nerve
injury. J Neurosci. 30:5754–5766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swift DL, Johannsen NM, Lavie CJ, Earnest
CP and Church TS: The role of exercise and physical activity in
weight loss and maintenance. Prog Cardiovasc Dis. 56:441–447. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foster-Schubert KE, Alfano CM, Duggan CR,
Xiao L, Campbell KL, Kong A, Bain CE, Wang CY, Blackburn GL and
McTiernan A: Effect of diet and exercise, alone or combined, on
weight and body composition in overweight-to-obese postmenopausal
women. Obesity (Silver Spring). 20:1–1638. 2012. View Article : Google Scholar
|
|
15
|
Kujala UM: Benefits of exercise therapy
for chronic diseases. Br J Sports Med. 40:3–4. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Xu J, Zhao W and Han HR: Measuring
self-care in persons with Type 2 diabetes: A systematic review.
Eval Health Prof. 39:131–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tajiria N, Yasuharaa T, Shingo T, Kondo A,
Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, et al:
Exercise exerts neuroprotective effects on Parkinson's disease
model of rats. Brain Res. 1310:200–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hutchinson KJ1, Gómez-Pinilla F, Crowe MJ,
Ying Z and Basso DM: Three exercise paradigms differentially
improve sensory recovery after spinal cord contusion in rats.
Brain. 127:1403–1414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perot PL Jr, Lee WA, Hsu CY, Hogan EL, Cox
RD and Gross AJ: Therapeutic model for experimental spinal cord
injury in the rat: I. Mortality and motor deficit. Cent Nerv Syst
Trauma. 4:149–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basso DM, Beattie MS, Bresnahan JC,
Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ,
Nockels R, et al: MASCIS evaluation of open field locomotor scores:
Effects of experience and teamwork on reliability. Multicenter
Animal Spinal Cord Injury Study. J Neurotrauma. 13:343–359. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaudet AD and Popovich PG: Extracellular
matrix regulation of inflammation in the healthy and injured spinal
cord. Exp Neurol. 258:24–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR,
Huang J and Ji RR: Role of the CX3CR1/p38 MAPK pathway in spinal
microglia for the development of neuropathic pain following nerve
injury-induced cleavage of fractalkine. Brain Behav Immun.
21:642–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guerrero AR, Uchida K, Nakajima H,
Watanabe S, Nakamura M, Johnson WE and Baba H: Blockade of
interleukin-6 signaling inhibits the classic pathway and promotes
an alternative pathway of macrophage activation after spinal cord
injury in mice. J Neuroinflammation. 9:402012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Einstein O and Ben-Hur T: The changing
face of neural stem cell therapy in neurological diseases. Arch
Neurol. 65:452–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuh SU, Cho YE, Yoon DH, Kim KN and Ha Y:
Functional recovery after human umbilical cord blood cells
transplantation with brain-derived neutrophic factor into the
spinal cord injured rat. Acta Neurochir (Wien). 147:985–992. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carlson NG, Wieggel WA, Chen J, Bacchi A,
Rogers SW and Gahring LC: Inflammatory cytokines IL-1 alpha, IL-1
beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin
through distinct pathways. J Immunol. 163:3963–3968.
1999.PubMed/NCBI
|
|
28
|
Jones TB, McDaniel EE and Popovich PG:
Inflammatory-mediated injury and repair in the traumatically
injured spinal cord. Curr Pharm Des. 11:1223–1236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
López-Vales R, Forés J, Verdú E and
Navarro X: Acute and delayed transplantation of olfactory
ensheathing cells promote partial recovery after complete
transection of the spinal cord. Neurobiol Dis. 21:57–68. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao ZM, Li HJ, Liu HY, Lu SH, Yang RC,
Zhang QJ and Han ZC: Intraspinal transplantation of CD34+ human
umbilical cord blood cells after spinal cord hemisection injury
improves functional recovery in adult rats. Cell Transplant.
13:113–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansilla E, Marin GH, Sturla F, Drago HE,
Gil MA, Salas E, Gardiner MC, Piccinelli G, Bossi S, Salas E, et
al: Human mesenchymal stem cells are tolerized by mice and improve
skin and spinal cord injuries. Transplant Proc. 37:292–294. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pal R, Gopinath C, Rao NM, Banerjee P,
Krishnamoorthy V, Venkataramana NK and Totey S: Functional recovery
after transplantation of bone marrow-derived human mesenchymal
stromal cells in a rat model of spinal cord injury. Cytotherapy.
12:792–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen HY and Boore JRP: Considering the
physiological and psychological consequences of spinal cord injury.
Br J Neuroscie. 1:225–232. 2006. View Article : Google Scholar
|
|
34
|
Driver HS and Taylorb SR: Exercise and
sleep. Sleep Med Rev. 4:387–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gorman PH, Scott W, York H, Theyagaraj M,
Price-Miller N, McQuaid J, Eyvazzadeh M, Ivey FM and Macko RF:
Robotically assisted treadmill exercise training for improving peak
fitness in chronic motor incomplete spinal cord injury: A
randomized controlled trial. J Spinal Cord Med. 39:32–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li QT and Verma IM: NF-kappaB regulation
in the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Janssen-Heininger YM, Poynter ME and
Baeuerle PA: Recent advances towards understanding redox mechanisms
in the activation of nuclear factor kappaB. Free Radic Biol Med.
28:1317–1327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zandi E, Rothwarf DM, Delhase M, Hayakawa
M and Karin M: The IkappaB kinase complex (IKK) contains two kinase
subunits, IKKalpha and IKKbeta, necessary for IkappaB
phosphorylation and NF-kappaB activation. Cell. 19:243–252. 1997.
View Article : Google Scholar
|