Introduction
Burn is a serious accidental injury. In particular,
burn caused by fire is a challenge in surgical treatment. According
to epidemiological statistics, approximately 450,000 burn patients
needed treatment in the United States in 2011, on increase of
approximately 340% compared with that in 1995 (1,2).
Therefore, how to effectively treat burns is a serious clinical
challenge. The molecular mechanisms after burn are very
complicated, especially the post-burn autophagy and wound healing,
which are key factors related to tissue reconstruction after burn.
Autophagy is an important process in vivo that is closely
related to substance and energy metabolisms in cells (3,4), which
plays an important role in many physiological and pathological
reactions and regulates various diseases and pathological reaction
processes such as tumor, inflammation and cell proliferation
(5). Beclin-1 is one of the
hallmarks of autophagy and plays an important role in autophagy,
which is considered a dynamic indicator of autophagy activity
(6,7). Burn wound healing involves cell
proliferation, differentiation and migration, granulation tissue
formation as well as extracellular matrix formation. c-Myc
proto-oncogene is considered to be a mediator of cell division and
plays an important role in wound healing, which can regulate tissue
cell proliferation and promote cell division, thus contributing to
tissue reconstruction (8).
Therefore, studying the post-burn expression levels of Beclin-1 and
c-Myc is of important guiding significance for the treatment of
burns. Intervention can be carried out in the changes of Beclin-1
and c-Myc expression, thereby inhibiting the adverse effects of
pathological responses activated due to abnormal expression on
post-burn tissue reconstruction.
Materials and methods
Experimental animals and grouping
A total of 48 Sprague-Dawley (SD) rats weighing
220±20 g (half male and half female) were purchased from Shanghai
Slack Laboratory Animal Co., Ltd. (Shanghai, China) license no.
SCXK 2014-0003. The above-mentioned 48 rats were randomly divided
into the normal group, the 3-day burn group, the 5-day burn group
and the 7-day burn group, with 12 rats in each group Rats were
housed in a temperature controlled room (21±2°C) on a 12-h
light/dark cycle (lights on at 06:00). All rats had free access to
water and food.
This study was approved by the Animal Ethics
Committee of Weifang People's Hospital Animal Center (Weifang,
China).
Experimental reagents
Main reagents used in this study included primary
antibodies anti-Beclin-1 and anti-c-Myc, immunohistochemical kits
(KIT-9710; Maxim, San Jose, CA, USA), AceQ reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
SYBR-Green Master mix kits (Q111-02/03) and HiScript II Q RT
Sperfect for qPCR [(+genomic deoxyribonucleic acid (gDNA) wiper]
kits (R223-01) (both from Vazyme Biotech Co., Ltd., Nanjing,
China).
Experimental equipments
Main experimental equipment used in this study were
an optical microscope (Leica DMI 4000B/DFC425C), a fluorescence
RT-qPCR instrument (ABI 7500), Image-lab image analysis system,
Image-Pro image analysis system (both from Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and a digital thermostatic water bath
kettle (Changzhou Guohua Electric Appliance Co., Ltd., Changzhou,
China).
Establishment of burn models
Rats were intraperitoneally injected with 7% chloral
hydrate, and the injection volume was 5 ml/kg. After successful
anesthesia, hair on the back of the rats was removed to expose the
skin. After disinfection, the back of the rats was immersed in the
digital thermostat water bath kettle for 10 sec, resulting in burn
wounds.
Treatment in each group
Burn models were prepared in the 3, 5 and 7-day burn
groups according to the above establishment method for burn models.
After successful modeling, wounds were given routine dressing
change and packing. Then, rats were sacrificed at 3, 5 and 7 days
after modeling, respectively, and skin tissues of burn wounds on
the back were collected for experiments. However, rats in the
normal group received no treatment and were sacrificed directly to
collect normal skin tissues on the back for experiments.
Material collection
After successful anesthesia, 6 rats in each group
were fixed with paraformaldehyde, and skin tissues on the back
(with an area of ~1 cm2) were collected and placed in 4%
paraformaldehyde, followed by fixation at 4°C for 48 h. Then,
paraffin tissue sections were made for immunohistochemical
detection. Skin tissues on the back (with an area of ~1
cm2) were directly taken from the remaining 6 rats and
placed in epoxy resin (EP) tubes for western blotting
detection.
Immunohistochemistry
Paraffin tissue sections (5 µm) were conventionally
dewaxed, hydrated, added with citric acid buffer, and heated in a
microwave oven for antigen retrieval. Then, sections were rinsed
with phosphate-buffer saline (PBS) solution and added with
endogenous peroxidase blocking agent for 10 min of incubation.
After that, sections were rinsed with PBS solution and added with
goat serum for 20 min of blocking. Then, serum-blocking solution
was removed, and anti-Beclin-1 primary antibody (1:200; cat. no.
ab62557) and anti-c-Myc primary antibody (1:200; cat. no. ab39688),
both from Abcam, Cambridge, MA, USA, were added for incubation
overnight at 4°C. After that, sections were rinsed with PBS
solution and incubated with secondary goat anti-rabbit (HRP) IgG
antibody (dilution, 1:500; cat. no. ab6721; Abcam) for 10 min.
Then, PBS solution was used for rinsing, and streptavidin
peroxidase was added for 10 min of incubation. Last, sections were
subjected to color development via diaminobenzidine (DAB),
counterstained with hematoxylin, mounted with neutral balsam,
observed under the microscope (Leica) and photographed.
Western blotting
Skin tissues stored at −20°C for standby application
were added with lysis solution, followed by ice bath for 60 min.
Then, tissues were centrifuged at 14,000 × g for 10 min, and
protein was quantified by bicinchoninic acid (BCA) assay. The
standard curve and optical density were measured via a microplate
reader (Bio-Rad) and the concentration of protein was calculated.
After protein was denatured, samples were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with
corresponding concentration. When marker protein reached to the
bottom of the glass plate, and sample protein was almost in a
straight line at the bottom, gel running was stopped. Then, the
sample was transferred onto a polyvinylidene fluoride (PVDF)
membrane for blocking, followed by washing with tetrapropyl benzene
sulfonate (TPBS) 3 times. After that, membrane was blocked with
blocking solution for 1.5 h, added with anti-Beclin-1 primary
antibody (1:1,000) and anti-c-Myc primary antibody (1:1,000),
rinsed with Tris-buffered saline with Tween-20 (TBST), and added
with secondary goat anti-rabbit (HRP) IgG antibody (dilution,
1:1,000; cat. no. ab6721; Abcam), USA), followed by rinsing with
TBST. After the secondary antibody was removed by TBST washing,
development was started. Finally, a membrane was placed in a
chemiluminescence reagent for 1 min of reaction, developed in the
dark and analyzed using the gel scanning imaging system.
RT-qPCR detection
Total ribonucleic acid (RNA) was extracted from
spare bone stored at −20°C using RNA extraction kits. The extracted
total RNA was reverse-transcribed into complementary
deoxyribonucleic acid (cDNA) through reverse transcription kits,
and the volume of the reaction system was 20 µl. Reaction
conditions: reaction at 51°C for 2 min, predenaturation at 96°C for
10 min, denaturation at 96°C for 10 sec, annealing at 60°C for 30
sec, for 40 cycles. Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as an internal control, and relative messenger RNA
(mRNA) expression levels of Beclin-1 and c-Myc were calculated.
Primer sequences are shown in Table
I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name |
| Primer sequences |
|---|
| Beclin-1 | Upstream: |
5′-CGGAATTCTATGGAAGGGTCTAAGACGTCC-3′ |
|
| Downstream: |
5′-CGGGATCCTCATTTGTTATAAAATTGTGAGGACA-3′ |
| c-Myc | Upstream: |
5′-ATCACAGCCCTCACTCAC-3′ |
|
| Downstream: |
5′-ACAGATTCCACAAGGTGC-3′ |
| GADPH | Upstream: |
5′-ACGGCAAGTTCAACGGCACAG-3′ |
|
| Downstream: |
5′-GAAGACGCCAGTAGACTCCACGAC-3′ |
Observation indexes
The expression levels of Beclin-1 and c-Myc in skin
tissues were detected by immunohistochemistry. The protein
expression levels of Beclin-1 and c-Myc in skin tissues were
detected through western blotting. The mRNA expression levels of
Beclin-1 and c-Myc in skin tissues were detected via RT-qPCR.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 software (IBM SPSS, Armonk, NY, USA) was used for statistical
analysis in this study. Measurement data were expressed as mean ±
standard deviation. Comparison between multiple groups was done
using One-way ANOVA test followed by post hoc test (Least
Significant Difference). The Chi-square test was used for
enumeration data.
Results
Expression levels of Beclin-1 and
c-Myc detected via immunohistochemistry
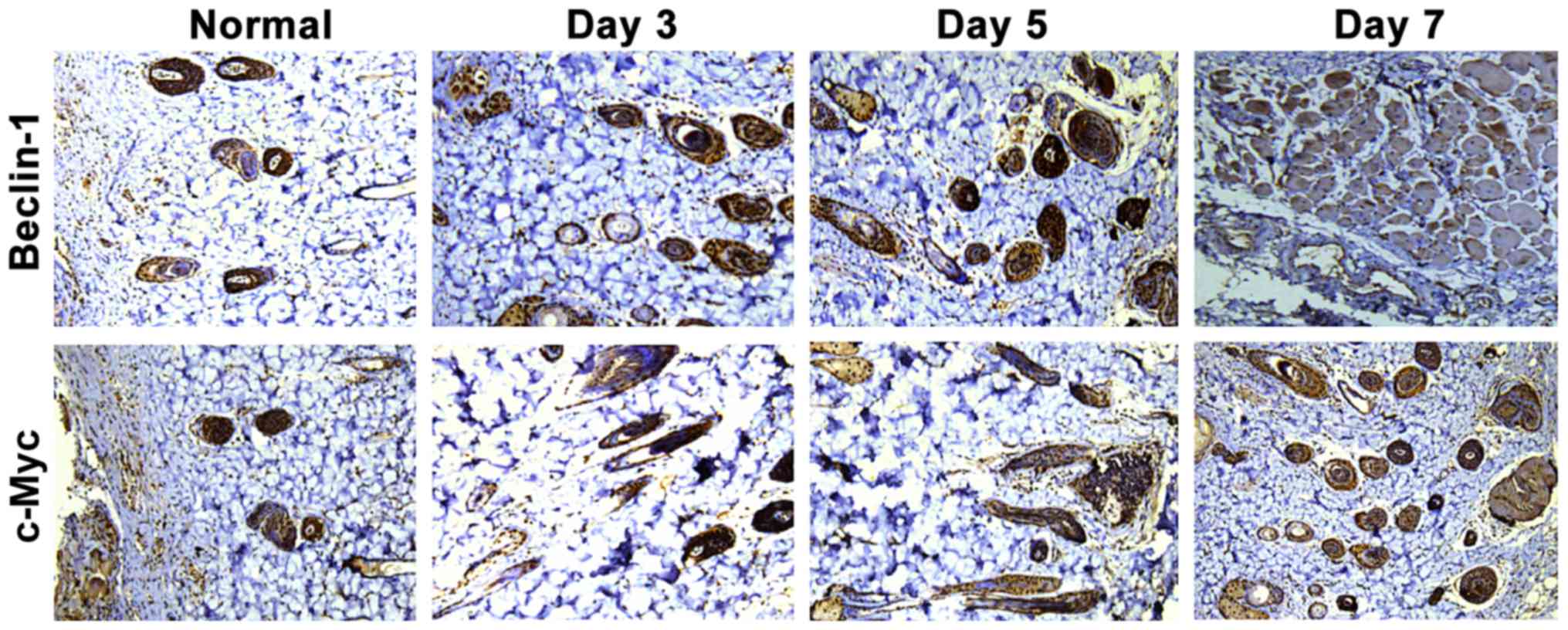
As shown in Fig. 1,
the positive expression of Beclin-1 and c-Myc were tan color. The
positive expression of Beclin-1 and c-Myc in the normal group were
fewer, while those in the three burn groups were significantly
increased, which showed statistically significant differences
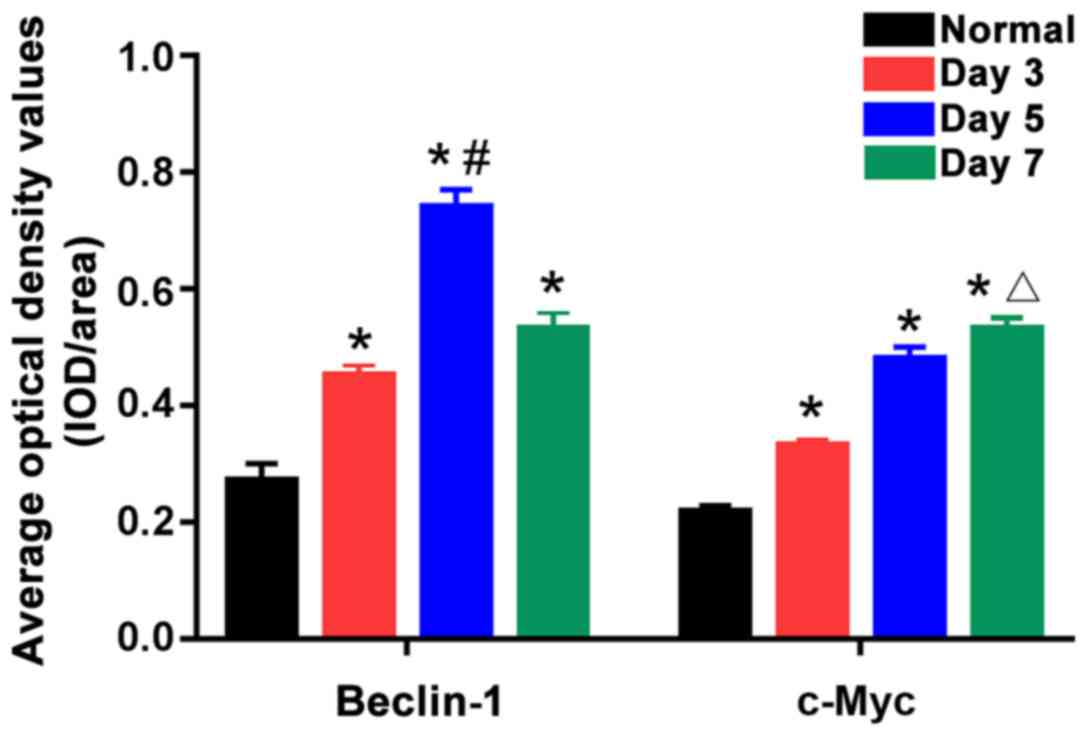
(P<0.05). According to statistical analysis of positive
expression (Fig. 2), the 5-day burn
group had the highest positive expression level of Beclin-1, which
was obviously higher than those in the 3- and 7-day burn groups,
showing statistically significant differences (P<0.05). In
addition, the positive expression level of c-Myc in the 7-day burn
group was the highest, which was evidently higher than those in the
3- and 5-day burn groups, showing statistically significant
differences (P<0.05). This suggests that after burn, the
expression levels of Beclin-1 and c-Myc in skin tissues are
gradually increased with the expression level of Beclin-1 reaching
the peak around the 5th day after burn and then beginning to
gradually decline, and that of c-Myc was the highest around the 7th
day after burn.
Protein expression levels of Beclin-1
and c-Myc detected by western blotting
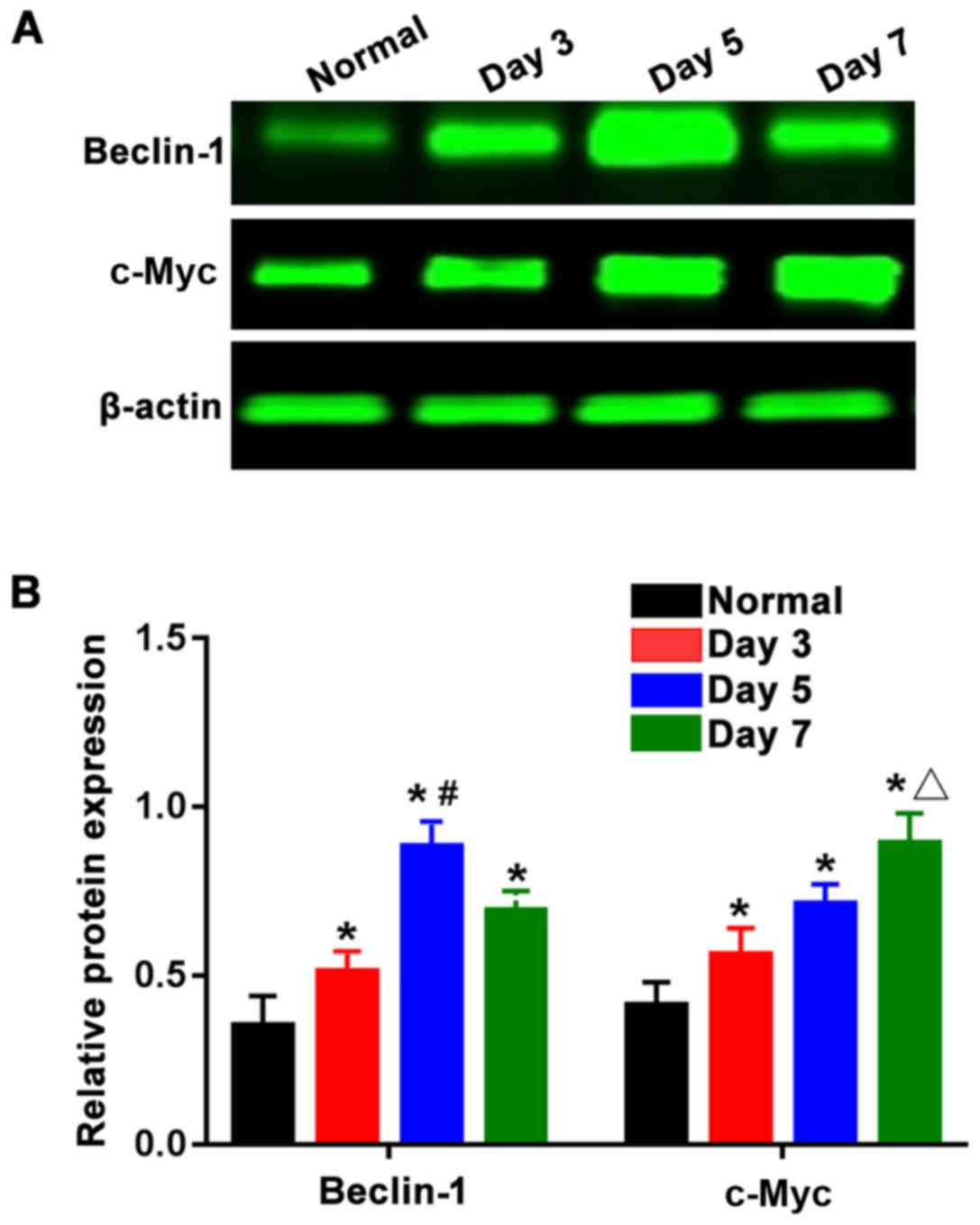
As shown in Fig. 3A,
the expression levels of Beclin-1 protein and c-Myc protein were
reduced in the normal group, while those in the three burn groups
were overtly increased, and the differences were statistically
significant (P<0.05). The expression levels of Beclin-1 and
c-Myc proteins were subjected to statistical analysis, and the
results (Fig. 3B) showed that the
Beclin-1 protein expression level in the 5-day burn group was the
highest, which was significantly greater than those in the 3-and
7-day burn groups, showing statistically significant differences
(P<0.05). The expression level of c-Myc protein in the 7-day
burn group was the highest and significantly higher than those in
the 3- and 5-day burn groups. This indicates that the expression
levels of Beclin-1 and c-Myc proteins in skin tissues are gradually
increased after burn with the Beclin-1 protein expression level
reaching the peak around the 5th day after burn and then beginning
to decline, and the c-Myc protein expression level was the highest
around the 7th day after burn.
Expression levels of Beclin-1 mRNA and
c-Myc mRNA detected by RT-qPCR
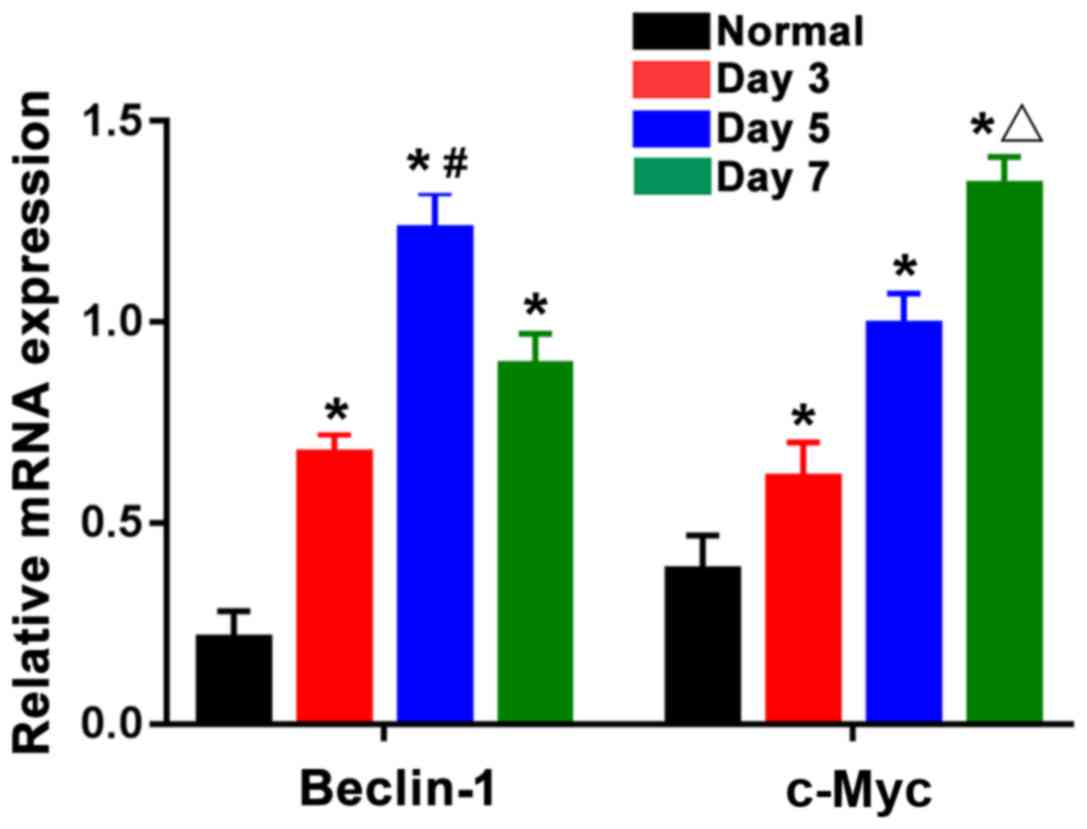
According to Fig. 4,
the mRNA expression levels of Beclin-1 and c-Myc were lower in the
normal group, while those in the three burn groups were distinctly
elevated, showing statistically significant differences
(P<0.05). The mRNA expression of Beclin-1 was the highest in the
5-day burn group, which was clearly higher than those in the 3- and
7-day burn groups, and the differences were statistically
significant (P<0.05). The mRNA expression of c-Myc in the 7-day
burn group was the highest and obviously higher than those in the
3- and 5-day burn groups. This suggests that the expression of
Beclin-1 and c-Myc mRNAs in skin tissues are gradually increased
after burn with the mRNA expression level of Beclin-1 reaching the
peak around the 5th day after burn and then beginning to gradually
decline, and that of c-Myc was the highest around the 7th day after
burn.
Discussion
Autophagy and cell proliferation in skin tissues
after burn are important factors affecting wound healing. Autophagy
is closely related to recycling and utilization of intracellular
macromolecular substances, removal of damaged tissues and
maintenance of the stability of intracellular environment (9,10).
Present studies have suggested that there is a close relationship
between autophagy and apoptosis. Although their characteristics and
pathological mechanisms are different, autophagy and apoptosis are
not separate from each other, have many common stimulating factors
and regulatory proteins, and are interrelated and complex (11). The process of autophagy exists in
physiological and pathological processes, such as injury and
disease. In the pathological processes of injury and disease,
intervention in autophagy can have a serious impact on cell
recovery process (12,13). Currently, studies have indicated that
as a mammalian homologue of the yeast autophagy-related protein 6
(ATG6)/vacuolar protein sorting-associated protein 30 (Vps30) gene,
Beclin-1 binds to its ligand and regulates the activity of
autophagy, thus playing an important role in autophagy (14). Further studies have found that
different domains of Beclin-1 bind to autophagy regulatory proteins
to form protein complexes, thus regulating autophagy (15,16).
This study showed that Beclin-1 expression is gradually increased
in skin tissues after burn, thereby regulating the process of
autophagy started in the cells. The level of autophagy is getting
higher and higher. The Beclin-1 expression reaches a peak on the
5th day after burn, and the autophagy level in cells also reaches
the peak, leading to autophagic cell death. Then, the expression
level is gradually decreased, and the level of autophagy begins to
recede.
Cell proliferation is also an important factor
affecting wound healing after burn. c-Myc is an important oncogene
that regulates cell division and proliferation. In addition, c-Myc
is an important mediator of mitosis, which can prompt cells in the
quiescent stage to rapidly transfer into the division stage, thus
promoting cell division and proliferation (17). Meanwhile, c-Myc is a downstream
substrate for various cell signaling pathways, which can act as a
transcription factor and transmit information in the nucleus so as
to regulate cell cycle, promote cell proliferation and block cell
differentiation (18). A study
suggested that c-Myc cannot only promote E2F to bind to
corresponding DNA by activating cyclin E/cyclin-dependent kinase 2
(CDK2) so as to mediate cell proliferation, but also regulate cell
cycle by regulating transferrin receptor-1 (TFRC1) so as to
regulate cell proliferation (19).
At the same time, c-Myc does not always lead to cell proliferation.
When the peripheral environment where cells are located allows the
proliferation of cells, c-Myc acts on the downstream signaling
pathways, releases the signal of growth and secretes a variety of
growth-related factors, so as to maintain cell proliferation. When
the signal promoting cell growth disappears, the overexpression of
c-Myc causes apoptosis, thus allowing cells to maintain homeostasis
of reproduction and apoptosis (20).
This study showed that the c-Myc expression begins to increase
after burn, indicating that local injured skin tissue cells begin
to proliferate and repair, and c-Myc expression on the 7th day
after burn is significantly increased. This indicates that skin
tissue cells have rapid proliferation, and damaged skin tissues
start a process of rapid repair. At the same time, conside ring the
expression regularity of Beclin-1, it was believed that autophagy
occurs in cells when the Beclin-1 expression is increased in the
early stage of the injury, causing a large number of cell death,
and autophagy reaches the highest level when the Beclin-1
expression reaches a peak on the 5th day after burn, which is not
conducive to cell proliferation, so it was observed that the c-Myc
expression levels on the 3rd and 5th day after burn were not high,
indicating that the c-Myc expression is inhibited by autophagy
caused by the high expression of Beclin-1. On the 7th day after
burn, the expression of Beclin-1 is significantly decreased, and
the level of autophagy is clearly decreased. Therefore, the
inhibition effect of autophagy on c-Myc is weakened. At the same
time, the environment where cells are located is favorable for the
proliferation of cells. Therefore, the expression of c-Myc is
increased, and massive cells begin to proliferate, promoting local
wound repair.
In conclusion, the expression of Beclin-1 and c-Myc
after burn has certain regularities, which can be used as effective
targets for the treatment of burns. At the same time, corresponding
intervention can be carried out for the expression features of
Beclin-1 and c-Myc after burn, namely, inhibit the expression of
Beclin-1 after burn to inhibit autophagy, and promote the
expression of c-Myc to benefit cell proliferation, thereby
promoting wound repair and healing.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CL analyzed and HL interpreted the data. HL
performed experiments. CL wrote the manuscript. YL carried out the
statistical analysis and CL edited the language. All authors have
read and approved the final study.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Weifang People's Hospital Animal Center (Weifang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshimura K, Suga H and Eto H:
Adipose-derived stem/progenitor cells: Roles in adipose tissue
remodeling and potential use for soft tissue augmentation. Regen
Med. 4:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomita K, Madura T, Sakai Y, Yano K,
Terenghi G and Hosokawa K: Glial differentiation of human
adipose-derived stem cells: Implications for cell-based
transplantation therapy. Neuroscience. 236:55–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanida I: Autophagy basics. Microbiol
Immunol. 55:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Czaja MJ, Ding WX, Donohue TM Jr, Friedman
SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, et
al: Functions of autophagy in normal and diseased liver. Autophagy.
9:1131–1158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doria A, Gatto M and Punzi L: Autophagy in
human health and disease. N Engl J Med. 368:1845–1846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Fan H, Zhou Y, Duan P, Zhao G and
Wu G: Knockdown of autophagy-related gene BECLIN1 promotes cell
growth and inhibits apoptosis in the A549 human lung cancer cell
line. Mol Med Rep. 7:1501–1505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruck A, Attonito J, Garces KT, Núnez L,
Palmisano NJ, Rubel Z, Bai Z, Nguyen KC, Sun L, Grant BD, et al:
The Atg6/Vps30/Beclin 1 ortholog BEC-1 mediates endocytic
retrograde transport in addition to autophagy in C. elegans.
Autophagy. 7:386–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clark DE, Li C, Wang W, Martin SK and
Suttie JM: Vascular localization and proliferation in the growing
tip of the deer antler. Anat Rec A Discov Mol Cell Evol Biol.
288:973–981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanida I: Autophagosome formation and
molecular mechanism of autophagy. Antioxid Redox Signal.
14:2201–2214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goehe RW, Bristol ML, Wilson EN and
Gewirtz DA: Autophagy, senescence, and apoptosis. Methods Mol Biol.
962:31–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Liu JH, Sui YX, Jin L, Yang Y, Lin
SM and Shi H: Beclin1 overexpression inhibitis proliferation,
invasion and migration of CaSki cervical cancer cells. Asian Pac J
Cancer Prev. 12:1269–1273. 2011.PubMed/NCBI
|
|
13
|
Mijaljica D, Prescott M and Devenish RJ:
Autophagy in disease. Methods Mol Biol. 648:79–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsunaga K, Saitoh T, Tabata K, Omori H,
Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe
T, et al: Two Beclin 1-binding proteins, Atg14L and Rubicon,
reciprocally regulate autophagy at different stages. Nat Cell Biol.
11:385–396. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wirawan E, Lippens S, Vanden Berghe T,
Romagnoli A, Fimia GM, Piacentini M and Vandenabeele P: Beclin1: A
role in membrane dynamics and beyond. Autophagy. 8:6–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahni S, Merlot AM, Krishan S, Jansson PJ
and Richardson DR: Gene of the month: BECN1. J Clin Pathol.
67:656–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Florin L, Hummerich L, Dittrich BT,
Kokocinski F, Wrobel G, Gack S, Schorpp-Kistner M, Werner S, Hahn
M, Lichter P, et al: Identification of novel AP-1 target genes in
fibroblasts regulated during cutaneous wound healing. Oncogene.
23:7005–7017. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui J, Waltman P, Le VH and Lewis EA: The
effect of molecular crowding on the stability of human c-MYC
promoter sequence I-motif at neutral pH. Molecules. 18:12751–12767.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalra N and Kumar V: c-Fos is a mediator
of the c-myc-induced apoptotic signaling in serum-deprived hepatoma
cells via the p38 mitogen-activated protein kinase pathway. J Biol
Chem. 279:25313–25319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DuHadaway JB, Sakamuro D, Ewert DL and
Prendergast GC: Bin1 mediates apoptosis by c-Myc in transformed
primary cells. Cancer Res. 61:3151–3156. 2001.PubMed/NCBI
|