Introduction
Inflammation is a process that is associated with
the adherence and invasion of leukocytes to injured or infected
tissues. It is modulated by a number of mediators including
cytokines, chemokines, prostaglandins and substance P (1). The central nervous system (CNS) was
reported to respond to peripheral inflammatory stimuli by
initiating a local inflammatory response, also known as
neuroinflammation (2). There are
numerous critical features in the development of neuroinflammation,
including glial activation, the accumulation of pro-inflammatory
cytokines and the expression of adhesion molecules (1,3,4).
Neuroinflammation is inflammation in the peripheral
nervous system and the CNS. Excessive inflammatory responses
activate microglia, leading to the release of pro-inflammatory
factors, including interleukin (IL)-1β, IL-6 and tumor necrosis
factor-α (TNF-α). In turn, pro-inflammatory factors can aggravate
neuroinflammatory reactions, neuronal degeneration and brain
function. A large number of clinical and animal studies have
demonstrated that neuroinflammation is closely associated with
mental and neurodegenerative diseases (5). Non-steroidal anti-inflammatory drugs
are commonly used to treat neuroinflammation (6,7). To the
best of the authors' knowledge, no previous studies have
investigated the effects of analgesic drugs on
neuroinflammation.
As a glycolipid that is a major component of the
outer leaflet of the outer membrane in Gram-negative bacteria,
lipopolysaccharide (LPS) is commonly applied in the study of
neuroinflammation in microglia (8,9). LPS
activates several cellular responses, which accelerate the release
of pro-inflammatory cytokines (10).
The BV-2 microglia cell line exhibits properties associated with
inflammation following treatment with 1 µg/ml LPS, although the
cell viability is not affected (11,12).
Therefore, LPS was adopted as the stimulant of inflammation in BV-2
cells in the present study.
Fentanyl, a high-potency opiate, is widely
prescribed to treat acute and chronic pain (13,14). The
mechanisms responsible for the analgesic effects of fentanyl have
been extensively investigated (15–17). It
has been well documented that inflammation results in pain
(18,19). It remains unknown whether fentanyl
attenuates neuroinflammation in BV-2, therefore the effect of
fentanyl on LPS-induced neuroinflammation in BV-2 cells and its
molecular mechanisms were investigated in the present study.
Materials and methods
Cell culture
BV-2 cells, purchased from the American Type Culture
Collection (Manassas, VA, USA), were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin in an incubator at the temperature of
37°C with 95% humidity and 5% CO2. Fentanyl
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in
0.1% dimethyl sulfoxide (DMSO) to avoid toxicity in BV-2 microglial
cells and a corresponding amount of 0.1% DMSO without fentanyl was
added to the cells of the control group. The cells were also kept
in the dark during each experiment.
MTT assay
BV-2 microglial cells were seeded in 96-well plates
at a density of 5×103 cells/well. Following treatment
with various concentrations of fentanyl (0.01, 0.1, 1 and 5 µmol/l)
for 24 h, cells were cultured in fresh DMEM supplemented with 10%
FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 5 mg/ml MTT
for another 4 h. The blue formazan products in cells were dissolved
in DMSO. The optical density of the reaction medium was determined
at 570 nm using a microplate reader.
ELISA
Levels of tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-10 in the supernatant of the BV-2 cell culture,
obtained by centrifugation (15 min; 1,000 × g; 2–8°C) were measured
using TNF-α (MTA00B), IL-1β (MLB00C) and IL-10 (M1000B) ELISA kits
(all R&D Systems, Inc., Minneapolis, MN, USA) in accordance
with the manufacturer's protocol. BV-2 microglial cells were seeded
in 96-well cell culture plates and fentanyl pretreatment was added
to the medium with or without 1 µg/ml LPS (from Escherichia
coli strain 055:B5; Sigma-Aldrich; Merck KGaA). The TNF-α level
was determined 6 h after LPS stimulation (20), while IL-1β and IL-10 levels were
determined 24 h after LPS stimulation (21).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The toll-like receptor (TLR)4 mRNA level in BV-2
cells reaches the highest level at 4 h after LPS stimulation, as
previously reported (22). Thus, in
the current study, the same time point was selected for RT-qPCR.
RNA was isolated from BV2 cells using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.). Analysis was performed using the Maxima
First Strand cDNA Synthesis kit for RT-qPCR with dsDNase
(Invitrogen; Thermo Fisher Scientific, Inc.). For the RT reaction
10X dsDNase Buffer (1 µl), dsDNase (1 µl), total RNA (1 µg) and
nuclease-free water to a total volume of 10 µl were incubated for 2
min at 37°C. Then, 5X Reaction mix (4 µl), Maxima Enzyme Mix (2 µl)
and nuclease-free water (4 µl) were added and the mixture was
incubated for 10 min at 25°C followed by 15 min at 50°C. Following
RT, qPCR was performed in 96-well plates with the ABI
PRISM® 7000 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The qPCR reaction
mixture contained the following: cDNA (0.5 µg), forward primer (0.5
µM), reverse primer (0.5 µM), 50X ROX reference dye (0.5 µl), 2X
master mix (25 µl) and water to 50 µl. The thermocycling conditions
were as follows: Initial denaturation 95°C for 15 min; followed by
40 cycles of denaturation at 94°C for 10 sec, annealing for GAPDH
at 62°C and for TLR4 at 55°C for 30 sec, extension at 72°C for 30
sec; and a final extension at 72°C for 10 min. The relative amount
of TLR4 was measured using the 2−∆∆Cq method (23). The primers were as follows: TLR4,
5′-TGGTTGCTGTTCTTATTCTGATTTG-3′ (forward) and
5′-GACCCATGAAATTGGCACTCAT-3′ (reverse); GAPDH,
5′-TCCTGCACCACCAACTGCTTAGCC-3′ (forward) and
5′-GTTCAGCTCTGGGATGACCTTGCC-3′ (reverse). GAPDH was used as the
endogenous control to normalize TLR4.
Western blot analysis
Cells were lysed with 0.5 ml
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) and the concentration of protein was
determined using an Enhanced BCA Protein Assay kit (P0010; Beyotime
Institute of Biotechnology). Protein samples (20 µg) from the
lysates were separated by 10% SDS-PAGE and blotted onto
polyvinylidene fluoride membranes. Membranes were blocked with 5%
skimmed milk or 5% bovine serum albumin (Beyotime Institute of
Biotechnology) for phosphorylated (p-) protein for 1 h at room
temperature. Membranes were then treated with primary antibodies
against TLR4 (1:1,000; sc-293072; Sigma-Aldrich; Merck KGaA),
GSK-3β (1:1,000; sc-71186; Sigma-Aldrich; Merck KGaA), p-GSK-3β
(1:1,000; sc-373800; Sigma-Aldrich; Merck KGaA) and β-actin
(1:1,000; sc-58673; Sigma-Aldrich; Merck KGaA) at 4°C overnight.
Following three rinses with Tris-buffered saline and Tween-20,
membranes were treated with horseradish peroxidase (HRP)-conjugated
secondary antibody (mouse anti-Armenian hamster IgG-HRP; 1:10,000;
sc-2789; Sigma-Aldrich; Merck KGaA) at room temperature for 1 h.
Bands were visualized by an enhanced chemiluminescence kit
(Sigma-Aldrich; Merck KGaA). β-actin was used as an endogenous
control. Image pro plus 6.0 (Media Cybernetics, Inc., Rockville,
MD, USA) was used for densitometry.
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed by one-way analysis of variance followed by the
Schefffe post-hoc test. Analyses were conducted using SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
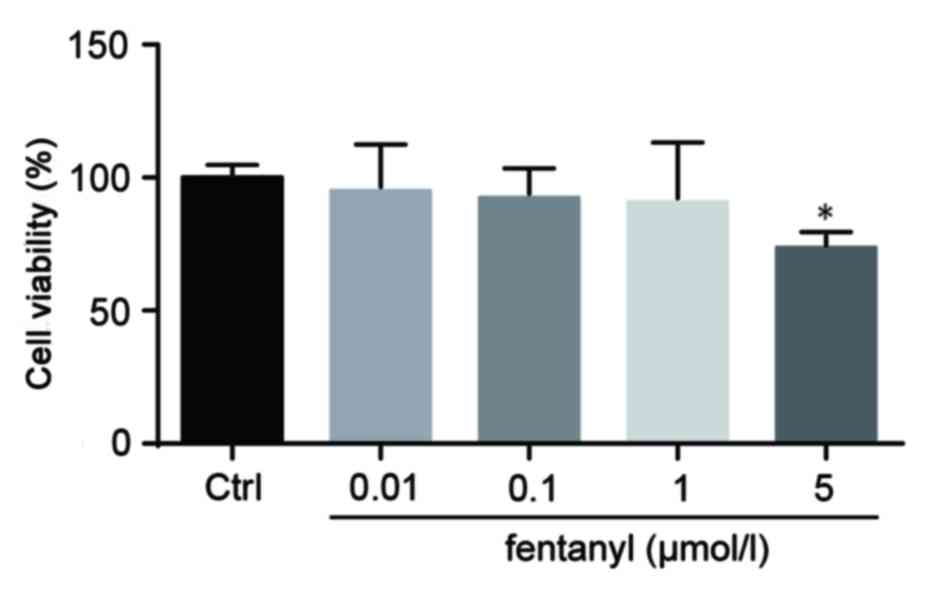
Low concentrations of fentanyl do not
induce cytotoxicity in BV-2 cells
The cell viability of BV-2 cells was assessed by an
MTT assay. BV-2 cells that were treated with 0.01, 0.1 or 1 µmol/l
fentanyl demonstrated no significant reduction in cell viability
compared with the control group. However, 5 µmol/l fentanyl
significantly decreased cell viability by 36% compared with the
control group (P<0.05; Fig. 1).
Consequently, 5 µmol/l fentanyl was selected for subsequent
experiments.
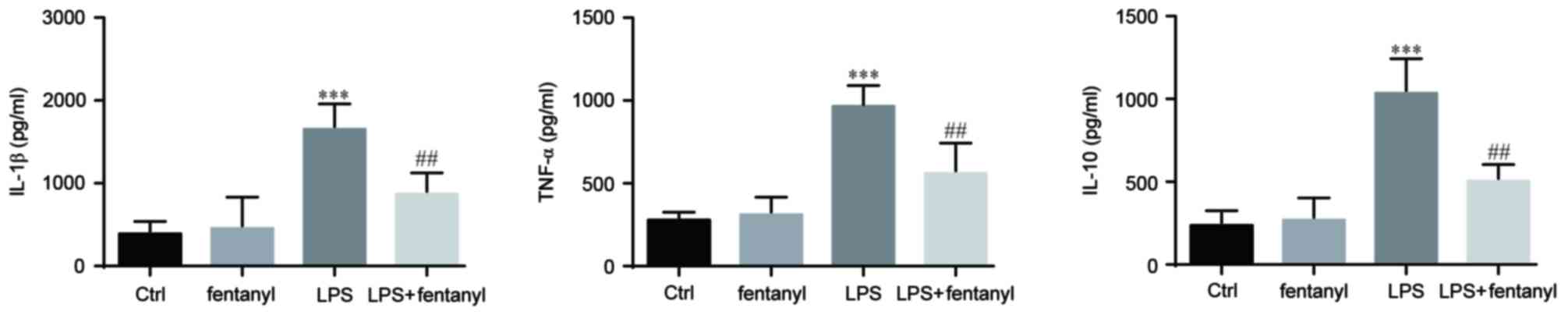
Fentanyl pretreatment suppresses the
LPS-induced elevation of IL-1β, TNF-α and IL-10 in BV-2 cells
The levels of IL-1β, TNF-α and IL-10 that were
released from BV-2 cells were assessed by ELISA (Fig. 2). Released cytokines were not
affected by treatment with 5 µmol/l fentanyl alone in BV-2 cells.
Compared with the control group, 1 µg/ml LPS significantly elevated
the release of IL-1β, TNF-α and IL-10 (P<0.001; Fig. 2). Pretreatment with 5 µmol/l fentanyl
significantly decreased the LPS-upregulated release of IL-1β, TNF-α
and IL-10 compared with the LPS stimulated group (P<0.01;
Fig. 2).
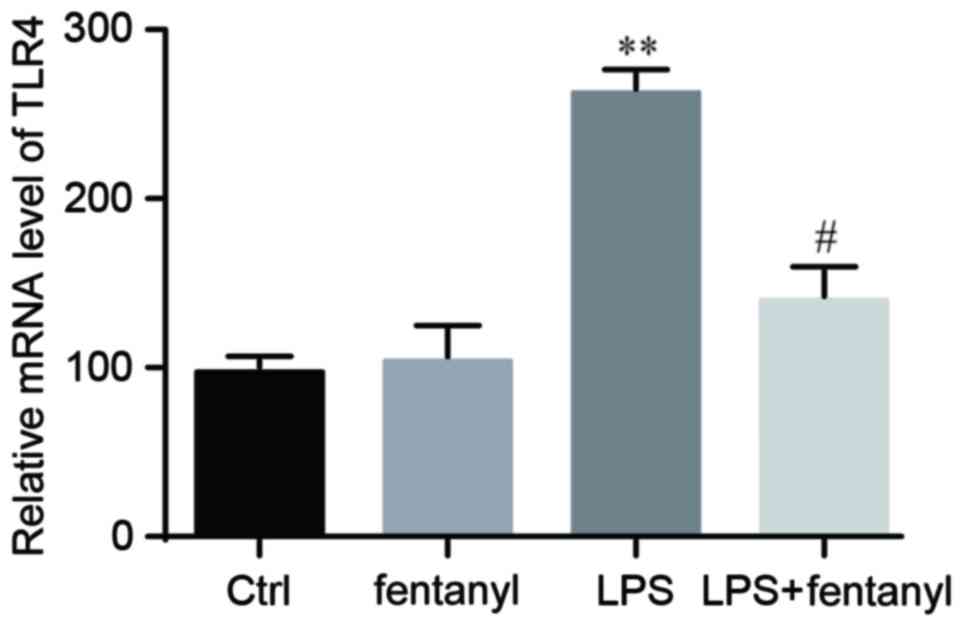
LPS-induced upregulation of TLR4 mRNA
level is suppressed by fentanyl
An RT-qPCR analysis was conducted to detect the mRNA
level of TLR4 in BV-2 cells. No significant differences in the mRNA
level of TLR4 were identified between the control group and the
fentanyl alone treatment group (Fig.
3). Compared with the control group, the mRNA level of TLR4 was
significantly elevated by LPS stimulation (P<0.01; Fig. 3). Fentanyl pretreatment significantly
decreased the LPS-induced upregulation of TLR4 mRNA by 38% compared
with the LPS stimulated group (P<0.05; Fig. 3).
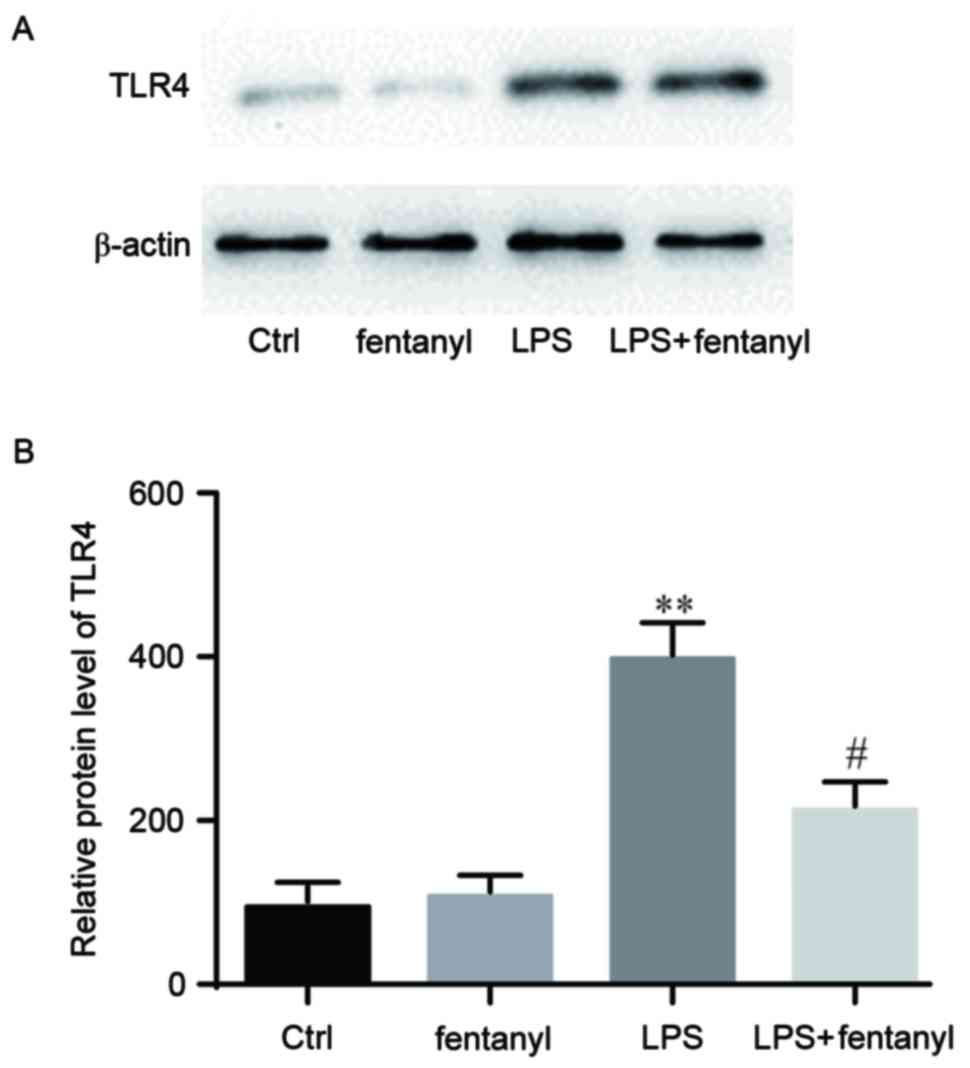
LPS-induced upregulation of the TLR4
protein level is repressed by fentanyl
A western blot analysis was performed to detect the
TLR4 protein level in BV-2 cells (Fig.
4A). No significant difference in the TLR4 protein level was
identified between the control group and the fentanyl alone
treatment group (Fig. 4B). The TLR4
protein level was significantly elevated by LPS stimulation
compared with the control group (P<0.01; Fig. 4B). Fentanyl pretreatment
significantly downregulated the LPS-induced increase of TLR4
protein level compared with the LPS stimulated group (P<0.05;
Fig. 4B).
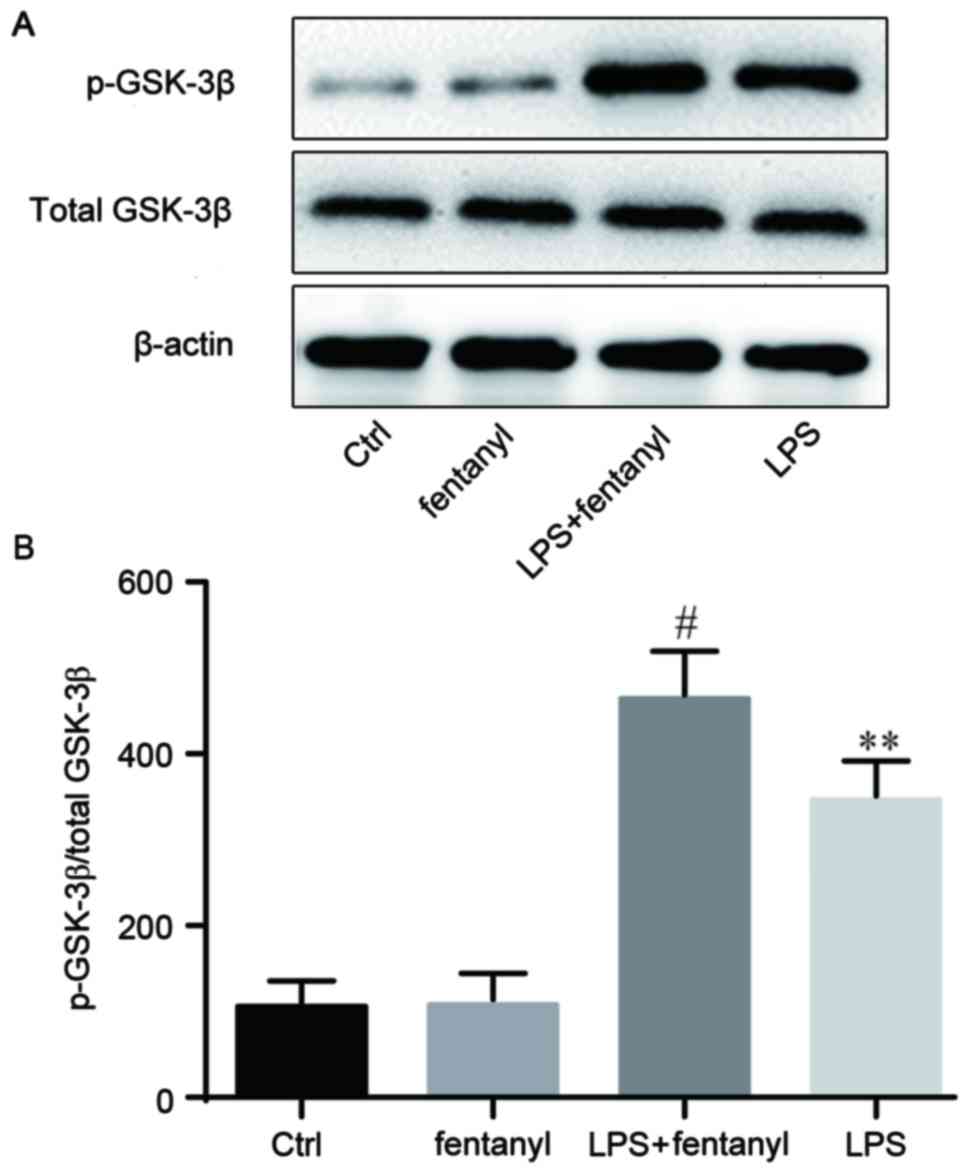
Fentanyl pretreatment further promotes
LPS-induced inactivation of GSK-3β
p-GSK-3β and total GSK-3β expression levels were
evaluated by western blot analysis (Fig.
5). No obvious differences in the total GSK-3β protein level
were identified between the four groups. No significant differences
in the p-GSK-3β protein level were identified between the control
group and the fentanyl alone treatment group. Compared with the
control group, LPS significantly increased the level of p-GSK-3β
protein (P<0.01; Fig. 5B), which
was further and significantly promoted by fentanyl pretreatment
compared with the LPS stimulated group (P<0.05; Fig. 5B).
Discussion
The current study revealed that pretreatment of 5
µmol/l fentanyl inhibited the LPS-induced release of IL-1β, TNF-α
and IL-10, as well as the mRNA and protein levels of TLR4 in BV-2
cells. This process was associated with an increased p-GSK-3β
protein level.
The upregulation of IL-1β and TNF-α is reported to
be stimulated by LPS, thus they are involved in the progression of
systemic inflammation and organ failure (24,25).
Conversely, IL-10 may have the ability to repress the synthesis of
multiple pro-inflammatory cytokines (26). The released levels of IL-1β, TNF-α
and IL-10 were detected in different treatment groups. The results
demonstrated that, compared with the control group, LPS elevated
the release of IL-1β, TNF-α and IL-10, which was inhibited by
fentanyl pretreatment. Emerging evidence demonstrated that fentanyl
serves an important role in regulating the levels of IL-1β, TNF-α
and IL-10 in LPS-induced neuroinflammation. However, the
corresponding molecular mechanisms that were responsible for the
aforementioned effects remained unclear. Consequently, further
experiments were performed to establish the molecular
mechanisms.
TLRs are primarily expressed by immune and
immune-like cells, including granulocytes, monocyte macrophages,
NK, T and B cells, and evolved to detect danger signals. TLR1, TLR2
and TLR4 are reported to respond to nerve injury, they are
upregulated in the CNS, and are positively associated with the
production of pro-inflammatory cytokines TNF-α and IL-1β (27). TLR4 knockout mice and rats that were
intrathecally administered with TLR4 antisense oligonucleotides
exhibited downregulated spinal microglia activation and spinal
pro-inflammatory cytokines, and reduced neuropathic pain (28). TLR4 is expressed specifically by
microglia (29), while LPS is an
agonist to TLR4 (30). In a previous
study, internalization of TLR4 generated microglial cells that were
less sensitive to LPS, resulting in decreased TNF-α production in
BV-2 cells (31). Elevated TLR4
expression was deemed to take part in the progression of
inflammation-associated organ injury (32). Thus, the mRNA and protein levels of
TLR4 in BV-2 cells were assessed in the current study. The results
demonstrated that, in comparison with the control group, TLR4
levels were higher in the LPS stimulated group. Fentanyl
pre-administration reduced the LPS-induced elevation of TLR4 levels
compared with the LPS stimulated group. Nonetheless, the downstream
molecule of TLR4 remained unknown.
Inactivation of GSK-3β was demonstrated to
negatively affect the release of pro-inflammatory cytokines in
response to TLR4 stimulation (33,34). The
protein levels of p-GSK-3β and total GSK-3β were examined in the
current study. The results revealed that the protein level of total
GSK-3β was not obviously different between the four groups.
However, the protein level of p-GSK-3β was higher in the LPS
stimulated group compared with the control group, and
pre-administration of fentanyl led to further elevation of p-GSK-3β
compared with the LPS stimulated group.
In summary, a possible novel treatment for
neuroinflammation was proposed. The results of the current study
suggested that fentanyl pre-treatment exhibits a protective role
during LPS-induced inflammation, via the targeting of the
TLR4/p-GSK-3β signaling pathway, ultimately inhibiting
pro-inflammatory cytokines. Neuroinflammation induced by surgery
serves an important role in the development of postoperative
cognitive dysfunction (POCD) and targeting the TLR4/p-GSK-3β
signaling pathway, which may provide a novel therapeutic approach
for the treatment of POCD.
Acknowledgements
The present study was supported by the commercial
sponsorship of SINCH Pharmaceuticals Tech. Co., Ltd..
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW, YJ and JL made substantial contributions to the
conception and design of the study, performed the experiments and
analyzed the data. JW, YJ and JL managed the literature searches
and figure preparations. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allan SM and Rothwell NJ: Inflammation in
central nervous system injury. Phil Trans R Soc Lond B Biol Sci.
358:1669–1677. 2003. View Article : Google Scholar
|
|
2
|
Yan A, Zhang Y, Lin J, Song L, Wang X and
Liu Z: Partial depletion of peripheral M1 macrophages reverses
motor deficits in MPTP-treated mouse by suppressing
neuroinflammation and dopaminergic neurodegeneration. Front Aging
Neurosci. 10:1602018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baltuch GS: Microglia as mediators of
inflammatory and degenerative diseases. Annu Rev Neurosci.
22:219–240. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucas SM, Rothwell NJ and Gibson RM: The
role of inflammation in CNS injury and disease. Br J Pharmacol. 147
Suppl 1:S232–S240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Theoharides TC, Asadi S and Patel AB:
Focal brain inflammation and autism. Neuroinflamm. 10:462013.
View Article : Google Scholar
|
|
6
|
Auriel E, Regev K and Korczyn AD:
Nonsteroidal anti-inflammatory drugs exposure and the central
nervous system. Handb Clin Neurol. 119:577–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bacchi S, Palumbo P, Sponta A and
Coppolino MF: Clinical pharmacology of non-steroidal
anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents
Med Chem. 11:52–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin L, Wu X, Block ML, Liu Y, Breese GR,
Hong JS, Knapp DJ and Crews FT: Systemic LPS causes chronic
neuroinflammation and progressive neurodegeneration. Glia.
55:453–462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilms H, Sievers J, Rickert U,
Rostami-Yazdi M, Mrowietz U and Lucius R: Dimethylfumarate inhibits
microglial and astrocytic inflammation by suppressing the synthesis
of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model
of brain inflammation. J Neuroinflamm. 7:302010. View Article : Google Scholar
|
|
10
|
Choi Y, Lee MK, Lim SY, Sung SH and Kim
YC: Inhibition of inducible NO synthase, cyclooxygenase-2 and
interleukin-1beta by torilin is mediated by mitogen-activated
protein kinases in microglial BV2 cells. British J Phamrmacol.
156:933–940. 2009. View Article : Google Scholar
|
|
11
|
Blasi E, Barluzzi R, Bocchini V, Mazzolla
R and Bistoni F: Immortalization of murine microglial cells by a
v-raf/v-myc carrying retrovirus. J Neuroimmunol. 27:229–237. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ooi YY, Ramasamy R, Rahmat Z, Subramaiam
H, Tan SW, Abdullah M, Israf DA and Vidyadaran S: Bone marrow
derived mesenchymal stem cells modulate BV2 microglia responses to
lipopolysaccharide. Int Immunopharmacol. 10:1532–1540. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nelson L and Schwaner R: Transdermal
fentanyl: Pharmacology and toxicology. J Med Toxicol. 5:230–241.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnston KD: The potential for mu-opioid
receptor agonists to be anti-emetic in humans: A review of clinical
data. Acta Anaesthesiol Scand. 54:132–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dahan A, Sarton E, Teppema L and Olievier
C: Sex-related differences in the influence of morphine on
ventilatory control in humans. Anesthesiology. 88:903–913. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarton E, Teppema L and Dahan A: Sex
differences in morphine-induced ventilatory depression reside
within the peripheral chemoreflex loop. Anesthesiology.
90:1329–1338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein C, Clark JD, Oh U, Vasko MR, Wilcox
GL, Overland AC, Vanderah TW and Spencer RH: Peripheral mechanisms
of pain and analgesia. Brain Res Rev. 60:90–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watkins LR, Maier SF and Goehler LE:
Immune activation: The role of pro-inflammatory cytokines in
inflammation, illness responses and pathological pain states. Pain.
63:289–302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McNally L, Bhagwagar Z and Hannestad J:
Inflammation, glutamate, and glia in depression: A literature
review. CNS Spectr. 13:501–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan SW, Ramasamy R, Abdullah M and
Vidyadaran S: Inhibitory effects of palm α-, γ- and δ-tocotrienol
on lipopolysaccharide-induced nitric oxide production in BV2
microglia. Cell Immunol. 271:205–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Łabuzek K, Liber S, Gabryel B and Okopień
B: AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside)
increases the production of toxic molecules and affects the profile
of cytokines release in LPS-stimulated rat primary microglial
cultures. Neurotoxicology. 31:134–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gui B, Su M, Chen J, Jin L, Wan R and Qian
Y: Neuroprotective effects of pretreatment with propofol in LPS
induced BV-2 microglia cells: Role of TLR4 and GSK-3β.
Inflammation. 35:1632–1640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crozier TA, Müller JE, Quittkatt D,
Weyland W, Sydow M, Wuttke W and Kettler D: Interleukin-1 beta and
interleukin-6-plasma concentrations in laparotomies. Interaction
with neuroendocrine secretion and postoperative temperature
regulation? Anaesthetist. 42:343–349. 1993.(In German).
|
|
25
|
Grivennikov SI, Tumanov AV, Liepinsh DJ,
Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN,
Förster I, Clausen BE, et al: Distinct and nonredundant in vivo
functions of TNF produced by t cells and macrophages/neutrophils:
Protective and deleterious effects. Immunity. 22:93–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marie C, Pitton C, Fitting C and Cavaillon
JM: IL-10 and IL-4 synergize with TNF-alpha to induce IL-1ra
production by human neutrophils. Cytokine. 8:147–151. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Owens T, Babcock AA, Millward JM and
Toft-Hansen H: Cytokine and chemokine inter-regulation in the
inflamed or injured CNS. Brain Res Brain Res Rev. 48:178–184. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morzaev D, Nicholson JD, Caspi T, Weiss S,
Hochhauser E and Goldenberg-Cohen N: Toll-like receptor-4 knockout
mice are more resistant to optic nerve crush damage than wild-type
mice. Clin Exp Ophthalmol. 43:655–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lehnardt S, Lachance C, Patrizi S,
Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ and
Vartanian T: The toll-like receptor TLR4 is necessary for
lipopolysaccharide-induced oligodendrocyte injury in the CNS. J
Neurosci. 22:2478–2486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hwang D: Modulation of the expression of
cyclooxygenase-2 by fatty acids mediated through toll-like receptor
4-derived signaling pathways. FASEB J. 15:2556–2564. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Willis LM, Bielinski DF, Fisher DR,
Matthan NR and Joseph JA: Walnut extract inhibits LPS-induced
activation of BV-2 microglia via internalization of TLR4: Possible
involvement of phospholipase D2. Inflamm. 33:325–333. 2010.
View Article : Google Scholar
|
|
32
|
Barsness KA, Arcaroli J, Harken AH,
Abraham E, Banerjee A, Reznikov L and McIntyre RC:
Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J
Physiol Regul Integr Comp Physiol. 287:R592–R599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu X, Paik PK, Chen J, Yarilina A,
Kockeritz L, Lu TT, Woodgett JR and Ivashkiv LB: IFN-gamma
suppresses IL-10 production and synergizes with TLR2 by regulating
GSK3 and CREB/AP-1 proteins. Immunity. 24:563–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodionova E, Conzelmann M, Maraskovsky E,
Hess M, Kirsch M, Giese T, Ho AD, Zöller M, Dreger P and Luft T:
GSK-3 mediates differentiation and activation of proinflammatory
dendritic cells. Blood. 109:1584–1592. 2007. View Article : Google Scholar : PubMed/NCBI
|