Introduction
A recent investigation by the China National Center
for Cardiovascular Diseases demonstrated that over 4.5 million
patients have heart failure, and this number will only trend upward
in the next 10 years (1). For
patients with end-stage heart failure, heart transplantation has
proven to be a successful therapeutic procedure (2,3) that
results in a dramatic improvement in patient survival. A great
development in this technique has been made over the past half
century; however, the field of heart transplantation is still
facing some serious challenges, such as the shortage of donor
organs, cardiac allograft vasculopathy and malignancy, and
ischemia/reperfusion (I/R) injury. These challenges can limit the
application and the success of heart transplantation (4,5). As an
independent risk factor, I/R injury is associated with early and
late survival as well as the subsequent delayed cardiac allograft
vasculopathy (6,7). Therefore, the development of more
effective protection to prevent the myocardium from I/R injury is
required to play a pivotal role in increasing the success of heart
transplantation.
Eugenol (4-allyl-2-methoxyphenol), mainly derived
from the essential oils of cloves, is a natural phenolic compound
with various desirable pharmacological functions (8). The mixture of zinc oxide-eugenol has
been applied as a temporary filling material in dentistry due to
its anesthetic and antimicrobial properties (9). Antiproliferative and pro-apoptotic
effects of eugenol have been reported on malignant melanoma cells
where eugenol was shown to have selective action on tumor cells but
did not interfere with normal cell growth (10). Other previous studies have
concentrated on the anti-diabetic and anti-hypertension effects of
eugenol in diabetic rats (11,12). In
different experimental models, the anti-inflammatory and
anti-oxidant properties of eugenol have been explored (13–15).
Eugenol exhibits a protective effect against I/R-induced liver
damage and exhibits anti-ischemic properties in
isoproterenol-induced myocardial infarction in rat models (16,17).
Thus, identifying the protective role of eugenol against donor
heart injury after heterotopic heart transplantation in rats will
provide direction for heart disease research in the future.
Materials and methods
Animals
Male SD rats weighing 250–300 g were obtained from
the Department of Experimental Animal Center, Third Xiangya
Hospital of Central South University (Changsha, China). The weight
of rats in the three groups showed in Table I. Animals were maintained in laminar
flow cages in a specific pathogen-free animal facility and provided
ad libitum access to standard rodent chow diet and filtered water.
The protocol was approved by the Animal Ethics Committee of Central
South University. All rat experiments were carried out in
accordance with the National Institute of Health Guide for the Care
and Use of Laboratory Animals.
 | Table I.The weight of rats in the three
groups. |
Table I.
The weight of rats in the three
groups.
|
| Treatment group
(g) |
|---|
|
|
|
|---|
| Rat group | Sham | Eugenol | Control |
|---|
| Sham | 280.10±14.59 | N/A | N/A |
| Donor rat | N/A | 287.10±14.38 | 277.00±15.53 |
| Recipient rat | N/A | 279.10±12.59 | 281.10±15.77 |
Experimental design
Animal grouping
Fifty male SD rats were randomly divided into a sham
group (n=10), a eugenol group (n=10 pairs, donor and recipient) and
a control group (n=10 pairs, donor and recipient). The weight of 5
subgroups had no significantly statistical differences. The
recipients of the eugenol group received an intraperitoneal
injection of 20 mg/kg eugenol (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 15 days, and a corresponding volume of
physiological saline was applied in the sham group and the control
group for 15 days. Then, all rats were fasted for 12 h, and water
was taken freely before the operation.
Heart harvest from donor rats
The donor rats of the eugenol and control groups
were subjected to 40 mg of chloral hydrate (Sigma-Aldrich; Merck
KGaA) per 100 g body weight of the rats by intraperitoneal
administration and no signs of peritonitis were observed during the
experiment, and then these rats were intravenously administered
sodium heparin 100 U per 100 g as previously described (18,19)
which did not show significant interference on the three groups of
anesthetic effects [data not shown]. The rats were euthanized by
terminal exsanguination and the aorta was identified, isolated,
ligated and perfused slowly with histidine-tryptophan-ketoglutarate
(HTK) solution (Custodiol; Dr Franz-Kohler Chemie GmbH, Hesse,
Germany) at 4°C until the heart stopped beating. The ascending
aorta, pulmonary artery and vena cava were transected, and the
hearts were stored in HTK solution at 4°C for 6 h before
transplantation.
Heterotopic heart transplantation
After receiving an intraperitoneal injection of
eugenol or physiological saline for 1 h, the recipients of the
eugenol and control groups were subjected to abdominal heterotopic
heart transplantation (20,21), while the sham group was subjected
only to coeliotomy without heterotopic heart transplantation. At
sacrifice, rats were fully anaesthetized as described above 3 h
after operation. The native hearts of the sham group and the
transplanted hearts of the eugenol and control groups, along with 3
ml peripheral blood sample per rat of the three groups, were
harvested for further analyses, and then 10–12 ml blood was
obtained by terminal exsanguination in the three groups. Rat death
was confirmed by the absence of corneal and pupillary reflexes and
an apnea test.
Dosage determination of eugenol
In our preliminary experiment, recipient rats were
randomly divided into four groups (5 rats/group) and received
different eugenol treatment (0, 2, 20, 200 mg/kg/day, respectively)
by intraperitoneal injection for 15 days. Heterotopic heart
transplantation (20,21) was performed in eugenol and control
groups. Results showed that rat weight of 200 mg/kg/day Eugenol
treatment group were lower than that of 0, 2, 20 mg/kg/day Eugenol
treatment groups (data not shown). The specific reasons are not
very clear. Furthermore, 200 mg/kg/day Eugenol treatment did not
show a stronger myocardial protection than 20 mg/kg/day Eugenol
treatment (data not shown). research shows 100 mg/kg/day Eugenol
treatment amplifies the injury via oxidant and inflammatory effects
(17). Therefore, we chose the
dosage of 20 mg/kg/day Eugenol treatment for our experiment.
Myocardial malondialdehyde (MDA)
content
Myocardial tissue homogenate was harvested from 100
mg of myocardial tissue and centrifuged at 1,600 × g for 10 min at
4°C, and the supernatant was snap-frozen at −80°C until analyzed.
The MDA concentrations of the liquid supernatants were detected by
a Lipid Peroxidation MDA Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer's
instructions.
Serum levels of cardiac troponin I
(cTnI), creatine kinase-MB (CK-MB), tumor necrosis factor-α (TNF-α)
and interleukin-6 (IL-6)
Before the hearts were collected, peripheral blood
was sampled from the three groups and centrifuged at 12,000 × g for
20 min. The supernatant was snap-frozen at −80°C until analyzed. A
commercial enzyme-linked immunosorbent assay (ELISA) kit was used
to identify serum levels of cTnI, CK-MB, TNF-α and IL-6 (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's instructions.
Western blotting
Right and left ventricular myocardial tissues from
each rat were separately harvested and lysed by RIPA buffer (CWbio,
Beijing, China) containing 0.1 mg/ml phenylmethanesulfonylfluoride
(PMSF; Keygen, Nanjing, China) and 1X protease inhibitor cocktail
(Roche Diagnostics, Mannheim, Germany). Protein concentration was
determined by a commercial BCA protein assay kit (Keygen). A total
of 50 µg of protein was separated by 10% SDS-PAGE electrophoresis
(Keygen), and samples were transferred to 0.45 µm polyvinylidene
fluoride (PVDF) membrane (EMD Millipore, Billerica, USA). The blots
were blocked with 5% skim milk in Tris-buffered saline with 0.1%
Tween-20 (TBST) for 1 h at room temperature and incubated with
primary antibodies (diluted 1:1,000) against cleaved PARP1 (Cell
Signaling Technology, Inc., Danvers, MA, USA), active Caspase-3
(ImmunoWay Biotechnology Company, Plano, TX, USA), BAX, BCL2 and
GAPDH (Wuhan Sanying Biotechnology, Wuhan, China) overnight at 4°C.
The membranes were washed with TBST and then incubated with
appropriate horseradish peroxidase (HRP)-conjugated secondary
antibody (diluted 1:5,000; Cell Signaling Technology, Inc.) for 1 h
at room temperature. After rinsing, the signal on the membrane was
detected by the enhanced chemiluminescence method. The average
protein band intensities of the right and left myocardial specimens
were calculated separately, and the results were presented as the
ratio of average protein band intensity to the GAPDH band intensity
average of left and right tissues.
Histopathology and TUNEL staining
Myocardial tissues were excised and fixed in 4%
paraformaldehyde/PBS at room temperature, embedded in paraffin,
sectioned at 5 µm and subjected to hematoxylin and eosin (H&E)
staining. The specimens were examined under a light microscope. To
detect apoptosis inside the myocardial samples,
terminal-deoxynucleotidyl transferase mediated nick end labeling
(TUNEL; Roche Diagnostics) was performed according to the
manufacturer's instructions. Five high-magnification fields (HPF,
magnification, ×400) were randomly selected and quantified from
each sample. All acquisitions were analyzed with Image-pro-plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All data were analyzed using SPSS Version 18
statistical software (SPSS, Inc, Chicago, IL, USA). Normally
distributed continuous variables were compared using ANOVA and
pairwise comparison among groups was done with LSD post hoc test.
The statistical results are expressed as the mean ± standard
deviation of 3 independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
Eugenol treatment decreases cTnI,
CK-MB, TNF-α and IL-6 expression in serum
cTnI and CK-MB are sensitive and specific indicators
of myocardial damage (22,23). Furthermore, I/R injury leads to the
early activation of inflammatory cytokines such as IL-6 and TNF-α
(24). In comparison with the sham
and control groups, heart transplantation treatment could
significantly increase the level of serum cTnI, CK-MB, TNF-α and
IL-6 in the eugenol and control groups (Fig. 1 and Table
II). Serum cTnI and CK-MB levels of the eugenol group
(5.89±0.77 and 20.16±2.07 ng/ml, respectively, n=3) were
significantly lower than those of the control group (7.34±1.02 and
22.92±1.68 ng/ml, respectively, n=3) (Table II). Serum TNF-α and IL-6 levels were
significantly decreased 3 h after the operation in the eugenol
group (115.48±16.13 and 135.77±16.31 pg/ml, respectively, n=3)
compared with those in the control group (145.09±20.50 and
172.80±20.41 pg/ml, respectively, n=3) (Table II).
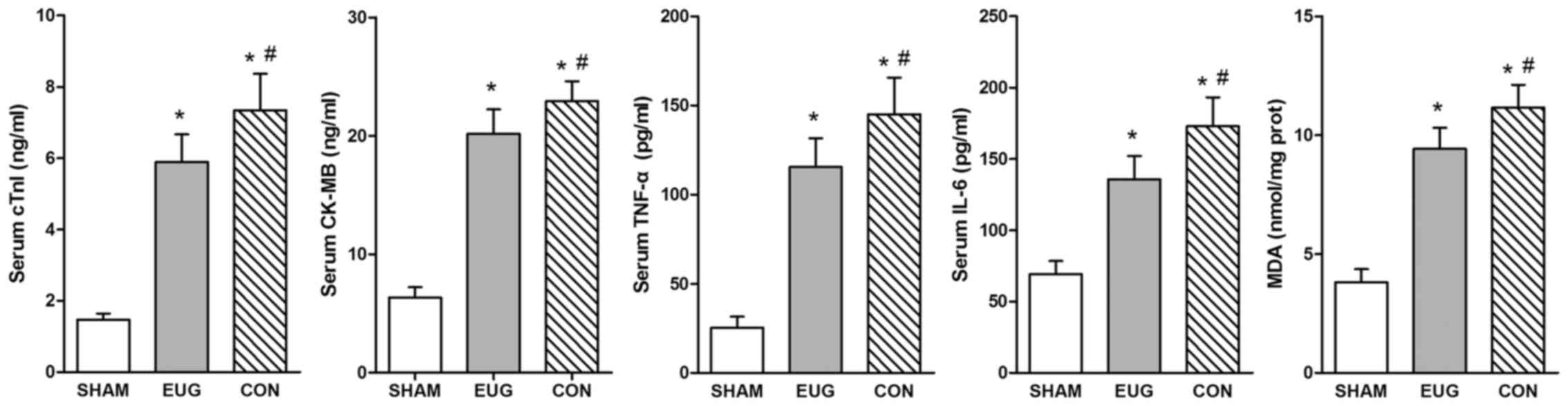 | Figure 1.Serum cTnI, CK-MB, TNF-α and IL-6
levels in the three groups. All statistical results are represented
as the mean ± standard deviation (n=3). *P<0.05 vs. the sham
group; #P<0.05 vs. the EUG group. CON, control group.
EUG, Eugenol; CON, control; IL, interleukin; TNF, tumor necrosis
factor; MDA, malondialdehyde; CK-MB, creatine kinase-MB; cTnI,
cardiac troponin I. |
 | Table II.Serum CK-MB, cTnI, TNF-a and IL-6
levels of the three groups. |
Table II.
Serum CK-MB, cTnI, TNF-a and IL-6
levels of the three groups.
|
| Treatment
group |
|---|
|
|
|
|---|
| Parameter | Sham | Eugenol | Control |
|---|
| cTnI (ng/ml) | 1.47±0.17 |
5.89±0.77a |
7.34±1.02a,b |
| CK-MB (ng/ml) | 6.34±0.87 |
20.16±2.07a |
22.92±1.68a,b |
| TNF-α (pg/ml) | 25.30±6.29 |
115.48±16.13a |
145.09±20.50a,b |
| IL-6 (pg/ml) | 69.26±9.11 |
135.77±16.31a |
172.80±20.41a,b |
| MDA
(nmol/mg.prot) | 3.81±0.56 |
9.42±0.89a |
11.16±0.96a,b |
MDA content
The myocardial MDA content of the sham group was
3.81±0.56 nmol per 1 mg protein (/mg.prot), which was significantly
higher than the levels of the eugenol and control groups (9.42±0.89
and 11.16±0.96 nmol/mg.prot, respectively, n=3) (Table I). Compared with the control group,
myocardial MDA content in the eugenol group was significantly
lower. These results serve as convincing evidence that eugenol
decreased oxidative stress reaction and oxygen free radical injury
after heart transplantation.
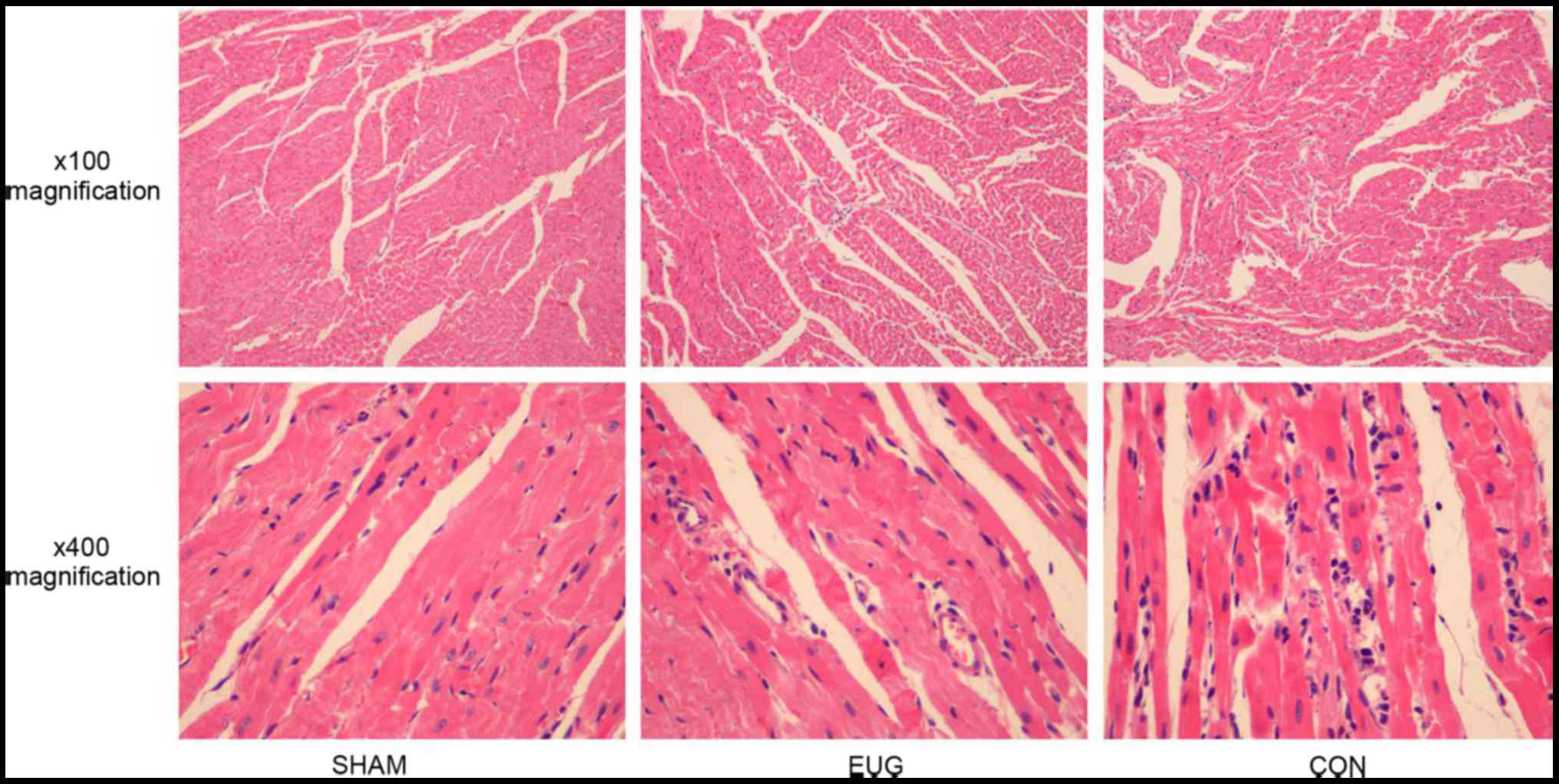
Histopathology
H&E-stained heart sections from the sham group
revealed that there was no obvious myocardial damage, the cardiac
muscle fibers were regular, and there was no edema between cells
(Fig. 2). In contrast, the control
group exhibited extensive myocardial injury, including irregularly
arranged cardiac muscle fibers and interstitial edema with
infiltration of inflammatory cells (Fig.
2). However, the severity of the injury in the eugenol group
was significantly less than that of the control group (Fig. 2).
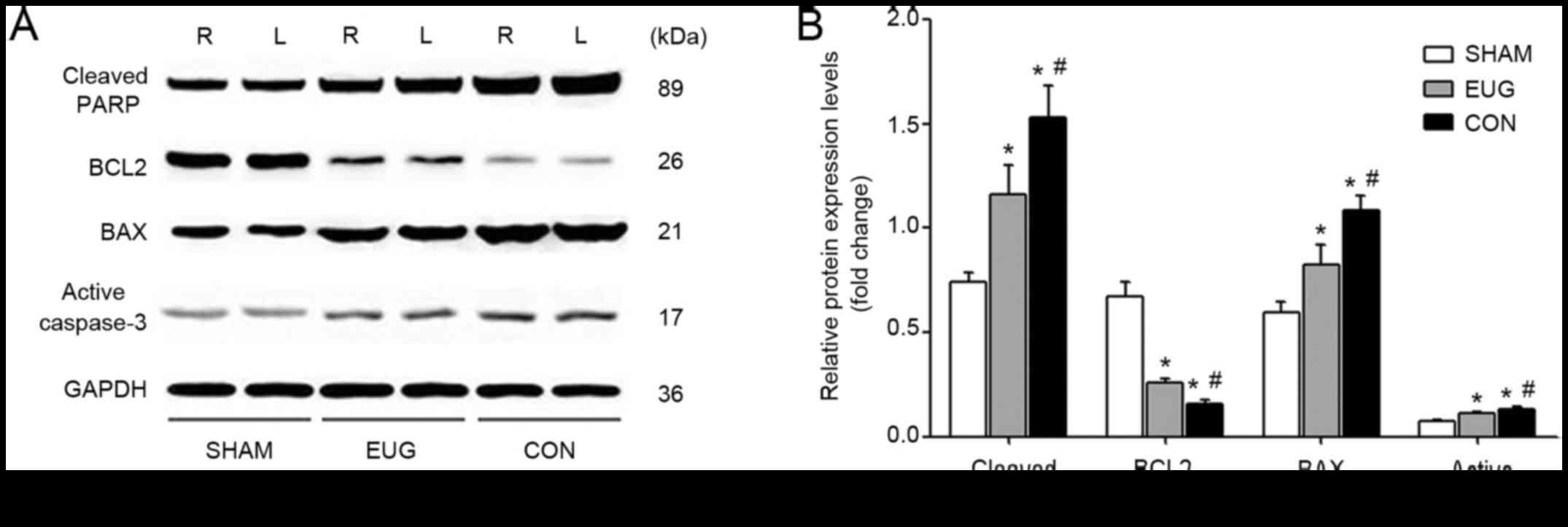
Eugenol treatment decreases the
expression of apoptotic proteins cleaved PARP1, BAX and active
Caspase-3 and increases the expression of anti-apoptotic protein
BCL2
To test whether eugenol treatment can reduce
myocardial cell apoptosis in a transplanted heart, we used the
Western blot method to measure protein expression levels of
apoptosis molecules in rat myocardial tissues, including cleaved
PARP1, BCL2, Bax and active Caspase-3. Our results showed that the
eugenol and control groups had significantly higher levels of
cleaved PARP1, BAX and active Caspase-3 and lower levels of BCL2
compared with the sham group (Fig.
3). This suggests that the heart transplantation treatment
induced early apoptosis of the cardiomyocytes. In comparison with
the sham group, the expressions of cleaved PARP1, BAX and active
Caspase-3 in the eugenol and control groups were significantly
increased, and the expression of BCL2 was significantly decreased
(Fig. 3), which suggests that the
eugenol treatment inhibited cardiomyocyte apoptosis in heart
transplantation.
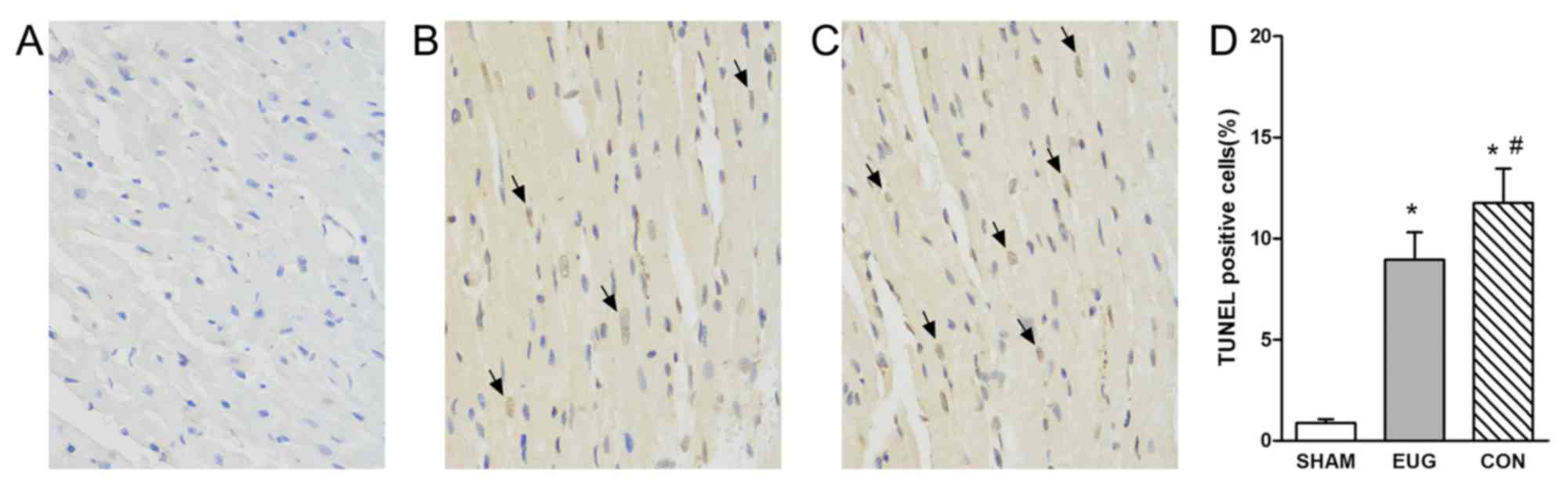
Eugenol treatment reduces
TUNEL-positive muscle cells
In addition to Western blot analysis, TUNEL staining
was used to characterize apoptosis, which provided knowledge about
myocardial damage based on DNA breaks. Compared with the sham group
(apoptosis rate, 0.88±0.19%), heart transplantation treatment
resulted in an increase in the percentage of TUNEL-positive cells
in the eugenol (apoptosis rate, 8.95±1.35%) and control (apoptosis
rate, 11.75±1.71%) groups (Fig. 4).
In comparison with the sham group, the percentage of TUNEL-positive
cells significantly increased in the eugenol and control groups
(Fig. 4). The reduction of
TUNEL-positive cells in the eugenol group compared with the control
group demonstrated the effects of eugenol on apoptosis.
Discussion
Our present research provided obvious evidence that
pretreatment with eugenol (20 mg/kg/day) in recipient rats before
heart transplantation protected the donor heart from myocardial
injury. A similar study showed the protection of liver I/R injury
by eugenol (17). However, our study
focused on the protection of a transplanted heart and the dosage of
eugenol (20 mg/Kg) which was higher than their effective dosage (10
mg/Kg) (17) showed significant
protective effect for the donor heart. Most importantly, their
experimental results showed the protective effect of eugenol on the
autologous liver which had been pretreated with eugenol for 15 days
(17), but our experiments showed
the protective effect of eugenol on the allogeneic heart which had
not been pretreated with eugenol. In clinical practice, it is
difficult to use eugenol to pretreat the donator for a long-term
which is not in accordance with ethical standards. What's more, our
study not only provided sufficient evidence to demonstrate that
eugenol can inhibit the lipid peroxidation and inflammatory
reaction as previous studies have shown (13–15) but
also for the first time demonstrated that eugenol can significantly
inhibit the production of markers of myocardial injury and
myocardial apoptosis in the transplanted heart.
Serum CK-MB and cTnI are biochemical markers of
myocardial injury with notable specificity and sensitivity
(25,26). These markers significantly increased
after heart transplantation in previous studies (24,27,28). The
same results were observed in our study, as serum CK-MB and cTnI
levels of the eugenol and control groups were obviously higher than
those of the sham group. Eugenol pretreatment led to decreases in
CK-MB and cTnI levels in serum. These data might reflect that
eugenol pretreatment could effectively reduce myocardial injury
after heart transplantation. The mechanism behind this outcome
might be related to the anti-inflammatory and anti-oxidative stress
effects of eugenol as reported by other studies (14,15,17).
Myocardial ischemia and hypoxia can increase the expression of
inflammatory cytokine IL-6 (29,30) and
subsequently activate various immune-related receptors to release
TNF-α from cardiac mast cells, macrophages and endothelial cells
(31,32). Warm reperfusion of grafts followed by
cold ischemia is a signal not only to recruit neutrophils but also
to increase their activation to release more inflammatory cytokines
(including IL-6 and TNF-α), which in turn augments the myocardial
tissue injury (28,33). Our study also revealed the
infiltration of inflammatory cells into the myocardial tissue and
the up-regulation of serum inflammatory cytokines 3 h after
operation in the eugenol and control groups. This finding was also
reported by several studies that noted inflammatory cytokine
production and mononuclear cell infiltration in different heart
transplantation models (24,34,35). The
speed and strength of lipid peroxidation could be directly
reflected by measuring the MDA content, which is the end product of
lipid peroxidation metabolism (26).
Compared with the control group, eugenol significantly
down-regulated myocardial MDA content. These results indicated that
pretreatment with eugenol could reduce oxidative stress reaction
and oxygen free radical damage as reported by other studies
(15,17).
Compared with the method of saline pretreatment, we
observed that pretreatment with intraperitoneal injection of
eugenol for recipients remarkably decreased the number of
TUNEL-positive cells in the sectioned left ventricular myocardium
of transplanted hearts. Furthermore, the level of apoptosis, as
assessed by protein expression detection of cleaved PARP1, Bcl-2,
Bax and active Caspase-3, was significantly inhibited in hearts
that were transplanted into recipients and received eugenol
pretreatment. Consequently, the fact that eugenol could effectively
inhibit myocardial apoptosis further confirmed that eugenol
exhibits a protection effect against myocardial injury. According
to our study, the mechanism of myocardial apoptosis inhibition by
eugenol might be related to the fact that eugenol reduced the
expression of inflammatory cytokines and eliminated the active
oxygen radicals.
Because we observed down-regulation of myocardial
injury markers and inflammatory cytokines as well as a reduction in
cardiac cell apoptosis, our data supported the protection effect of
eugenol against transplanted heart injury in the process of heart
transplantation. However, there were still some limitations in our
study. We only studied myocardial injury and apoptosis after
eugenol treatment, and other parameters such as pharmacokinetics,
myocardial function, immune rejection or other clinical events in
eugenol treatment need to be tested in the future.
In conclusion, according to our present study,
eugenol exerts protective effects on the transplanted heart in rat
heterotopic heart transplantation through alleviating myocardial
edema, down-regulating the myocardial inflammatory response and
inhibiting myocardial apoptosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81472774).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DW and WF conceived and designed the study. WF, CL,
YJ and LY performed the experiments. WF and ZJ wrote the paper. LJ,
QX and LH wrote the paper, revised the manuscript and given final
approval of the version to be published. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Ethics
Committee of Central South University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang W, Hu SS, Kong LZ, Gao RL, Zhu ML,
Wang WY, Wu ZS, Chen WW, Yang JG, Ma LY, et al: Summary of report
on cardiovascular diseases in China, 2012. Biomed Environ Sci.
27:552–558. 2014.PubMed/NCBI
|
|
2
|
Singh TP, Milliren CE, Almond CS and
Graham D: Survival benefit from transplantation in patients listed
for heart transplantation in the United States. J Am Coll Cardiol.
63:1169–1178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H, Zhang X, Zheng X, Lan Z, Shi J,
Jiang J, Zwiep T, Li Q, Quan D, Zhang ZX and Min W: Prevention of
allograft rejection in heart transplantation through concurrent
gene silencing of TLR and Kinase signaling pathways. Sci Rep.
6:338692016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu H, Xie M, Xu L, Zheng X, Yang Y and Lv
X: The protective role of interleukin-18 binding protein in a
murine model of cardiac ischemia/reperfusion injury. Transpl Int.
28:1436–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tonsho M, Michel S, Ahmed Z, Alessandrini
A and Madsen JC: Heart transplantation: Challenges facing the
field. Cold Spring Harb Perspect Med. 4:a0156362014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luciani GB, Forni A, Rigatelli G,
Chiominto B, Cardaioli P, Mazzucco A and Faggian G: Myocardial
protection in heart transplantation using blood cardioplegia:
12-year outcome of a prospective randomized trial. J Heart Lung
Transplant. 30:29–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wood KJ and Goto R: Mechanisms of
rejection: Current perspectives. Transplantation. 93:1–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pramod K, Ansari SH and Ali J: Eugenol: A
natural compound with versatile pharmacological actions. Nat Prod
Commun. 5:1999–2006. 2010.PubMed/NCBI
|
|
9
|
Markowitz K, Moynihan M, Liu M and Kim S:
Biologic properties of eugenol and zinc oxide-eugenol. A clinically
oriented review. Oral Surg Oral Med Oral Pathol. 73:729–737. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pisano M, Pagnan G, Loi M, Mura ME,
Tilocca MG, Palmieri G, Fabbri D, Dettori MA, Delogu G, Ponzoni M
and Rozzo C: Antiproliferative and pro-apoptotic activity of
eugenol-related biphenyls on malignant melanoma cells. Mol Cancer.
6:82007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mnafgui K, Kaanich F, Derbali A, Hamden K,
Derbali F, Slama S, Allouche N and Elfeki A: Inhibition of key
enzymes related to diabetes and hypertension by Eugenol in vitro
and in alloxan-induced diabetic rats. Arch Physiol Biochem.
119:225–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srinivasan S, Sathish G, Jayanthi M,
Muthukumaran J, Muruganathan U and Ramachandran V: Ameliorating
effect of eugenol on hyperglycemia by attenuating the key enzymes
of glucose metabolism in streptozotocin-induced diabetic rats. Mol
Cell Biochem. 385:159–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nam H and Kim MM: Eugenol with antioxidant
activity inhibits MMP-9 related to metastasis in human fibrosarcoma
cells. Food Chem Toxicol. 55:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peixotoneves D, Lealcardoso JH and Jaggar
JH: Eugenol dilates rat cerebral arteries by inhibiting smooth
musclecell voltage-dependent calcium channels. J Cardiovasc
Pharmacol. 64:401–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yogalakshmi B, Viswanathan P and Anuradha
CV: Investigation of antioxidant, anti-inflammatory and
DNA-protective properties of eugenol in thioacetamide-induced liver
injury in rats. Toxicology. 268:204–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mnafgui K, Hajji R, Derbali F, Gammoudi A,
Khabbabi G, Ellefi H, Allouche N, Kadri A and Gharsallah N:
Anti-inflammatory, antithrombotic and cardiac remodeling preventive
effects of eugenol in isoproterenol-induced myocardial infarction
in wistar rat. Cardiovasc Toxicol. 16:336–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Motteleb DM, Abd El, Selim SA and Mohamed
AM: Differential effects of eugenol against hepatic inflammation
and overall damage induced by ischemia/re-perfusion injury. J
Immunotoxicol. 11:238–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Xia Y, Zhu B, Hu X, Xu S, Chen L,
Papadimos TJ, Wang W, Wang Q and Xu X: Measurement of the efficacy
of 2% lipid in reversing bupivacaine- induced asystole in isolated
rat hearts. BMC Anesthesiol. 14:602014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gherardini G, Haegerstrand A, Matarasso A,
Gurlek A, Evans GR and Lundeberg T: Cell adhesion and short-term
patency in human endothelium preseeded 1.5-mm
polytetrafluoroethylene vascular grafts: An experimental study.
Plast Reconstr Surg. 99:472–478. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ono K and Lindsey ES: Improved technique
of heart transplantation in rats. J Thorac Cardiovasc Surg.
57:225–229. 1969.PubMed/NCBI
|
|
21
|
Wakayama K, Fukai M, Yamashita K, Kimura
T, Hirokata G, Shibasaki S, Fukumori D, Haga S, Sugawara M, Suzuki
T, et al: Successful transplantation of rat hearts subjected to
extended cold preservation with a novel preservation solution.
Transpl Int. 25:696–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Croal BL, Hillis GS, Gibson PH, Fazal MT,
El-Shafei H, Gibson G, Jeffrey RR, Buchan KG, West D and
Cuthbertson BH: Relationship between postoperative cardiac troponin
i levels and outcome of cardiac surgery. Circulation.
114:1468–1475. 2007. View Article : Google Scholar
|
|
23
|
Yuan Z, Li H, Qi Q, Gong W, Qian C, Dong
R, Zang Y, Li J, Zhou M, Cai J, et al: Plasma levels of growth
differentiation factor-15 are associated with myocardial injury in
patients undergoing off-pump coronary artery bypass grafting. Sci
Rep. 6:282212016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee S, Huang CS, Kawamura T, Shigemura N,
Billiar TR, Nakao A and Toyoda Y:
Histidine-tryptophan-ketoglutarate or celsior: Which is more
suitable for cold preservation for cardiac grafts from older
donors? Ann Thorac Surg. 91:755–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rittoo D, Jones A, Lecky B and Neithercut
D: Elevation of cardiac troponin T, but not cardiac troponin I, in
patients with neuromuscular diseases: Implications for the
diagnosis of myocardial infarction. J Am Coll Cardiol.
63:2411–2420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuang J, Sun Y, Wang W, Ke H and Ye H:
Myocardial apoptosis and injury of donor hearts kept in completely
beating status with normothermic blood perfusion for transplants.
Int J Clin Exp Med. 8:5767–5773. 2015.PubMed/NCBI
|
|
27
|
Li Z, Galli U, Becker LE, Bruns H,
Nickkolgh A, Hoffmann K, Karck M and Schemmer P: Sulforaphane
protects hearts from early injury after experimental
transplantation. Ann Transplant. 18:558–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alamran FF and Shahkolahi M: Oxytocin
ameliorates the immediate myocardial injury in heart transplant
through down regulation of the neutrophil dependent myocardial
apoptosis. Transplant Proc. 45:2506–2512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamauchitakihara K, Ihara Y, Ogata A,
Yoshizaki K, Azuma J and Kishimoto T: Hypoxic stress induces
cardiac myocyte-derived interleukin-6. Circulation. 91:1520–1524.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Q, Li J, Jubair S, Wang D, Luo Y, Fan
D and Janicki JS: Sparstolonin B attenuates hypoxia-induced
apoptosis, necrosis and inflammation in cultured rat left
ventricular tissue slices. Cardiovasc Drugs Ther. 28:433–439. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaczorowski DJ, Nakao A, Mollen KP,
Vallabhaneni R, Sugimoto R, Kohmoto J, Tobita K, Zuckerbraun BS,
McCurry KR, Murase N and Billiar TR: Toll-like receptor 4 mediates
the early inflammatory response after cold ischemia/reperfusion.
Transplantation. 84:1279–1287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reil JC, Gilles S, Zahler S, Brandl A,
Drexler H, Hültner L, Matrisian LM, Welsch U and Becker BF:
Insights from knock-out models concerning postischemic release of
TNFalpha from isolated mouse hearts. J Mol Cell Cardiol.
42:133–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Itoh S, Kimura N, Axtell RC, Velotta JB,
Gong Y, Wang X, Kajiwara N, Nambu A, Shimura E, Adachi H, et al:
Interleukin-17 accelerates allograft rejection by suppressing
regulatory T cell expansion. Circulation. 124 11 Suppl:S187–S196.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sang HL, Lee S, Noda K, Kawamura T, Tanaka
Y, Shigemura N, Nakao A and Toyoda Y: Adenosine injection prior to
cardioplegia enhances preservation of senescent hearts in rat
heterotopic heart transplantation. Eur J Cardiothorac Surg.
43:1202–1208. 2013. View Article : Google Scholar : PubMed/NCBI
|