Introduction
Acute lung injury (ALI) and the more severe form of
ALI, acute respiratory distress syndrome (ARDS), typically have
high morbidity and mortality (1,2).
Lipopolysaccharide (LPS) derived from Gram-negative bacteria is a
key pathogenic factor in the progression of ALI, (1,2). It has
been reported that LPS induces the inflammatory response by binding
LPS receptors and accessory proteins, thereby promoting the
production of pro-inflammatory mediators (1–4). A
number of studies have identified the important role of Toll-like
receptor 4 (TLR4) in LPS-induced inflammation (5–7). TLR4
has been reported to activate the nuclear factor (NF)-κB signaling
pathway, causing the transcription of pro-inflammatory cytokines,
including tumor necrosis factor (TNF)-α (8).
MicroRNAs (miRs or miRNAs) are a class of small
non-coding RNAs ~21–25 nucleotides in length that are found in
almost all genomes (9,10). It has been suggested that miRNAs
repress gene expression at the post-transcriptional level by
binding to the 3′ untranslated regions (3′UTRs) (11). Abnormal miRNA expression has been
reported in a number of diseases, including cystic fibrosis,
asthma, ALI and ARDS (12,13). MiR-34a levels are significantly
higher in the lungs of neonatal rats exposed to hyperoxia compared
with normal controls and miR-34a suppression improves the pulmonary
phenotype and bronchopulmonary dysplasia-associated pulmonary
arterial hypertension (14).
Furthermore, plasma miR-200c-3p levels are much higher in patients
with severe pneumonia compared with healthy controls (15).
MiRNAs have been detected in a variety of sources,
including tissues, blood and body fluids (16,17).
They are stable and resistant to different sample handling
conditions and, as such, may have potential as biomarkers for the
progression of a number of diseases (18,19).
Recently, miRNAs have been demonstrated to be potential biomarkers
for cancer as well as cardiovascular and rheumatic diseases
(17–19). Plasma miR-155 and miR-146a may be
used as novel biomarkers to predict the mortality and treatment
outcome of severe sepsis and sepsis-induced acute lung injury
(20).
MiR-140 has been reported to be aberrantly expressed
in a number of tumor types, including glioma and gastric cancer
(21,22). However, the specific role of miR-140
in the progression of ALI has never been explored. The aim of the
present study was to measure the expression of miR-140 and assess
the underlying mechanism by which it affects the progression of
ALI.
Materials and methods
Study population
Briefly, 50 mechanically ventilated patients with
ALI (mean age, 48.57±16.55 years; sex ratio, male:female, 26:24;
comorbidities, none) admitted to the Zhongnan Hospital of Wuhan
University (Wuhan, China) were recruited from September 2016 to
March 2017. A total of 20 healthy subjects (mean age: 45.25±10.24
years; sex ratio, 1:1; comorbidities, none) were recruited from
September 2016 to March 2017 and screened at the same hospital.
Screening consisted of history, physical examination, routine blood
investigation, electrocardiogram and spirometry. At the baseline,
the mean forced expiratory volume and forced vital capacity were
4.2 (0.5 l) and 5.2 (0.75 l), respectively. Patients were included
if they met the criteria for ALI, without sepsis (ALI criteria was
established in accordance with the definition and diagnostic
criteria of the ARDS Berlin in 2012). Upon admission to hospital,
all patients, within the onset of 24 h, accepted oral tracheal
intubation with ventilator-assisted respiration. The right
subclavian vein catheter was also retained. Patients were excluded
from the present study if they exhibited thoracic deformity,
pneumothorax, severe myocardial ischemia, intracranial
hypertension, cerebral blood supply insufficiency, hemodynamic
instability or chronic organ failure. Patients were also excluded
if they were <18 years of age. The study was approved by the
local research ethics committee of Zhongnan Hospital of Wuhan
University (Wuhan, China) and written informed consent was obtained
from the legal representative of each patient or the subjects
themselves in the control group. The research was carried out in
compliance with the Declaration of Helsinki.
Animals
Male Wistar rats were supplied by SPF (Beijing)
Biotechnology Co., Ltd. (Beijing, China). Rats were housed at
23±2°C with a 12-h light/dark cycle in an air-conditioned room with
50±10% relative humidity. A normal rat pellet diet and water were
supplied ad libitum. Rats were randomly allocated to
different experimental groups. All animal procedures were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals of Zhongnan Hospital of Wuhan University (23). Ethical approval was granted by the
local ethics committee of Zhongnan Hospital of Wuhan University
(Wuhan, China).
LPS (Escherichia coli serotype K235) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rats
were randomly divided into the control and LPS groups (n=6 in each
group). Rats in the control group received saline (0.5 ml)
intraperitoneally (IP). Rats in the LPS group received LPS (0.5
mg/kg, IP). Rats were anesthetized with ketamine + xylazine 20 h
later, following which blood was sampled and lung tissues were
harvested. Tissues were flash frozen in liquid nitrogen and stored
at −70°C for use in the myeloperoxidase assay, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting.
Histological assessment
Lung tissue samples were fixed in 4%
phosphate-buffered neutral formalin at room temperature for 20 min,
embedded in paraffin and cut into 5 µm thick sections. Samples were
then deparaffinized, rehydrated using a descending alcohol series
and microwave-heated for 30 min in sodium citrate buffer (Beijing
Solarbio Science & Technology, Co., Ltd., Beijing, China) at
100°C for antigen retrieval. Sections were subsequently incubated
with 0.3% hydrogen peroxide/phosphate-buffered saline at room
temperature for 30 min. Samples were incubated with hematoxylin
(Beijing Solarbio Science & Technology, Co., Ltd.) at room
temperature for 5 min. Then, the slides were washed with
ddH2O for 3 min and further stained with eosin for 2 min
at room temperature. Samples were washed a second time with
ddH2O for 3 min. Slides were visualized using light
microscopy (magnification, ×40; Olympus CK40, Olympus Corporation,
Tokyo, Japan).
Cell culture
A549 human lung adenocarcinoma epithelial cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Ham's F12 nutrient medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA). 293T cells were
purchased from the Peking Union Medical College Cell Culture Center
(Beijing, China) and cultured in Dulbecco's modified Eagle's medium
(Hyclone; GE Healthcare Life Sciences). THP1 cells (American Type
Culture Collection, Manassas, VA, USA) were cultured in RPMI-1640
medium (Hyclone; GE Healthcare Life Sciences). Cells were cultured
in the appropriate medium supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
U ml−1 penicillin and 100 U ml−1 streptomycin
at 37°C.
RNA extraction
Total RNA was isolated from the whole blood samples
(5 ml, collected in tubes containing EDTA) or epithelial cells
using RNAVzol LS (Vigorous Biotechnology Beijing Co., Ltd.,
Beijing, China) according to the manufacturer's protocol. The
concentration and the purity of RNA samples were determined by
measuring the optical density (OD) 260/OD280.
RT-qPCR
A total of 1 µg RNA was reverse transcribed using
Moloney Murine Leukemia Virus (MMLV) reverse transcription enzyme
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with specific
primers. The temperature protocol used for RT was as follows 72°C
for 10 min; 42°C for 60 min, 72°C for 5 min and 95°C for 2 min. To
quantify the relative mRNA levels, qPCR was performed using SYBR
Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in
an iCycleriQ real-time PCR detection system. The PCR amplifications
were performed in a 10 µl reaction system containing 5 µl SYBR
Green Supermix, 0.4 µl forward primer, 0.4 µl reverse primer, 2.2
µl double distilled H2O and 2 µl template cDNA.
Thermocycling conditions were as follows: 95°C for 10 min followed
by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Relative mRNA
expression was normalized to U6 using the 2−∆∆Cq method
(24). Primer sequences are as
follows: miR-140-5p-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTACCA-3′; U6-RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATG-3′;
miR-140-5p, forward 5′-GCGCGCAGUGGUUUUACCCUA-3′; U6, forward
5′-GCGCGTCGTGAAGCGTTC-3′; universal reverse primer,
5′-GTGCAGGGTCCGAGGT-3′.
Western blotting
Total proteins were isolated from lung tissues or
A549 cells using a total protein extraction kit (Beijing Solarbio
Science & Technology Co., Ltd.) and collected following
centrifugation at 12,000 × g for 30 min at 4°C. A BCA protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) was used to determine
the protein concentration. A total of 20 µg protein was separated
using 12% SDS-PAGE, transferred onto polyvinylidene difluoride
(PVDF) membranes and blocked with 5% fat-free milk at room
temperature for 2 h. Membranes were incubated with primary
antibodies against TLR4 (cat. no. ab22048; 1:1,000; Abcam,
Cambridge, UK), phosphorylated (p)-nuclear factor (NF)-κB (cat. no.
3033; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
NF-κB (cat. no. 8242; 1:1,000; Cell Signaling Technology, Inc.) and
anti-GAPDH (2118; 1:5,000; Cell Signaling Technology, Inc.) at 4°C
overnight. Membranes were subsequently incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (both 1:5,000;
cat. no. ZB-2301; Beijing Zhongshan Golden Bridge Biotechnology
Co., Beijing, China) for 2 h at room temperature, followed by three
washes with TBST. Enhanced chemiluminescence (EMD Millipore,
Billerica, MA, USA) was used to determine the protein
concentrations according to the manufacturer's protocol. Signals
were detected using a Super ECL Plus Kit (Nanjing KeyGen Biotech
Co., Ltd.) and quantitative analysis was performed using UVP
software (UVP LLC, Upland, CA, USA). Relative protein expressions
were normalized to GAPDH. All experiments were repeated three
times. ImageJ 1.43b software (National Institutes of Health,
Bethesda, MD, USA) was used for densitometry analysis.
Transfection
MiR-140 mimics, inhibitors, TLR4 siRNA were obtained
from Guangzhou RiboBio Co., Ltd. (Guangzhou, China) and transfected
into A549 and 293T cells was performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. Cells were collected for subsequent
experimentation following the 48 h transfection.
ELISA
Serum or cell lysates were homogenized in lysis
buffer (50 mmol/l Tris-HCl; 300 mmol/l NaCl; 5 mmol/l EDTA; 1%
Triton X-100; and 0.02% sodium azide) containing a protease
inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) and then
centrifuged at 16,000 × g for 15 min at 4°C. Levels of TNF-α (cat.
no. DTA00D), interleukin (IL)-6 (cat. no. D6050) and IL-1β (cat.
no. 201-LB) in the supernatant were measured using ELISA (all
R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. Samples were read at a 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Luciferase reporter assay
The TLR4 3′UTR segments containing the miR-140
binding sites were amplified using PCR. To amplify TLR3 3′UTR, the
genome DNA from A549 cells were extracted using an EasyPure Genomic
DNA kit (cat. no. EE101-02, Beijing TransGen Biotech Co., Ltd.,
Beijing, China). PCR was performed using a 2×EasyTaq PCR SuperMix
kit (cat. no. AS111-02; Beijing TransGen Biotech Co., Ltd.). The
DNA polymerase used was part of this kit. 2 µl genome DNA (2 µl), 1
µl forward primer, 1 µl reverse primer, 25 µl 2×EasyTaq PCR
SuperMix, and 20 µl nuclease-free water were mixed. The
thermocycling conditions were as follows: 94°C for 5 min, followed
by 40 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 30
sec, and then 72°C 10 min. The primers used were as follows:
TLR4-3′UTR forward, 5′-GGCTCCTGATGCAAGATGCCCCT-3′ and reverse,
5′-CTGCCTTGAATACCTTCACACGT-3′; GAPDH forward,
5′-CTGAGCACCAGGTGGTCTC-3′ and GAPDH reverse,
5′-CATGACAAGGTGCGGCTCC-3′. Oligonucleotides were then inserted into
a pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega
Corp., Madison, WI, USA) and sequenced to confirm there were no
mutations. 293 cells were seeded in 24-well plates and
co-transfected with miR-140 mimics or negative control miR with
recombinant pmirGLO. The relative Luciferase activity was measured
using the Dual-Luciferase Reporter Assay System (Promega Corp.)
following 48 h co-transfection.
Statistical analysis
Data are presented as the mean ± standard deviation.
Two-tailed unpaired student's t-tests were used for comparisons
between two groups. Analysis of variance followed by Tukey's post
hoc test were used for multiple group comparisons with SPSS 13
(SPSS, Inc., Chicago, IL, USA). Receiver operator characteristic
(ROC) curves were used to assess the potential of miR-140 as a
biomarker, and the area under curve (AUC) was recorded. P<0.05
was considered to indicate a statistically significant
difference.
Results
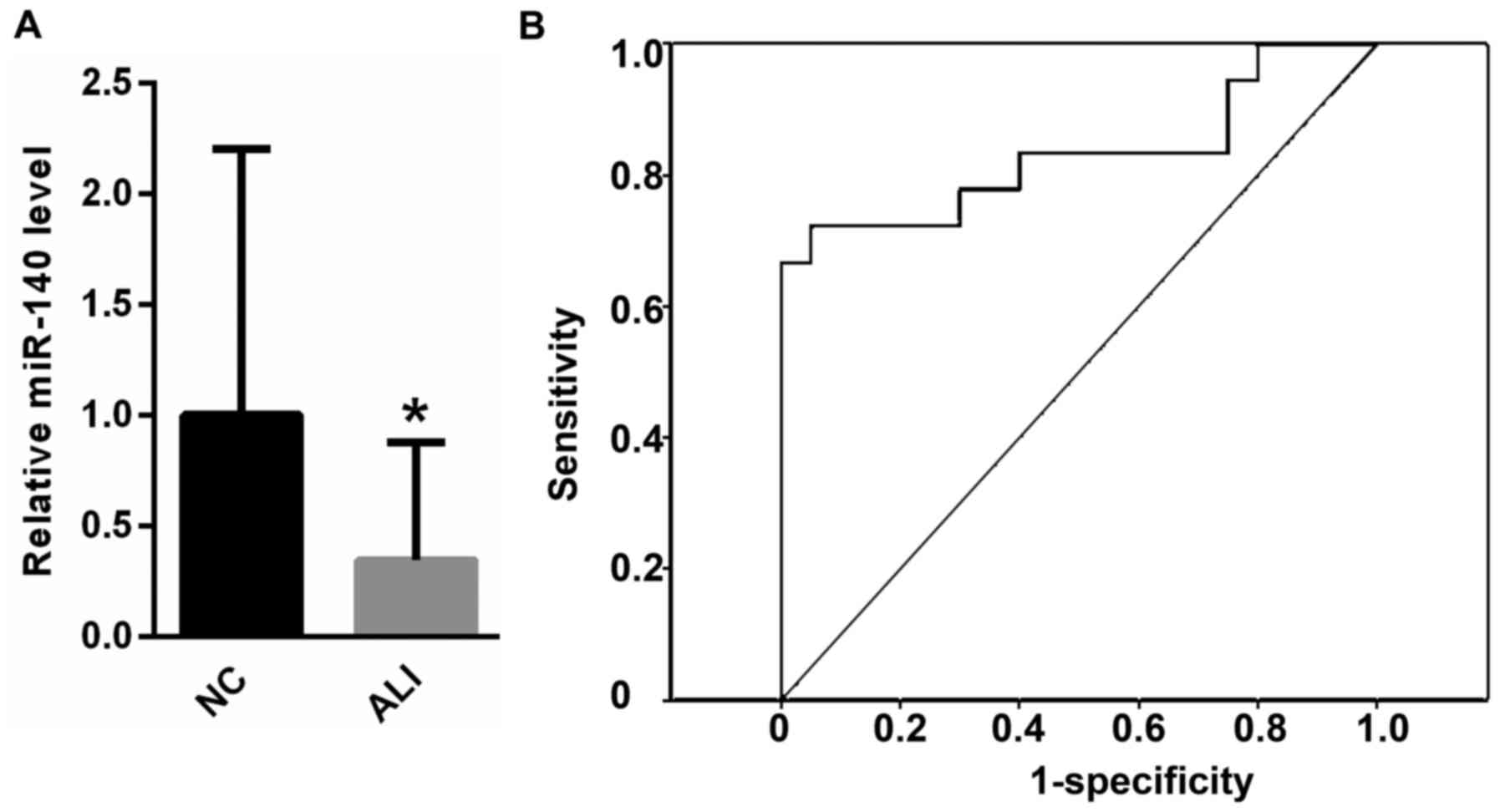
Peripheral blood miR-140 was lower in
patients with ALI
As shown in Fig. 1A,
peripheral blood miR-140 was much lower in patients with ALI
compared with healthy controls. In addition, ROC analysis revealed
that peripheral blood miR-140 could be used to differentiate
subjects with ALI from healthy controls, with an ROC curve area of
0.935 (95% confidence interval: 0.817–1.000; P<0.0001; Fig. 1B).
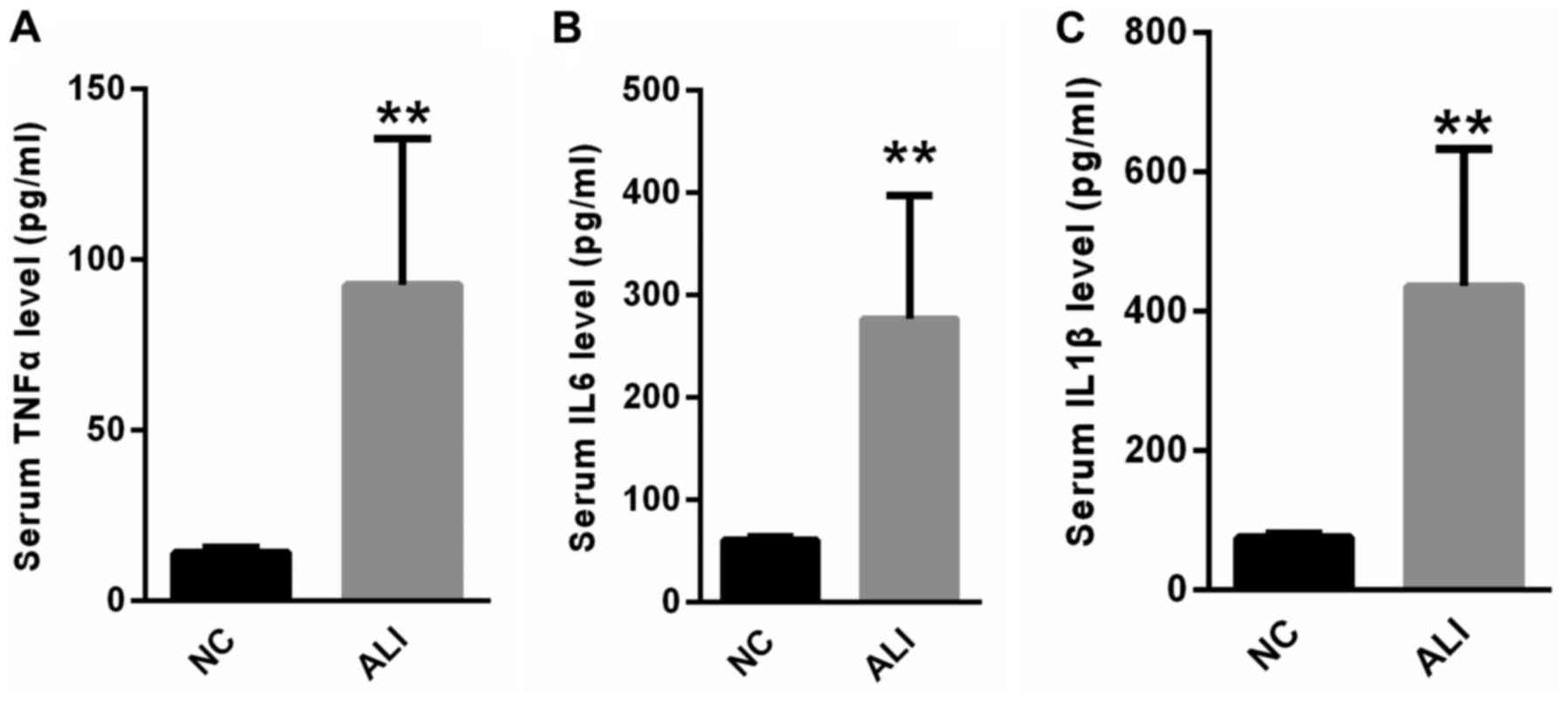
Serum levels of inflammatory factors
are higher in patients with ALI
The serum levels of inflammatory factors, including
TNF-α, IL-6 and IL-1β were measured. Compared with the healthy
controls, serum TNF-α, IL-6 and IL-1β levels were significantly
increased in patients with ALI (Fig.
2).
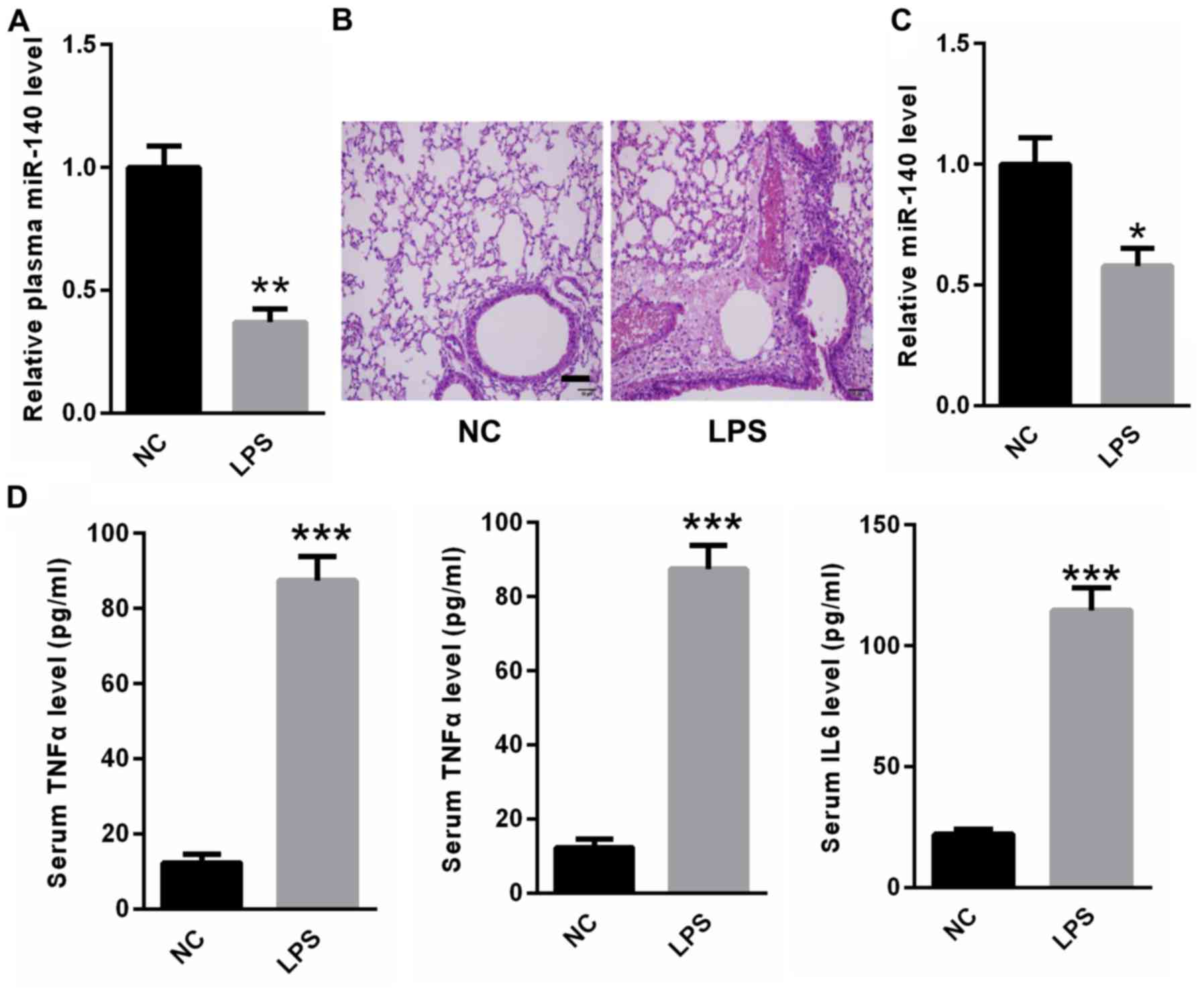
MiR-140 was lower in the peripheral
blood and lung tissues of rats with LPS-induced ALI
LPS-induced ALI rat models were established. Real
time PCR analysis indicated that miR-140 expression was decreased
in the peripheral blood of rats with LPS-induced ALI compared with
control rats (Fig. 3A). H&E
staining revealed that rats in the LPS group developed lung
inflammation, hemorrhaging and alveolar septal thickening compared
with the normal control rats (Fig.
3B). In addition, the expression of miR-140 was decreased in
the lung tissues of rats with ALI compared with control rats
(Fig. 3C). ELISA results indicated
that serum TNF-α, IL-6 and IL-1β were significantly upregulated in
rats with ALI compared with control rats (Fig. 3D). These findings suggest a potential
correlation between miR-140 and ALI-associated inflammatory
responses.
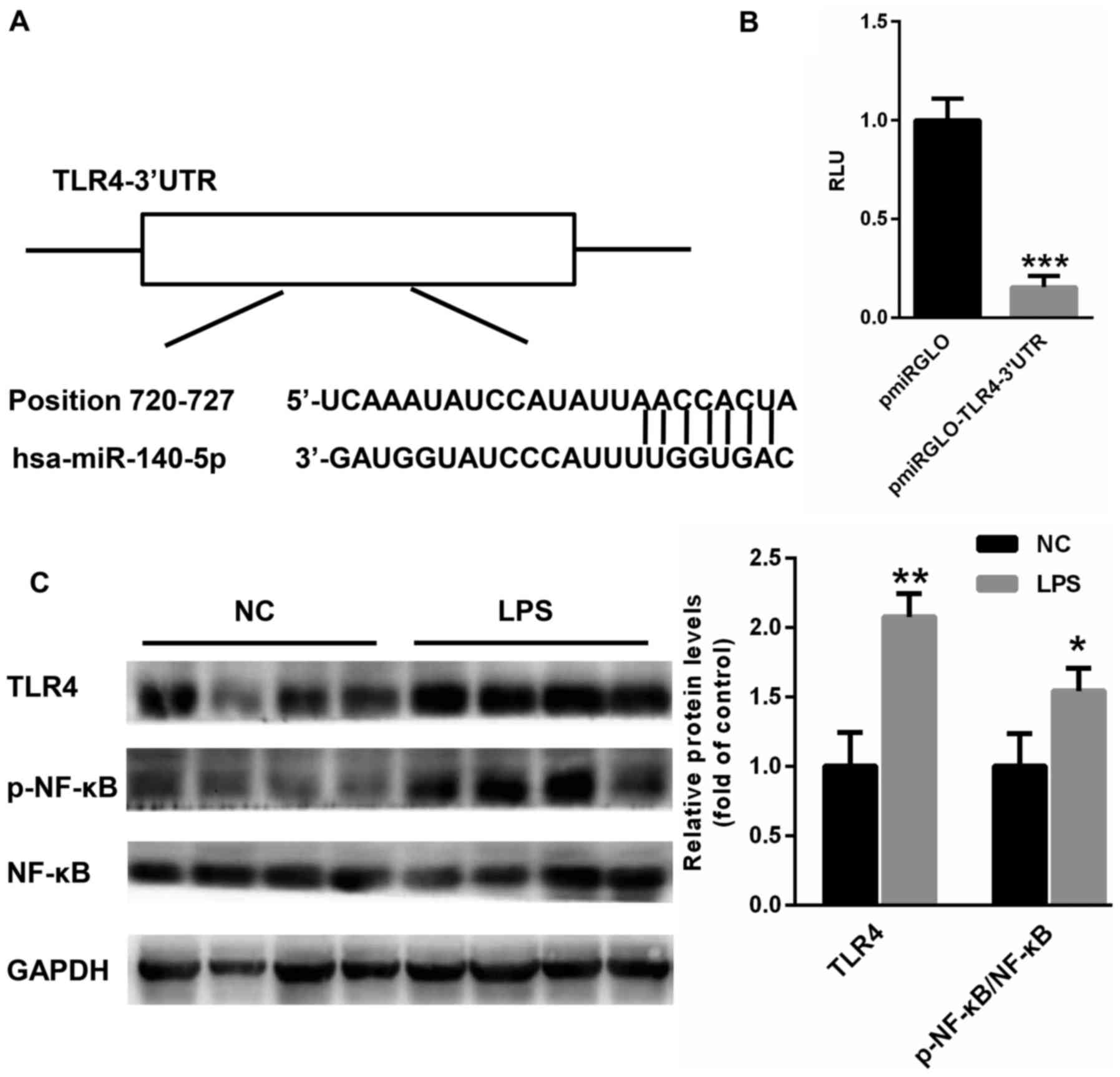
TLR4 is a target gene of miR-140
To explore the potential mechanism by which miR-140
regulates the progression of ALI, potential target genes of miR-140
were searched using TargetScan (http://www.targetscan.org/vert_71/). Interestingly, a
conserved binding site of miR-140 was identified on the 3′UTR of
TLR4 (Fig. 4A). This 3′UTR region
was cloned into pmirGLO plasmids. A dual luciferase reporter assay
suggested that miR-140 significantly suppressed the relative
luciferase activity of pmirGLO-TLR4-3′UTR (Fig. 4B), suggesting that TLR4 is a target
gene of miR-140. Furthermore, western blotting results revealed
that TLR4 expression and NF-κB phosphorylation were much higher in
the lung tissues of rats with ALI (Fig.
4C), suggesting a negative correlation between miR-140 and TLR4
expression.
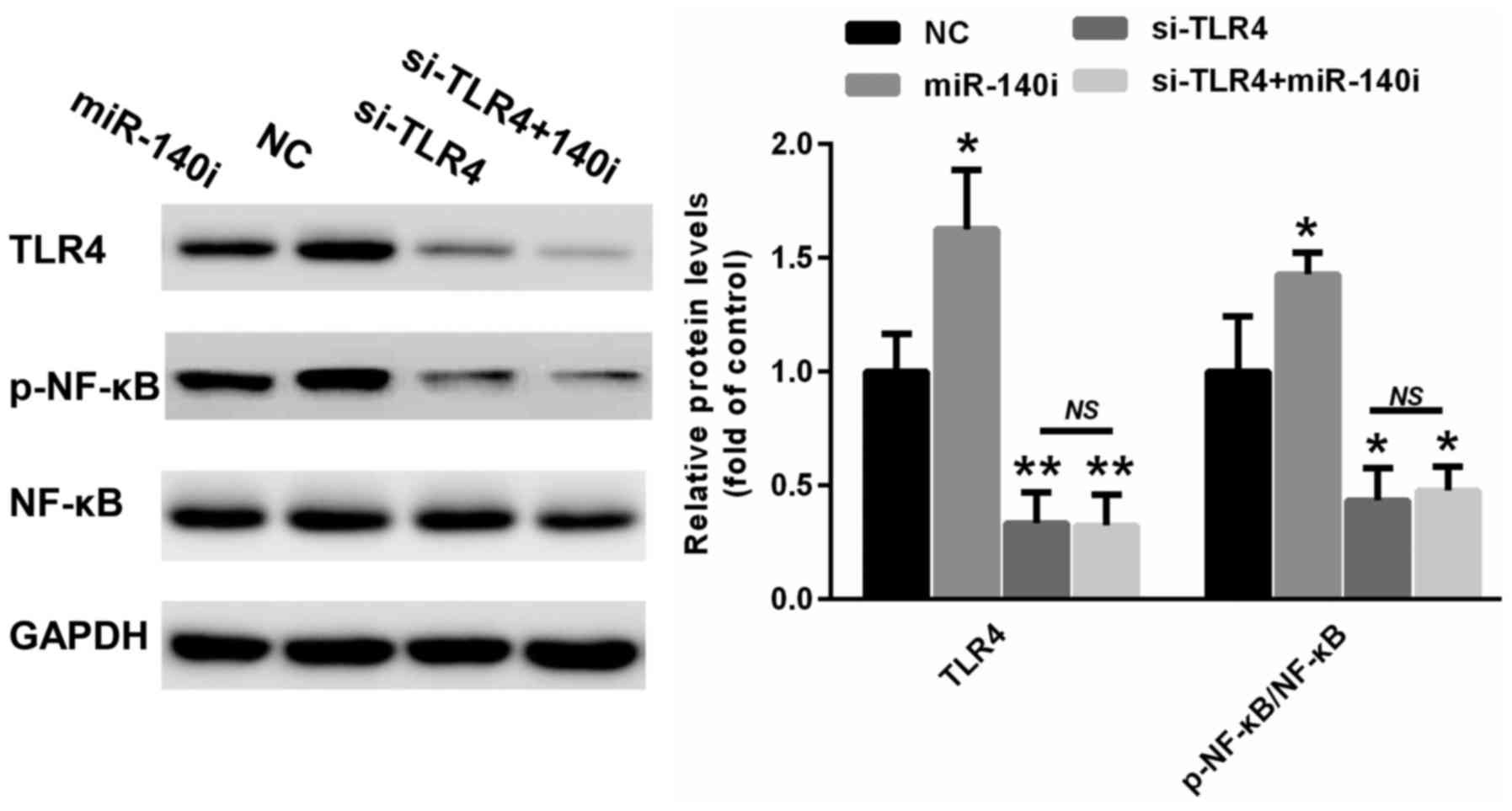
TLR4 knockdown could reverse miR-140
inhibition-induced inflammatory response
To determine the role of TLR4 in the miR-140-induced
inflammatory response, a specific siRNA targeting TLR4 was
selected. As shown in Fig. 5,
miR-140 inhibition increased the expression of TLR4 and enhanced
the phosphorylation of NF-κB. In contrast, TLR4 silencing could
suppress NF-κB phosphorylation in A549 cells transfected with
miR-140 inhibitor (Fig. 5). These
data suggest that the reduction of the miR-140-induced inflammatory
response was mainly achieved through targeting TLR4.
Discussion
ALI is a common complication of sepsis, which often
results in mortality due to a lack of effective pharmacological
interventions (25,26). It is therefore important to explore
effective prognosis prediction and treatment strategies for this
disease (27). It has been widely
reported that TLRs serve important roles in the progression of lung
diseases (26). Microbe-derived LPS
is suggested to be a major element involved in the development of
sepsis (28). In general, LPS
triggers inflammatory responses by activating TLRs, which then
recruit neutrophils to the inflammatory site (25,29).
However, when the defensive reactions are pathologically
over-stimulated, acute hyper-inflammation can be induced and
further exacerbates injury in the lung (30).
In inflamed tissues, TLR4 overexpression promotes
neutrophil migration into the LPS-stimulated lungs (30,31).
TLR4 interacts with the adaptor protein MYD88 and activates NF-κB
signaling, thereby inducing TNF-α production in LPS-induced ALI
(26). In line with previous
studies, the results of the present study demonstrate that TLR4
expression is increased in the lungs of rats treated with LPS.
Meanwhile, serum levels of TNF-α, IL-6 and IL-1β were significantly
increased in LPS-treated rats compared with normal controls. These
data indicated that LPS-induced ALI rat models had been
successfully established.
A number of studies have reported that changes in
miRNA expression are correlated with the immune response and
inflammatory lung diseases, including ALI (12,32). In
the past decade, some studies have focused on the potential
application of circulating miRNAs as novel prognostic and
therapeutic biomarkers (33,34). miRNAs target multiple genes and may
affect different signaling pathways (35,36).
miRNAs may suppress inflammation pathway genes and lead to abnormal
changes in the acute inflammatory response in patients under
mechanical ventilation (35,36). In 2008, miRs were first identified in
human blood (37), suggesting the
potential application of peripheral blood or serum microRNAs as
biomarkers for neoplastic and non-neoplastic diseases (37,38).
In the focus of the present study was miR-140, which
is poorly understood in the progression of ALI. The results
revealed that miR-140 is lower in the peripheral blood of patients
with ALI than in healthy subjects. ROC analysis indicated that
miR-140 could be used to screen ALI patients from healthy controls.
Furthermore, decreased miR-140 expression was observed in the
plasma and lungs of rats with ALI compared with controls. As miRNA
mainly exerts its effects through target genes, bioinformatics was
used to establish TLR4 as a target gene of miR-140 in the
progression of ALI. TLR4 silencing was demonstrated to suppress the
phosphorylation of NF-κB even in A549 cells transfected with
miR-140 inhibitor. Taken together, these results indicate that
miR-140 downregulation and increased TLR4 signaling activation may
serve important roles in the lungs following LPS-triggered
inflammation.
In conclusion, to the best of our knowledge the
present study demonstrated that miR-140 was decreased in the
progression of ALI for the first time, with further study revealing
that TLR4 was a target gene of miR-140. However, miRNA expression
is dynamic due to the changing cellular environment. Hence, it is
important to further explore the underlying network by which
miR-140 is associated with the development of inflammatory lung
disease. Furthermore, future study is necessary to fully elucidate
whether miR-140 could be used as a therapeutic target for patients
with ALI.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhongnan
Hospital of Wuhan University (Wuhan, China; grant. no.
ZHWU-20160825).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL performed the experiments and analyzed the data.
JW, HW, PG, CW, and YW performed part of the RT-qPCR experiments.
ZZ designed the experiments, analyzed the data and gave final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Zhongnan Hospital of Wuhan University (Wuhan,
China) and all the patients have provided written informed consent
for this study.
Patient consent for publication
Informed consent for participation in the study or
use of their tissue was obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng J, Wang DX, Liang AL, Tang J and
Xiang DK: Effects of baicalin on alveolar fluid clearance and
α-ENaC expression in rats with LPS-induced acute lung injury. Can J
Physiol Pharmacol. 95:122–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fragoso IT, Ribeiro EL, Gomes FO, Donato
MA, Silva AK, Oliveira AC, Araújo SM, Barbosa KP, Santos LA and
Peixoto CA: Diethylcarbamazine attenuates LPS-induced acute lung
injury in mice by apoptosis of inflammatory cells. Pharmacol Rep.
69:81–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsia TC and Yin MC: Post-intake of S-ethyl
cysteine and S-methyl cysteine improved LPS-induced acute lung
injury in mice. Nutrients. 8:pii: E5072016. View Article : Google Scholar
|
|
4
|
Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu
C, Zhang C, Liu J, Li YY, Zhang F, et al: Activation of MTOR in
pulmonary epithelium promotes LPS-induced acute lung injury.
Autophagy. 12:2286–2299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang WC, Lai CL, Liang YT, Hung HC, Liu
HC and Liou CJ: Phloretin attenuates LPS-induced acute lung injury
in mice via modulation of the NF-κB and MAPK pathways. Int
Immunopharmacol. 40:98–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang YJ, Back MJ, Fu Z, Lee JH, Won JH, Ha
HC, Lee HK, Jang JM, Choi JM and Kim DK: Protective effect of
sesquiterpene lactone parthenolide on LPS-induced acute lung
injury. Arch Pharm Res. 39:1716–1725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim J, Jeong SW, Quan H, Jeong CW, Choi JI
and Bae HB: Effect of curcumin (Curcuma longa extract) on
LPS-induced acute lung injury is mediated by the activation of
AMPK. J Anesth. 30:100–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang L, Zhang L, Kang K, Fei D, Gong R,
Cao Y, Pan S and Zhao M and Zhao M: Resveratrol ameliorates
LPS-induced acute lung injury via NLRP3 inflammasome modulation.
Biomed Pharmacother. 84:130–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Qiu X, Jiang H, Han Y, Wei D and Liu
J: Downregulation of miR-181a protects mice from LPS-induced acute
lung injury by targeting Bcl-2. Biomed Pharmacother. 84:1375–1382.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Z and You Z: Mesenchymal stem cells
alleviate LPS-induced acute lung injury in mice by
MiR-142a-5p-controlled pulmonary endothelial cell autophagy. Cell
Physiol Biochem. 38:258–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao Z, Yuan Y and Liao Q: Alleviation of
lipopolysaccharides-induced acute lung injury by MiR-454. Cell
Physiol Biochem. 38:65–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao J, Tang J, Chen Q, Tang D, Liu M, Luo
M, Wang Y, Wang J, Zhao Z, Tang C, et al: miR-429 regulates
alveolar macrophage inflammatory cytokine production and is
involved in LPS-induced acute lung injury. Biochem J. 471:281–291.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao H, Gao F, Xie G and Liu Z:
Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary
endothelial cells during acute lung injury through suppressing
MiR-4262. Cell Physiol Biochem. 37:759–767. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Syed M, Das P, Pawar A, Aghai ZH, Kaskinen
A, Zhuang ZW, Ambalavanan N, Pryhuber G, Andersson S and Bhandari
V: Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in
neonatal lungs. Nat Commun. 8:11732017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Du J, Yu X, Xu J, Huang F, Li X,
Zhang C, Li X, Chang J, Shang D, et al: miRNA-200c-3p is crucial in
acute respiratory distress syndrome. Cell Discov. 3:170212017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Xia M, Chen D, Wu F, Lv Z, Zhan Q,
Jiao Y, Wang W, Chen G and An F: Profiling of downregulated
blood-circulating miR-150-5p as a novel tumor marker for
cholangiocarcinoma. Tumour Biol. 37:15019–15029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Tang S, Ji-Yan C, Huang C, Li J,
Cai AP and Feng YQ: Circulating miR-92a expression level in
patients with essential hypertension: A potential marker of
atherosclerosis. J Hum Hypertens. 31:200–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coskunpinar E, Cakmak HA, Kalkan AK,
Tiryakioglu NO, Erturk M and Ongen Z: Circulating miR-221-3p as a
novel marker for early prediction of acute myocardial infarction.
Gene. 591:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan R, Wang G, Xu Z, Zhao H, Chen H, Han
Y, Wang B, Zhou J, Hu H, Guo Z, et al: Up-regulated circulating
miR-106a by DNA methylation promised a potential diagnostic and
prognostic marker for gastric cancer. Anticancer Agents Med Chem.
16:1093–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han Y, Li Y and Jiang Y: The prognostic
value of plasma MicroRNA-155 and MicroRNA-146a level in severe
sepsis and sepsis-induced acute lung injury patients. Clin Lab.
62:2355–2360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui Y, Yi L, Zhao JZ and Jiang YG: Long
noncoding RNA HOXA11-AS functions as miRNA sponge to promote the
glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol.
36:822–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y,
Zhang T, Khaliq J and Li Y: miR-140-5p suppresses the
proliferation, migration and invasion of gastric cancer by
regulating YES1. Mol Cancer. 16:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Z, Zhou D, Xie X, Wang S, Wang Z, Zhao
W, Xu H and Zheng L: Cross-talk between macrophages and atrial
myocytes in atrial fibrillation. Basic Res Cardiol. 111:632016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carvalho JL, Britto A, de Oliveira AP,
Castro-Faria-Neto H, Albertini R, Anatriello E and Aimbire F:
Beneficial effect of low-level laser therapy in acute lung injury
after i-I/R is dependent on the secretion of IL-10 and independent
of the TLR/MyD88 signaling. Lasers Med Sci. 32:305–315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu C, Chen G, Yang W, Xu Y, Xu Y, Huang X,
Liu J, Feng Y, Xu Y and Liu B: Hyaluronan ameliorates LPS-induced
acute lung injury in mice via Toll-like receptor (TLR) 4-dependent
signaling pathways. Int immunopharmacol. 28:1050–1058. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen C, Wang YL, Wang CY and Zhang ZZ:
Effect of TLR-4 and HO-1 on acute lung injury induced by
hemorrhagic shock in mice. Chin J Traumatol. 11:78–83. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cabrera-Perez J, Babcock JC, Dileepan T,
Murphy KA, Kucaba TA, Badovinac VP and Griffith TS: Gut microbial
membership modulates CD4 T cell reconstitution and function after
sepsis. J Immunol. 197:1692–1698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barsness KA, Arcaroli J, Harken AH,
Abraham E, Banerjee A, Reznikov L and McIntyre RC:
Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J
Physiol Regul Integr Comp Physiol. 287:R592–R599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sodhi CP, Jia H, Yamaguchi Y, Lu P, Good
M, Egan C, Ozolek J, Zhu X, Billiar TR and Hackam DJ: Intestinal
epithelial TLR-4 activation is required for the development of
acute lung injury after trauma/hemorrhagic shock via the release of
HMGB1 from the gut. J Immunol. 194:4931–4939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tianzhu Z and Shumin W: Esculin inhibits
the inflammation of LPS-induced acute lung injury in mice via
regulation of TLR/NF-κB pathways. Inflammation. 38:1529–1536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CC, Yuan JR, Wang CF, Yang N, Chen J,
Liu D, Song J, Feng L, Tan XB and Jia XB: Anti-inflammatory effects
of phyllanthus emblica L on benzopyrene-induced precancerous lung
lesion by regulating the IL-1β/miR-101/Lin28B signaling pathway.
Integr Cancer Ther. 16:505–515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK,
Pritchard CC, Gibson DF, Mitchell PS, Bennett CF,
Pogosova-Agadjanyan EL, Stirewalt DL, et al: Argonaute2 complexes
carry a population of circulating microRNAs independent of vesicles
in human plasma. Proc Natl Acad Sci USA. 108:5003–5008. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kroh EM, Parkin RK, Mitchell PS and Tewari
M: Analysis of circulating microRNA biomarkers in plasma and serum
using quantitative reverse transcription-PCR (qRT-PCR). Methods.
50:298–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang K, Guo S, Zhang T, Yang Y, Zhao G,
Shaukat A, Wu H and Deng G: Downregulation of TLR4 by miR-181a
provides negative feedback regulation to lipopolysaccharide-induced
inflammation. Front Pharmacol. 9:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brogaard L, Larsen LE, Heegaard PMH,
Anthon C, Gorodkin J, Dürrwald R and Skovgaard K: IFN-λ and
microRNAs are important modulators of the pulmonary innate immune
response against influenza A (H1N2) infection in pigs. PLoS One.
13:e01947652018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng HH, Mitchell PS, Kroh EM, Dowell AE,
Chéry L, Siddiqui J, Nelson PS, Vessella RL, Knudsen BS, Chinnaiyan
AM, et al: Circulating microRNA profiling identifies a subset of
metastatic prostate cancer patients with evidence of
cancer-associated hypoxia. PLoS One. 8:e692392013. View Article : Google Scholar : PubMed/NCBI
|