Introduction
Knee osteoarthritis (KOA) is a major cause of
disability among the elderly (1).
Inflammation of the synovial joint plays a key role in the
progression of KOA and also results in pain and disability
(2). Furthermore, increased
inflammation and pain can decrease the use of the knee extensor
muscles thereby relieving the joint load related to knee function
(3,4). Thus, it is common for muscle atrophy to
occur beginning at the onset of knee OA (5). It has been well established that the
knee extensor muscles mainly function as a key regulators in the
maintenance of daily walking activities (6). However, the underlying mechanism by
which inflammation is modulated in the skeletal muscles (knee
extensors) of KOA patients is poorly understood.
In response to insult and/or injury, inflammation is
induced and participates in cell injury (7). Abnormal expression of inflammatory
factors, including interleukin-1β (IL-1β) and tumor necrosis factor
α (TNFα), has been widely reported in KOA patients (8). Within muscle, p65 NF-κB signaling is
one of the key signaling pathways that upregulates cytokine gene
expression, including TNFα, IL-1β, IL-6, and MCP-1 (7,9).
Undoubtedly, activation of p65 NF-κB signaling in muscle may
decrease muscle strength and function (10,11).
Upstream stimulating factor 1 (USF1), which is a 43-kDa protein, is
a key member of the eukaryotic evolutionarily conserved basic
helix-loop-helix-leucine zipper transcription factor family
(12). USF1 has been reported to be
involved in multiple biological processes, including cell
proliferation and lipogenesis, by binding the E-box regulatory
elements (CANNTG) (13–15). The mechanism of the problem of muscle
movement caused by KOA is not clear. Previous studies have shown
that upstream stimulatory factor (USF1) plays a key role in various
muscle cells. For instance, USF1 is shown to regulate human
cGMP-dependent protein kinase I gene expression in vascular smooth
muscle cells, thereby maintaining smooth muscle cell relaxation,
growth, and differentiation (16).
Furthermore, USF1 is demonstrated to modulate the expression of
osteopontin in cultured vascular smooth muscle cells and might
promote initial osteopontin expression observed post carotid injury
in vivo (17). In skeletal
muscle, USF1 is shown to increase PGC-1alpha promoter activation
(18). However, whether USF1 is
abnormally expressed in the muscle tissues of KOA patients has
never been explored.
The current study mainly aims to evaluate the
expression pattern and underlying mechanism of action of USF1 in
the muscle tissues of KOA patients, which may shed light on the
prevention and treatment of KOA.
Materials and methods
Patient samples
In the current study, twenty patients (10 men and 10
women) with diagnosed KOA and five control individuals (3 men and 2
female) were recruited from Hongqi Hospital Affiliated with
Mudanjiang Medical University. These patients were scheduled for
knee replacement surgery and able to walk at least forty-five
meters independently (without the use of walking aids). Patients
were excluded if they had uncontrolled systemic disease
(non-musculoskeletal conditions that would make testing difficult
and uncomfortable for the participants, such as chronic obstructive
airway disease or congestive heart failure) or a preexisting
neurologic or other orthopedic condition affecting walking. The
study protocol was approved by the Human Research Ethics Committees
of Hongqi Hospital Affiliated with Mudanjiang Medical University.
All of the participants were informed about the nature of the study
and signed a consent form prior to participation. The details for
all participants are listed in Table
I.
 | Table I.Basic physical characteristics of KOA
patients and healthy controls. |
Table I.
Basic physical characteristics of KOA
patients and healthy controls.
| Characteristics | Control | KOA | P-value |
|---|
| Age (years) | 67.8±5.6 | 65.8±9.4 | >0.05 |
| Height (cm) | 168.3±8.7 | 171.3±10.9 | >0.05 |
| Weight (kg) | 67.88±20.33 | 73.24±8.7 | >0.05 |
| BMI
(kg/m2) | 27.6±1.3 | 28.9±2.4 | >0.05 |
| Muscle strength
(Nm) | 143.5±26.5 | 83.5±11.5 | <0.001 |
Cell culture
Primary human skeletal muscle cells were purchased
from Procell (CP-H095, Wuhan, China, http://www.procell.com.cn/view/2244.html). The cells
were cultured in specific complete medium for human skeletal muscle
cells (CM-H095; Procell, Wuhan, China) supplemented with 10%
heat-inactivated fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 100 U/ml penicillin and streptomycin in
25-cm2 culture flasks at 37°C in a humidified atmosphere
with 5% CO2.
Determination of muscle strength
The strength of the knee extensor muscle group was
determined in the affected legs of the 20 patients with KOA and in
the leg from which the muscle biopsy specimen was obtained in the 7
control subjects. A portable nonextendable strain gauge (load cell)
was used to measure muscle strength for this study. The strain
gauge was attached to the subject's leg using a webbing strap with
a Velcro fastener. The subject sat in a tall chair with a strap
around the lower leg 10 cm above the ankle joint, and the hip and
knee joint angles were positioned at 90 degrees. The distance from
the knee joint to the strap around the ankle was measured with a
tape measure and was used for the calculation of torque [force (N)
xdistance (m)]. Each subject exerted maximal force against the
strap assembly for 3 sec. Three trials were recorded for each
subject, and the highest score was used for the analysis.
Muscle biopsy
Resting muscle samples were isolated from the vastus
lateralis, as previously described (19). In brief, the muscle samples from KOA
patients were collected during their knee replacement surgery ~5 cm
proximal to the suprapatellar pouch. The biopsies were taken after
the skin was incised and prior to knee joint capsule incision with
no trauma to the muscle or the joint at that time (19).
Protein extraction and western blot
analysis
Skeletal muscle (30 mg) was extracted using RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) and was collected following centrifugation at
12,000 × g for 30 min at 4°C. A bicinchoninic protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to determine the
protein concentration. A total of 15 µg protein was loaded per
lane, separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. The membranes were blocked with 8% non-fat
dry milk at 4°C overnight. Following three washes with PBS with
Tween 20 (5 min/wash), the membranes were incubated with the
following primary antibodies at 4°C overnight: p-p65 (#3033,
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), p65
(#8242, 1:1,000; Cell Signaling Technology, Inc.), anti-IκBα
(#4812, 1:1,000; Cell Signaling Technology, Inc.) USF1 (ab125020,
1:1,000; Abcam, Cambridge, MA, USA) and GAPDH (cat. no. 5174;
1:1,000; Cell Signaling Technology, Inc.). Following several washes
with TBST, the membranes were incubated with horseradish-peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) or
HRP-conjugated mouse antigoat IgG (ZF-0311, all 1:5,000; Zhongshan
Gold Bridge Biological Technology Co., Beijing, China) for 2 h at
room temperature and then washed followed by detection with
enhanced chemiluminescent substrate (EMD Millipore, Billerica, MA,
USA). GAPDH was used as an internal control. ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used for
density analysis.
Adenoviral vector construction
The adenovirus vectors overexpressing USF1 (Ad-USF1)
or negative control (NC) (Ad-NC) were constructed by GenChem
(Shanghai, China). For the transfection of adenovirus vectors into
primary human skeletal muscle cells, the cells were seeded at a
density of 106 cells/well in 6-well plate. At 80%
confluence, Ad-USF1 and Ad-NC were transfected into primary human
skeletal muscle cells at 30 multiplicity of infection (MOI) for 48
h. Then, the cells were collected for further study.
Enzyme-linked immunosorbent assay
(ELISA)
Muscle tissue or cell lysates were centrifuged at
16,000 × g for 15 min at 4°C, and supernatants were used to
quantify the levels of TNF-α (cat no. DTA00C; Human TNF-α
Quantikine ELISA kit), IL-6, (cat no. D6050; Human IL-6 Quantikine
ELISA kit), IL-1β (cat no. DLB50; Human IL-1 beta/IL-1F2 Quantikine
ELISA kit), and IL-8 (cat no. D8000C; Human IL-8/CXCL8 Quantikine
ELISA kit) by way of a sandwich ELISA following the manufacturers'
protocols (R&D Systems, Minneapolis, MN, USA). Samples were
read at a 450 nm wavelength using a microplate reader (Model 3550;
Thermo Fisher Scientific, Inc.).
Immunohistochemistry
Muscle tissues samples from KOA patients or control
were cut into 5 µm. Then, the slices were fixed in 4%
phosphate-buffered neutral formalin at room temperature for 20 min,
embedded in paraffin and cut into 5-µm thick sections, followed by
deparaffinizition, descending alcohol series of rehydration, and
microwave-heating in sodium citrate buffer (Solarbio Science &
Technology Co., Ltd.) at 100°C for 30 min for antigen retrieval.
Sections were subsequently incubated with 0.3% hydrogen
peroxide/phosphate-buffered saline for 30 min. The sections were
incubated with a primary anti-p-p65 antibody (#3033; Cell Signaling
Technology, Inc.,) or anti-IκBα (#4812, Cell Signaling Technology,
Inc.) at a 1:50 dilution and 4°C overnight. Detection of the
primary antibody was performed via incubation with a horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(ZDR-5036, Zhongshan Gold Bridge Biological Technology Co.,) for 1
h at room temperature and visualized with a 3,3′-Diaminobenzidine
substrate. Stained cells were counted in 5 random fields using
light microscopy (magnification, 40×, Olympus CK40; Olympus
Corporation, Tokyo, Japan).
Immunofluorescence
Primary human skeletal muscle cells
(~1×106) cells were cultured in a 6-well plate for 24 h
with glass coverslips. After that, the cells were transfected with
Ad-NC or Ad-USF1 for 48 h. Then, the cells on the coverslips were
fixed in 4% paraformaldehyde for 30 min at room temperature. The
samples were washed three times in PBS for 5 min and fluorescence
intensity was examined using a fluorescence microscope (Olympus
Corporation) at a magnification of ×100.
Statistical analysis
Data are presented as the mean ± standard deviation.
To compare the two groups, two-tailed unpaired Student's t-test was
performed. For multiple group comparisons, one-way analysis of
variance followed by Tukey's post hoc test were used. Statistical
tests were performed using SPSS software (version 13.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Decreased muscle strength was
identified in the muscle tissues of KOA patients
The basic physical characteristics are shown in
Table I. No significance was found
in the age, height, weight and BMI of patients with KOA or healthy
controls. By contrast, lower muscle strength was identified in KOA
patients than in healthy controls (Table
I).
Increased inflammatory factors in the
vastus lateralis muscle tissues of KOA patients
Next, we determined the inflammatory factors in the
vastus lateralis muscle tissues of KOA patients and control
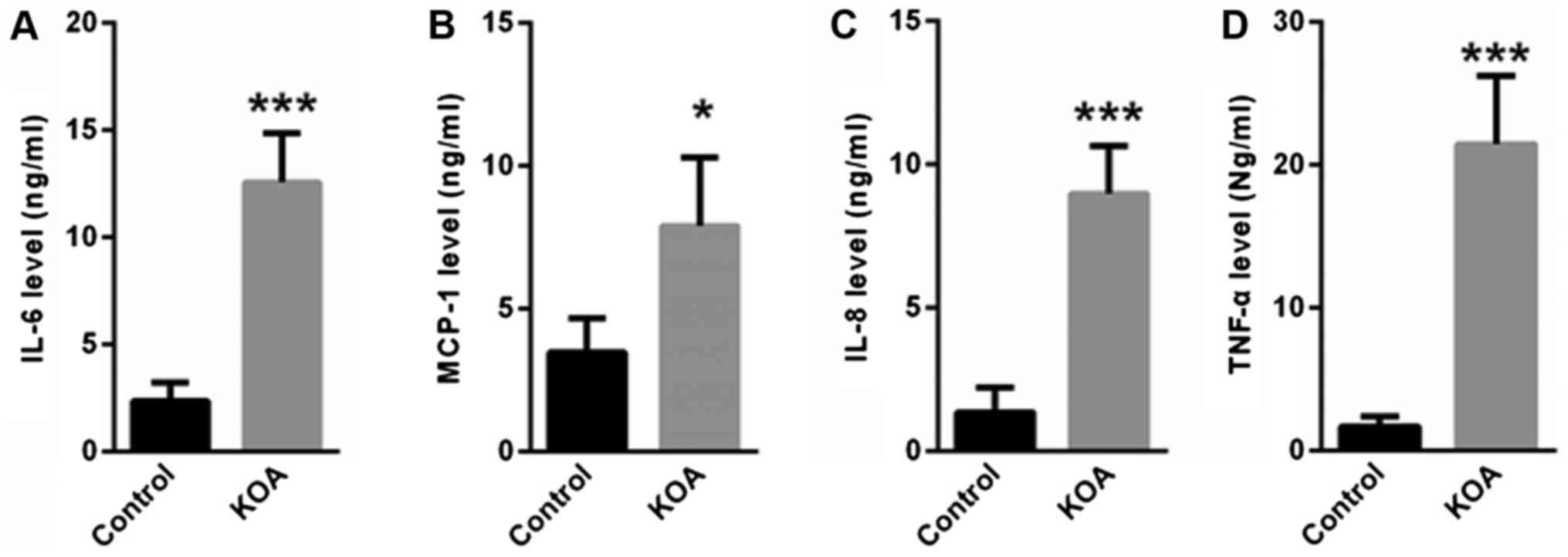
individuals. ELISA showed that the levels of IL-6, MCP-1, IL-8 and
TNFα were significantly increased in the muscle tissues of KOA
patients compared with control individuals (Fig. 1).
NF-κB signaling is activated in the
vastus lateralis muscle tissues of KOA patients
p65 NF-κB signaling is a key signaling pathway that
upregulates cytokine gene expression, including TNFα, IL-1β, IL-6,
and MCP-1, in muscle tissues (7,9). Thus,
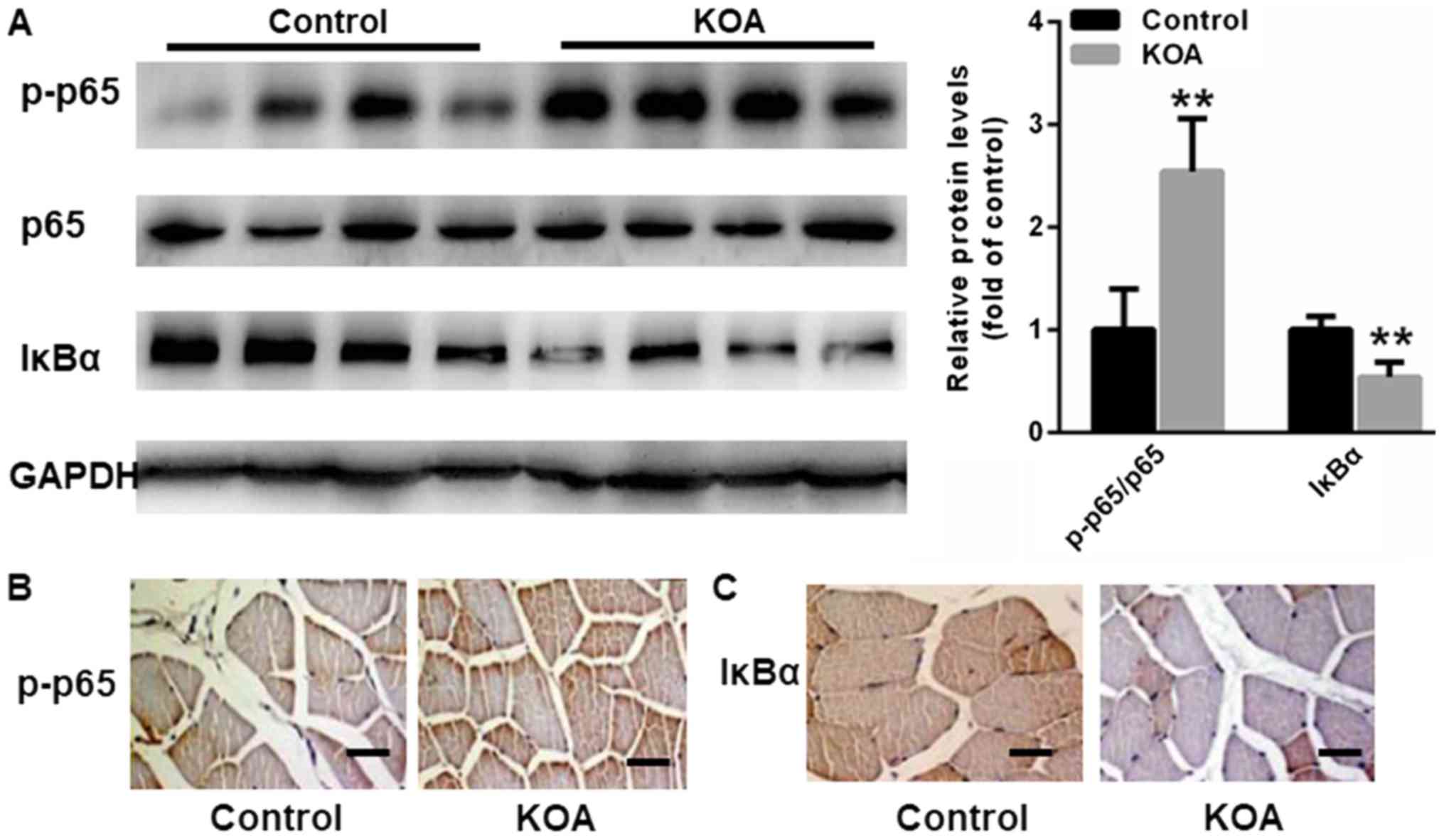
we analyzed p65 NF-κB activation in the muscle tissues of KOA
patients and control individuals. Western blot assays indicated
that p65 NF-κB was significantly increased in the muscle tissues of
KOA patients compared with control individuals, while IκBα, an
inhibitor of NF-κB, was shown to be decreased in the muscle tissues
of KOA patients (Fig. 2). We also
analyzed histological changes of p-p65 and IκBα in skeletal muscle.
In line with the findings of western blot, p-p65 was found to be
enhanced in the muscle tissues of KOA patients compared with
control individuals, but IκBα was decreased in the muscle tissues
of KOA patients (Fig. 2B and C).
Upregulation of USF1 in the vastus
lateralis muscle tissues of KOA patients
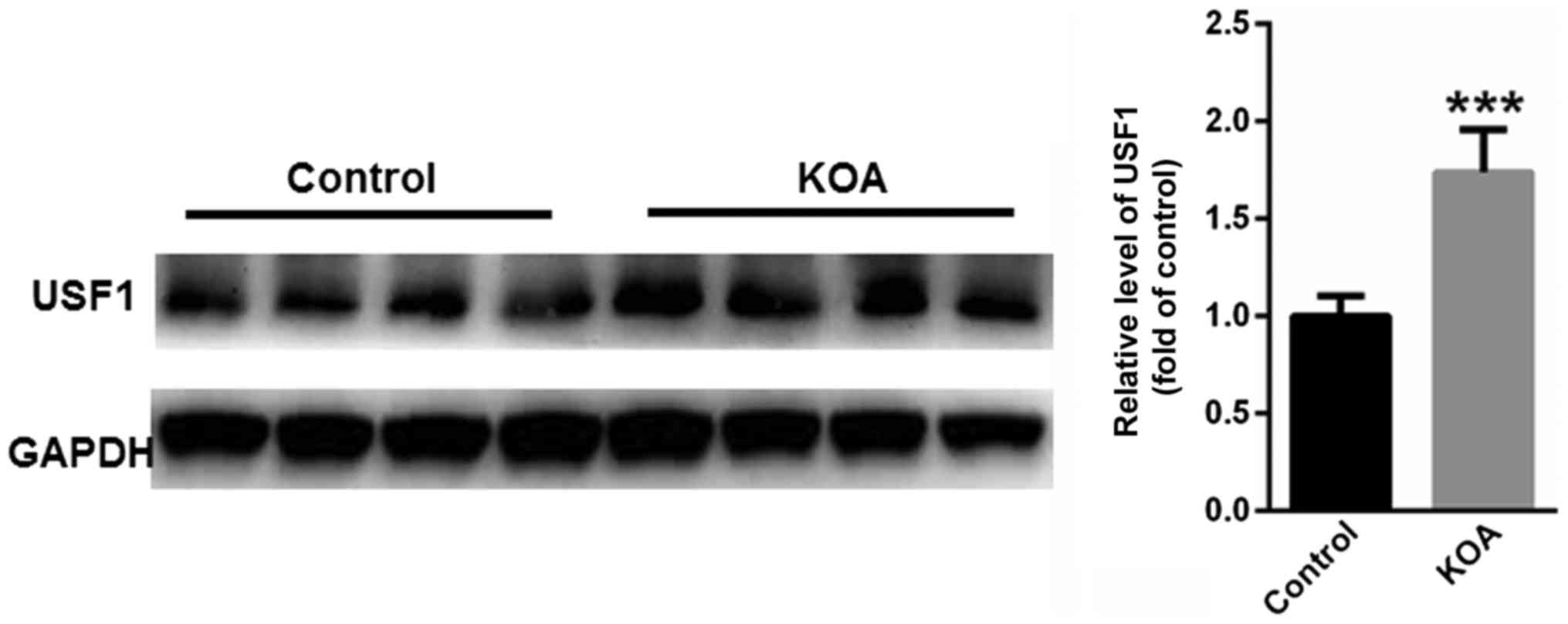
Furthermore, we evaluated the expression of USF1 in
muscle tissues of KOA patients. Compared with control individuals,
the protein levels of USF1 were significantly enhanced in the
muscle tissues of KOA patients (Fig.
3).
USF1 activates NF-κB signaling in
primary human skeletal muscle cells
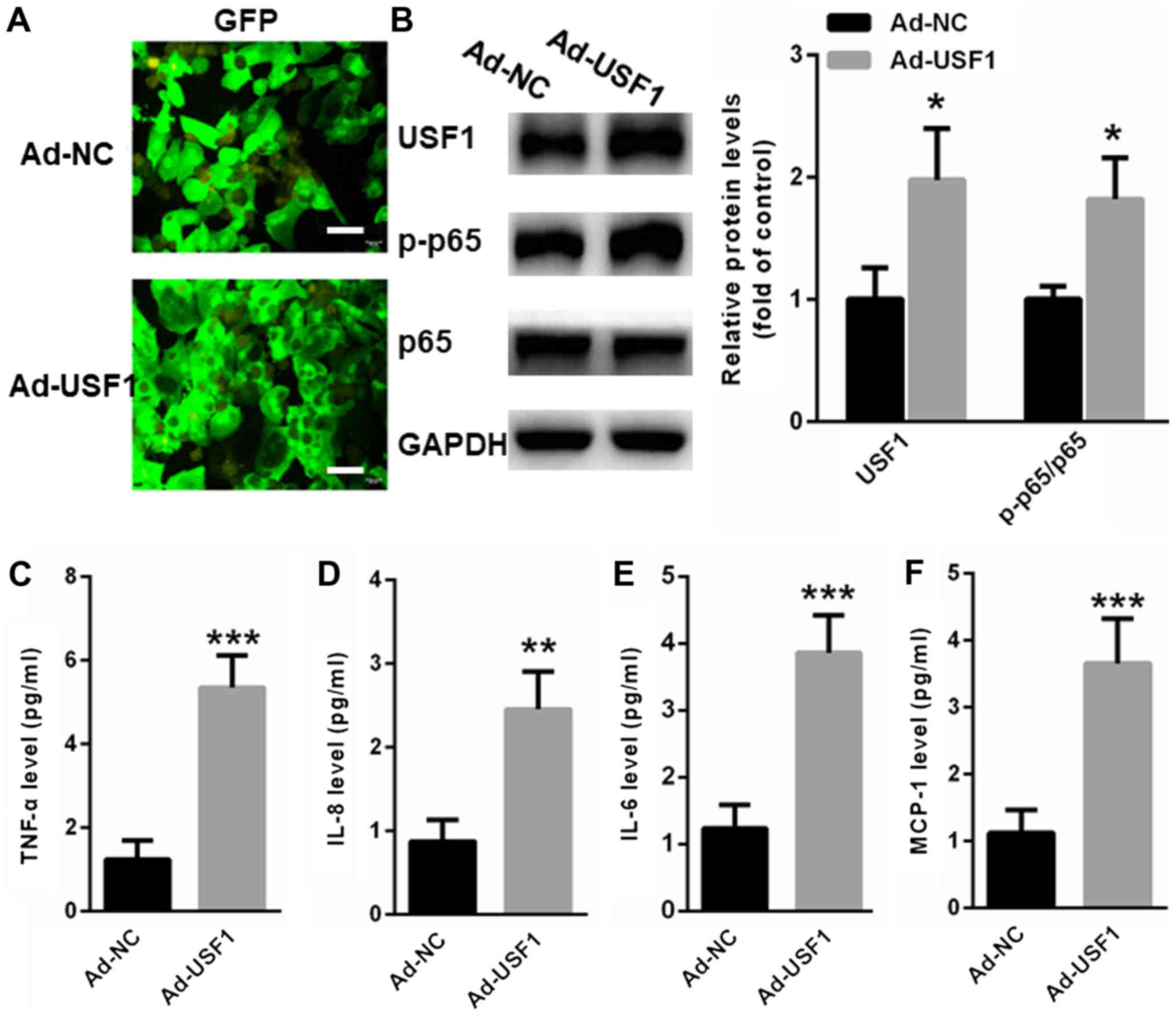
To further explore whether USF1 activates NF-κB
signaling in primary human skeletal muscle cells, adenovirus
vectors overexpressing USF1 or NC were transfected into primary
human skeletal muscle cells for 48 h. As shown in Fig. 4A, the transfection efficiency was
similar between Ad-NC or Ad-USF1 in in primary human skeletal
muscle cells. Western blot assays indicated that overexpression of
USF1 significantly induced the transcription of p65 NF-κB signaling
(Fig. 4B). Moreover, an ELISA assay
revealed upregulation of inflammatory factors, including TNFα
(Fig. 4C), IL-8 (Fig. 4D), IL-6 (Fig. 4E) and MCP-1 (Fig. 4F).
Discussion
The progression of KOA is accompanied by injury of
the entire joint structure and increased inflammation in the joint
(20,21). Impaired muscle strength and
dysfunction are common features in the affected legs and likely
decrease the quality of life among patients with knee OA (22,23).
Thus, it is important to improve inflammation-induced impairments
in muscle strength among patients with KOA.
Muscle weakness is a typical characteristic in
patients with KOA (24,25). In accordance with previous findings,
our data showed that muscle strength was significantly decreased in
KOA patients compared with controls. Increasing evidence has
indicated that inflammatory responses can induce significant
changes in the cellular microenvironment that then result in the
survival, repair and maintenance of muscle cells (21,26). In
KOA patients, it has been reported that the levels of
pro-inflammatory cytokines are significantly increased and muscle
mass is obviously decreased (27,28). Our
data showed that several inflammatory factors, including TNFα,
IL-8, IL-6 and MCP-1, were associated with reduced muscle strength
in KOA patients. These observations suggest that the enhancement of
proinflammatory molecules within the muscle tissues may impair
physical function among KOA patients.
Increased NF-κB activity in injured muscle fibers is
widely reported to diminish the myogenic potential of their
associated satellite cells (29).
Furthermore, the p105/p50 subunit in NF-κB knockout mice has been
demonstrated to be partially resistant to muscle atrophy (30). Thus, we evaluated the activation of
NF-κB signaling in the vastus lateralis muscle tissues of KOA
patients compared with controls. Not surprisingly, NF-κB signaling
was significantly activated in the muscle tissues of KOA patients
compared with control individuals. Thus, it is of great importance
to elucidate the underlying cellular mechanisms that regulate
inflammatory signaling in the muscle tissues of KOA patients,
thereby providing a novel therapeutic method for treating KOA.
In skeletal muscle, the transcription of the mouse
type Iα (RIα) subunit of the cAMP-dependent protein kinase begins
at the alternative noncoding first exons 1a and 1b (31). A previous study has indicated that
the regulation of the promoter upstream of exon 1a (Pa) depends on
two adjacent E boxes (E1 and E2) in intact muscle (31). More importantly, USF1 is an important
transcription factor that binds the E-box elements in the promoter
region of muscle-specific genes (32,33).
However, the expression pattern of USF1 in the muscle tissues of
KOA patients has never been reported. For the first time, we showed
that USF1 was increased in the muscle tissues of KOA patients
compared with control. More importantly, overexpression of USF1 in
primary human skeletal muscle cells significantly increased the
activation of NF-κB signaling as well as the levels of
pro-inflammatory factors. Thus, our data showed that USF1 activated
NF-κB signaling in muscle tissues of KOA patients, which was then
involved in inflammation-induced muscle weakness.
To our knowledge, this is the first study to explore
a relationship between USF1 and NF-κB activation-induced
inflammatory responses in muscle tissues of KOA patients, with
findings aimed at improving the inflammatory response and
preventing physical disability. However, we have to admit that some
limitations exist in the current study. For instance, how the
expression of USF1 was upregulated in the muscle tissues of KOA
patients. In addition, whether other signaling pathways are
involved in the correlation between USF1 and inflammation response
in muscle tissues of KOA patients deserves further exploration. In
the future, we will carry out deep research on the above questions
thereby fully elucidating the underlying mechanism by which USF1 is
modulated in the progression of KOA.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
Mudanjiang Medical University (MDJ-20160432).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS performed the experiments and analyzed the data.
HL, BL, ZY, WW, HL, JS and SL performed the IHC staining and
western blot experiments. MZ designed the experiments, analyzed the
data and gave final approval of the version to be published. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Hongqi Hospital Affiliated with Mudanjiang
Medical University (Mudanjiang City, China) and all the patients
have provided written informed consent for this study.
Patient consent for publication
Informed consent for participation in the study or
use of their tissue was obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Michael JW, Schlüter-Brust KU and Eysel P:
The epidemiology, etiology, diagnosis, and treatment of
osteoarthritis of the knee. Dtsch Arztebl Int. 107:152–162.
2010.PubMed/NCBI
|
|
2
|
Richter F, Natura G, Löser S, Schmidt K,
Viisanen H and Schaible HG: Tumor necrosis factor causes persistent
sensitization of joint nociceptors to mechanical stimuli in rats.
Arthritis Rheum. 62:3806–3814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SK, Kobsar D and Ferber R:
Relationship between lower limb muscle strength, self-reported pain
and function, and frontal plane gait kinematics in knee
osteoarthritis. Clin Biomech (Bristol, Avon). 38:68–74. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macías-Hernández SI, Miranda-Duarte A,
Ramírez-Mora I, Cortés-González S, Morones-Alba JD, Olascoaga-Gómez
A, Coronado-Zarco R, Soria-Bastida MLA, Nava-Bringas TI and
Cruz-Medina E: Knee muscle strength correlates with joint cartilage
T2 relaxation time in young participants with risk factors for
osteoarthritis. Clin Rheumatol. 35:2087–2092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruhdorfer A, Wirth W and Eckstein F:
Association of knee pain with a reduction in thigh muscle
strength-a cross-sectional analysis including 4553 osteoarthritis
initiative participants. Osteoarthritis Cartilage. 25:658–666.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farrokhi S, Voycheck CA, Gustafson JA,
Fitzgerald GK and Tashman S: Knee joint contact mechanics during
downhill gait and its relationship with varus/valgus motion and
muscle strength in patients with knee osteoarthritis. Knee.
23:49–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarkar D and Fisher PB: Molecular
mechanisms of aging-associated inflammation. Cancer Lett.
236:13–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peake J, Della Gatta P and Cameron-Smith
D: Aging and its effects on inflammation in skeletal muscle at rest
and following exercise-induced muscle injury. Am J Physiol Regul
Integr Comp Physiol. 298:R1485–R1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Russell AP: Molecular regulation of
skeletal muscle mass. Clin Exp Pharmacol Physiol. 37:378–384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buch A, Carmeli E, Boker LK, Marcus Y,
Shefer G, Kis O, Berner Y and Stern N: Muscle function and fat
content in relation to sarcopenia, obesity and frailty of old
age-an overview. Exp Gerontol. 76:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mishra SK and Misra V: Muscle sarcopenia:
An overview. Acta Myol. 22:43–47. 2003.PubMed/NCBI
|
|
12
|
Zhang L, Handel MV, Schartner JM, Hagar A,
Allen G, Curet M and Badie B: Regulation of IL-10 expression by
upstream stimulating factor (USF-1) in glioma-associated microglia.
J Neuroimmunol. 184:188–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheung E, Mayr P, Coda-Zabetta F, Woodman
PG and Boam DS: DNA-binding activity of the transcription factor
upstream stimulatory factor 1 (USF-1) is regulated by
cyclin-dependent phosphorylation. Biochem J. 344:145–152. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naukkarinen J, Gentile M, Soro-Paavonen A,
Saarela J, Koistinen HA, Pajukanta P, Taskinen MR and Peltonen L:
USF1 and dyslipidemias: Converging evidence for a functional
intronic variant. Hum Mol Genet. 14:2595–2605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rada-Iglesias A, Ameur A, Kapranov P,
Enroth S, Komorowski J, Gingeras TR and Wadelius C: Whole-genome
maps of USF1 and USF2 binding and histone H3 acetylation reveal new
aspects of promoter structure and candidate genes for common human
disorders. Genome Res. 18:380–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sellak H, Choi C, Browner N and Lincoln
TM: Upstream stimulatory factors (USF-1/USF-2) regulate human
cGMP-dependent protein kinase I gene expression in vascular smooth
muscle cells. J Biol Chem. 280:18425–18433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malyankar UM, Hanson R, Schwartz SM,
Ridall AL and Giachelli CM: Upstream stimulatory factor 1 regulates
osteopontin expression in smooth muscle cells. Exp Cell Res.
250:535–547. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irrcher I, Ljubicic V, Kirwan AF and Hood
DA: AMP-activated protein kinase-regulated activation of the
PGC-1alpha promoter in skeletal muscle cells. PLoS One.
3:e36142008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gustafsson T, Osterlund T, Flanagan JN,
von Waldén F, Trappe TA, Linnehan RM and Tesch PA: Effects of 3
days unloading on molecular regulators of muscle size in humans. J
Appl Physiol 1985. 109:721–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huebner JL, Landerman LR, Somers TJ, Keefe
FJ, Guilak F, Blumenthal JA, Caldwell DS and Kraus VB: Exploratory
secondary analyses of a cognitive-behavioral intervention for knee
osteoarthritis demonstrate reduction in biomarkers of adipocyte
inflammation. Osteoarthritis Cartilage. 24:1528–1534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neogi T, Guermazi A, Roemer F, Nevitt MC,
Scholz J, Arendt-Nielsen L, Woolf C, Niu J, Bradley LA, Quinn E and
Law LF: Association of joint inflammation with pain sensitization
in knee osteoarthritis: The multicenter osteoarthritis study.
Arthritis Rheumatol. 68:654–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henricsdotter C, Ellegaard K, Klokker L,
Bartholdy C, Bandak E, Bartels EM, Bliddal H and Henriksen M:
Changes in ultrasound assessed markers of inflammation following
intra-articular steroid injection combined with exercise in knee
osteoarthritis: Exploratory outcome from a randomized trial.
Osteoarthritis Cartilage. 24:814–821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Veronesi F, Giavaresi G, Maglio M, Scotto
d'Abusco A, Politi L, Scandurra R, Olivotto E, Grigolo B, Borzì RM
and Fini M: Chondroprotective activity of N-acetyl phenylalanine
glucosamine derivative on knee joint structure and inflammation in
a murine model of osteoarthritis. Osteoarthritis Cartilage.
25:589–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rantanen T, Volpato S, Ferrucci L,
Heikkinen E, Fried LP and Guralnik JM: Handgrip strength and
cause-specific and total mortality in older disabled women:
Exploring the mechanism. J Am Geriatr Soc. 51:636–641. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoeksma AF, ter Steeg AM, Nelissen RG, van
Ouwerkerk WJ, Lankhorst GJ and de Jong BA: Neurological recovery in
obstetric brachial plexus injuries: an historical cohort study. Dev
Med Child Neurol. 46:76–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franceschi C, Capri M, Monti D, Giunta S,
Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M,
et al: Inflammaging and anti-inflammaging: A systemic perspective
on aging and longevity emerged from studies in humans. Mech Ageing
Dev. 128:92–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taekema DG, Westendorp RG, Frölich M and
Gussekloo J: High innate production capacity of tumor necrosis
factor-alpha and decline of handgrip strength in old age. Mech
Ageing Dev. 128:517–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gopinath SD and Rando TA: Stem cell review
series: Aging of the skeletal muscle stem cell niche. Aging Cell.
7:590–598. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai D, Frantz JD, Tawa NE Jr, Melendez PA,
Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ and
Shoelson SE: IKKbeta/NF-kappaB activation causes severe muscle
wasting in mice. Cell. 119:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hunter RB and Kandarian SC: Disruption of
either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy.
J Clin Invest. 114:1504–1511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barradeau S, Imaizumi-Scherrer T, Weiss MC
and Faust DM: Muscle-regulated expression and determinants for
neuromuscular junctional localization of the mouse RIalpha
regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad
Sci USA. 98:5037–5042. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whetstine JR, Witt TL and Matherly LH: The
human reduced folate carrier gene is regulated by the AP2 and sp1
transcription factor families and a functional 61-base pair
polymorphism. J Biol Chem. 277:43873–43880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Apone S and Hauschka SD: Muscle gene E-box
control elements. Evidence for quantitatively different
transcriptional activities and the binding of distinct regulatory
factors. J Biol Chem. 270:21420–21427. 1995. View Article : Google Scholar : PubMed/NCBI
|