Introduction
Acute myeloid leukemia (AML) is a heterogeneous
disease involving hematopoietic stem cells. Various clonal
disorders of AML result from the failure of differentiation and
uncontrolled proliferation of cells. It is estimated that ~15% of
adults with AML carry the t(8;21) (q22;q22) chromosomal
translocation (1,2) which fuses the runt related
transcription factor 1 (AML1, also known as RUNX1) and runt
related transcription factor 1 translocation partner 1 (ETO,
otherwise known as RUNX1T1 or MTG8) genes to produce
an AML1/ETO chimeric protein (3,4). The
AML1/ETO fusion gene is associated with ~6% of M1 morphology
cases of AML, according to the French-American-British
classification system, and with up to 92% of M2 morphology cases
(5).
To date, the methods available for the detection of
the AML1/ETO fusion gene include reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
(6,7)
and fluorescence in situ hybridization (FISH) (8). However, these methods require expensive
laboratory equipment and well-trained personnel, and these
represent barriers to the widespread use of these methods,
particularly in developing countries. Therefore, rapid, sensitive
and affordable diagnostic methods, which can be used in
resource-limited settings are urgently required.
Loop-mediated isothermal amplification (LAMP) was
developed by Notomi et al (9). Briefly, LAMP can amplify nucleic acids
within ~1 h and it has a detection limit of <10 copies. This
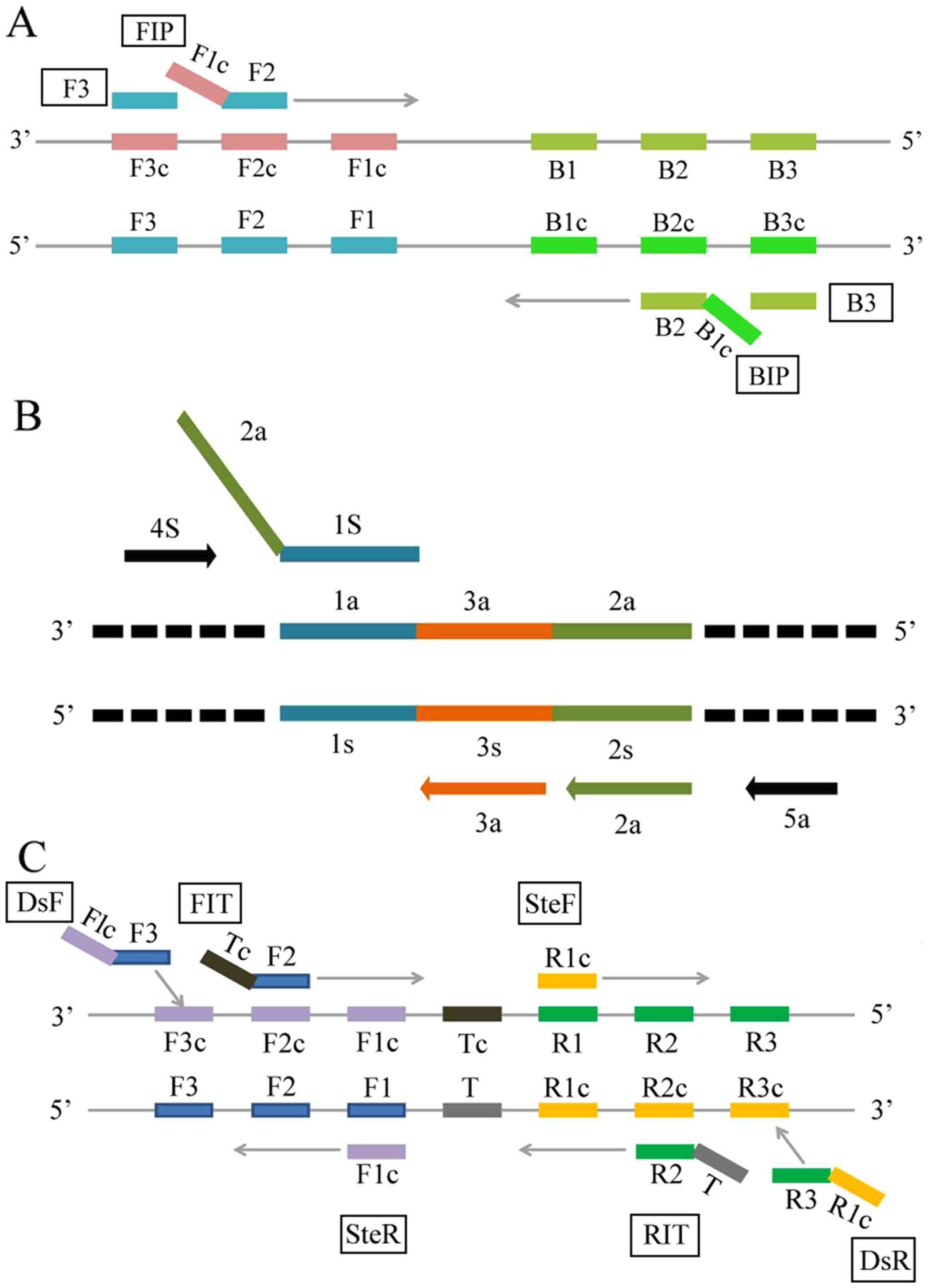
method uses four specially designed primers, which recognize six
regions of a target DNA (Fig. 1A).
Advantages of the LAMP assay include its ease of operation, its
high degree of specificity and sensitivity, and its rapid and
simple procedure compared with PCR methods. Another isothermal DNA
amplification method is cross-priming amplification (CPA), which
was developed by Ustar Biotechnologies (Hangzhou, China). CPA
relies on the use of DNA polymerase for strand displacement
activity (10) and it is
characterized by a high sensitivity of amplification with the use
of five primers (Fig. 1B).
Furthermore, the CPA method does not require a DNA denaturation
step, which makes the CPA method suitable for performing diagnostic
tests in resource-poor settings. More recently, isothermal
multiple-self-matching-initiated amplification (IMSA) has been
developed, which is a method that utilizes an isothermal DNA
amplification system. This method was developed by Ding et
al and has been used to detect human enterovirus 71 and
coxsackievirus A16 (11). A total of
three primer pairs are required to recognize seven regions of a
target DNA sequence in this assay (Fig.
1C). The IMSA method only requires a simple heating device,
results are easily obtained with several detection formats, and
Ding et al (12) have been
improving the visual diagnosis for this method.
In the present study, three rapid and sensitive
isothermal amplification methods were used to detect the
AML1/ETO fusion gene: LAMP, CPA and IMSA. The
sensitivity, specificity and practical use of each method for the
detection of the AML1/ETO fusion gene were evaluated
and compared. To the best of our knowledge, the present study is
the first demonstration of the capacity for these newly developed
methods to be applied to AML, which have the potential to
facilitate AML-M2 monitoring in developing countries.
Materials and methods
Reagents
The following reagents were purchased as indicated:
Bst 2.0 DNA polymerase large fragment (New England Biolabs,
Guangzhou Ltd., Guangzhou, China), Avian Myeloblastosis Virus
Reverse Transcriptase (Promega Corporation, Madison, WI, USA),
dNTPs (TransGen Biotech Co., Ltd., Beijing, China), RNAiso Plus
(Takara Biotechnology Co., Ltd., Dalian, China), betaine
(Sigma-Aldrich; EMD Millipore, Billerica, MA, USA), SYBR-Green I
(SG I) fluorescent dye (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China), PCR SuperMix (TransGen Biotech Co.,
Ltd.), and primers synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China).
Primer design
According to the sequences available for the AML1
gene (GenBank: KJ890835.1), the ETO gene (GenBank: X79990.1), and
the AML1/ETO fusion gene (GenBank: S78158.1), primers for
the LAMP method were designed with the assistance of Primer
explorer v 4.0 (http://primerexplorer.jp/e/). Primers for the CPA and
IMSA methods were also designed according to the principles of
these methods (Table I) (10,11).
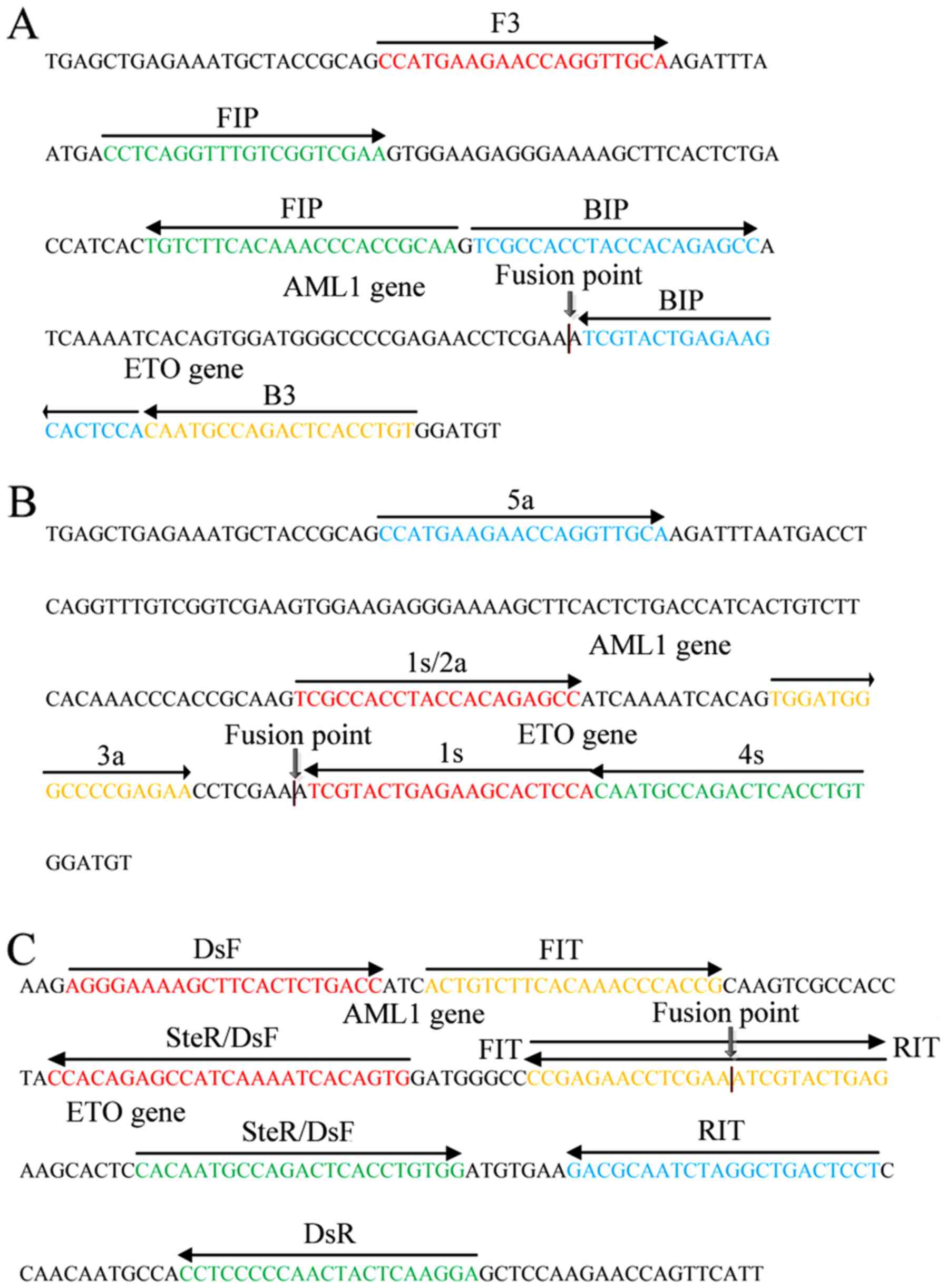
Three primers, BIP for LAMP, 1s for CPA, and RIT/FIT for IMSA, were
designed to recognize binding sites at the fusion point of the
AML1/ETO fusion gene (Fig.
2A-C). The primers used for PCR and FISH probes were
synthesized as previously described (Table I) (13).
 | Table I.Primers used in LAMP, CPA, IMSA and
reverse transcription-polymerase chain reaction assays. |
Table I.
Primers used in LAMP, CPA, IMSA and
reverse transcription-polymerase chain reaction assays.
| Assay | Primer | Sequence
(5′-3′) |
|---|
| LAMP | F3 |
CCATGAAGAACCAGGTTGCA |
|
| B3 |
ACAGGTGAGTCTGGCATTG |
|
| FIP |
TTGCGGTGGGTTTGTGAAGACA-CCTCAGGTTTGTCGGTCGA |
|
| BIP |
TCGCCACCTACCACAGAGCC-TGGAGTGCTTCTCAGTACGA |
| CPA | 1s |
TCGCCACCTACCACAGAGCC-TGGAGTGCTTCTCAGTACGA |
|
| 2a |
TCGCCACCTACCACAGAGCC |
|
| 3a |
TGGATGGGCCCCGAGAA |
|
| 4s |
ACAGGTGAGTCTGGCATTG |
|
| 5a |
CCATGAAGAACCAGGTTGCA |
| IMSA | DsF |
CACTGTGATTTTGATGGCTCTGTGG-AGGGAAAAGCTTCACTCTGACC |
|
| DsR |
CACAATGCCAGACTCACCTGTGG-TCCTTGAGTAGTTGGGGGAGG |
|
| FIT |
CTCAGTACGATTTCGAGGTTCTCGG-ACTGTCTTCACAAACCCACCG |
|
| RIT |
CCGAGAACCTCGAAATCGTACTGAG-AGGAGTCAGCCTAGATTGCGTC |
|
| SteF |
CACAATGCCAGACTCACCTGTGG |
|
| SteR |
CACTGTGATTTTGATGGCTCTGTGG |
RNA isolation
Mononuclear cells were isolated from bone marrow
(BM) and peripheral blood (PB) samples with Lymphoprep reagent (TBD
Science, Tianjin, China) and were stored at −80°C. Total RNA from
the BM and PB samples was extracted with TRIzol reagent (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol.
Each RNA sample was eluted in a final volume of 30-µl RNase-free
water.
LAMP, CPA and IMSA assays
Initially, the LAMP, CPA and IMSA assays were each
performed using a total volume of 25 µl, which included aliquots
from an isothermal master mix that contained 1.5 µl Bst 2.0 DNA
polymerase (8 U/µl), 1 µl AMV enzyme, 0.5 M betaine, 1.0 mM dNTPs,
2.5 µl of 10× ThermoPol buffer (New England Biolabs, Guangzhou
Ltd.), and 2 µl template. For the LAMP reaction, the primer mix
consisted of F3 and B3 primers at 0.2 µM, and FIP and BIP primers
at 2 µM. The primer mix for CPA consisted of the 1s primer at 2.0
µM, the 2a/3a primers at 0.8 µM, and the 4s/5a primers at 0.4 µM.
For IMSA, the concentration of the outer primers, DsF/DsR, were at
0.2 µM, whereas the FIT/RIT and SteF/SteR primer sets were each at
0.8 µM. In parallel, ddH2O was used as a negative
control in these assays. All assays were performed with triplicate
samples.
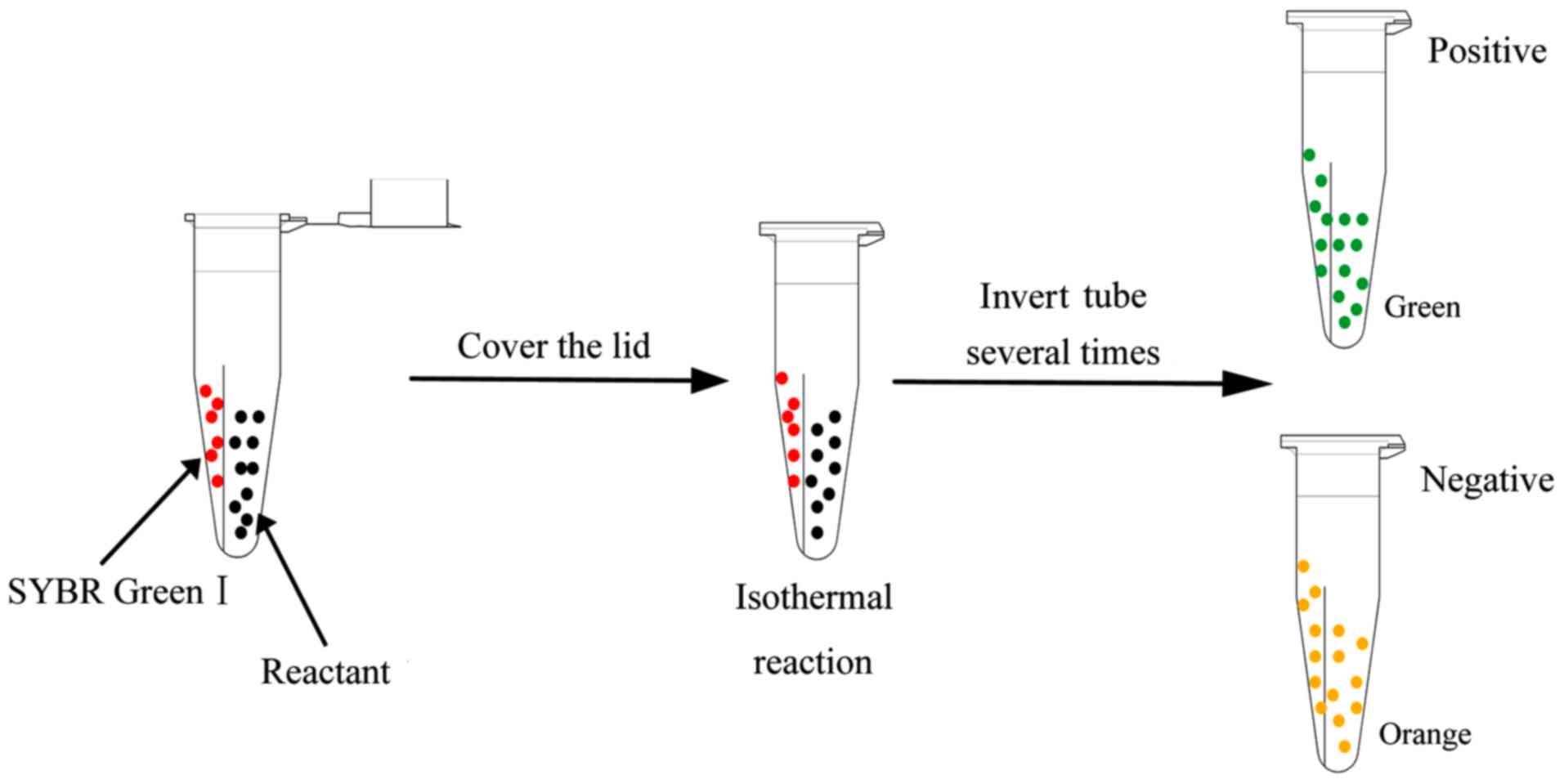
The reactant mixture and 1.0 µl of 2,000X SGI
fluorescent dye were added into relatively separated spaces within
0.2-ml isothermal amplification microcentrifuge (IAM) tubes. The
IAM tubes were inverted several times to mix the reactant mixture
and SGI. When the reaction was complete, the samples were directly
observed under visible light or UV light without opening the tubes
(Fig. 3). To terminate the
reactions, the tubes were incubated at 85°C for 5 min.
RT-PCR analysis
The RT-PCR assay was performed in a final volume of
25 µl containing 0.75 µl AML1/ETO forward primer (10 µM), 0.75 µl
of AML1/ETO reverse primer (10 µM), 0.25 µl of AML1/ETO
probe (10 µM), 2 µl template, 12.5 µl of PCR SuperMix, and 8.75 µl
of deionized water. A LightCycler 480 (Roche Diagnostics GmbH,
Mannheim, Germany) was used for amplification with the following
cycling conditions: Initial denaturation for 30 sec at 95°C,
followed by 40 cycles consisting of 30 sec at 95°C, 15 sec at 60°C,
and 10 sec at 72°C. Fluorescence was visualized at the end of the
annealing/extension step.
Optimization of the LAMP, CPA and IMSA
assays
The LAMP, CPA and IMSA reactions were optimized with
regard to the temperature, Bst polymerase and Mg2+
concentrations, and incubation time. Briefly, the mixtures were
incubated for 60 min at 60, 61, 62, 63, 64, or 65°C to identify the
optimal reaction temperature for the protocol mentioned above.
Optimization of the Bst polymerase concentration was achieved by
evaluating polymerase concentrations of 6, 8, 10, and 12 units,
respectively. Various concentrations of Mg2+ were used
(1.0, 2.0, and 3.0 mM). When the reaction conditions had been
optimized in terms of the temperature and Bst polymerase and
Mg2+ concentrations, various amplification times were
assessed (15, 30, 45, 60, 75, and 90 min).
Specificity of the LAMP, CPA and IMSA
assays
Between October 2015 and May 2016, 5 BM or PB
samples (2 males and 3 females) were collected from patients with
primary leukemia aged between 17 and 52 years old at the Affiliated
Hospital of Guangdong Medical University (Zhanjiang, China). These
patients had AML (AML1/ETO), chronic myelogenous leukemia
[breakpoint cluster region (BCR)/abelson (ABL)], acute
promyelocytic leukemia [promyelocytic leukemia (PML)/retinoic acid
receptor-α (RARα)], 11q23/mixed lineage leukemia (MLL) leukemia or
acute lymphocytic leukemia [(E2A/PBX homebox 1 (PBX1)]. RNA was
extracted from these samples and was used to evaluate specificity
of the LAMP, CPA and IMSA assays for detecting the AML1/ETO
fusion gene. RNA samples from healthy individuals were used as
controls. These samples were subjected to LAMP, CPA and IMSA
assays, which were performed under the optimal conditions
identified above.
Sensitivity of the LAMP, CPA and IMSA
assays
To assess the sensitivity of the LAMP, CPA and IMSA
assays for the AML1/ETO fusion gene, an
AML1/ETO-positive plasmid was serially diluted
(106, 105, 104, 103,
102, 50, 25, 10, and 5 copies/tube) and detected in the
LAMP, CPA and IMSA and RT-PCR assays described above. Assays at
each dilution were performed in triplicate.
Detection of clinical specimens
Between October 2015 and May 2016, 41 BM or PB
samples (20 males and 21 females) were collected from patients with
suspected primary acute leukemia aged between 17 and 63 years old
at the Affiliated Hospital of Guangdong Medical University. These
samples were subjected to LAMP, CPA and IMSA assays. RT-PCR assays
were also performed on these samples.
Ethics statement
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the Ethics Committee of the Affiliated Hospital of
Guangdong Medical University, and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards. No experiments involving animals were performed in the
present study. Informed consent was obtained from all of the
individuals who were involved in the study.
Results
Amplification of the AML1/ETO fusion
gene by LAMP, CPA and IMSA assays
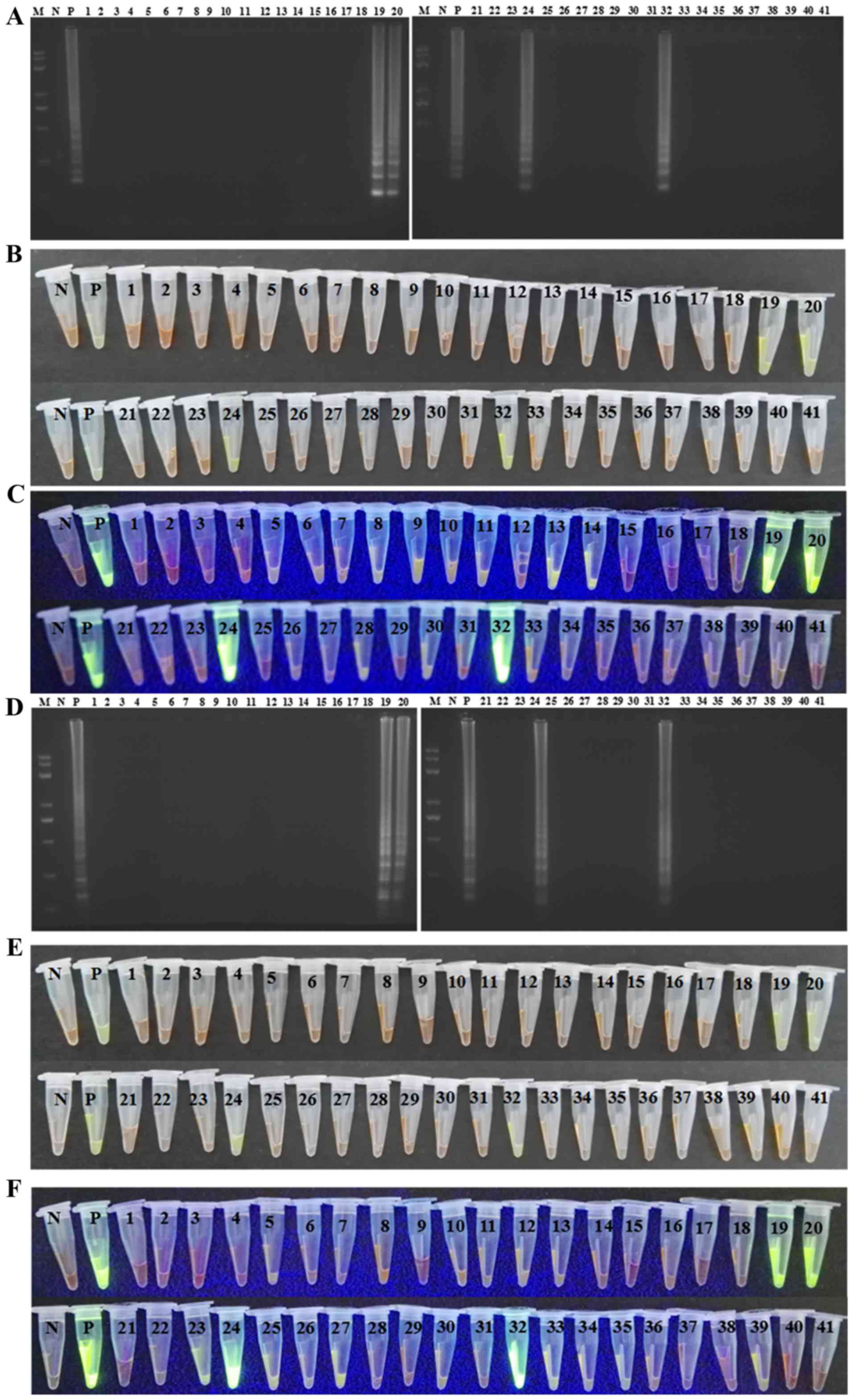
Positive samples in the LAMP, CPA and IMSA reactions
exhibited a ladder-like pattern with electrophoretic separation on
a 2.0% agarose gel. By contrast, this pattern was absent in the
control samples (Fig. 4A-C). When
the reaction products were mixed with SGI fluorescent dye, the
positive samples changed color from orange to green, whereas the
negative controls remained orange in color (Fig. 4D-F). Under UV light, the positive
samples exhibited a green fluorescent signal, whereas the negative
controls remained dark orange in color (Fig. 4G-I).
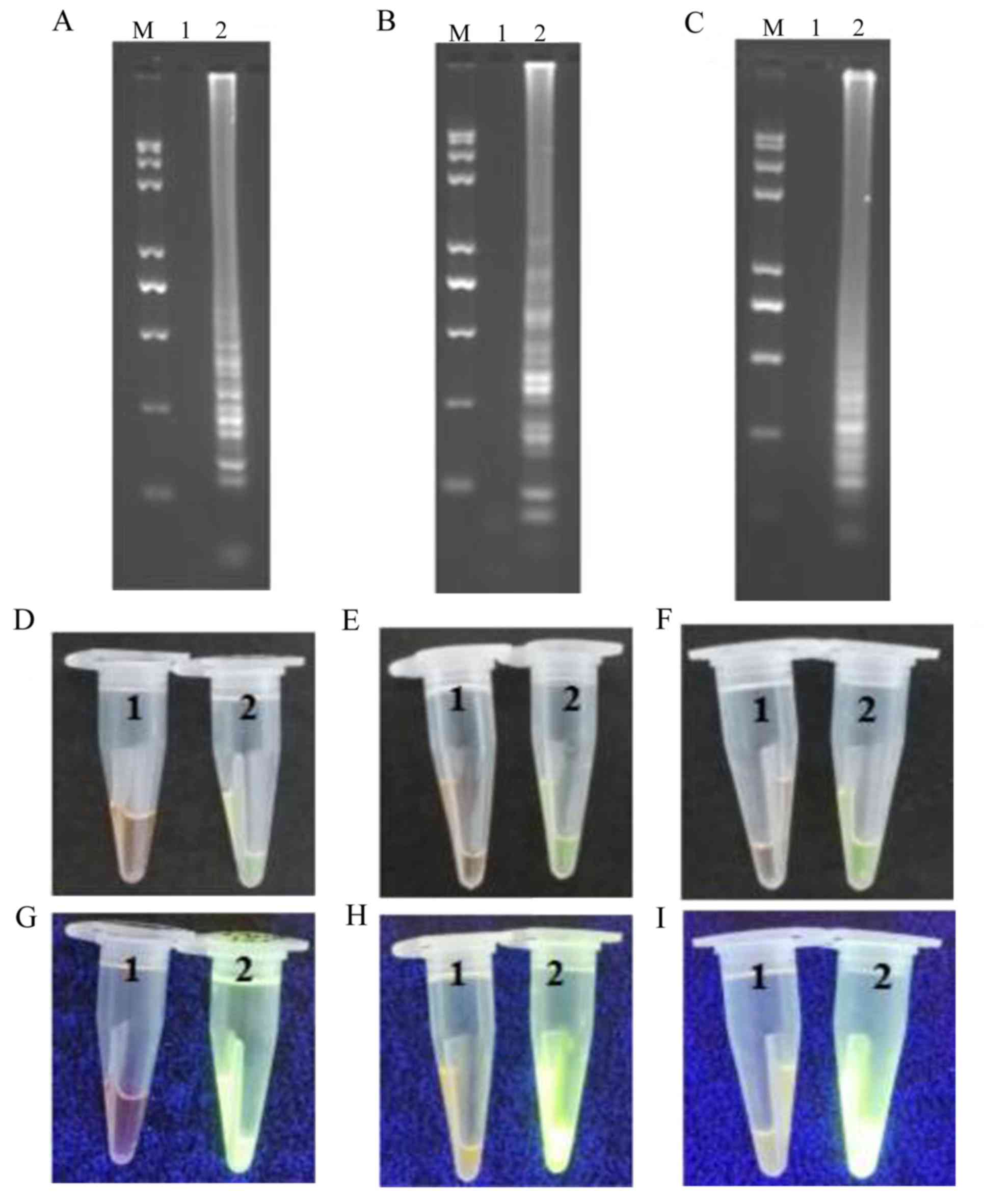 | Figure 4.Representative isothermal reaction
assays of negative controls and positive samples subjected to LAMP,
CPA and IMSA methods to detect the AML1/ETO fusion
gene. Reaction contents of the tubes for (A) LAMP, (B) CPA and (C)
IMSA assays were subjected to agarose gel electrophoresis and UV
visualization of the products. Images of reaction tubes for (D)
LAMP, (E) CPA and (F) IMSA under visible light. Images of reaction
tubes for (G) LAMP, (H) CPA and (I) IMSA under UV light. Images
were captured following the addition of SYBR-Green I. LAMP,
loop-mediated isothermal amplification; CPA, cross-priming
amplification; IMSA, isothermal multiple-self-matching-initiated
amplification; M, Trans 2K plus II DNA marker; 1, negative control;
2, positive detection of AML1/ETO fusion gene.
AML1, runt related transcription factor 1; ETO, runt
related transcription factor 1 translocation partner 1. |
Optimization of the assays
The optimal temperatures for the LAMP, CPA and IMSA
assays were determined by varying the temperature of the assays
between 60 and 65°C for 60 min and using a plasmid containing the
AML1/ETO fusion gene as a template. For the LAMP (Fig. 5A-C), CPA (Fig. 5-D-F), and IMSA (Fig. 5G-I) assays, the optimal temperature
was 60°C (Fig. 5A, E and I). The
optimal concentrations of Bst DNA polymerase and Mg2+ in
the three assays were 8 U/tube (Fig. 5B,
F and J), and 2.0, 3.0, and 2.0 mM (Fig. 5C, G and K), respectively. The optimal
reaction time for the LAMP and CPA assays was 45 min, and the
optimal reaction time was 30 min for the IMSA assay (Fig. 5D, H and L).
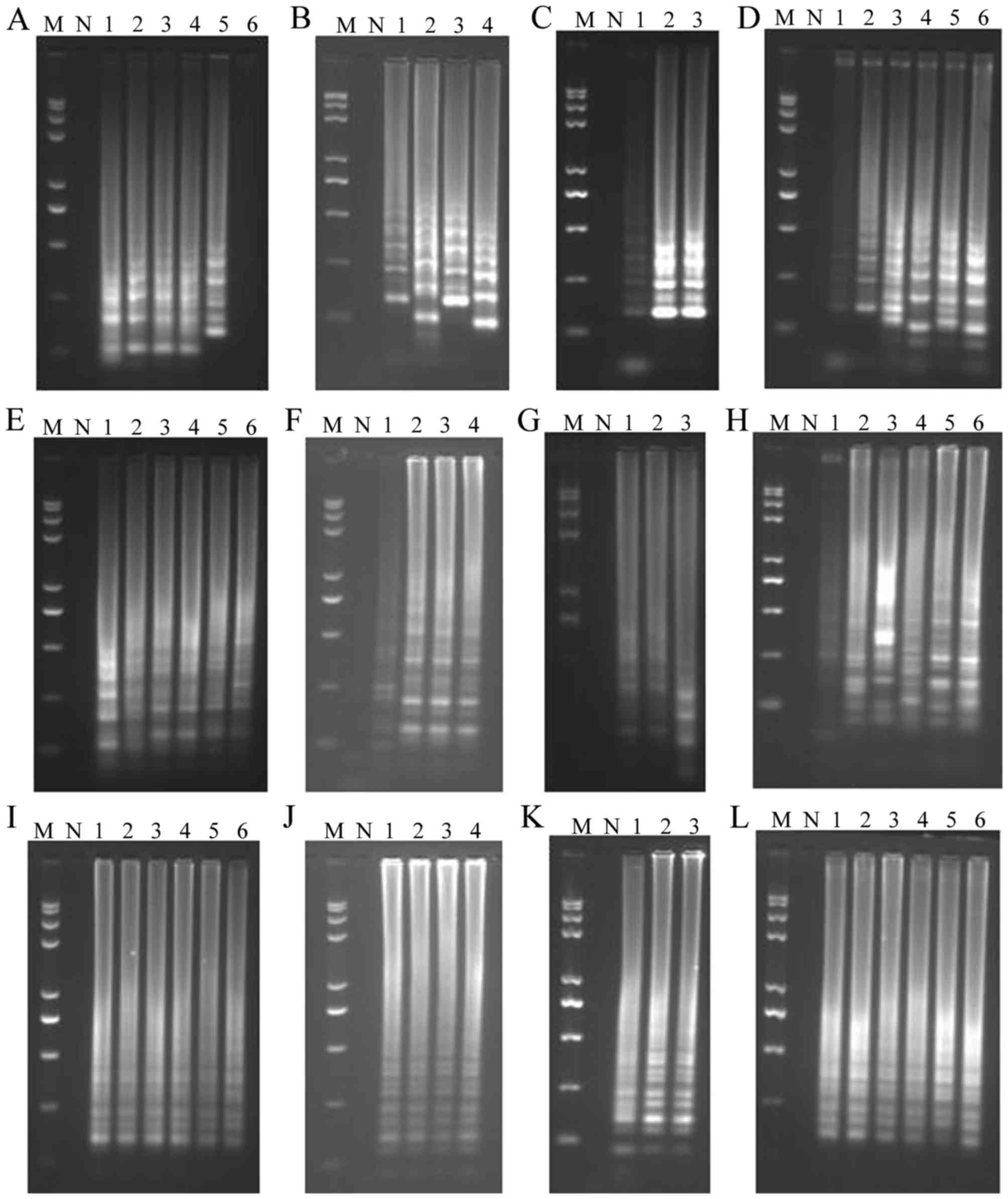 | Figure 5.Optimization of LAMP, CPA and IMSA
assay conditions for detection of the AML1/ETO fusion gene.
Optimization of (A) temperature, (B) Bst enzyme
concentration, (C) Mg2+ concentration and (D)
amplification time in the LAMP assay. Optimization of (E)
temperature, (F) Bst enzyme concentration, (G)
Mg2+ concentration and (H) amplification time in the CPA
assay. Optimization of (I) temperature, (J) Bst enzyme
concentration, (K) Mg2+ concentration and (L)
amplification time in the IMSA assay. Optimization of temperature
was performed at 60, 61, 62, 63, 64, and 65°C for samples 1–6,
respectively; optimization of Bst enzyme concentration was
performed with 6.0, 8.0, 10, and 12 units of enzyme for samples
1–4, respectively; optimization of Mg2+ concentration
was performed with 1.0, 2.0, and 3.0 mM Mg2+ in samples
1–3, respectively; optimization of amplification time was performed
for 15, 30, 45, 60, 75, and 90 min for samples 1–6, respectively.
LAMP, loop-mediated isothermal amplification; CPA, cross-priming
amplification; IMSA, isothermal multiple-self-matching-initiated
amplification; M, Trans 2K plus II DNA marker; N, negative
control. |
Assay specificity
The specificity of the LAMP, CPA, and IMSA methods
for detecting the AML1/ETO fusion gene was determined by
assaying samples containing other fusion genes of hematologic
malignancies, including BCR/ABL, PML/RARA,
11q23/MLL and E2A/PBX1. Samples from healthy
individuals were used as controls. Amplification products were only
observed in the reaction tubes that contained the target region of
the AML1/ETO fusion gene. In addition, the three isothermal
assays achieved 100% specificity for the target fusion gene, and
this was comparable to the detection specificity achieved with PCR
analysis (Fig. 6A-I).
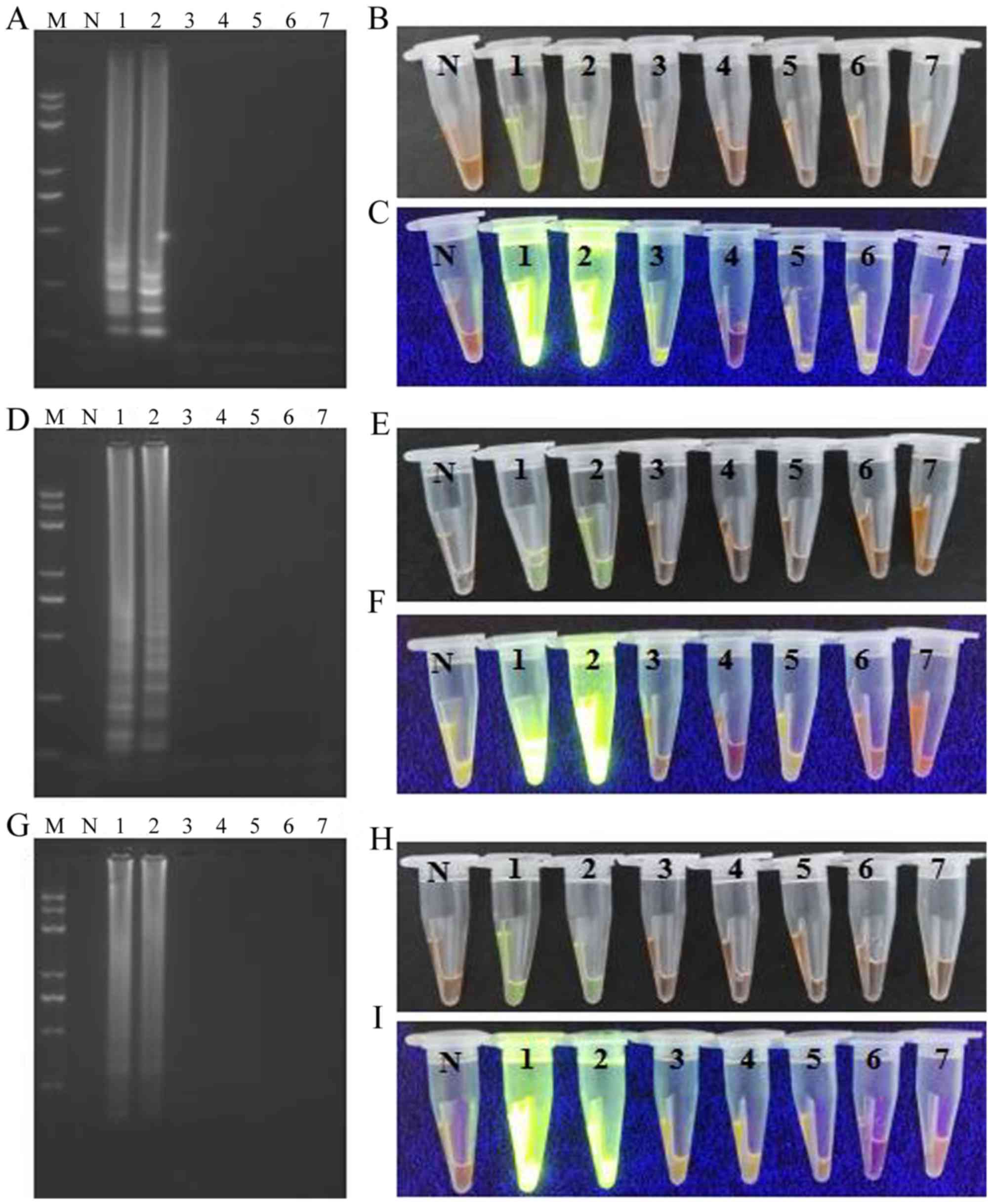 | Figure 6.Specificity test for the LAMP, CPA
and IMSA assays. LAMP assay: (A) Agarose gel electrophoresis of
products obtained; (B) visualization of reactions performed with
various samples (1–7) and SYBR-Green I under visible light; (C)
visualization of reactions performed with various samples (1–7) and
SYBR-Green I under UV light. CPA assay: (D) agarose gel
electrophoresis of products obtained; (E) visualization of
reactions performed with various samples (1–7) and
SYBR-Green I under visible light; (F) visualization of reactions
performed with various samples (1–7) and
SYBR-Green I under UV light. IMSA assay: (G) agarose gel
electrophoresis of products obtained; (H) visualization of
reactions performed with various samples (1–7) and
SYBR-Green I under visible light; (I) visualization of reactions
performed with various samples (1–7) and
SYBR-Green I under UV light. M, Trans 2K plus II DNA marker; N,
negative control; LAMP, loop-mediated isothermal amplification;
CPA, cross-priming amplification; IMSA, isothermal
multiple-self-matching-initiated amplification;
AML1/ETO, runt related transcription factor 1/runt
related transcription factor 1 translocation partner 1; 1,
AML1/ETO plasmid; 2, AML1/ETO fusion gene sample; 3,
chronic myelogenous leukemia (breakpoint cluster region/abelson)
sample; 4, acute promyelocytic leukemia (promyelocytic
leukemia/retinoic acid receptor-α) sample; 5, 11q23/mixed lineage
leukemia leukemia sample; 6, acute lymphocytic leukemia/PBX homebox
1 sample; 7, healthy sample as the template. |
Assay sensitivity
A plasmid containing the AML1/ETO fusion gene
was serially diluted. Various dilutions of the plasmid were then
subjected to LAMP, CPA, and IMSA assays to evaluate sensitivity.
RT-PCR analysis was also performed on the diluted samples for
comparison. It was observed that as few as 10 plasmid copies/tube
were detected in the IMSA assay, whereas 50 plasmid copies/tube was
the detection limit for the LAMP, CPA and PCR assays (Fig. 7A-J). These results demonstrated that
the IMSA assay exhibited increased sensitivity in detecting the
AML1/ETO fusion gene compared with the LAMP, CPA and RT-PCR
assays.
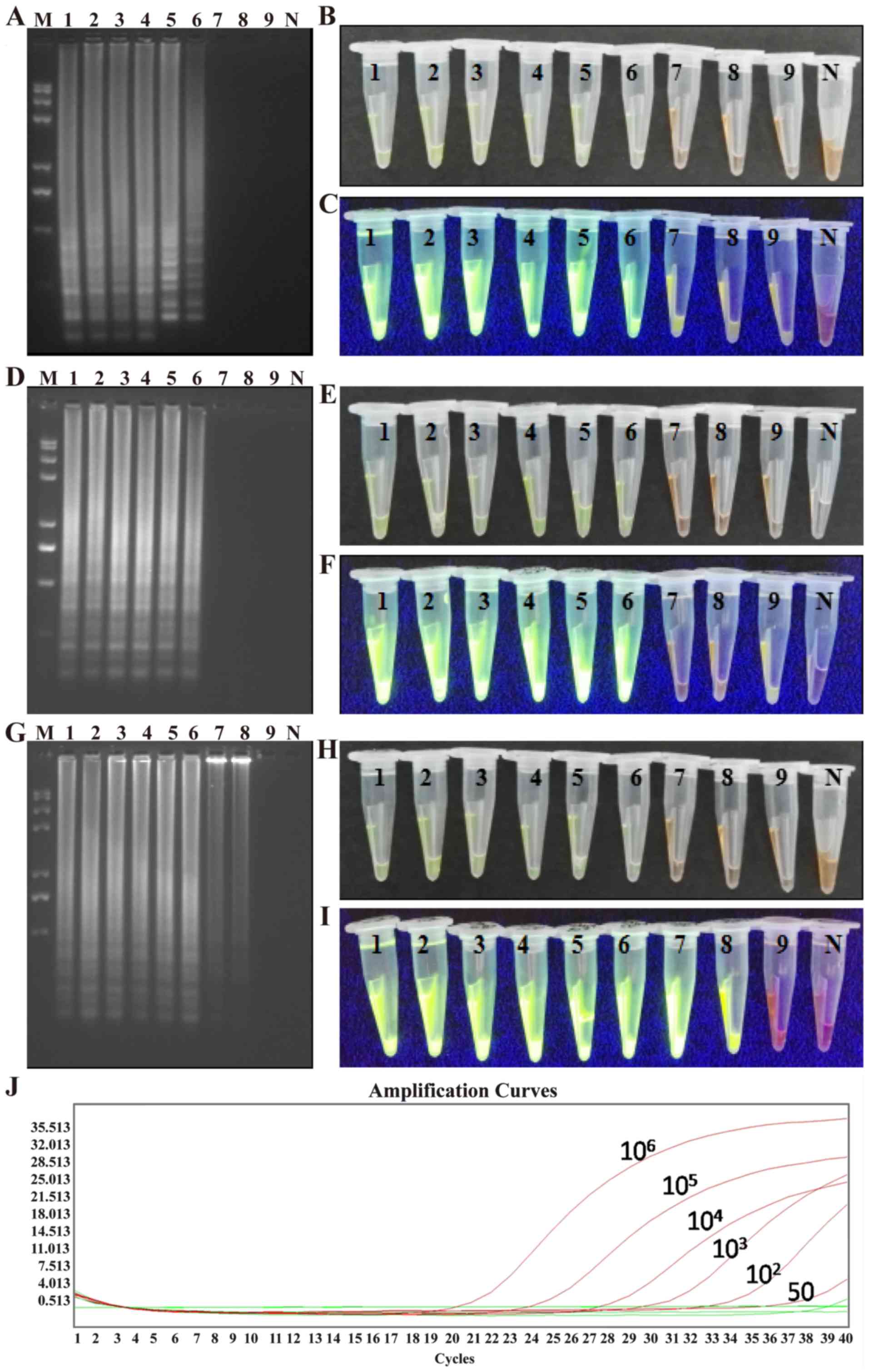 | Figure 7.Sensitivity test for the LAMP, CPA
and IMSA assays. LAMP assay: (A) Agarose gel electrophoresis of the
products; (B) visualization of reactions performed with serially
diluted ABL1-ETO fusion gene plasmids (1–9) and
SYBR-Green I under visible light; (C) visualization of reactions
performed with various fusion gene samples (1–9) and
SYBR-Green I under UV light. CPA assay: (D) agarose gel
electrophoresis of the products; (E) visualization of reactions
performed with serially diluted ABL1-ETO fusion gene plasmids
(1–9)
and SYBR-Green I under visible light; (F) visualization of
reactions performed with various fusion gene samples (1–9) and
SYBR-Green I under UV light. IMSA assay: (G) agarose gel
electrophoresis of the products; (H) visualization of reactions
performed with serially diluted ABL1-ETO fusion gene plasmids
(1–9)
and SYBR-Green I under visible light; (I) visualization of
reactions performed with various fusion gene samples (1–9) and
SYBR-Green I under UV light. (J) Reverse transcription-polymerase
chain reaction amplification curves for samples 1–9. LAMP,
loop-mediated isothermal amplification; CPA, cross-priming
amplification; IMSA, isothermal multiple-self-matching-initiated
amplification; M, Trans 2K plus II DNA marker; 1–9, 106,
105, 104, 103, 102, 50,
25, 10, and 5 plasmid copies/tube, respectively; N, negative
control. |
Detection of clinical samples with
LAMP, CPA and IMSA assays
A total of 41 BM or PB samples were subjected to
LAMP, CPA and IMSA assays to detect the AML1/ETO fusion
gene. In parallel, the same samples were subjected to RT-PCR
assays. The positive detection rate for all three assays was 9.76%,
and these results were in agreement with those from the RT-PCR
assays (Fig. 8A-J).
Discussion
AML is a hyperplastic disease involved in the
proliferation and abnormal differentiation of leukemia cells in BM
and PB, and this can be a life-threatening condition (14). The crude incidence and mortality
rates of leukemia in China in 2009 were 5.68/105 and
4.28/105, respectively. In addition, the incidence and
mortality rates of myeloid leukemia were significantly higher than
those for lymphoid leukemia. The incidence of AML is closely
associated with the formation of oncogenic fusion genes from
chromosomal translocation events, and they often represent
molecular biological markers of AML (2). Therefore, the ability to detect and
monitor fusion genesis is of importance to patients with AML. To
date, the fusion genes of AML mainly include: PML-RARA,
AML1-ETO, CBFβ-MYH11, MLL/AF9, DEK/CAN,
nucleophosmin (NPM)/myeloid leukemia factor 1 (MLF),
E2A/PBX1, and translocation liposarcoma
(TLS)/ETS-related gene (ERG), and these are of
significance for the diagnosis and treatment of AML.
Among the fusion genes mentioned above, the
AML1/ETO fusion gene is recognized as a classic molecular
marker for AML-M2. Previously, RT-PCR was the gold standard
for rapid and sensitive detection of the AML1/ETO fusion
gene in leukemia. However, RT-PCR requires ~4-5 h due to separation
of the reverse transcription step and the amplification step. In
the present study, these two steps were performed simultaneously,
which shortened the RT-PCR protocol by 1–1.5 h. In addition, unlike
conventional RT-PCR, the amplification products of LAMP, CPA and
IMSA assays do not require any further manipulation as they can be
visualized with fluorescent dyes, including calcein and SGI, or
directly analyzed with a real-time turbidimeter. Calcein was the
first dye used in isothermal reactions based on its ability to
release Mn2+ upon complexation with pyrophosphate during
DNA synthesis (15,16). Therefore, this dye can be added into
a reaction system prior to an isothermal reaction, and
cross-contamination does not occur to affect the reaction. However,
calcein exhibits reduced sensitivity compared with the SGI
fluorescent dye and a real-time turbidimeter (11,17). The
use of a real-time turbidimeter also means that LAMP, CPA and IMSA
assays can be quantitative techniques (18). However, turbidimeters are not widely
available in developing countries due to their cost. Previously,
SGI was the most common fluorescent dye used for isothermal
reactions due to its high sensitivity and low cost. However, this
dye is added following the reaction endpoint (19,20), and
the opening of a reaction tube increases the potential for
cross-contamination (21). In the
present study, novel IAM tubes were used in the LAMP, CPA, and IMSA
reactions that were initially performed. The circumference of these
tubes was such that the isothermal reaction liquid was added and
remained distinct from the added SGI fluorescent dye. When the
isothermal reaction was initiated, the IAM tube was inverted
several times to completely mix the isothermal reaction liquid and
the SGI fluorescent dye without opening the cover, therefore,
minimising cross-contamination (Fig.
3).
To the best of our knowledge, the present study is
the first to compare LAMP, CPA and IMSA assays in terms of
detection of the AML1/ETO fusion gene. In addition, the
diagnostic performance of these three thermal amplification assays
was compared with that achieved with RT-PCR. In terms of
sensitivity, the LAMP and CPA assays exhibited comparable
sensitivity to RT-PCR (50 copies/tube). However, the IMSA assay
exhibited increased sensitivity (10 copies/tube) of detection, and
this result is in agreement with the results of a previous study,
which demonstrated that the IMSA assay was more sensitive than the
LAMP assay in the detection of human enterovirus 71 (11).
Primer specificity is important in isothermal
assays. To distinguish the AML1/ETO fusion gene from other
fusion genes, specific primer sets were designed for the LAMP, CPA
and IMSA assays, which were all based on a region common to the
AML1 gene (GenBank: KJ890835.1), the ETO gene
(GenBank: X79990.1), and the AML1-ETO fusion gene (GenBank:
S78158.1). Specifically, BIP, 1s, and RIT/FIT primers,
respectively, were designed to bind the fusion point of the
AML1/ETO fusion gene. To determine the specificity of these
primers, the AML1/ETO fusion gene and four other fusion
genes associated with malignant tumors of the hematological system
were assayed in parallel. A high degree of specificity in detection
of the AML1/ETO fusion gene was observed, and no
false-positive results were obtained. Therefore, the LAMP, CPA and
IMSA assays exhibited high specificity for the AML1/ETO
target. Of note, only four fusion genes were selected to serve as
negative controls in the evaluation of assay specificity due to the
limited availability of other fusion genes. It was hypothesized
that primers with increased specificity prevent the detection of
other fusion genes as false-positives, and this can be assessed in
future investigations by assaying additional fusion genes to
provide data to further characterize the sensitivity of isothermal
assays.
A practical evaluation of the LAMP, CPA and IMSA
assays was performed with detection of the AML1/ETO fusion
gene in 41 clinical samples. An RT-PCR assay of these samples was
performed in parallel, and 4/41 clinical samples were identified as
positive for the AML1/ETO fusion gene. The LAMP, CPA and
IMSA assays produced the same result. Taken together, these results
confirmed that the LAMP, CPA and IMSA assays, which were optimized
in the present study, exhibited comparable sensitivity and
specificity to RT-PCR assays in detecting the AML1/ETO
fusion gene. Furthermore, these results demonstrated the
reliability of these assays for use with clinical samples, and the
ease of applying these methods in small hospitals with
non-specialized laboratories may eliminate delays in waiting for
results from reference hematological centers. However, LAMP, CPA
and IMSA assays are more expensive than RT-PCR assays (~20.00, vs.
~5.00 Yuan, respectively). As the RT-PCR assay is a more
established method, there are numerous commercially available PCR
kits, and this has reduced the cost of the RT-PCR method.
In conclusion, the results of the present study
demonstrated that the LAMP, CPA and IMSA isothermal assays, which
were optimized in the present study, exhibited high specificity in
detecting the AML1/ETO fusion gene. They exhibited no
cross-reactivity with other fusion genes, and their clinical
accuracy was equivalent to that of an RT-PCR assay, which was
performed in parallel. These assays represent rapid, sensitive and
specific tools, which can be applied in poorly equipped
laboratories. However, these assays do not currently represent
quantitative assays for the detection of minimal residual disease
during follow-up. It is considered possible to generate
quantitative versions of the LAMP, CPA and IMSA assays for the
detection of the AML1/ETO fusion gene, and these are
currently under evaluation.
Acknowledgements
Not applicable.
Funding
Financial support for the present study was provided
by grants from the Science and Technology Planning Project of
Guangdong Province, China (grant nos. 2014A020212300 and
2016A020215149) and the Science and Technology Special Competitive
Allocation Project of Zhanjiang City (grant no. 2016A06003).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZY designed the study and revised the manuscript. WL
designed the primers and revised the manuscript. HL performed the
experiments and drafted the manuscript. RW analyzed the data and
revised the manuscript. YZ analyzed the data and figures.
Ethics approval and consent to
participate
All procedures involving human participants were in
accordance with the ethical standards of the Ethics Committee of
the Affiliated Hospital of Guangdong Medical University, and with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. Informed consent was obtained from
all of the individuals involved.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Salmon JM, Bots M, Vidacs E, Stanley KL,
Atadja P, Zuber J and Johnstone RW: Combining the differentiating
effect of panobinostat with the apoptotic effect of arsenic
trioxide leads to significant survival benefit in a model of
t(8;21) acute myeloid leukemia. Clin Epigenetics. 7:22015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okuda T, Cai Z, Yang S, Lenny N, Lyu CJ,
van Deursen JM, Harada H and Downing JR: Expression of a knocked-in
AML1-ETO leukemia gene inhibits the establishment of normal
definitive hematopoiesis and directly generates dysplastic
hematopoietic progenitors. Blood. 91:3134–3143. 1998.PubMed/NCBI
|
|
3
|
Saia M, Termanini A, Rizzi N, Mazza M,
Barbieri E, Valli D, Ciana P, Gruszka AM and Alcalay M: AML1/ETO
accelerates cell migration and impairs cell-to-cell adhesion and
homing of hematopoietic stem/progenitor cells. Sci Rep.
6:349572016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peterson LF and Zhang DE: The 8;21
translocation in leukemogenesis. Oncogene. 23:4255–4262. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumari P, Lingappa Kavitha B, Obula Reddy
C, Mangalagowri M, Madhumathi DS, Mahadeva Prasad M, Raghavendra
HV, Premalata CS, Lakshmaiah KC and Mir Mazloumi SH: A rare
cytogenetic presentation of acute myeloid leukemia (AML-M2). Acta
Med Iran. 50:827–830. 2012.PubMed/NCBI
|
|
6
|
Gabert J, Beillard E, van der Velden VH,
Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela
JM, Cavé H, et al: Standardization and quality control studies of
‘real-time’ quantitative reverse transcriptase polymerase chain
reaction of fusion gene transcripts for residual disease detection
in leukemia-a Europe against cancer program. Leukemia.
17:2318–2357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang ZD, Qin YZ, Liu YR, Xu LP, Liu DH,
Liu KY and Huang XJ: Monitoring AML1-ETO mRNA levels by real-time
quantitative RT-PCR in t(8;21) acute myeloid leukemia patients
after hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue
Za Zhi. 29:672–675. 2008.(In Chinese). PubMed/NCBI
|
|
8
|
Cui C, Shu W and Li P: Fluorescence in
situ hybridization: Cell-based genetic diagnostic and research
applications. Front Cell Dev Biol. 4:892016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Notomi T, Okayama H, Masubuchi H, Yonekawa
T, Watanabe K, Amino N and Hase T: Loop-mediated isothermal
amplification of DNA. Nucleic Acids Res. 28:E632000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu G, Hu L, Zhong H, Wang H, Yusa S, Weiss
TC, Romaniuk PJ, Pickerill S and You Q: Cross priming
amplification: Mechanism and optimization for isothermal DNA
amplification. Sci Rep. 2:2462012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding X, Nie K, Shi L, Zhang Y, Guan L,
Zhang D, Qi S and Ma X: Improved detection limit in rapid detection
of human enterovirus 71 and coxsackievirus A16 by a novel reverse
transcription-isothermal multiple-self-matching-initiated
amplification assay. J Clin Microbiol. 52:1862–1870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding X, Wu W, Zhu Q, Zhang T, Jin W and Mu
Y: Mixed-dye-based label-free and sensitive dual fluorescence for
the product detection of nucleic acid isothermal
multiple-self-matching-initiated amplification. Anal Chem.
87:10306–10314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Viehmann S, Teigler-Schlegel A, Bruch J,
Langebrake C, Reinhardt D and Harbott J: Monitoring of minimal
residual disease (MRD) by real-time quantitative reverse
transcription PCR (RQ-RT-PCR) in childhood acute myeloid leukemia
with AML1/ETO rearrangement. Leukemia. 17:1130–1136. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niessen L and Vogel RF: Detection of
Fusarium graminearum DNA using a loop-mediated isothermal
amplification (LAMP) assay. Int J Food Microbiol. 140:183–191.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao X, Li P, Xu J, Zhang M, Ren R, Liu G
and Yang X: Rapid and sensitive detection of didymella bryoniae by
visual loop-mediated isothermal amplification assay. Front
Microbiol. 7:13722016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li F, Yan W, Long L, Qi X, Li C and Zhang
S: Development and application of loop-mediated isothermal
amplification assays for rapid visual detection of cry2Ab and cry3A
genes in genetically-modified crops. Int J Mol Sci. 15:15109–15121.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu X, Li X, Mo Z, Jin F, Wang B, Zhao H,
Shan X and Shi L: Rapid identification of Chikungunya and Dengue
virus by a real-time reverse transcription-loop-mediated isothermal
amplification method. Am J Trop Med Hyg. 87:947–953. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ke Y, Wang Y, Wang Z, Du X, Huang L and
Chen Z: Sensitive and rapid detection of blaNDM-1 in clinical
samples by isothermal cross-priming amplification. J Microbiol
Methods. 95:215–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wozniakowski G, Niczyporuk JS,
Samorek-Salamonowicz E and Gawel A: The development and evaluation
of cross-priming amplification for the detection of avian reovirus.
J Appl Microbiol. 118:528–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandra A, Keizerweerd AT and Grisham MP:
Detection of puccinia kuehnii causing sugarcane orange rust with a
loop-mediated isothermal amplification-based assay. Mol Biotechnol.
58:188–196. 2016. View Article : Google Scholar : PubMed/NCBI
|