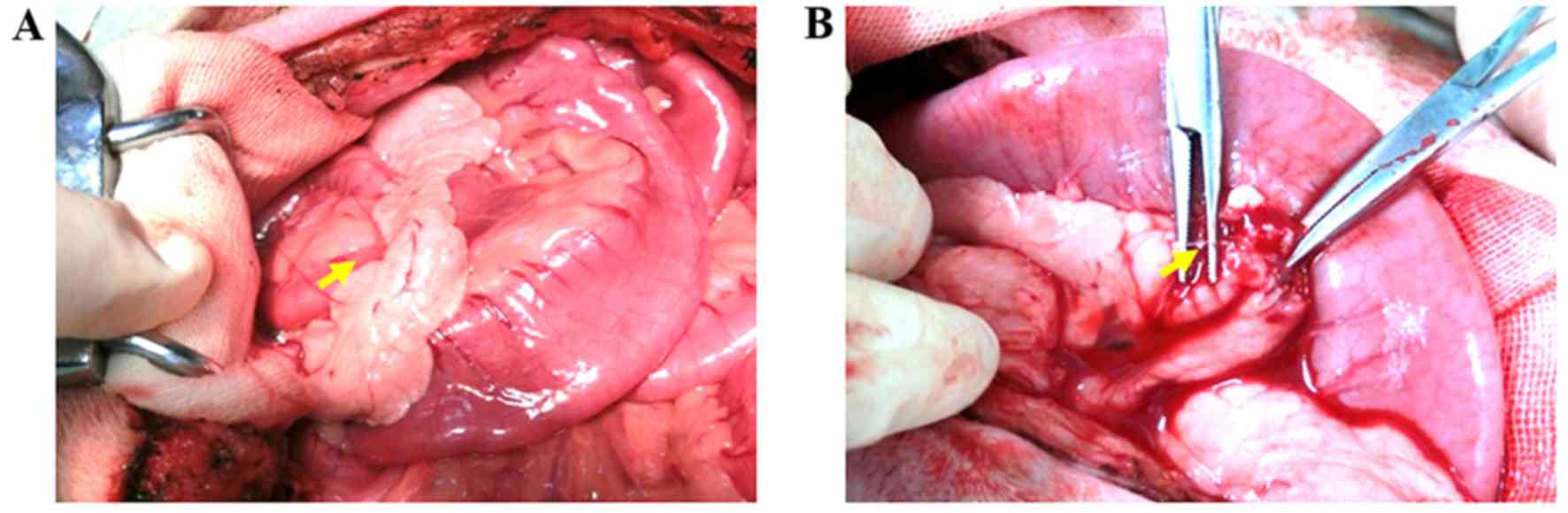
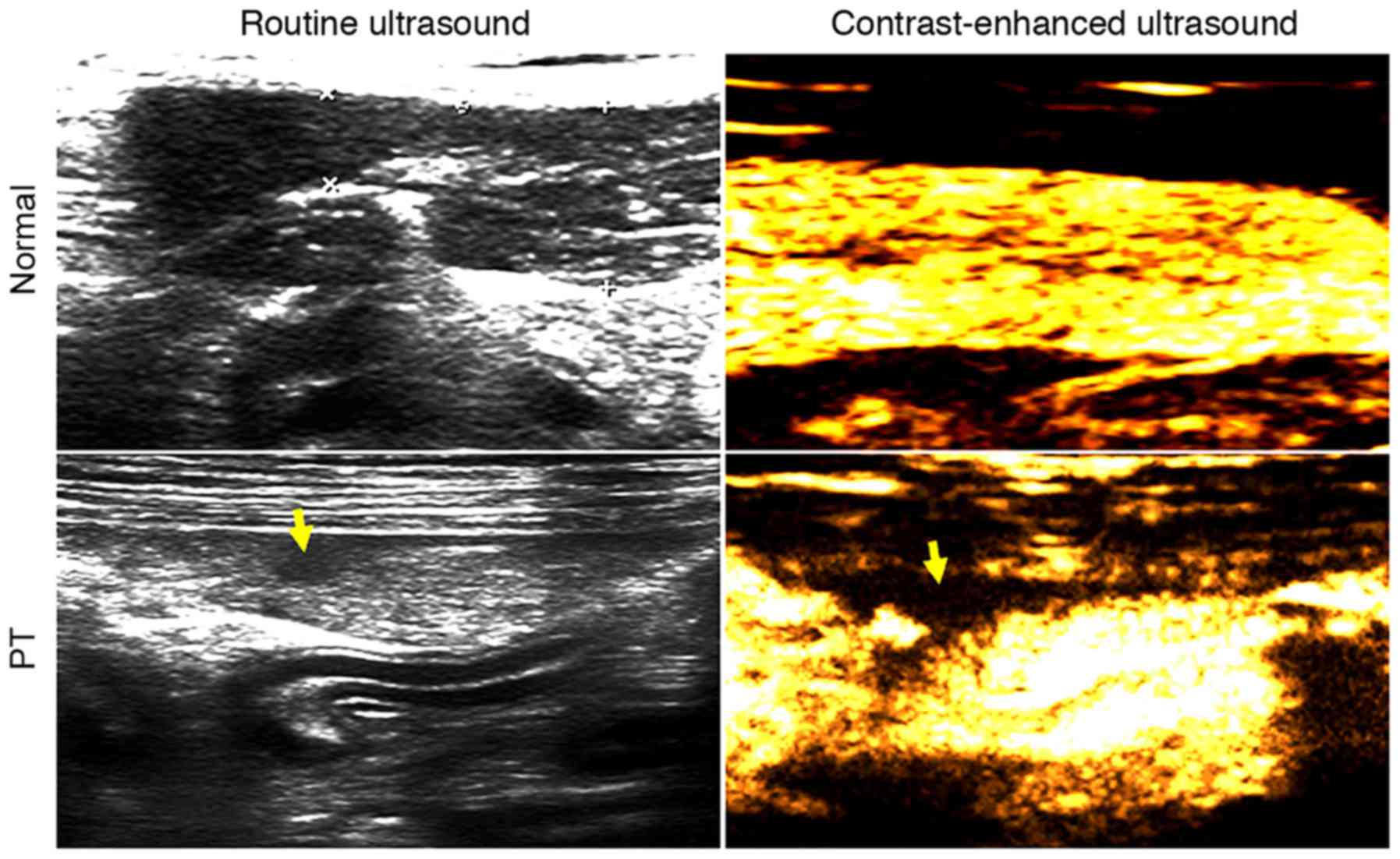
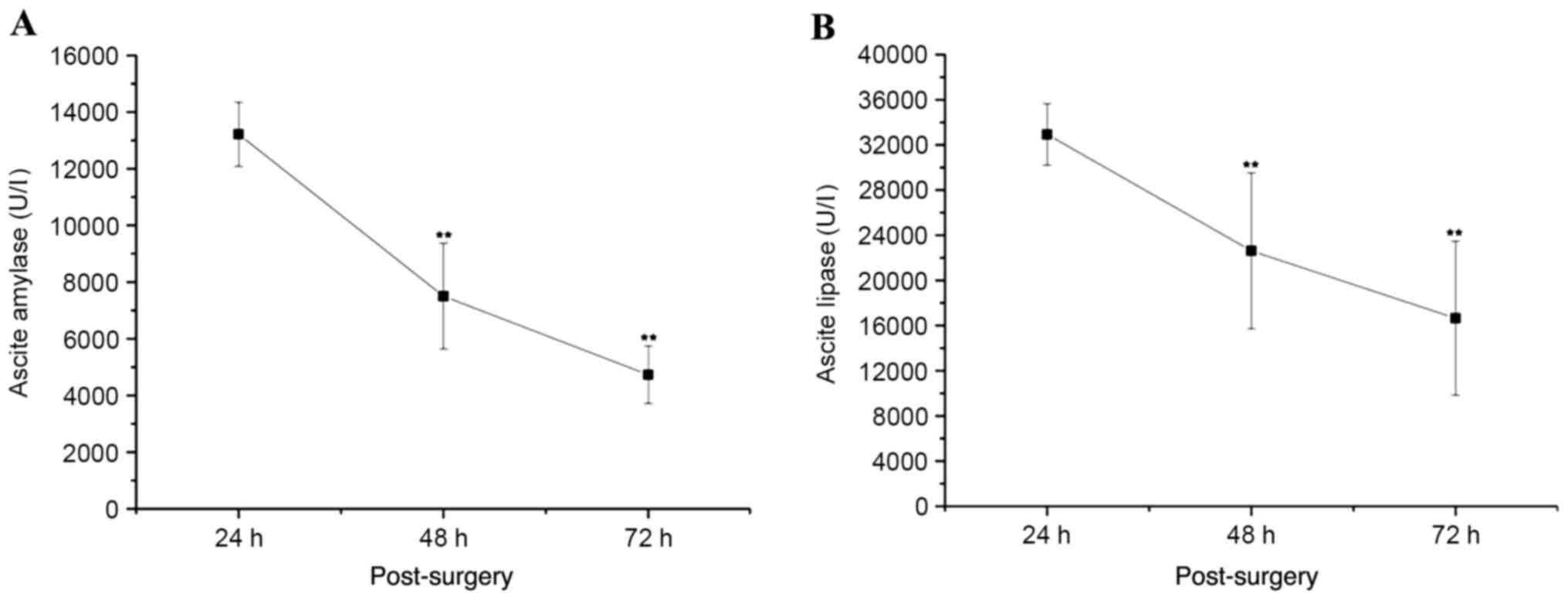
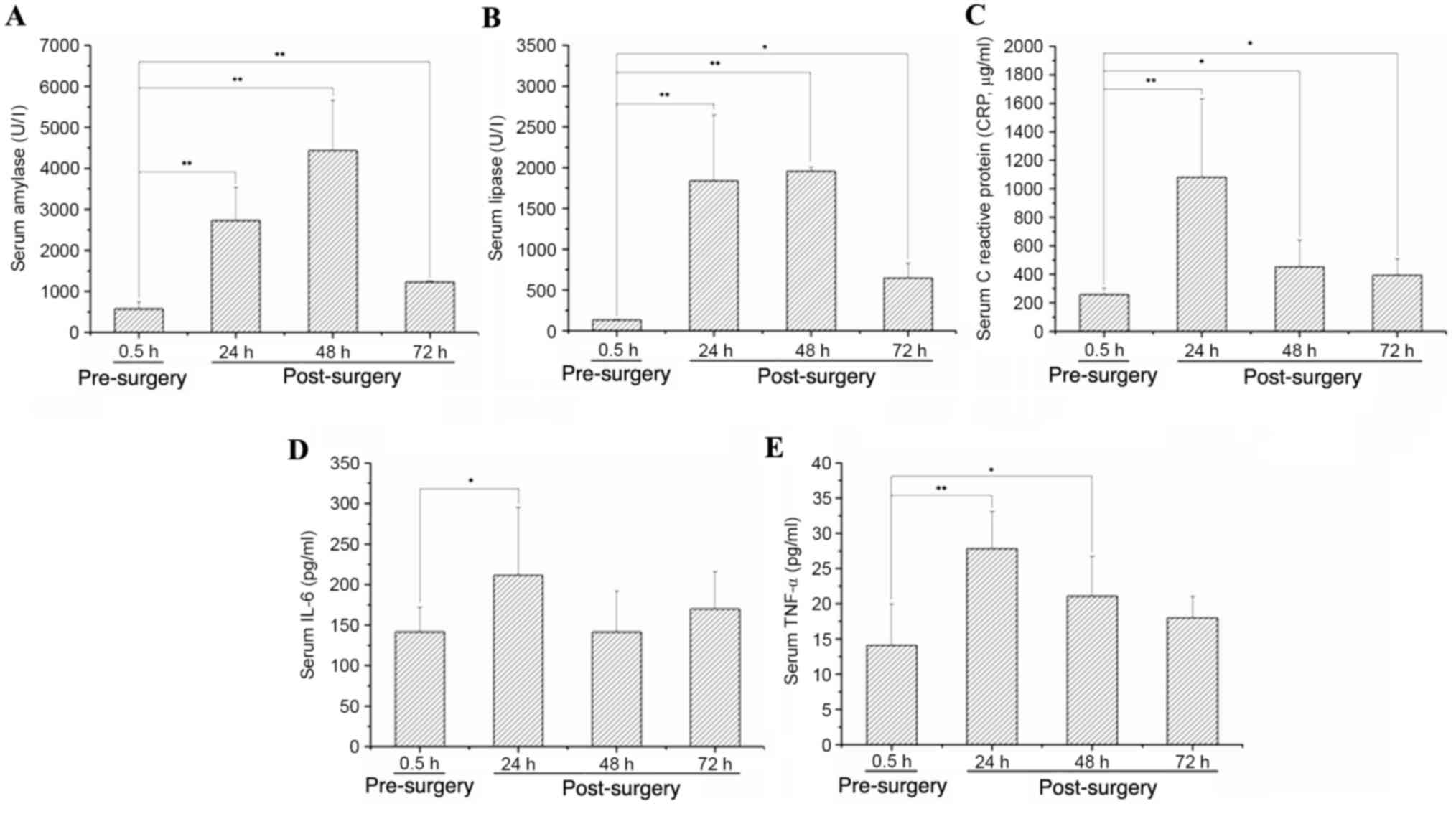
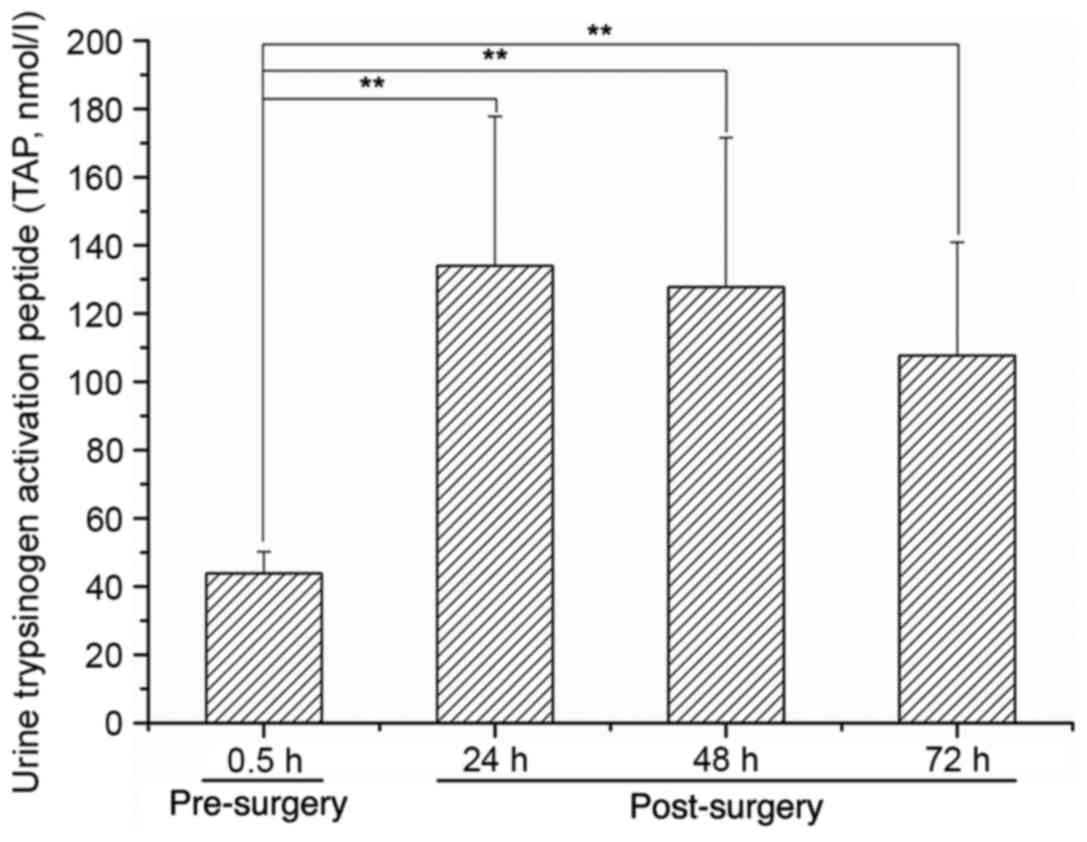
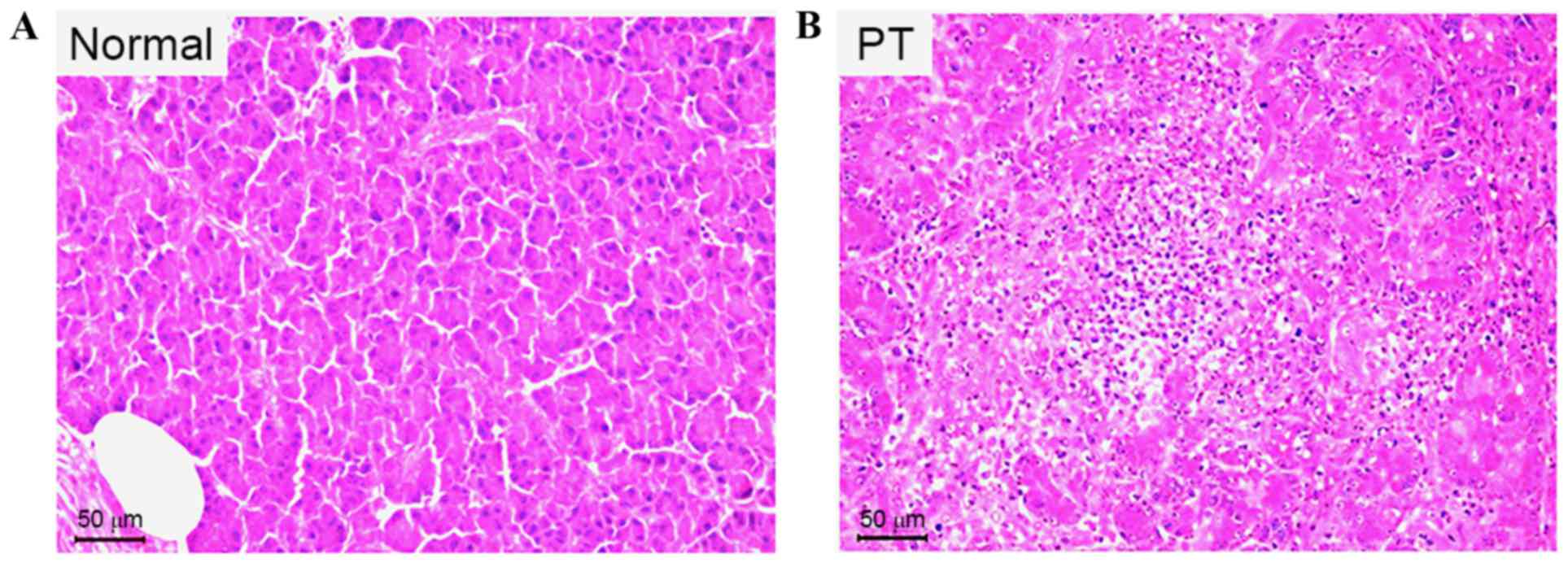
|
1
|
Lv F, Tang J, Luo Y, Nie Y, Liang T, Jiao
Z, Zhu Z and Li T: Emergency contrast-enhanced ultrasonography for
pancreatic injuries in blunt abdominal trauma. Radiol Med.
119:920–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cirillo RL Jr and Koniaris LG: Detecting
blunt pancreatic injuries. J Gastrointest Surg. 6:587–598. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toro A, Cavallaro A, Mannino M, Cappello
G, Politi A and Di Carlo I: Pancreatic injury in a blunt abdominal
trauma treated by a conservative approach with
Tachosil®. Minerva Chir. 67:461–463. 2012.PubMed/NCBI
|
|
4
|
Heuer M, Hussmann B, Lefering R, Taeger G,
Kaiser GM, Paul A and Lendemans S; Trauma Registry of the DGU:
Pancreatic injury in 284 patients with severe abdominal trauma:
Outcome, course, and treatment algorithm. Langenbecks Arch Surg.
396:1067–1076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hasanovic J, Agic M, Rifatbegovic Z,
Mehmedovic Z and Jakubovic-Cickusic A: Pancreatic injury in blunt
abdominal trauma. Med Arch. 69:130–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goh E and Chen EM: Traumatic pancreatic
transection from blunt abdominal trauma. CJEM. 16:502–503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rui-Wu D, Guang-Yu C, Fa-Qun H, Zu H,
Hong-Tao Y, Hong-Yin L, Tao W, Ning L, Li-Jun T and Li-Ping C: Cell
cycle characteristics of the pancreas in an animal model of
isolated pancreatic trauma. J Trauma Acute Care Surg. 76:784–790.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen M, Walsh RM and Goldblum JR:
Application of a new collagen-based sealant for the treatment of
pancreatic injury. Surg Laparosc Endosc Percutan Tech. 14:181–185.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Q, Tang J, Lv FQ, Zhang Y, Jiao ZY,
Liu Q and Luo YK: Evaluation of blunt pancreatic injury with
contrast-enhanced ultrasonography in comparison with
contrast-enhanced computed tomography. Exp Ther Med. 5:1461–1465.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou P, Wang L, Huang S, Fu C, He H, Hong
M, Su S and Li S: Beagle dogs have low susceptibility to BJ94-like
H9N2 avian influenza virus. Infect Genet Evol. 31:216–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore EE, Gogbil TH, Malangoni MA,
Jurkovich GJ, Champion HR, Gennarelli TA, McAninch JW, Pachter HL,
Shackford SR and Trafton PG: Organ injury scaling II: Pancreas,
duodenum, small bowel, colon, and rectum. J Trauma. 30:1427–1429.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao H, Song Q, Lv F, Wang S, Wang Y, Li X,
Luo Y, Mei X and Tang J: Protection provided by a gabexate mesylate
thermo-sensitive in situ gel for rats with grade III pancreatic
trauma. Gut and liver. 11:156–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teh SH, Sheppard BC, Mullins RJ, Schreiber
MA and Mayberry JC: Diagnosis and management of blunt pancreatic
ductal injury in the era of high-resolution computed axial
tomography. Am J Surg. 193:641–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang G, Yang ZG, Yao J, Deng W, Zhang S,
Xu HY and Long QH: Differentiation between tuberculosis and
leukemia in abdominal and pelvic lymph nodes: Evaluation with
contrast-enhanced multidetector computed tomography. Clinics (Sao
Paulo). 70:162–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tal S, Pollak L and Berkovitz N:
Contrast-enhanced computed tomography as a necessary scan in acute
stroke: A case series. J Stroke Cerebrovasc Dis. 24:1548–1554.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An JS, Moon SH, Chun SY, Kim JH, Koh DH,
Yoon JH and Jeon TY: Value of CT for ERCP endoscopists to identify
the type of gastroenteric anastomosis in patients with previous
subtotal gastrectomy. Hepatogastroenterology. 61:916–919.
2014.PubMed/NCBI
|
|
17
|
Finkelmeier F, Tal A, Ajouaou M, Filmann
N, Zeuzem S, Waidmann O and Albert J: ERCP in elderly patients:
Increased risk of sedation adverse events but low frequency of
post-ERCP pancreatitis. Gastrointest Endosc. 82:1051–1059. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrescu I, Bratu AM, Petrescu S, Popa BV,
Cristian D and Burcos T: CT vs. MRCP in choledocholithiasis
jaundice. J Med Life. 8:226–231. 2015.PubMed/NCBI
|
|
19
|
Botsford A, McKay K, Hartery A and Hapgood
C: MRCP imaging of duplicate gallbladder: A case report and review
of the literature. Surg Radiol Anat. 37:425–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGahan JP, Horton S, Gerscovich EO,
Gillen M, Richards JR, Cronan MS, Brock JM, Battistella F, Wisner
DH and Holmes JF: Appearance of solid organ injury with
contrast-enhanced sonography in blunt abdominal trauma: Preliminary
experience. AJR Am J Roentgenol. 187:658–666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan MX, Li R, Zhang XH, Tang CL, Guo YL,
Guo DY and Luo MK: Factors affecting the enhancement patterns of
intrahepatic cholangiocarcinoma (ICC) on contrast-enhanced
ultrasound (CEUS) and their pathological correlations in patients
with a single lesion. Ultraschall Med. 37:609–618. 2016.PubMed/NCBI
|
|
22
|
Sessa B, Trinci M, Ianniello S, Menichini
G, Galluzzo M and Miele V: Blunt abdominal trauma: Role of
contrast-enhanced ultrasound (CEUS) in the detection and staging of
abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med.
120:180–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv F, Tang J, Li W, Zhang H, Wang W and
Yang L: Hemostatic agents injected directly into hepatic injury
sites for liver trauma hemorrhage under the guidance of
contrast-enhanced ultrasound: An animal experiment. Ultrasound Med
Biol. 34:1604–1609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang J, Lv F, Li W, Zhang H, Luo Y, An L
and Li T: Percutaneous injection of hemostatic agents for severe
blunt hepatic trauma: An experimental study. Eur Radiol.
18:2848–2853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv F, Tang J, Luo Y, Nie Y, Jiao Z, Li T
and Zhou X: Percutaneous treatment of blunt hepatic and splenic
trauma under contrast-enhanced ultrasound guidance. Clin Imaging.
36:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu HX, Weskott HP, Liu JB and Zheng RQ:
Contrast-enhanced ultrasound. Biomed Res Int.
2015:8650282015.PubMed/NCBI
|
|
27
|
Meloni MF, Smolock A, Cantisani V, Bezzi
M, D'Ambrosio F, Proiti M, Lee F, Aiani L, Calliada F and Ferraioli
G: Contrast enhanced ultrasound in the evaluation and percutaneous
treatment of hepatic and renal tumors. Eur J Radiol. 84:1666–1674.
2015. View Article : Google Scholar : PubMed/NCBI
|