Introduction
The pancreas is located deep in the retroperitoneal
space, and has significant endocrine and exocrine functions
(1,2). When the pancreas is damaged, the
clinical symptoms are often overshadowed by symptoms and signs of
other organs, which may cause a delay in its diagnosis and
treatment, and often induces severe complications, such as
traumatic pancreatitis, pancreatic abscess, pseudocyst and
pancreatic fistula (3). As the
popularity of expressways and motor vehicles increases, and with
the rapid development of construction and other types of industry,
the occurrence of accidents has increased the morbidity of
pancreatic trauma (PT) (4). It has
been reported that the morbidity of PT is ~45%, and the mortality
of PT is typically 10–30%, even reaching 60% when diagnosis and
treatment are delayed (5,6). Therefore, PT is one of the most
intractable diseases of abdominal trauma, and is the subject of
considerable attention. However, due to the particular
characteristics of the anatomical site and physiological functions
of the pancreas, the diversity of PT injury factors, and the lack
of effective drug treatments and classical animal models, the study
of PT is challenging and is lagging behind that of other abdominal
parenchymal organs, such as the liver and spleen. The lack of a
classical animal model is particularly limiting to the study of
PT.
A few reliable animal models of PT have been
reported, that have been prepared using various experimental
approaches to mimic clinical PT. In one model, a BIM-III
multifunctional biological impact machine was used to establish a
rat PT model, in which bloody ascites were observed, in addition to
increased amylase levels in the serum and ascites, and hyperemia,
edema and necrosis of the pancreas (7). In another model, following the sharp
separation of the distal pancreas and hemostasis with minimal
cauterization, a pig PT model was established that exhibited severe
inflammation, fibrosis and necrosis (8). In a third model, the pancreas was
squeezed in front of the spine using hemostatic forceps to
establish a minipig model with various degrees of PT, and increased
levels of amylase and lipase in the serum (9). Although these models reflect the
disease symptoms of PT, they have certain limitations, such as
model instability, difficult procedures, unsatisfactory levels of
damage and poor clinical applicability.
Beagle dogs were selected as experimental subjects
in the present study in view of the anatomical structure and
physiological functions of dogs being much more closer to those of
humans than are those of pigs and rats, in addition to these dogs
having a docile temperament, good compliance and being easy to
experiment on (10). Theoretically,
ultrasound examination should provide good imaging results due to
the relatively thin abdominal walls of dogs. However, the ability
of ultrasound to display the pancreas is poor for dogs, and the
pancreatic echo is not clearly distinguishable. The dog thorax is
quite deep, and the pancreas is freely located in the abdominal
cavity, with its left lobe positioned deeply and covered by the
liver and stomach at the front, and so body surface ultrasound is
not able to detect it. In addition, the right lobe of the pancreas
is deeply located in the intestinal cavity in close dorsal
proximity to the duodenum, and is readily shielded by the
intestinal canal and disturbed by gas, and body surface ultrasound
is also not able to detect it. In the present study, the duodenum
intestinal wall was fixed to the abdominal wall making the right
lobe move closer to the abdominal wall to avoid shielding of the
thorax and the disturbing effects of gas in the intestinal tract,
and thereby to improve the display rate and image quality of the
pancreas during ultrasound examination.
Materials and methods
Experimental animals
In total, 5 female Beagle dogs (weight, 10–12 kg and
23–24 months of age) were provided by the National Institutes of
Health for the Care of Laboratory Animals [license number, SCXK
(Beijing, China) 2011–0003]. The dogs were housed at a temperature
of 24–28°C, humidity of 45–60% and a light-dark cycle of 12-h with
ad libitum access to food and water.
Establishment of a Beagle model of
grade III PT
After fasting for 18 h, the Beagles were
anesthetized with a forelimb injection of pentobarbital sodium (3%;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a preoperative
dose of 30 mg/kg, and then fixed on an operating table to perform
endotracheal intubation. A gastric tube was inserted and linked to
an apparatus for vacuum aspiration and gastrointestinal
decompression. Heparin (300 IU/kg; Sigma-Aldrich; Merck KGaA) was
then administered by intravenous injection followed by
intraoperative anesthetization with 3% pentobarbital sodium at a
dose of 3 mg/kg.
When the Beagles were anesthetized, the abdominal
hair was removed by shaving, and the skin was sterilized prior to a
medial incision being made to expose the pancreatic tissue through
layered laparotomy. Subsequently, focal parenchyma trauma combined
with main pancreatic duct damage, where the pancreatic parenchyma
was distanced 7 cm from the pylorus, was created using hemostatic
forceps, and the depth of the focus was half of the anteroposterior
diameter of the pancreas. After this, the incision was sutured
layer by layer, using a needle and 4-0 Mousse thread with a needle
layer by layer, and an intravenous injection of antibiotic was
subsequently administered. According to the Organ Scaling Committee
of the American Association for the Surgery of Trauma, pancreatic
damage with rupture of the main pancreatic duct may be used to
mimic grade III PT (11). The study
protocol was approved by the Chinese People's Liberation Army
General Hospital Ethics Committee (Beijing, China).
Routine ultrasound and
contrast-enhanced ultrasound (CEUS) examination
Following the modeling of PT, the focus location,
scope, shape, boundary and internal echo were observed immediately
using routine ultrasound. Subsequently, CEUS examination of the
abdomen was performed using a color Doppler diagnostic apparatus
(CX50; Philips Healthcare, DA Best, The Netherlands; probe, L12-3,
3–12 MHz) as follows. The probe was placed on the right side of the
abdomen and the inside of the abdominal wall, and the device
condition was set to provide abdominal contrast imaging over time.
Simultaneously, 0.5 ml SonoVue (Bracco S.p.A., Milan, Italy) was
injected by an intravenous bolus injection technique, followed by
fast flushing of the injection tubing with 5.0 ml normal saline.
Subsequently, dynamic images of the pancreas were recorded through
continuous examination to observe the focal trauma location, scope,
shape and boundary, and to determine whether or not contrast agent
filling, retention and overflow occurred. If the procedure was
repeated, the interval time between examinations was >10 min.
The probe was isolated by a sterile protective membrane under
sterile conditions.
After reviving the Beagles, ~1.0 ml bucinnazine
hydrochloride injection (100 mg/2 ml; Shenyang First Pharmaceutical
Co., Ltd., Shenyang, China) was injected into the coxal muscle.
When the vital signs were stable and no bleeding was observed, the
Beagles were each returned to a single cage, and fasted for 18 h
with continuous observation for 3 days. In addition, the general
condition and survival counts of the dogs were recorded at 0.5 h
pre-surgery, and 24, 48 and 72 h post-surgery, and samples of
ascites, whole blood, urine and pancreatic tissues were collected
during surgery and used for subsequent examinations.
Measurement of ascites amylase and
lipase levels
At 24, 48 and 72 h post-surgery, ascites were
collected and centrifuged at 7,200 × g for 30 min at room
temperature. Following this, assays of amylase and lipase
expression were conducted using a dog amylase ELISA kit (cat. no.
C016) and dog lipase ELISA kit (cat. no. A054; both from Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). The data were
recorded at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and analyzed using Origin 9.5 software
(http://www.originlab.com/).
Measurement of serum amylase, lipase,
C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis
factor-α (TNF-α) levels
At 0.5 h pre-surgery, and 24, 48 and 72 h
post-surgery, samples of whole blood were collected and centrifuged
at 4,500 × g for 30 min at room temperature to separate the serum
for measurement of amylase, lipase, CRP, IL-6 and TNF-α levels. For
the amylase assay, a dog amylase ELISA kit (cat. no. C016) was used
and results were recorded at 450 nm using a microplate reader.
Similarly, dog lipase ELISA (cat. no. A054; both from Nanjing
Jiancheng Bioengineering Institute), and dog CRP ELISA (cat. no.
F28110), dog IL-6 ELISA (cat. no. F28180) and dog TNF-α ELISA kits
(cat. no. F28330; all from Shanghai Westang Bio-Tech Co., Ltd.,
Shanghai, China) were used and results were recorded at 450 nm
using microplate reader. The assays were conducted according to the
manufacturers' instructions, and data were analyzed using Origin
9.5 software.
Measurement of urinary trypsinogen
activation peptide (TAP) levels
At 0.5 h pre-surgery, and 24, 48 and 72 h
post-surgery, urine samples were collected and centrifuged at 7,200
× g for 30 min at room temperature followed by examination of the
expression of TAP using a dog TAP ELISA kit (cat. no. CSB-E13461c;
Wuhan Cusabio Biotech Co., Ltd., Wuhan, China). The data were
recorded at 450 nm using a microplate reader and analyzed using
Origin 9.5 software.
Hematoxylin and eosin (H&E)
staining
At 72 h post-surgery, the surviving Beagles were
sacrificed for examination of the changes induced in the pancreas.
Thus, pancreatic tissues were collected and fixed with 10%
paraformaldehyde prior to performing H&E staining as previously
described (12). Slides were
deparaffinized and rehydrated, and frozen or vibratome sections
were mounted on slides and rehydrated. The sections were slightly
overstained with hematoxylin for 3–5 min, according to the
thickness of the section (3–5 µm) and fixed using 4%
paraformaldehyde (for up to 20 min if the solution was not fully
fixed), and excess stain was removed using tap water. The sections
were differentiated and destained for a few sec in acidic alcohol
until the sections appeared red, which typically took four or five
dips. The sections were then briefly rinsed in tap water to remove
the acid. Bicarbonate was applied for ~2 min until the nuclei were
sharply visible in blue. The hematoxylin-stained slides from the
final tap water rinse were placed in 70% ethanol for 3 min and then
in eosin for 2 min. Finally, the slides were subjected to three
washes with 95% ethanol for 5 min and then transferred to the first
absolute ethanol of the clearing series. Images were captured using
a brightfield microscope connected to a CCD camera at ×20 and ×40
magnification.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed with one-way analysis
of variance using SPSS software (version 21.0; http://spss.en.softonic.com/; IBM SPSS, Armonk, NY,
USA), and Student's t-tests were performed for comparisons of two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of a Beagle model of
grade III PT is verified by ultrasound and general condition
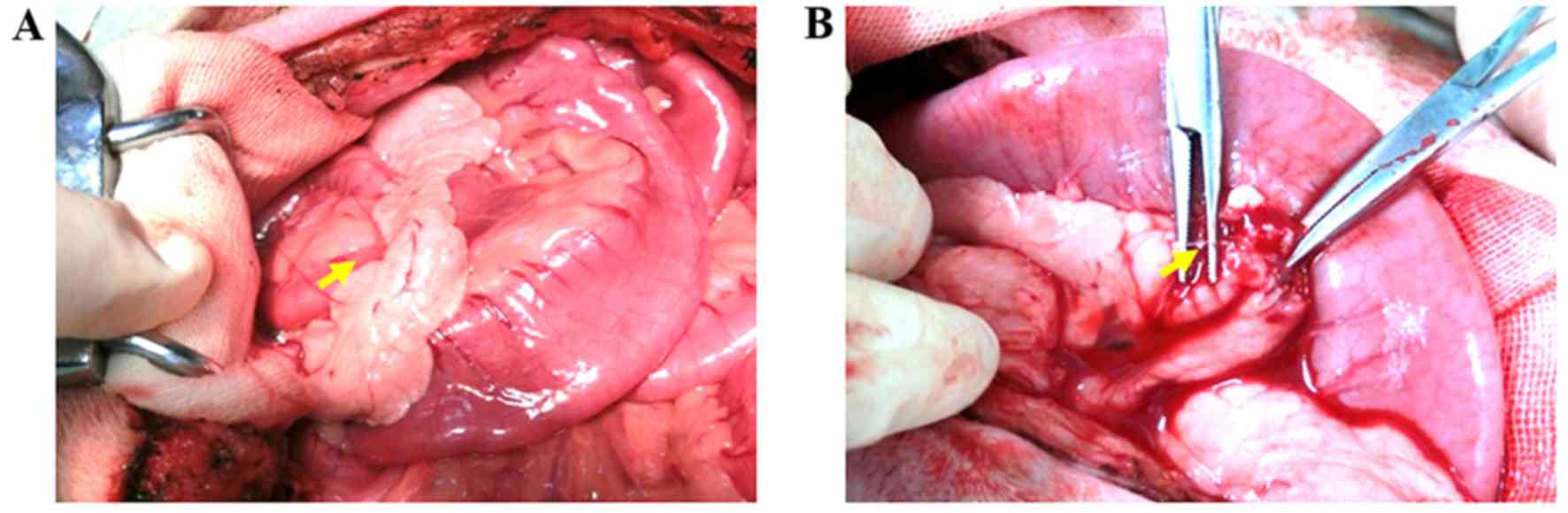
In order to establish the grade III PT model, the
main pancreatic duct was exposed as indicated by the yellow arrow
in Fig. 1A, and divided using
hemostatic scissors as shown by the yellow arrow in Fig. 1B to correctly establish the grade III
PT model. After modeling, all of Beagles were surviving, lethargic
and laid passively and laterally at 6 h, were slowly moving at 12
h, and had begun to eat again at 24 h, although they frequently
vomited.
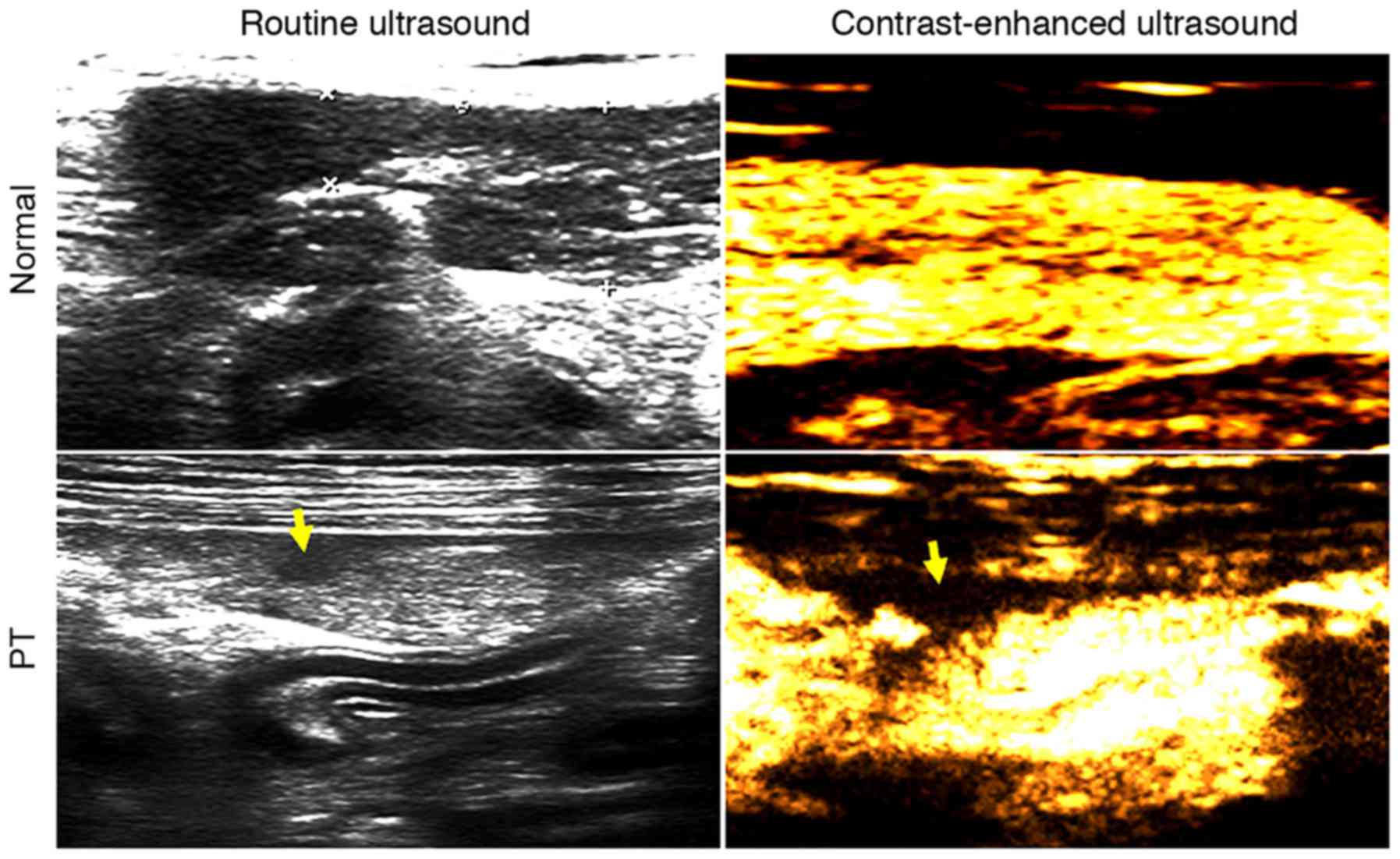
When examined by routine ultrasound, the pancreas
was completely displayed, with locally uneven echo, an obscured
boundary at the lower echo region and irregularly shaped PT
(Fig. 2). As the time after the
modeling surgery increased, the margins of the focal trauma tended
to become smoother, and its shape remained irregular with lower or
no echo (data not shown). CEUS detected a large region of focal
trauma, with a depth greater than one-half of the anteroposterior
diameter of the pancreas, with a clear boundary, clear capsular
rupture and trauma from active bleeding (Fig. 2).
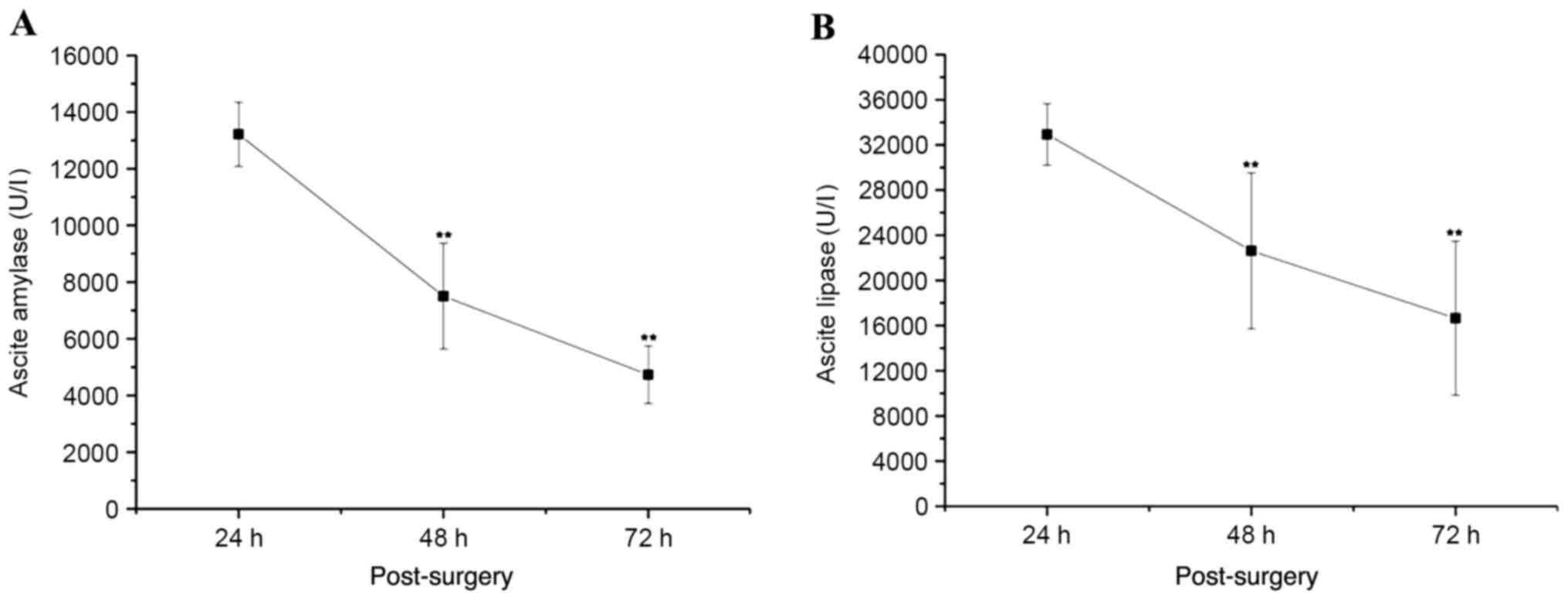
Amylase and lipase levels of ascites
peak at 48 h and decrease at 72 h after PT
The volume of ascites detected by ultrasound was
increased to a peak at 48 h after the PT modeling surgery and
decreased at 72 h post-surgery (Table
I). The expression of amylase and lipase in the ascites reached
the highest levels at 24 h post-surgery, and then significantly
decreased at 48 and 72 h post-surgery (P<0.01 vs. 24 h
post-surgery; Fig. 3). At 72 h after
the surgery, the expression of amylase and lipase in the ascites
was >3-fold indicating pancreatic fistula.
 | Table I.Volume of ascites detected by
ultrasound in vivo. |
Table I.
Volume of ascites detected by
ultrasound in vivo.
| Time
post-surgery | Ascites dimensions
(cm) |
|---|
| 24 h | 2.33×0.6×3.1 |
| 48 h | 3.41×0.91×3.6 |
| 72 h | 2.6×1.8×2.7 |
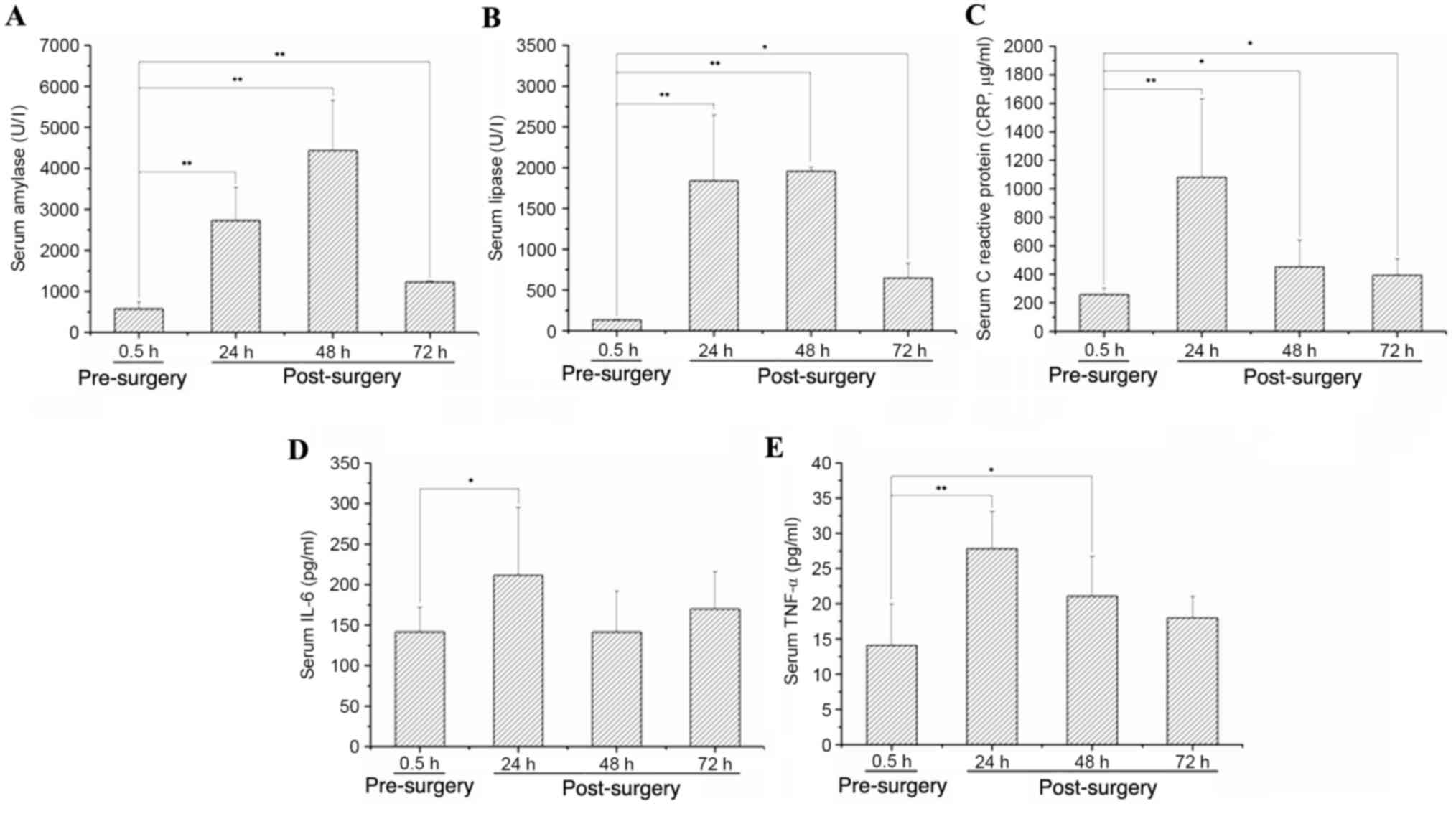
Serum amylase, lipase, CRP, IL-6 and
TNF-α levels increase after PT and then decrease
The expression levels of serum amylase and lipase
increased after the PT modeling surgery, peaking at 48 h after the
trauma to the pancreas and then decreasing (P<0.05 vs. 0.5 h
pre-surgery; Fig. 4A and B). The
expression levels of serum CRP, IL-6 and TNF-α were also increased
after the PT modeling surgery, peaking at 24 h post-surgery and
then decreasing (P<0.05 vs. 0.5 h pre-surgery; Fig. 4C-E).
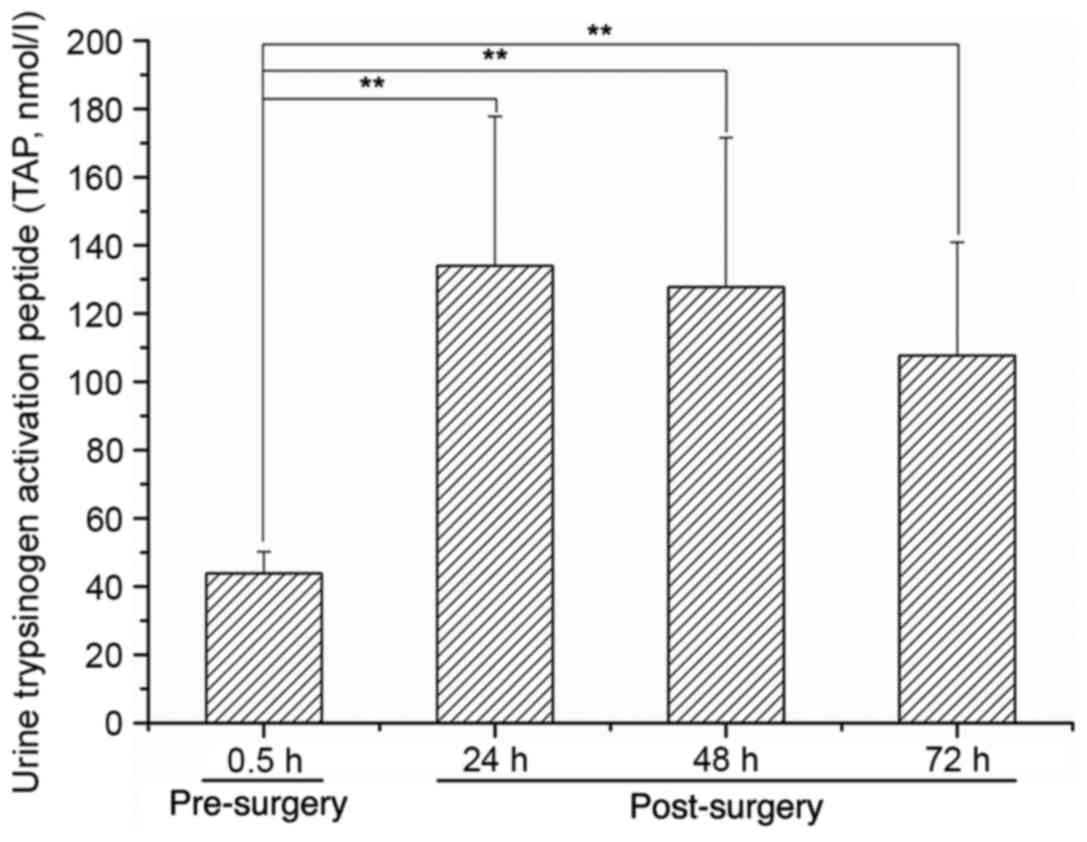
Urinary expression of TAP peaks at 24
h after PT and then decreases
The urinary expression of TAP was increased to a
peak level at 24 h after the PT modeling surgery, and then
decreased at 48 and 72 h post-surgery (P<0.01 vs. 0.5 h
pre-surgery; Fig. 5).
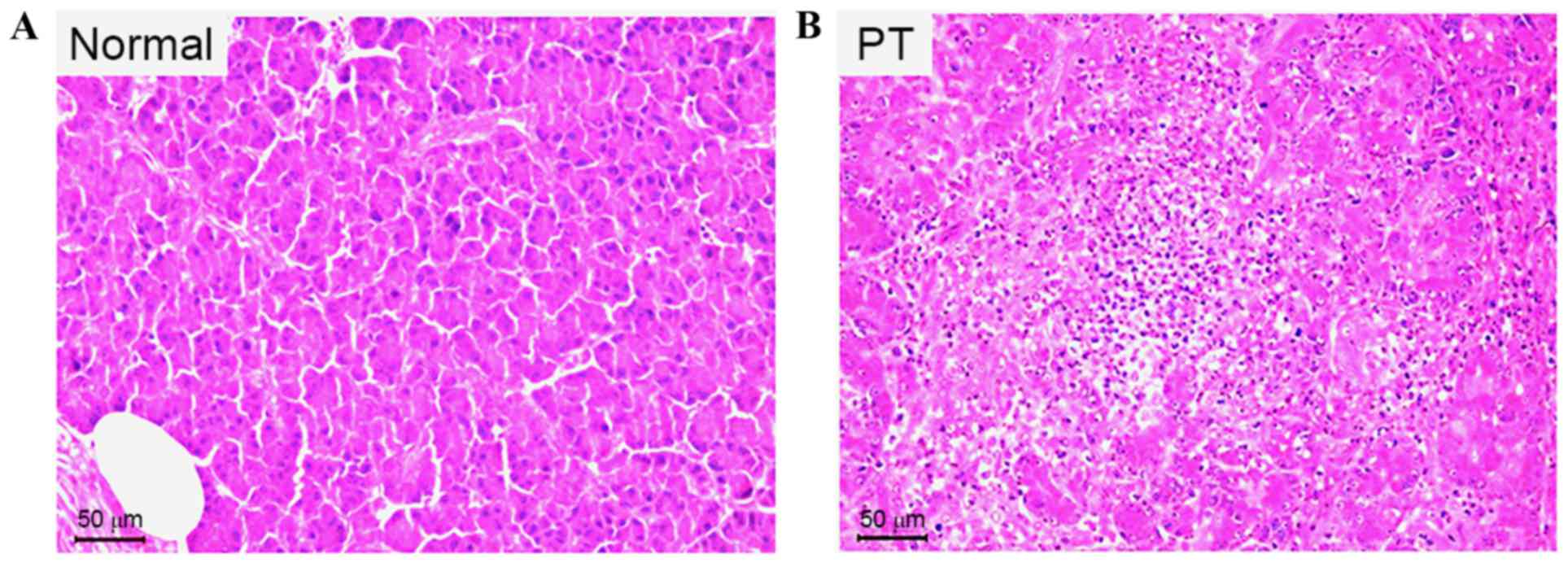
PT models exhibit loosely distributed
cells, with severely damaged acini, hyperchromatic nuclei and
inflammatory cell invasion
The H&E staining results demonstrate that in the
pancreas of a normal Beagle dog, the pancreatic cells were tightly
distributed, with regular acini, no hyperchromatic nuclei and no
inflammatory cell invasion (Fig. 6).
However, at 72 h after the trauma to the pancreas, pancreatic cells
were loosely distributed, and displayed damaged acini,
hyperchromatic nuclei and severe inflammatory cell invasion
(Fig. 6).
Discussion
In this study, a Beagle model of grade III PT was
established, which involved transection of the main pancreatic duct
and pancreatic parenchyma, 7 cm distant from the pylorus. After
modeling, the volume of ascites and the levels of amylase and
lipase it contained were increased to peak levels at 24 h
post-surgery and then decreased. Serum amylase and lipase levels
were increased to their highest levels at 48 h post-surgery and
then decreased, whereas serum CRP, IL-6 and TNF-α levels peaked at
24 h post-surgery and then decreased. Urinary TAP levels were
elevated in this model, peaking at 24 h post-surgery, and then
decreased slightly at 48 and 72 h post-surgery. When compared with
normal pancreatic cells, pancreatic tissue specimens examined 72 h
after the induction of trauma exhibited loosely distributed
pancreatic cells, with damaged acini, hyperchromatic nuclei and
severe inflammatory cell invasion. All of these observations
indicated that the Beagle model of grade III PT was correctly
established, and was easy to prepare, with clear and reliable
grading, good repeatability and controllability, and without
damaging effects on the liver and intestinal canal.
Several imaging evaluation approaches for PT have
been reported, including ultrasound, computed tomography (CT),
endoscopic retrograde cholangiopancreatography (ERCP) and magnetic
resonance cholangiopancreatography (MRCP) (13). Among these, ultrasound, CT and MRCP
are noninvasive examination techniques. CT, particularly
contrast-enhanced CT (CECT), has a high sensitivity and
specificity, and is able to guide the grading of PT. It is one of
the most frequently used methods for clinical PT examination.
However, it is not suitable for patients with aberrant hemodynamics
or contrast agent anaphylaxis, and radiation damage and
inconvenient follow-up have limited its further clinical
application (14,15). ERCP has become a significant
treatment means due to the advantages of being minimally invasive
and quick to use with few complications. It not only displays
pancreatic duct and biliary ducts, but can also be used for stent
implantation and inner drainage. However, ERCP may increase the
risk of iatrogenic traumatic pancreatitis (16,17).
MRCP has become a preferred diagnostic method for
pancreaticobiliary disease due to being noninvasive, lacking
radioactive damage potential and not requiring the use of a
contrast agent. However, the longer time it takes to perform and
its high expense have limited its application in PT (18,19).
Ultrasound has a significant application value in emergency
medicine due to its characteristics of providing real-time images
rapidly, safely, inexpensively and conveniently. It enables the
early examination of ascites, and repeated observation of their
changes can help to verify the state of the illness. However, the
sensitivity of ultrasound is relatively low for the diagnosis of PT
(20).
CEUS utilizes the nonlinear characteristics and
backscattering of blood gas microbubbles to obtain
contrast-enhanced images (21,22). In
CEUS, contrast agent microbubbles as a blood tracer are injected
intravenously to display the blood perfusion characteristics of
normal and abnormal tissues. This technique, which is widely used
in clinical imaging examinations, has the characteristics of
practicability, noninvasiveness and convenience, and has wide
applicability for the diagnosis of abdominal parenchymal organ
trauma, such as that affecting the liver, spleen and kidney. Over
the past 20 years, we have enacted CEUS examination of the liver,
spleen and kidney, and significantly increased the trauma
diagnostic rate (23–25). In addition, CEUS examination of PT
has attracted considerable attention, and demonstrated increasing
relevance for PT foci (26,27). In the present study, routine
ultrasound and CEUS were selected for the observation and
evaluation of the Beagle model of grade III PT. After simple
preparation of the intestinal cavity, the display rate of the
pancreas by routine ultrasound was 100%. At 24, 48 and 72 h after
the modeling procedure, the PT focus could be clearly displayed,
and manifested an irregular area of low to no echo, with a depth
greater than half of the anteroposterior diameter of the pancreas,
and a blurred edge to the low echo area. CEUS clearly revealed
disruption of the pancreatic capsule, the edge and scope of the
trauma focus, trauma to tissues surrounding the pancreas and active
bleeding. The trauma focus manifested no perfusion or a little low
enhancement at the arterial and venous phases. At the post-surgery
follow-up examination, the Beagles did not require narcosis, and
could be quickly and noninvasively examined using a bedside
machine. All of these observations indicated that CEUS was a
preferable image detection method for this Beagle model of grade
III PT.
To elucidate the reliability and availability of
this PT model, levels of amylase and lipase in the ascites,
amylase, lipase, CRP, IL-6 and TNF-α levels in the serum, and TAP
levels in the urine were examined by ELISA. Additionally,
pancreatic pathology was examined by H&E staining. At 24, 48
and 72 h after modeling, in comparison with their pre-surgery
values, all of the aforementioned indices were increased, peaking
at 24–48 h after modeling, and decreased thereafter. Among these,
urinary TAP levels exhibited significant differences at 24, 48 and
72 h after modeling in comparison with the pre-surgery level, which
indicated that urine TAP may be a sensitive test index for grade
III PT in Beagles. All animals developed ascites, with elevated
levels of amylase and lipase, which were >3-fold higher than the
normal reference value and indicated that trauma of the pancreatic
parenchyma and pancreatic duct led to pancreatic fluid spillover.
No inflammatory response or bleeding necrosis were evident in the
abdominal wall and duodenum suture, which indicated that the suture
and fixation methods used created little damage, as well as being
easy to use. The trauma resulted in damage to the pancreatic acinar
cells, with interstitial edema, blood extravasation and
inflammatory cell infiltration. This indicates that the grade III
Beagle model of PT was correctly established. However, this study
also has certain limitations, such as the small sample size, risk
of infection following laparotomy and short-term observation
period, and therefore the future studies should increase the sample
size and prolong the observation time to confirm the veracity and
effectiveness.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81327003).
References
|
1
|
Lv F, Tang J, Luo Y, Nie Y, Liang T, Jiao
Z, Zhu Z and Li T: Emergency contrast-enhanced ultrasonography for
pancreatic injuries in blunt abdominal trauma. Radiol Med.
119:920–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cirillo RL Jr and Koniaris LG: Detecting
blunt pancreatic injuries. J Gastrointest Surg. 6:587–598. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toro A, Cavallaro A, Mannino M, Cappello
G, Politi A and Di Carlo I: Pancreatic injury in a blunt abdominal
trauma treated by a conservative approach with
Tachosil®. Minerva Chir. 67:461–463. 2012.PubMed/NCBI
|
|
4
|
Heuer M, Hussmann B, Lefering R, Taeger G,
Kaiser GM, Paul A and Lendemans S; Trauma Registry of the DGU:
Pancreatic injury in 284 patients with severe abdominal trauma:
Outcome, course, and treatment algorithm. Langenbecks Arch Surg.
396:1067–1076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hasanovic J, Agic M, Rifatbegovic Z,
Mehmedovic Z and Jakubovic-Cickusic A: Pancreatic injury in blunt
abdominal trauma. Med Arch. 69:130–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goh E and Chen EM: Traumatic pancreatic
transection from blunt abdominal trauma. CJEM. 16:502–503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rui-Wu D, Guang-Yu C, Fa-Qun H, Zu H,
Hong-Tao Y, Hong-Yin L, Tao W, Ning L, Li-Jun T and Li-Ping C: Cell
cycle characteristics of the pancreas in an animal model of
isolated pancreatic trauma. J Trauma Acute Care Surg. 76:784–790.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen M, Walsh RM and Goldblum JR:
Application of a new collagen-based sealant for the treatment of
pancreatic injury. Surg Laparosc Endosc Percutan Tech. 14:181–185.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Q, Tang J, Lv FQ, Zhang Y, Jiao ZY,
Liu Q and Luo YK: Evaluation of blunt pancreatic injury with
contrast-enhanced ultrasonography in comparison with
contrast-enhanced computed tomography. Exp Ther Med. 5:1461–1465.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou P, Wang L, Huang S, Fu C, He H, Hong
M, Su S and Li S: Beagle dogs have low susceptibility to BJ94-like
H9N2 avian influenza virus. Infect Genet Evol. 31:216–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore EE, Gogbil TH, Malangoni MA,
Jurkovich GJ, Champion HR, Gennarelli TA, McAninch JW, Pachter HL,
Shackford SR and Trafton PG: Organ injury scaling II: Pancreas,
duodenum, small bowel, colon, and rectum. J Trauma. 30:1427–1429.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao H, Song Q, Lv F, Wang S, Wang Y, Li X,
Luo Y, Mei X and Tang J: Protection provided by a gabexate mesylate
thermo-sensitive in situ gel for rats with grade III pancreatic
trauma. Gut and liver. 11:156–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teh SH, Sheppard BC, Mullins RJ, Schreiber
MA and Mayberry JC: Diagnosis and management of blunt pancreatic
ductal injury in the era of high-resolution computed axial
tomography. Am J Surg. 193:641–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang G, Yang ZG, Yao J, Deng W, Zhang S,
Xu HY and Long QH: Differentiation between tuberculosis and
leukemia in abdominal and pelvic lymph nodes: Evaluation with
contrast-enhanced multidetector computed tomography. Clinics (Sao
Paulo). 70:162–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tal S, Pollak L and Berkovitz N:
Contrast-enhanced computed tomography as a necessary scan in acute
stroke: A case series. J Stroke Cerebrovasc Dis. 24:1548–1554.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
An JS, Moon SH, Chun SY, Kim JH, Koh DH,
Yoon JH and Jeon TY: Value of CT for ERCP endoscopists to identify
the type of gastroenteric anastomosis in patients with previous
subtotal gastrectomy. Hepatogastroenterology. 61:916–919.
2014.PubMed/NCBI
|
|
17
|
Finkelmeier F, Tal A, Ajouaou M, Filmann
N, Zeuzem S, Waidmann O and Albert J: ERCP in elderly patients:
Increased risk of sedation adverse events but low frequency of
post-ERCP pancreatitis. Gastrointest Endosc. 82:1051–1059. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrescu I, Bratu AM, Petrescu S, Popa BV,
Cristian D and Burcos T: CT vs. MRCP in choledocholithiasis
jaundice. J Med Life. 8:226–231. 2015.PubMed/NCBI
|
|
19
|
Botsford A, McKay K, Hartery A and Hapgood
C: MRCP imaging of duplicate gallbladder: A case report and review
of the literature. Surg Radiol Anat. 37:425–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGahan JP, Horton S, Gerscovich EO,
Gillen M, Richards JR, Cronan MS, Brock JM, Battistella F, Wisner
DH and Holmes JF: Appearance of solid organ injury with
contrast-enhanced sonography in blunt abdominal trauma: Preliminary
experience. AJR Am J Roentgenol. 187:658–666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan MX, Li R, Zhang XH, Tang CL, Guo YL,
Guo DY and Luo MK: Factors affecting the enhancement patterns of
intrahepatic cholangiocarcinoma (ICC) on contrast-enhanced
ultrasound (CEUS) and their pathological correlations in patients
with a single lesion. Ultraschall Med. 37:609–618. 2016.PubMed/NCBI
|
|
22
|
Sessa B, Trinci M, Ianniello S, Menichini
G, Galluzzo M and Miele V: Blunt abdominal trauma: Role of
contrast-enhanced ultrasound (CEUS) in the detection and staging of
abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med.
120:180–189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv F, Tang J, Li W, Zhang H, Wang W and
Yang L: Hemostatic agents injected directly into hepatic injury
sites for liver trauma hemorrhage under the guidance of
contrast-enhanced ultrasound: An animal experiment. Ultrasound Med
Biol. 34:1604–1609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang J, Lv F, Li W, Zhang H, Luo Y, An L
and Li T: Percutaneous injection of hemostatic agents for severe
blunt hepatic trauma: An experimental study. Eur Radiol.
18:2848–2853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv F, Tang J, Luo Y, Nie Y, Jiao Z, Li T
and Zhou X: Percutaneous treatment of blunt hepatic and splenic
trauma under contrast-enhanced ultrasound guidance. Clin Imaging.
36:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu HX, Weskott HP, Liu JB and Zheng RQ:
Contrast-enhanced ultrasound. Biomed Res Int.
2015:8650282015.PubMed/NCBI
|
|
27
|
Meloni MF, Smolock A, Cantisani V, Bezzi
M, D'Ambrosio F, Proiti M, Lee F, Aiani L, Calliada F and Ferraioli
G: Contrast enhanced ultrasound in the evaluation and percutaneous
treatment of hepatic and renal tumors. Eur J Radiol. 84:1666–1674.
2015. View Article : Google Scholar : PubMed/NCBI
|