Introduction
As an important signal transduction molecule in
cells of the spinal dorsal horn, protein kinase C (PKC) has an
extremely complex biological function. It is also distributed
widely throughout the body and many studies (1,2) have
verified that it has a close relationship with pain-induced
mechanisms. It plays an important role in pain-induced inflammatory
factor adjustment along afferent and efferent nerves and in the
expression of multiple targeting proteins. Dexmedetomidine is an
adrenergic receptor stimulant with high α2 specificity that was
discovered recently, which features less interference for systemic
hemodynamics, weak respiratory depression effects, increased
responsiveness, stable pharmacokinetics in vivo, as well as
high bioavailability. Dexmedetomidine also has anxiolytic,
anticonvulsion and anti-epileptic effects, and it has become one of
the primary narcotic and adjuvant drugs in clinic usage, with a
wide application potential (3,4). Some
researches (5,6) have pointed out that dexmedetomidine has
a certain analgesic effect on chronic neuropathic pain, but its
related mechanism has not been elucidated yet. Therefore, this
study aimed to estimate the influence of intrathecal injection of
dexmedetomidine on the behavioral impact and analgesic effect in
rat models with chronic neuropathic pain, as well as the role that
the expression of PKC in the spinal dorsal horn plays in the
analgesic mechanisms of dexmedetomidine. It is hypothesized that
the results of this study may provide a frame of reference for the
approach, dosage and effect of dexmedetomidine when applied in
chronic neuropathic pain.
Materials and methods
Experimental animals
A total of 35 heathy and clean adult male Sprague
Dawley rats were selected, which were bought from Shanghai
Bioengineering Animal Experimental Center (Shanghai, China), with
their weight ranging from 200 to 250 g. The rats had a free diet
and were kept in an environment with temperatures ranging from 22
to 25°C, humidity of about 55% and a day-night ratio of 1:1. After
adapting to the environment for a week, the rats were allocated
into experimental grouping. Five rats were randomly chosen as the
blank control group and the rest were prepared as experimental
models with chronic constriction injury, which were randomly
divided into the the model and observation groups which received
treatment (n=15, each group).
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China).
Preparation for models with chronic
neuropathic pain
After successful anesthesia by intraperitoneal
injection with pentobarbital sodium (40 mg/kg), the rats were fixed
in lateral position and had their skin cut along the thighbone, 1
cm away from the femoral inferior segment. Blunt dissection was
applied to completely expose the right sciatic nerve and the
surrounding tissues, approximately 8 mm before free main trunk and
bifurcation. Chromium-infused catgut was used to ligature the
sciatic nerve 4 times from a position 2 mm away from the nerve
initial point, with a space of 1 mm between two tunnels. Normal
saline was injected locally for washing, and then the muscle,
fascia, subcutaneous tissue and skin were sutured interruptedly.
When some certain behaviors, including paw withdrawal and tail
licking appeared in the rats, the positive reaction of pain was
accepted as the standard for successful modeling.
Experimental methods
The observation group was injected with 10 µl
dexmedetomidine intrathecally, while the model group was injected
with the equivalent amount of saline. Behavioral outcomes were
evaluated by a cumulative and a motor function score at the time of
injection, and then 3 and 5 days afterwards. In addition,
mechanical withdrawal threshold (MWT) and thermal withdrawal
latency (TWL) were applied for the determination of pain threshold.
Immunohistochemistry staining method was used to test the positive
expression of PKC in the spinal dorsal horn and western blot
analysis was used for detection of the PKC quantitative levels of
expression.
Intrathecal injection
Two hours after preparing the models, the rats
inhaled 3% isoflurane for anesthesia and were fixed in a prone
position. L3-L4 space was chosen as the cut location and the length
of longitudinal incision was 3 cm, while the muscle tissue was
separated by blunt separation, with exposure of the L3-L4 crest
space. A 25G needle was then used to penetrate the ligamentum
flavum and the spinal dura mater, with puncture success being
indicated by the discharge of cerebrospinal fluid. A catheter was
inserted slowly for 8 cm through the spinal dura mater and the open
end was fixed on L1-L2, while the other end was extracted from the
neck and back through a subcutaneous tunnel, with 2 cm exposed for
tube occlusion and fixation. When the anesthesia lost efficacy, the
rats were injected with 10 µl dexmedetomidine or normal saline.
Successful anesthesia was indicated if two legs were not load
bearing, without an escape reaction within 10 sec and recovery
after 20 min.
Detection methods
Behavior ability score: A cumulative score method
was calculated according to the hindpaw touchdown and load level of
rats, with hindpaw oppression whitening representing load bearing.
A score of 2 meant that the hindpaw did not touch the ground and
bore no load; 1, indicated that the hindpaw did not bear load but
could touch the ground; and 0, meant that the hindpaw touched the
ground and bore a load. An interval of 5 min was applied for each
scoring. The final cumulative score was the accumulation of
difference in score values between the operative and the healthy
side within 1 h. In terms of motor function score, a score of 0
showed that motor function did not change and the rats could walk
normally; 1, indicated a mild limitation of motor function, and
plantar stimulation could result in normal paw withdrawal; 2,
demonstrated that autonomic movement ability was weakened, with a
moderate limitation of motor function; the rats could walk
normally, but paw withdrawal response caused by plantar stimulation
declined; and 3, meant that autonomic movement was completely
limited and the rats could not walk, with obvious paw pull
phenomenon and no response to plantar stimulation.
Pain threshold detection
The rats were placed on a metal grid by MWT and
covered by organic glass shade. The grid was raised and, after 3
min was allotted for adaptation, a fiber threadlet was used to
vertically stimulate the middle part of rats' pelma from below,
with continuous increases in stimulation intensity. The stimulus
thresholds were recorded when leg lift and licking of feet was
observed. Each rat was measured 5 times, with an interval of 2 min
between measures, to obtain the average. The rats were placed in an
organic glass box by TWL, which was put on the glass plate. After 3
min for adaptation, an infrared light beam was directed toward the
middle planta of the rats' operation side, until escape behavior
was observed. The basic intensity of thermal stimulus was set at 10
sec and automatic cutout configured at 20 sec. Each rat was
measured 5 times, with an interval of 2 min, to obtain an average
time.
Immunohistochemical staining
method
Tissue sections of the spinal dorsal horn were
prepared conventionally to a thickness of 5 µm, and with xylene
dewaxing, gradient ethanol hydration and antigen retrieval. Three
per cent H2O2 solution was added and the
sample was incubated for 20 min under 27°C and then a normal goat
serum working solution was applied and it was incubated for another
20 min under 27°C. Rabbit anti-mouse PKC monoclonal antibody
(dilution, 1:2,000; cat. no. AF1576; Jiangsu Beyotime Science and
Technology Ltd., Jiangsu, China) was applied, which was placed in a
wet box set at 4°C and kept overnight for incubation.
Immunoglobulin G (IgG) from normal rats replaced the primary
antibody as a negative control. Goat anti-rabbit IgG polyclonal
antibody (dilution, 1:500; cat. no. A0208, Jiangsu Beyotime Science
and Technology Ltd.) was applied, which was put in the wet box at
27°C and incubated for 20 min. Horseradish peroxidase was added to
mark the streptavidin working solution (Jiangsu Beyotime Science
and Technology Ltd.), which was put in the wet box and incubated
for 20 min under 27°C, with 5 min of phosphate-buffered saline
(PBS) vibration and washing carried out three times. DAB
coloration, hematoxylin redyeing, hydrochloric acid alcohol
differentiation, ammonium hydroxide blue, dehydration of gradient
ethanol, xylene transparency, neutral balsam sealing, airing in
room temperature and observation under optical microscope (Olympus,
Tokyo, China) were then conducted sequentially. The results were as
follows: The semi-quantitative method was applied according to the
staining intensity and proportion of stained cells. The yellow
staining of cytoplasm or karyon became dark brown, which was
regarded as positive. As for staining intensity, a score of 0
indicated no positive staining; 1, indicated weak staining; 2,
indicated moderate intensity staining; and 3, indicated strong
staining. The ratio of positive cells ≤5% was designated as a score
of 0; 6–25% as 1; 26–50% as 2; 51–75% as 3; and >75% as 4. The
arithmetic product between two scores of 0–3 represented a negative
and 4–12 represented a positive outcome.
Western blot analysis
RIPA lysate was added to the tissue homogenate of
spinal dorsal horn tissue and total cellular protein was extracted.
Coomassie brilliant blue method was conducted for rough ration.
Before detection of protein content, antibody β-actin was applied
for protein dosage standardization detection of each sample. A
total of 30 µg total protein was selected to proceed with
electrophoretic separation using 8% SDS-PAGE and the separation
zone was transferred to a polyvinylidene fluoride membrane, with
the addition of mouse anti-rat PKC monoclonal antibody (dilution,
1:2,000; cat. no. P5704; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and left overnight. Then, rabbit anti-mouse polyclonal
secondary antibody (dilution, 1:500; cat. no. SAB3701221
Sigma-Aldrich; Merck KGaA) was added and incubated for 4 h, with
PBS washing and ECL coloration. The results were scanned and saved,
and gel-imaging software Lab Works4.5 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used for semi-quantitative
analysis, which was visualized using integral optical density.
Statistical analysis
Software SPSS 20.0 (IBM Corp., Armonk, NY, USA) was
applied for statistical analysis and measurement data was presented
as mean ± standard deviation (SD). Single-factor analysis ANOVA was
used for the comparison of data among three groups, and t-test was
applied for the comparison of data between two groups. Comparisons
of data obtained at different time points were conducted using
variance analysis of overall measurement. A P<0.05 was
considered to indicate a statistically significant difference.
Results
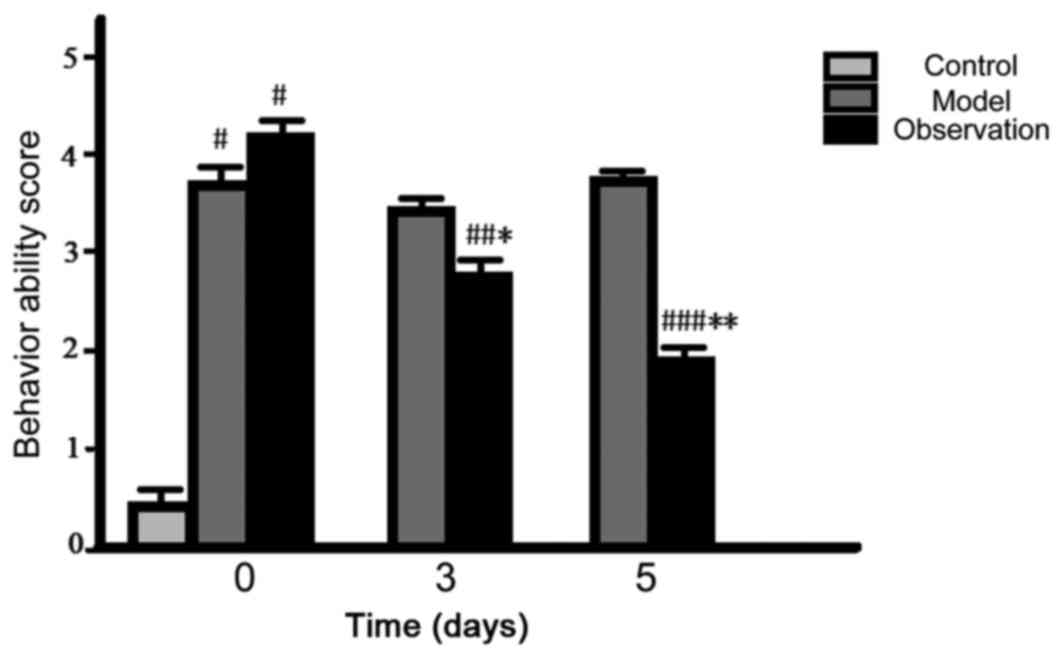
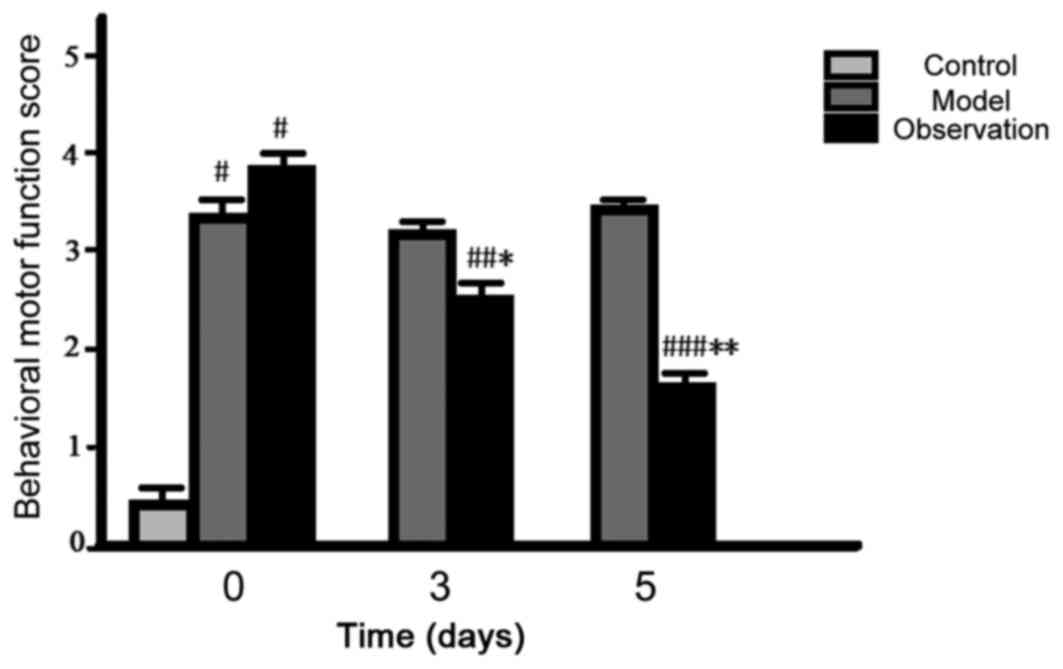
Comparison of behavior ability
core
After preparing the models, the initial cumulative
and motor function score of the model and the observation group
increased and the differences were statistically significant
(P<0.05). The values of the cumulative and motor function score
for 3 and 5 days decreased in the observation group (P<0.05),
while the values of the model group did not change noticeably
(P>0.05) (Figs. 1 and 2).
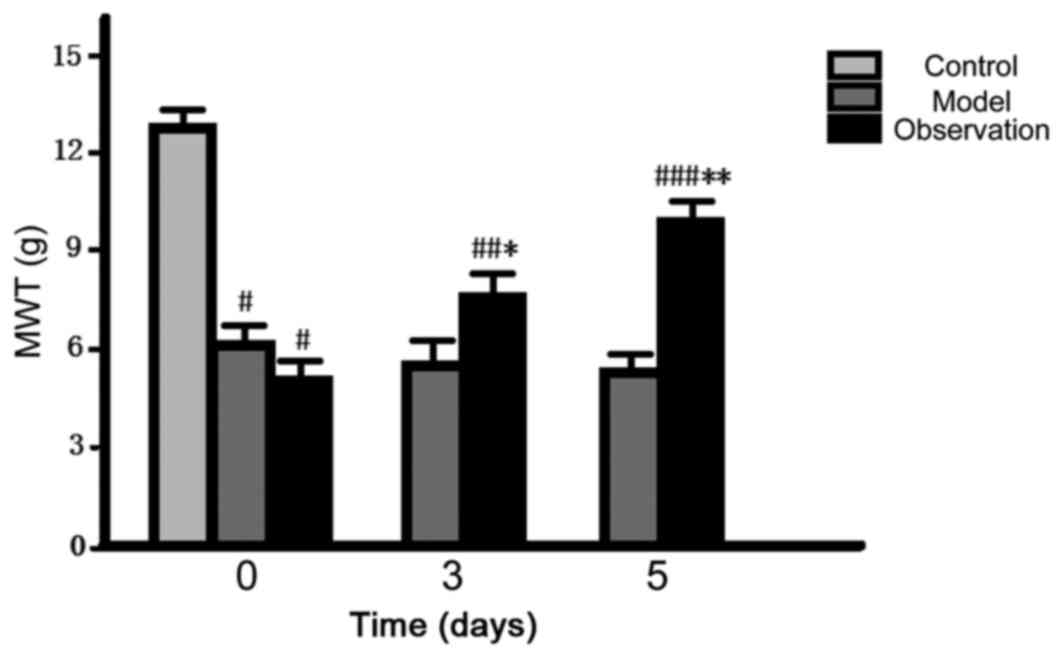
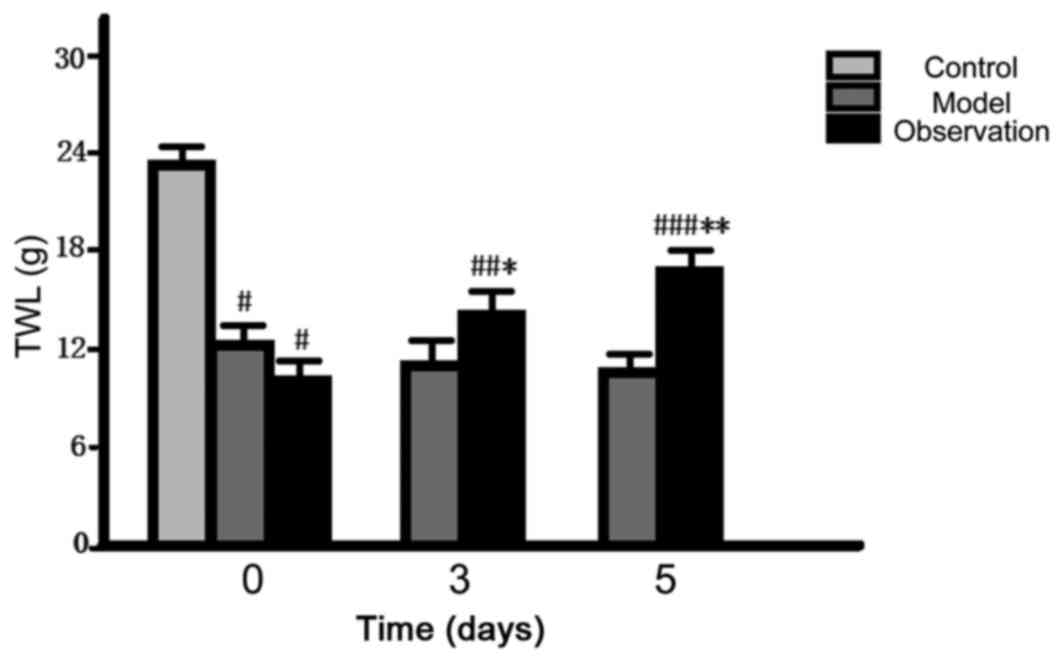
Comparison of pain threshold
After preparing the models, the initial values of
MWT and TWL of the model and the observation group decreased and
the differences were statistically significant (P<0.05). When it
came to 3 and 5 d, the values of MWT and TWL of the observation
group gradually increased (P<0.05), but there was no noticeable
change in the model group (P>0.05) (Figs. 3 and 4).
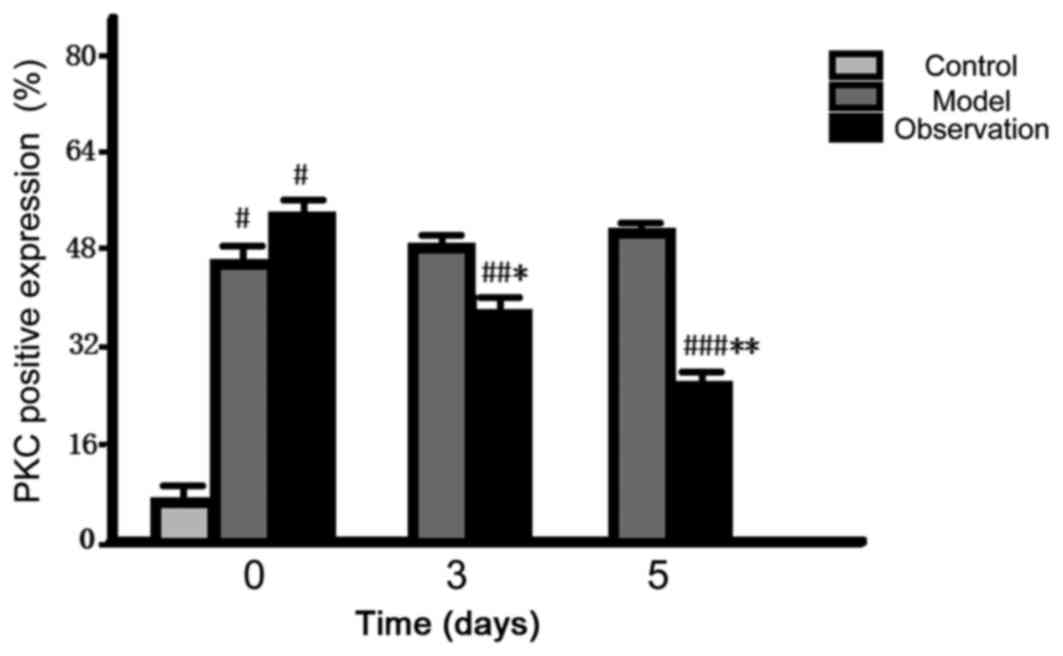
Comparison of PKC positive expression
in spinal dorsal horn
After preparing the models, the initial positive
expression of PKC in the spinal dorsal horn of the model and the
observation group rose dramatically and the differences were
statistically significant (P<0.05). As for the data at 3 and 5
d, the positive expression of the observation group reduced
gradually (P<0.05); however, there was no change in the model
group (P>0.05) (Fig. 5).
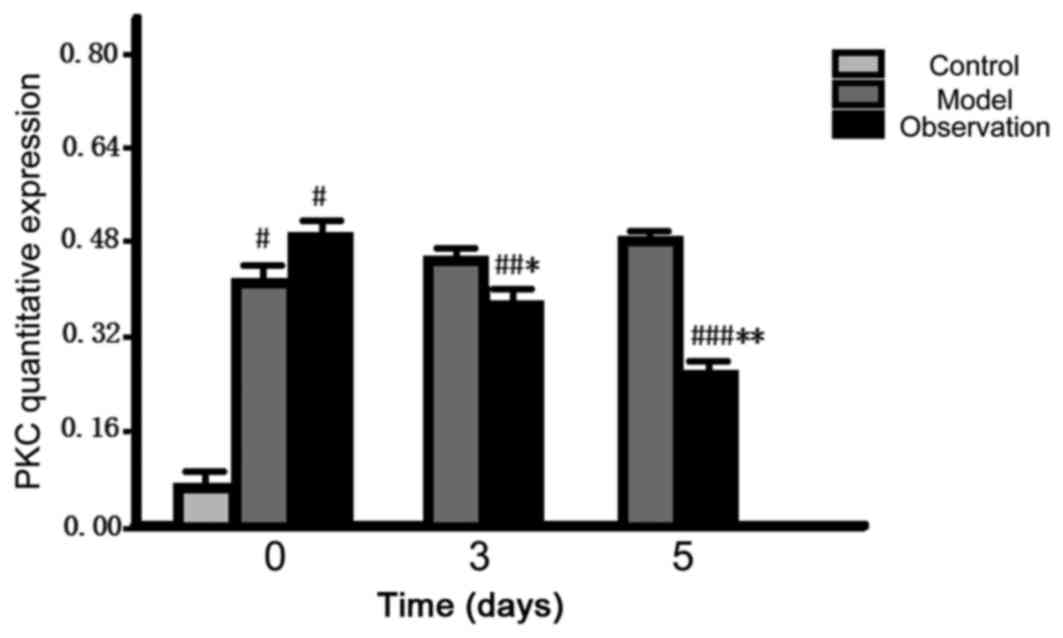
Comparison of PKC quantitative
expression levels in spinal dorsal horn
After preparing the models, the initial quantitative
expression level of PKC in the spinal dorsal horn of the model and
the observation group rose dramatically and the differences were
statistically significant (P<0.05). As for the data at 3 and 5
d, the quantitative expression levels in the observation group
reduced gradually (P<0.05); however, there was no change in the
model group (P>0.05) (Fig.
6).
Discussion
PKC belongs to a group of phospholipid-dependent
enzymes, which can be activated by Ca2+. Activated PKC
participates in many biological effects, such as cell
proliferation, apoptosis, skelemin remodeling, ion channel and
others (7). PKC plays an important
role as a secondary messenger in the central nervous system
conduction. External stimulus signals or ligand molecules combine
with NMDA receptors to activate Ca2+ channels, leading
to continuous gathering of Ca2+ in cells. After
combining with Ca2+, the conformation of PKC changes,
the catalytic area starts and PKC is activated (8). Pain can stimulate nerves and release
substance P, which activates the spinal dorsal horn NK1 receptor,
which then activates phospholipase C. After hydrolyzing the
phospholipid composition of the neuron membrane, diacylglycerol is
released, which is a recognized potent activating factor for PKC
(9). A study (10) has shown that PKC positive cells are
distributed mainly among the superficial layer of the spinal dorsal
horn, and are involved in the signal transmission of the central
nervous system damage and the adjustment of the central
sensitization mechanism. Overall, there are 12 subtypes of PKC in
animals whose functions and structures vary from each other
(11).
In the central nervous system, dexmedetomidine has
an effect on the locus coeruleus nucleus and plays an important
role in sedation and hypnosis via antagonism of the sympathetic
nerve activity under the combined action of the central and
peripheral neurotransmitters (12).
A research study (13) has indicated
that dexmedetomidine could inhibit the activity of the signal
transduction pathway of the dorsal horn neurons, decrease
Ca2+ influx and reduce the release of neurotransmitters.
Presently, dexmedetomidine is widely used in perioperative
anesthesia and postoperative analgesia. In addition, it also has
benefits in reducing postoperative cognitive impairment (14). According to the findings of this
study, it was concluded that, after preparing the models, the
initial cumulative and motor function score of the model and
observation groups increased, while the values of MWT and TWL
decreased. This suggests that the behavior ability and pain
threshold of rats with chronic neuropathic pain has changed. When
it came to 3 and 5 days, the cumulative and motor function score of
the observation group gradually declined, while the values of MWT
and TWL gradually increased. However, there was no obvious change
in the model group. This indicates that dexmedetomidine may improve
the behavior ability of rats with chronic neuropathic pain and
increase the pain threshold. Our further experiments showed that
after preparing the models, the initial positive expression of PKC
in the spinal dorsal horn of the model and observation groups rose
dramatically, and the quantitative expression level in the
observation group was also on the rise. As for the data from 3 and
5 days, the positive and quantitative expression levels in the
observation group were reduced gradually. However, there was no
notable change in the model group. Therefore, we consider that
intrathecal injection with dexmedetomidine may improve the
behavioral ability of rats with chronic neuropathic pain and
decrease their pain level, an effect that may be associated with
the inhibition of PKC expression in the spinal dorsal horn.
Some studies have also supported the theory that the
aberration of dexmedetomidine could be realized by inhibiting the
apoptosis levels of the spinal dorsal horn neurons (15). By activating the α2 adrenergic
receptor, dexmedetomidine may inhibit the reaction of the central
nervous system, reduce the release of noradrenaline and thereby
decrease the stress reaction of organism, so as to generate the
effects of analgesia, sedation and antianxiety (16). This sedation effect simulates normal
sleep, which implies a state from which one can be readily awoken.
The novel findings of this study lie in providing a frame of
reference for approaches in the medical application, dosage and
effects of dexmedetomidine in treating chronic neuropathic pain.
However, it did not thoroughly analyze the expression levels and
the effects and actions of the different PKC subtypes. Furthermore,
due to limitations of time in the present study, the potential
long-term intervention effects of chronic neuropathic pain also
require further verification.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL helped with the immunohistochemical staining. WZ
performed the western blot analysis. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ritter DM, Ho C, O'Leary ME and
Covarrubias M: Modulation of Kv3.4 channel N-type inactivation by
protein kinase C shapes the action potential in dorsal root
ganglion neurons. J Physiol. 590:145–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan X, Yadav R, Gao M and Weng HR:
Interleukin-1beta enhances endocytosis of glial glutamate
transporters in the spinal dorsal horn through activating protein
kinase C. Glia. 62:1093–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garrity AG, Botta S, Lazar SB, Swor E,
Vanini G, Baghdoyan HA and Lydic R: Dexmedetomidine-induced
sedation does not mimic the neurobehavioral phenotypes of sleep in
sprague dawley rat. Sleep. 38:73–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Funai Y, Pickering AE, Uta D, Nishikawa K,
Mori T, Asada A, Imoto K and Furue H: Systemic dexmedetomidine
augments inhibitory synaptic transmission in the superficial dorsal
horn through activation of descending noradrenergic control: An in
vivo patch-clamp analysis of analgesic mechanisms. Pain.
155:617–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu B, Zhang WS, Yang JL, Xu H, Deng XM and
Zhang YQ: Dexmedetomidine blocks thermal hyperalgesia and spinal
glial activation in rat model of monoarthritis. Acta Pharmacol Sin.
31:523–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang W, Lee J, Park J and Joo J:
Dexmedetomidine versus remifentanil in postoperative pain control
after spinal surgery: A randomized controlled study. BMC
Anesthesiol. 15:212015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Velázquez KT, Mohammad H and Sweitzer SM:
Protein kinase C in pain: Involvement of multiple isoforms.
Pharmacol Res. 55:578–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bu F, Tian H, Gong S, Zhu Q, Xu GY, Tao J
and Jiang X: Phosphorylation of NR2B NMDA subunits by protein
kinase C in arcuate nucleus contributes to inflammatory pain in
rats. Sci Rep. 5:159452015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JYV, Tillu DV, Quinn TL, Mejia GL, Shy
A, Asiedu MNK, Murad E, Schumann AP, Totsch SK, Sorge RE, et al:
Spinal dopaminergic projections control the transition to
pathological pain plasticity via a D1/D5-mediated mechanism. J
Neurosci. 35:6307–6317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mo G, Grant R, O'Donnell D, Ragsdale DS,
Cao CQ and Séguéla P: Neuropathic Nav1.3-mediated sensitization to
P2X activation is regulated by protein kinase C. Mol Pain.
7:142011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melemedjian OK, Tillu DV, Asiedu MN,
Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S and Price TJ: BDNF
regulates atypical PKC at spinal synapses to initiate and maintain
a centralizedchronic pain state. Mol Pain. 9:122013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Hang LH, Wang H, Shao DH, Xu YG, Cui
W and Chen Z: Intranasally administered adjunctive dexmedetomidine
reduces perioperative anesthetic requirements in general
anesthesia. Yonsei Med J. 57:998–1005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You HJ, Lei J, Xiao Y, Ye G, Sun ZH, Yang
L and Niu N: Pre-emptive analgesia and its supraspinal mechanisms:
Enhanced descending inhibition and decreased descending
facilitation by dexmedetomidine. J Physiol. 594:1875–1890. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni J, Wei J, Yao Y, Jiang X, Luo L and Luo
D: Effect of dexmedetomidine on preventing postoperative agitation
in children: A meta-analysis. PLoS One. 10:e01284502015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eskandr A and Maseeh SA: The effect of
dexmedetomidine on lumbar epidural injection for failed back
surgery syndrome. Anesthesiol Res Pract.
2016:71980482016.PubMed/NCBI
|
|
16
|
Iirola T, Aantaa R, Laitio R, Kentala E,
Lahtinen M, Wighton A, Garratt C, Ahtola-Sätilä T and Olkkola:
Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine
in critically ill patients. Crit Care. 15:R2572011. View Article : Google Scholar : PubMed/NCBI
|