Introduction
Premature ovarian failure refers to the occurrence
of hypergonadotropic hypogonadism, amenorrhea and infertility in
females <40 years of age (1). It
is thought that 1–3% of females will be affected by this disease in
their lifetime (2). Ovarian
transplantation between monozygotic twins is an effective treatment
for premature ovarian failure; however, the clinical application of
this technique is difficult due to the limited supply of ovarian
tissues (3). One of the main
challenges in treating premature ovarian failure is that its
pathogenesis remains unclear (4). As
such, focused research into the underlying mechanism responsible
for the pathogenesis of premature ovarian failure is required to
develop more effective treatments for this disease.
Long non-coding (lnc)RNAs are a group of non-coding
RNAs >200 nt in length that serve important roles in the
pathogenesis of various diseases (5). The functionality of lncRNA HOTAIR has
been well studied in malignant tumor models (6), while the development and recovery of
premature ovarian failure has been reported to be associated with
certain lncRNAs (7). LncRNA HOTAIR
is upregulated in ovarian cancer and serves a role in the
regulation of tumor growth and metastasis (8,9).
However, the functionality of lncRNA HOTAIR in patients with
premature ovarian failure has not yet been reported. The aim of the
present study was to investigate the role of lncRNA HOTAIR in
premature ovarian failure. The results revealed that lncRNA HOTAIR
overexpression may improve premature ovarian failure by
upregulating the expression of Notch-1. The present study provides
a further insight into the involvement of HOTAIR in ovarian
diseases and suggests a potential target for the treatment of
premature ovarian failure.
Materials and methods
Patients
A total of 69 women with spontaneous premature
ovarian failure were recruited at the Department of Reproductive
Medicine, People's Hospital of Dezhou (Dezhou, China) between
January 2015 and January 2017. Premature ovarian failure was
defined as a follicle stimulating hormone result of >30 IU/l
when measured twice with an interval of 4 weeks (10). Patient age ranged from 19 to 40
years, with a mean age of 29.1±4.3 years. Patients with other
ovarian diseases or patients that had been treated in other
hospitals prior to admission to the People's Hospital of Dezhou
were excluded. A total of 48 healthy women (age range, 21–40 years;
mean age, 29.8±5.1 years) were enrolled as a control group. The
present study was approved by the Ethics Committee of the People's
Hospital of Dezhou and all participants provided signed informed
consent.
Total RNA extraction
Ovarian tissues were harvested from each participant
via a fine needle aspiration biopsy. Fasting blood samples were
collected on the morning after admission. Blood samples were stored
at room temperature for 2 h, followed by centrifugation at 1,200 ×
g for 20 min at room temperature to collect the serum. TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to extract total RNA from serum samples and ovarian tissues.
Ovarian tissues were ground in liquid nitrogen prior to the
addition of TRIzol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A NanoDrop™ 2000 Spectrophotometer (Thermo Fisher
Scientific, Inc.) was used to measure the quality of RNA samples.
Samples with an A260/A280 ratio between 1.8 and 2.0 were subjected
to RT for cDNA synthesis using an iScript™ cDNA Synthesis kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR reaction
system was prepared using SYBR® Green Real-Time PCR
Master mix and performed on an ABI 7500 System (both from Thermo
Fisher Scientific, Inc.). Primers used were as follows: HOTAIR,
forward 5′-GGTCCTGCTCCGCTTCGCAG-3′ and reverse
5′-ACGCCCCTCCTTCCTCTCGC-3′; β-actin, forward
5′-GACCTCTATGCCAACACAGT-3′ and reverse 5′-AGTACTTGCGCTCAGGAGGA-3′.
Thermocycling conditions were as follows: 95°C for 55 sec followed
by 40 cycles of 95°C for 15 sec and 60°C for 50 sec. Data were
quantified using the 2−ΔΔCq method (11). The relative expression of HOTAIR was
normalized to β-actin.
Cells and culture
The normal hamster ovarian cell lines Lec8 (cat. no.
CRL-1737™) and CHO (cat. no. CRL-9096™) were obtained from the
American Type Culture Collection (Manassas, VA, USA). Lec8 cells
were cultured in α minimum essential medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). CHO cells were
cultured with Iscove's modified Dulbecco's medium (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS. All cells were cultured in
an atmosphere containing 5% CO2 at 37°C. Cells in the
logarithmic growth phase were harvested for subsequent
experiments.
Construction of HOTAIR overexpression
cell lines
HOTAIR cDNA was amplified from cDNA of ovarian
tissues using primers (Shanghai GenePharma Co., Ltd., Shanghai,
China) carrying EcoRI restriction enzyme sites at the
flanking ends. The EcoRI-EcoRI fragment containing
HOTAIR cDNA was inserted into GV299 lentiviral vectors (Shanghai
GeneChem Co., Ltd.) to establish HOTAIR overexpression vector. Lec8
and CHO cells were cultured overnight until 80–90% confluence was
reached, following which they were transfected with vectors using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Empty vectors without HOTAIR cDNA were used as
negative controls. Cells were cultured in an atmosphere containing
5% CO2 at 37°C for 24 h prior to subsequent
experiments.
MTT assay
Lec8 and CHO cells were harvested and single cell
suspensions were prepared using RPMI1640 (Gibco; Thermo Fisher
Scientific, Inc.). Cell suspensions were diluted to give a final
cell density of 5×104 cells/ml. Notch was inhibited by 1
µg/ml L685458 (Sigma-Aldrich; Merck KGaA). A total of 100 µl cell
suspension was seeded in each well of a 96-well plate. Cells were
incubated at 37°C for 8 h, followed by the addition of 10 µl MTT
(Sigma-Aldrich; Merck KGaA) in each well. Following incubation for
4 h at 37°C, formazan was dissolved by adding dimethyl sulfoxide.
Absorbance was measured at 570 nm using a microtiter plate reader.
Values were normalized to control cells.
Western blotting
Total proteins were extracted from Lec8 and CHO
cells using radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.). Protein samples were quantified using the BCA
method. A total of 30 µg protein/lane was separated by 10% SDS-PAGE
and transferred onto polyvinylidene fluoride membranes. Blocking
was performed by incubating with 5% skimmed milk at room
temperature for 1 h. Membranes were washed in TBST (3% Tween-20)
and incubated with primary antibodies, including rabbit anti-Notch1
antibody (1:2,000; ab65297) and anti-GAPDH antibody (1:1,000;
ab9485) (both from Abcam, Cambridge, UK), overnight at 4°C.
Membranes were washed with TBST (3% Tween-20) and further incubated
with anti-rabbit horseradish peroxidase-conjugated immunoglobulin g
secondary antibodies (1:1,000; MBS435036; MyBioSource, San Diego,
CA, USA) at room temperature for 1 h. Membranes were subsequently
washed with TBST (3% Tween-20) and signals were detected using
enhanced chemiluminescence (Sigma-Aldrich; Merck KGaA). The
relative expression of Notch-1 was normalized to endogenous control
GAPDH using ImageJ (version 1.37; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
all statistical analyses and data are expressed as the mean ±
standard deviation. Comparisons between two groups were made using
t tests and multiple group comparisons were made using one-way
analysis of variance and the least significant difference post hoc
test. Receiver operator characteristic (ROC) analysis was used to
evaluate the diagnostic value of HOTAIR expression for premature
ovarian failure and data was reported as the mean percentage and
95% confidence interval (CI). The cut-off value for HOTAIR in
ovarian tissues was 0.1221. The cut-off value for HOTAIR in serum
was 0.1140. P<0.05 was considered to indicate a statistically
significant differences.
Results
HOTAIR expression in ovarian tissues
from patients with premature ovarian failure and healthy
controls
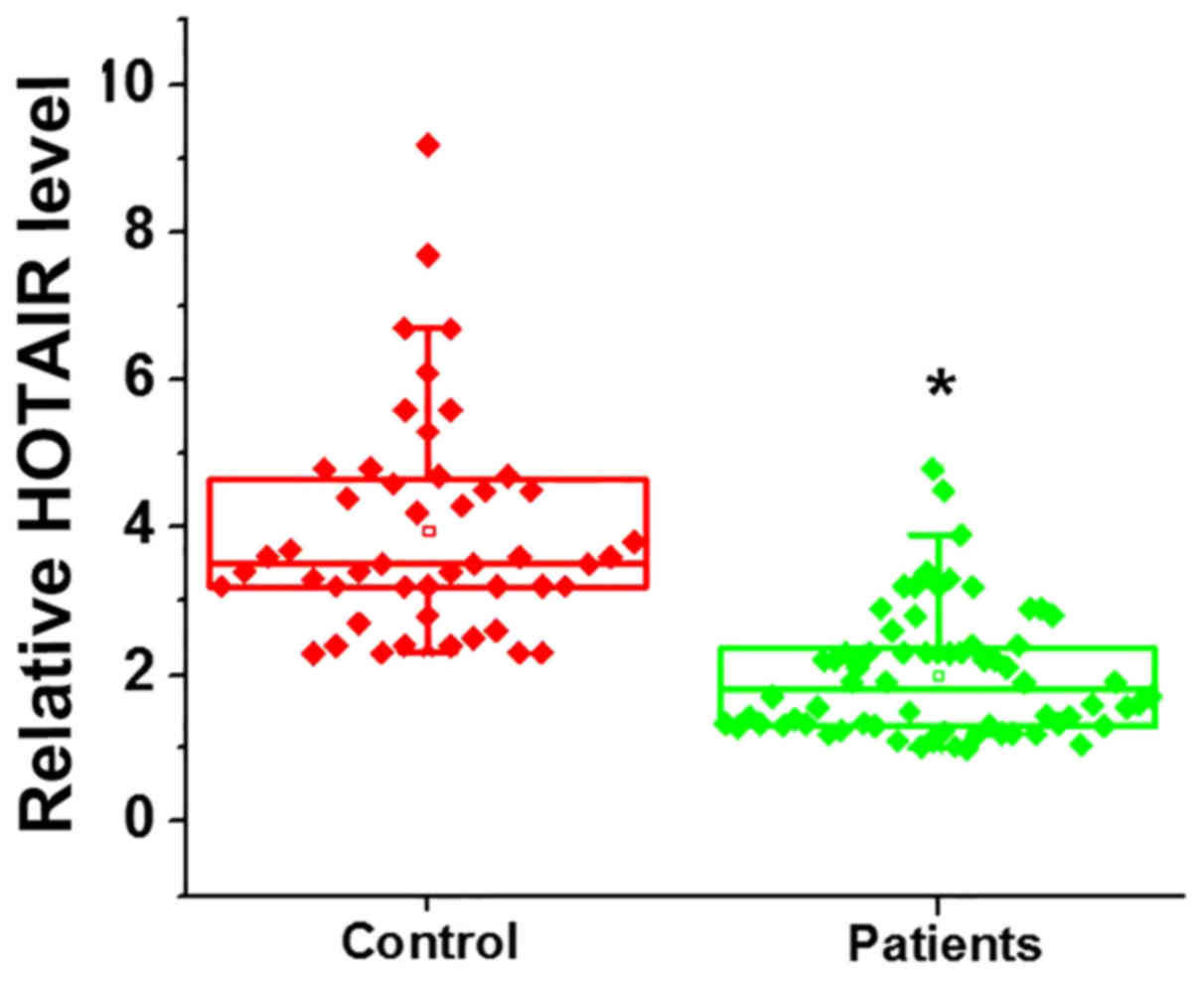
HOTAIR expression in ovarian tissues from patients
with premature ovarian failure and healthy controls was assessed
using RT-qPCR. The expression of HOTAIR was significantly decreased
in patients with premature ovarian failure compared with the
control group, indicating that HOTAIR downregulation may serve a
role in the pathogenesis of premature ovarian failure (Fig. 1; P<0.05).
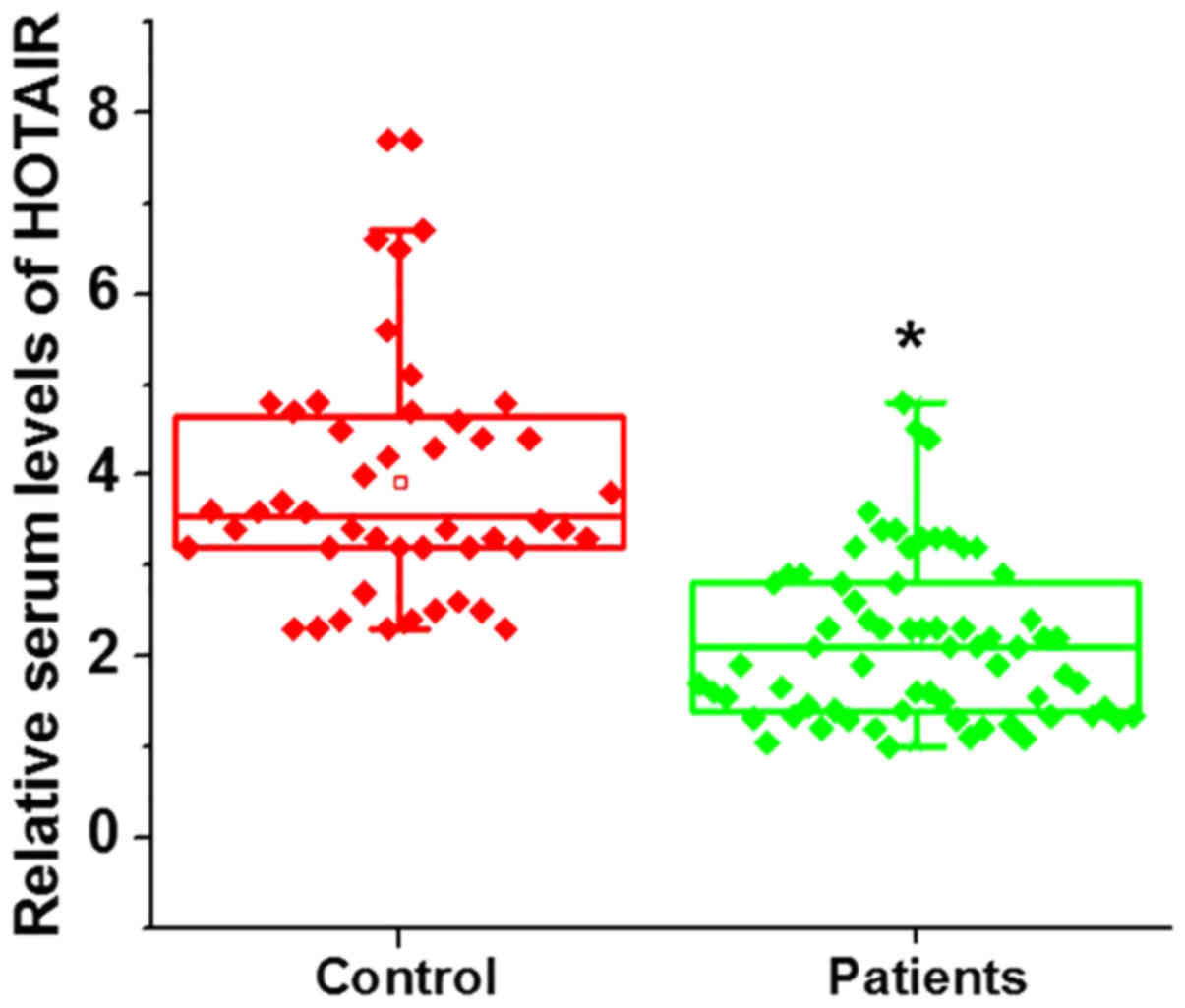
Serum HOTAIR expression in patients
with premature ovarian failure and healthy controls
Serum HOTAIR expression was significantly lower in
patients with premature ovarian failure compared with the control
group (Fig. 2; P<0.05).
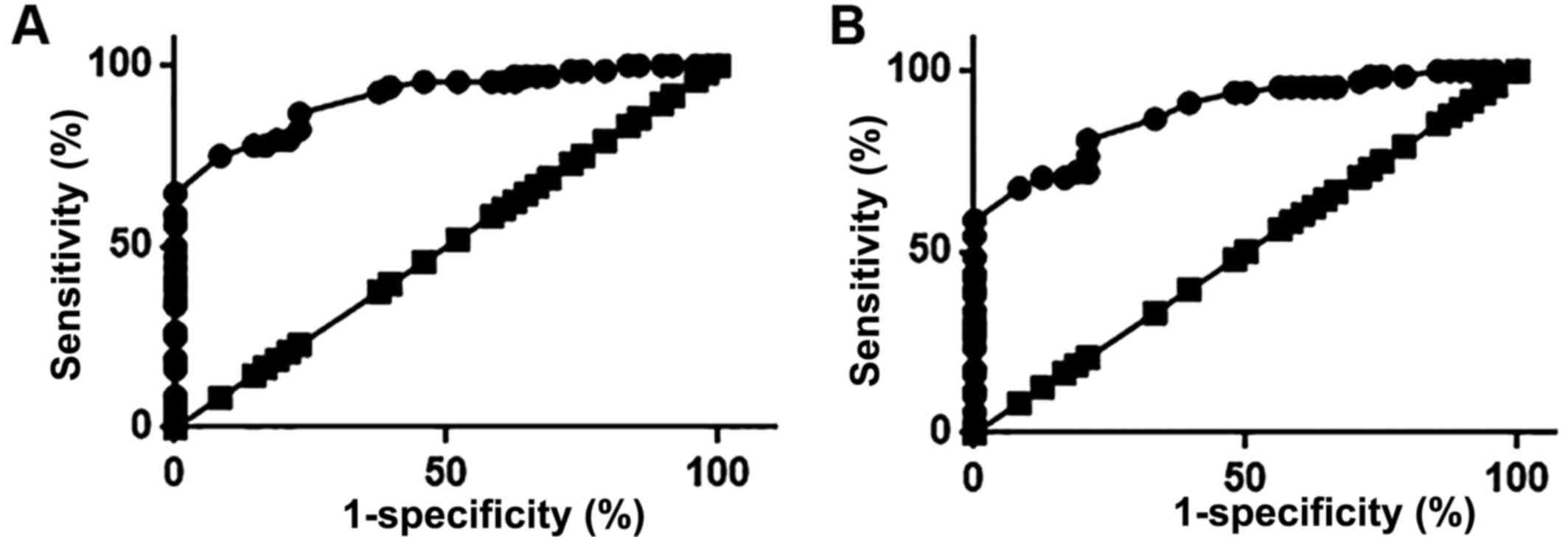
Diagnostic value of HOTAIR expression
in ovarian tissues and sera for premature ovarian failure
ROC analysis was performed to evaluate the
diagnostic value of serum and ovarian tissue HOTAIR expression for
premature ovarian failure. The area under the curve (AUC) for
ovarian tissue HOTAIR expression in the diagnosis of premature
ovarian failure was 0.9119, with a 95% CI of 0.8620–0.9619
(Fig. 3A; P<0.0001). Furthermore,
the AUC for serum HOTAIR in the diagnosis of premature ovarian
failure was 0.8868, with a 95% CI of 0.8295–0.9441 (P<0.0001;
Fig. 3B). These results suggest that
HOTAIR expression in the ovarian tissues and serum can be used to
accurately predict the risk of premature ovarian failure. Although
the diagnostic accuracy of HOTAIR expression in ovarian tissues is
higher than that of serum HOTAIR, measuring serum HOTAIR is less
invasive.
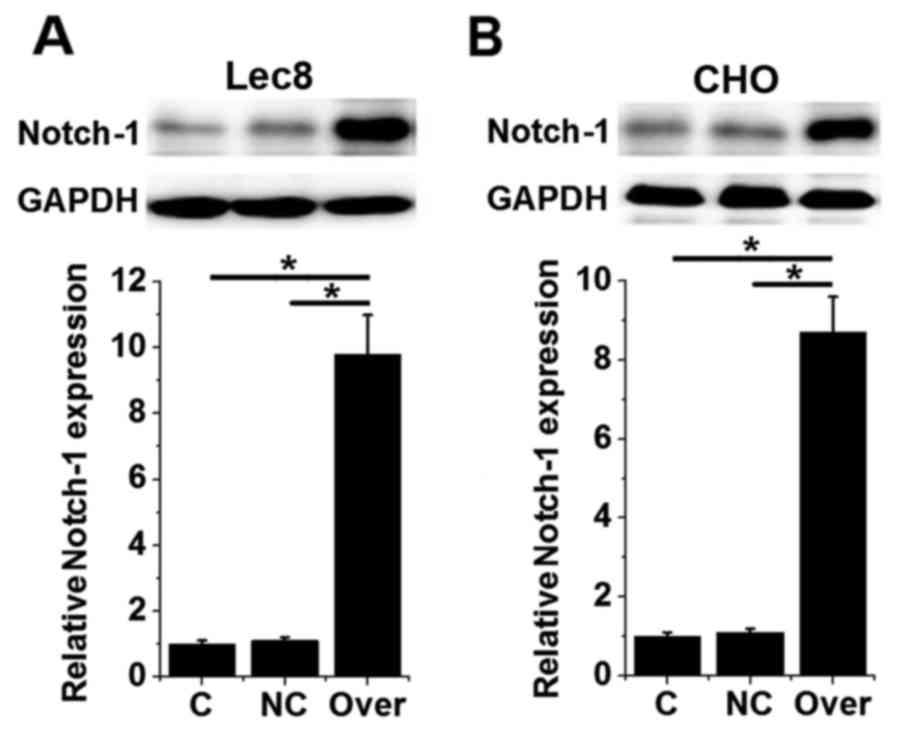
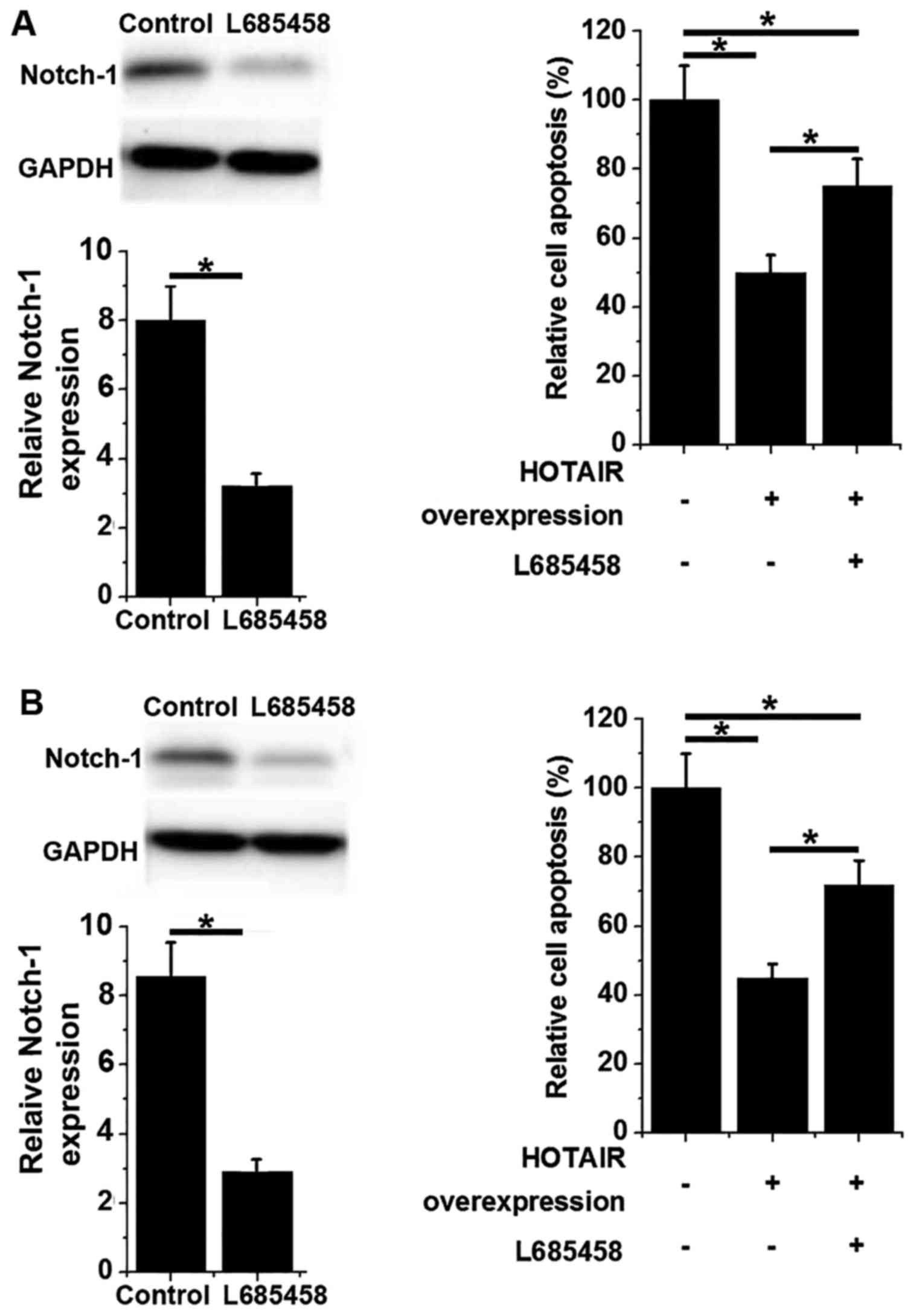
HOTAIR overexpression promotes Notch-1
expression in two normal hamster ovarian cell lines
It has been reported that lncRNA HOTAIR is able to
activate the Notch signaling pathway (12). In the present study, HOTAIR
overexpression was induced in hamster ovarian cells and Notch-1
expression was assessed using western blotting. No significant
differences were observed in the expression of Notch-1 protein
between control (untransfected) cells and negative control
(transfected with empty vectors) cells (Fig. 4). However, the expression of Notch-1
protein was significantly increased in HOTAIR-overexpressing Lec8
and CHO cells compared with the control and negative control groups
(P<0.05; Fig. 4). These data
suggest that HOTAIR overexpression is able to upregulate the
expression of Notch-1.
HOTAIR overexpression inhibits the
apoptosis of hamster ovarian cells
Notch-1 overexpression has been reported to inhibit
the apoptosis of various cancers, while activation of Notch-1
signaling has been demonstrated to be an effective treatment for
premature ovarian failure (13). In
the present study, HOTAIR overexpression significantly inhibited
the apoptosis of hamster ovarian cells compared with control cells
(P<0.05; Fig. 5). However,
treatment with Notch inhibitor L685458 (a Notch inhibitor)
significantly inhibited Notch-1 expression and increased cell
apoptosis (P<0.05; Fig. 5). These
results suggest that Notch-1 overexpression is able to inhibit the
apoptosis of ovarian cells, possibly by activating the Notch
signaling pathway.
Discussion
Abnormal lncRNA HOTAIR expression has been reported
in the development of various diseases (8,9). In a
gastric cancer study, the expression of HOTAIR was demonstrated to
be upregulated in tumor tissues compared with adjacent healthy
tissues and it was revealed that HOTAIR promoted cancer development
and increased chemoresistance cisplatin (14). LncRNA HOTAIR overexpression has also
been observed in liver cancer and was demonstrated to be
responsible for the increased growth rate of tumor cells (15). Furthermore, HOTAIR overexpression is
associated with the development of ovarian cancer (16). In the present study, HOTAIR
expression was demonstrated to be significantly reduced in ovarian
tissues and serum samples from patients with premature ovarian
failure compared with healthy controls. These results indicate that
lncRNA HOTAIR downregulation may serve a role in the pathogenesis
of premature ovarian failure.
The occurrence and development of disease is
typically accompanied by changes in the blood, and so blood testing
is performed to aid diagnosis (17).
A previous study reported that serum levels of anti-Müllerian
hormone are correlated with early ovarian aging in young and
infertile women; these levels can be used to predict accelerated
oocyte loss, but not unfavorable pregnancy outcomes (18). To the best of our knowledge, no
accurate and effective biomarker for premature ovarian failure has
previously been reported. In the present study, ROC curve analysis
revealed that HOTAIR expression in ovarian tissues and sera could
accurately predict the risk of premature ovarian failure. Although
the diagnostic accuracy of HOTAIR expression in ovarian tissues was
higher compared with serum HOTAIR, serum HOTAIR is easier to
measure using less invasive techniques. HOTAIR expression is
generally upregulated in ovarian diseases, including cancer
(16), and so HOTAIR downregulation
may be used to distinguish premature ovarian failure from other
ovarian diseases.
The results of a previous study revealed that
activation of the Notch-1 signaling pathway is important for the
recovery of premature ovarian failure in mice (13). It is also well known that HOTAIR
interacts with the Notch pathway to exert its biological functions
(19). In the present study, Notch-1
was significantly increased in cells overexpressing HOTAIR,
suggesting that HOTAIR may also serve a role in premature ovarian
failure by interacting with the Notch pathway. Notch-1
overexpression has previously been demonstrated to inhibit
apoptosis in certain cells (20),
while Notch-1 downregulation inhibits cell growth and promotes
apoptosis (21). In the present
study, HOTAIR overexpression significantly inhibited the apoptosis
of hamster ovarian cells, while treatment with Notch inhibitor
significantly increased cell apoptosis. These results suggest that
Notch-1 overexpression may ameliorate premature ovarian failure by
inhibiting the apoptosis of ovarian cells, at least in part via
activation of the Notch signaling pathway.
In conclusion, HOTAIR expression is downregulated in
ovarian tissues and serum samples from patients with premature
ovarian failure compared with healthy controls. Both ovarian
tissues and sera may be used to accurately predict the risk of
premature ovarian failure. HOTAIR overexpression promotes Notch-1
protein expression and inhibits apoptosis in hamster ovarian cells,
effects that are reversed by treatment with Notch inhibitor
L685458. In conclusion, the results of the present study suggest
that lncRNA HOTAIR overexpression is able to improve premature
ovarian failure by upregulating the expression of Notch-1. The
present study is limited due to the small sample size and further
research is required to confirm these results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WZ and LD conceived and designed the study. WZ
performed experiments and analyzed data. LD interpreted the data
and drafted the manuscript. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of People's Hospital of Dezhou and all participants
provided signed informed consent.
Patient consent for publication
Signed informed consent was obtained from all
participants and/or guardians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jankowska K: Premature ovarian failure.
Prz Menopauzalny. 16:51–56. 2017.PubMed/NCBI
|
|
2
|
Kovanci E and Schutt AK: Premature ovarian
failure: Clinical presentation and treatment. Obstet Gynecol Clin
North Am. 42:153–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silber SJ, Lenahan KM, Levine DJ, Pineda
JA, Gorman KS, Friez MJ, Crawford EC and Gosden RG: Ovarian
transplantation between monozygotic twins discordant for premature
ovarian failure. N Engl J Med. 353:58–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luisi S, Orlandini C, Regini C, Pizzo A,
Vellucci F and Petraglia F: Premature ovarian insufficiency: From
pathogenesis to clinical management. J Endocrinol Invest.
38:597–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Gao L, Guo X, Shi X, Wu H, Song F
and Wang B: A network based method for analysis of lncRNA-disease
associations and prediction of lncRNAs implicated in diseases. PLoS
One. 9:e877972014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai B, Song XQ, Cai JP and Zhang S:
HOTAIR: A cancer-related long non-coding RNA. Neoplasma.
61:379–391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong Y, Liu T, Wang S, Chi H, Chen C and
Zheng J: Cyclophosphamide promotes the proliferation inhibition of
mouse ovarian granulosa cells and premature ovarian failure by
activating the lncRNA-Meg3-p53-p66Shc pathway. Gene. 596:1–8. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu JJ, Lin YY, Ye LC, Ding JX, Feng WW,
Jin HY, Zhang Y, Li Q and Hua KQ: Overexpression of long non-coding
RNA HOTAIR predicts poor patient prognosis and promotes tumor
metastasis in epithelial ovarian cancer. Gynecol Oncol.
134:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G
and Hua KQ: The long non-coding RNA HOTAIR promotes the
proliferation of serous ovarian cancer cells through the regulation
of cell cycle arrest and apoptosis. Exp Cell Res. 333:238–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cartwright B, Robinson J, Seed PT,
Fogelman I and Rymer J: Hormone replacement therapy versus the
combined oral contraceptive pill in premature ovarian failure: A
randomized controlled trial of the effects on bone mineral density.
J Clin Endocrinol Metab. 101:3497–3505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong C, Liu S, Lv Y, Zhang C, Gao H, Tan L
and Wang H: Long non-coding RNA HOTAIR regulates proliferation and
invasion via activating Notch signalling pathway in retinoblastoma.
J Biosci. 41:677–687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu TE, Wang S, Zhang L, Guo L, Yu Z, Chen
C and Zheng J: Growth hormone treatment of premature ovarian
failure in a mouse model via stimulation of the Notch-1 signaling
pathway. Exp Ther Med. 12:215–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan J, Dang Y, Liu S, Zhang Y and Zhang G:
LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by
targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumor Biol.
37:16345–16355. 2016. View Article : Google Scholar
|
|
15
|
Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu
H and Lu D: LncRNA HOTAIR promotes human liver cancer stem cell
malignant growth through downregulation of SETD2. Oncotarget.
6:27847–27864. 2015.PubMed/NCBI
|
|
16
|
Teschendorff AE, Lee SH, Jones A, Fiegl H,
Kalwa M, Wagner W, Chindera K, Evans I, Dubeau L, Orjalo A, et al:
HOTAIR and its surrogate DNA methylation signature indicate
carboplatin resistance in ovarian cancer. Genome Med. 7:1082015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pankla R, Buddhisa S, Berry M, Blankenship
DM, Bancroft GJ, Banchereau J, Lertmemongkolchai G and Chaussabel
D: Genomic transcriptional profiling identifies a candidate blood
biomarker signature for the diagnosis of septicemic melioidosis.
Genome Biol. 10:R1272009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin PY, Huang FJ, Kung FT, Chiang HJ, Lin
YJ, Lin YC and Lan KC: Evaluation of serum anti-mullerian hormone
as a biomarker of early ovarian aging in young women undergoing
IVF/ICSI cycle. Int J Clin Exp Pathol. 7:6245–6253. 2014.PubMed/NCBI
|
|
19
|
Lee M, Kim HJ, Kim SW, Park SA, Chun KH,
Cho NH, Song YS and Kim YT: The long non-coding RNA HOTAIR
increases tumour growth and invasion in cervical cancer by
targeting the Notch pathway. Oncotarget. 7:44558–44571.
2016.PubMed/NCBI
|
|
20
|
Shelly LL, Fuchs C and Miele L: Notch-1
inhibits apoptosis in murine erythroleukemia cells and is necessary
for differentiation induced by hybrid polar compounds. J Cell
Biochem. 73:164–175. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J
and Sarkar FH: Down-regulation of Notch-1 contributes to cell
growth inhibition and apoptosis in pancreatic cancer cells. Mol
Cancer Ther. 5:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|