Introduction
Diabetes mellitus, also known as diabetes, is a
chronic disease characterized by abnormally high blood glucose
levels over a prolonged period (1).
With changes in lifestyle and diet, including the popularization of
a western-style diet, the incidence of diabetes is increasing
(2), seriously affecting patient
health and lifestyle. Abnormally high glucose levels in humans may
lead to multiple severe complications (3). As one of the major cardiac
complications of diabetes, diabetic cardiomyopathy is also one of
the leading causes of morbidity and mortality in patients with
diabetes (4). At present, the
treatment of diabetic cardiomyopathy is limited by the pathogenesis
remaining unclear (5). Therefore,
in-depth investigation of the mechanism of onset, development and
progression of diabetic cardiomyopathy is required.
Studies on the pathogenesis of diabetic
cardiomyopathy have revealed that its development is a complex and
multi-step process with various internal and external factors
involved (6). Long non-coding RNAs
(lncRNAs) are a group of RNA transcripts composed of more than 200
nucleotides that lack protein-coding ability (7). The involvement of lncRNAs in
cardiomyocyte viability, which is a major factor in diabetic
cardiomyopathy (8), has been
observed in previous studies (9,10).
Homeobox transcript antisense RNA (HOTAIR) is a well-studied lncRNA
with critical roles in numerous human diseases, particularly in
multiple types of cancer (11). A
recent study reported that expression level of HOTAIR determines
cardiac function in a sepsis mouse model, indicating the possible
involvement of HOTAIR in heart disease (12). However, to the best of our knowledge,
the involvement of HOTAIR in diabetic cardiomyopathy has not been
reported. Therefore, the current study investigated the function of
HOTAIR in diabetic cardiomyopathy and observed that HOTAIR improves
the viability of cardiomyocytes by activating the phosphoinositide
3-kinase (PI3K)/Akt pathway. The findings of the current study may
have implications for the diagnosis and treatment of diabetic
cardiomyopathy.
Patients and methods
Subjects
A total of 56 patients with diabetic cardiomyopathy
were recruited from March 2015 to March 2017 in The First Hospital
of Shanxi Medical University (Taiyuan, China). Diagnostic criteria
of diabetic cardiomyopathy were as follows: i) Confirmed diabetes;
ii) clinical manifestations of heart failure; iii) cardiac
enlargement with impaired cardiac systolic function, or diastolic
dysfunction with no enlarged heart; iv) heart failure caused by
heart disease such as hypertension heart disease, coronary heart
disease and rheumatic valvular heart disease was excluded; v)
biopsy of the heart confirmed microangiopathy and positive periodic
acid-Schiff staining. Diagnostic criteria of diabetes were as
follows: i) 2-h postprandial blood glucose, ≥11.1 mmol/l; ii)
fasting blood glucose, ≥7.0 mmol/l; iii) HbA1c, ≥6.5%. All patients
exhibited congestive heart failure and/or arrhythmias and/or angina
pectoris. Inclusion criteria were as follows: i) Patients diagnosed
with diabetic cardiomyopathy and treated for the first time in The
First Hospital of Shanxi Medical University; ii) patients and their
families willing to participate in the study; iii) patients
understood the whole experimental protocol and could cooperate with
the researchers. Exclusion criteria were as follows: i) Patients
with other types of cardiomyopathy; ii) patients with other
diabetic complications; iii) patients treated in other hospitals
prior to admission. The recruited patients included 30 males and 26
females, and the ages ranged from 37 to 72 years, with a mean age
of 55.2±10.1 years. At the same time, 44 patients with diabetes but
without cardiomyopathy and 42 healthy controls were also included.
The 44 patients with diabetes included 23 males and 21 females aged
between 34 and 72 years, with a mean age of 53.1±8.4 years.
Patients with diabetes complicated with other diseases were not
included. The 42 healthy controls included 23 males and 19 females,
and the ages ranged from 36 to 69 years, with a mean age of
53.7±8.2 years. All healthy controls exhibited normal physical
conditions. No significant differences in age or sex were
identified among the three groups of patients. No significant
differences in body mass index (BMI) were identified between
patients with diabetes and patients with diabetic cardiomyopathy,
while BMI was significantly higher in patients with diabetes and
patients with diabetic cardiomyopathy compared with healthy
controls. The Ethics Committee of The First Hospital of Shanxi
Medical University approved the study, and all patients provided
written informed consent.
Specimen collection
Myocardial biopsy was performed in patients with
diabetic cardiomyopathy to confirm the disease. Myocardial biopsy
was also performed on all patients with diabetes and healthy
controls to identify any existing heart diseases, but heart
diseases were finally excluded from these patients. Whole blood (20
ml) was obtained from each participant on the day of admission.
Serum samples were prepared by keeping the blood at room
temperature for 2 h, followed by centrifugation at 1,000 × g for 20
min at room temperature. Serum samples were stored in liquid
nitrogen until use. Myocardial tissues were washed with ice-cold
PBS and also stored in liquid nitrogen until use.
Cell line and cell culture
Human cardiomyocyte cell line AC16 was purchased
from EMD Millipore (Billerica, MA, USA). AC16 cells were cultured
in Dulbecco's modified Eagle's medium supplemented with 1%
antibiotics (penicillin and streptomycin) and 12% fetal bovine
serum (all Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in an
incubator (37°C, 5% CO2/95% air). Cells were harvested
at logarithmic growth phase for subsequent experiments. To
investigate the effects of high glucose on HOTAIR expression and
Akt phosphorylation in human cardiomyocytes, human cardiomyocytes
were treated with different concentrations of D-glucose (20, 40 and
60 mM; Sigma-Aldrich; Merck KGaA) for 48 h in an incubator (37°C,
5% CO2/95% air) prior to subsequent experiments. To
investigate the role of PI3K inhibition on cell viability, cells
were treated with PI3K inhibitor LY294002 (10 µM; Cell Signaling
Technology, Inc., Danvers, MA, USA) for 24 h in an incubator (37°C,
5% CO2/95% air) prior to subsequent experiments
Cell transfection
HOTAIR cDNA (HPRM54622; GeneCopoeia, Inc.,
Rockville, MD, USA) was inserted into pIRSE2-EGFP vector (Clontech
Laboratories, Inc., Mountainview, CA, USA) to construct the HOTAIR
expression vector. Cells were cultured overnight to reach 70–80%
confluence, and transfection was performed using Lipofectamine 2000
reagent (11668-019; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) to transfect 10 nM vector into 5×105
cells. Empty pIRSE2-EGFP vector was used as a negative control. An
overexpression rate >150% was confirmed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
prior to subsequent experiments.
MTT assay
Cell suspension with a cell density of
4×104 cells/ml was prepared, and 100 µl cell suspension
containing 4×104 cells was added into each well of a
96-well plate. Then, 40 mM D-glucose was added. Cells were cultured
in an incubator (37°C, 5% CO2) for 6 h, then 10 µl MTT
was added into each well. Following the addition of MTT, cells were
cultured for a further 4 h. The formazan product was dissolved in
dimethyl sulfoxide. Optical density values were measured at 570
nm.
RT-qPCR
Myocardial tissues were ground in liquid nitrogen,
followed by the addition of TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) to extract total RNA. TRIzol reagent was
also mixed with serum and in vitro cultured cells to extract
total RNA. A NanoDrop™ 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.) was used to test RNA quality, and only
those with a A260/A280 ratio between 1.8 and 2.0 were used to
synthesize cDNA through RT using SuperScript III Reverse
Transcriptase kit (Thermo Fisher Scientific, Inc.); the thermal
conditions were as follows: 25°C for 5 min, 55°C for 20 min and
75°C for 15 min. PCR reactions were performed using
SYBR® Green Real-Time PCR Master Mixes (Thermo Fisher
Scientific, Inc.) with the primers listed below: human HOTAIR
forward, 5′-GGCGGATGCAAGTTAATAAAAC-3′ and reverse,
5′-TACGCCTGAGTGTTCACGAG-3′; human β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
PCR reaction conditions were as follows: 95°C for 30 sec, followed
by 40 cycles of 95°C for 20 sec and 60°C for 35 sec. Data were
processed using the 2−ΔΔCq method (13), and expression of HOTAIR was
normalized to endogenous control β-actin.
Western blot analysis
Radioimmunoprecipitation assay buffer (Cell
Signaling Technology, Inc.) was mixed with in vitro cultured
cells to extract total protein, followed by quantification using
the BCA method. Electrophoresis was performed using 10% SDS-PAGE
with 20 µg protein from each sample. Proteins were transferred to a
PVDF membrane, followed by blocking with 5% skimmed milk at room
temperature for 2 h. Following washing, membranes were incubated
with primary antibodies, including rabbit anti-Akt antibody (cat.
no. ab126811), anti-p-Akt (phospho T308; cat. no. ab38449) and
anti-GAPDH (cat. no. ab8245; all 1:2,000; Abcam), overnight at 4°C.
Following washing, anti-rabbit IgG-horseradish peroxidase secondary
antibody (1:1,000; MBS435036; MyBioSource, Inc., San Diego, CA,
USA) was incubated with the membranes at room temperature for 1 h.
Following washing, enhanced chemiluminescence (Sigma-Aldrich; Merck
KGaA) was used to detect signals. Image J v1.6 software (National
Institutes of Health, Bethesda, MD, USA) was used to normalize
relative expression of each protein to endogenous control
GAPDH.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to
perform statistical analysis. All experiments were performed in
triplicate. Categorical data were compared by chi-square test.
Continuous data are presented as mean ± standard deviation and were
compared among multiple groups by one-way analysis of variance
followed by least significant difference post-hoc test. Receiver
operating characteristic (ROC) curve analysis was performed to
evaluate the diagnostic value of expression level of HOTAIR in
myocardial tissues (cutoff value, 2.33) and serum (cutoff value,
2.45) for diabetic cardiomyopathy. For the analysis of the
association between expression level of HOTAIR and
clinicopathological data of patients with diabetic cardiomyopathy,
patients with smoking habits were defined as patients who smoked
>3 cigarettes per day and >3 days per week, and patients with
drinking habits were defined as patient who drank >2 times per
week. P<0.05 was considered to indicate a statistically
significant difference.
Results
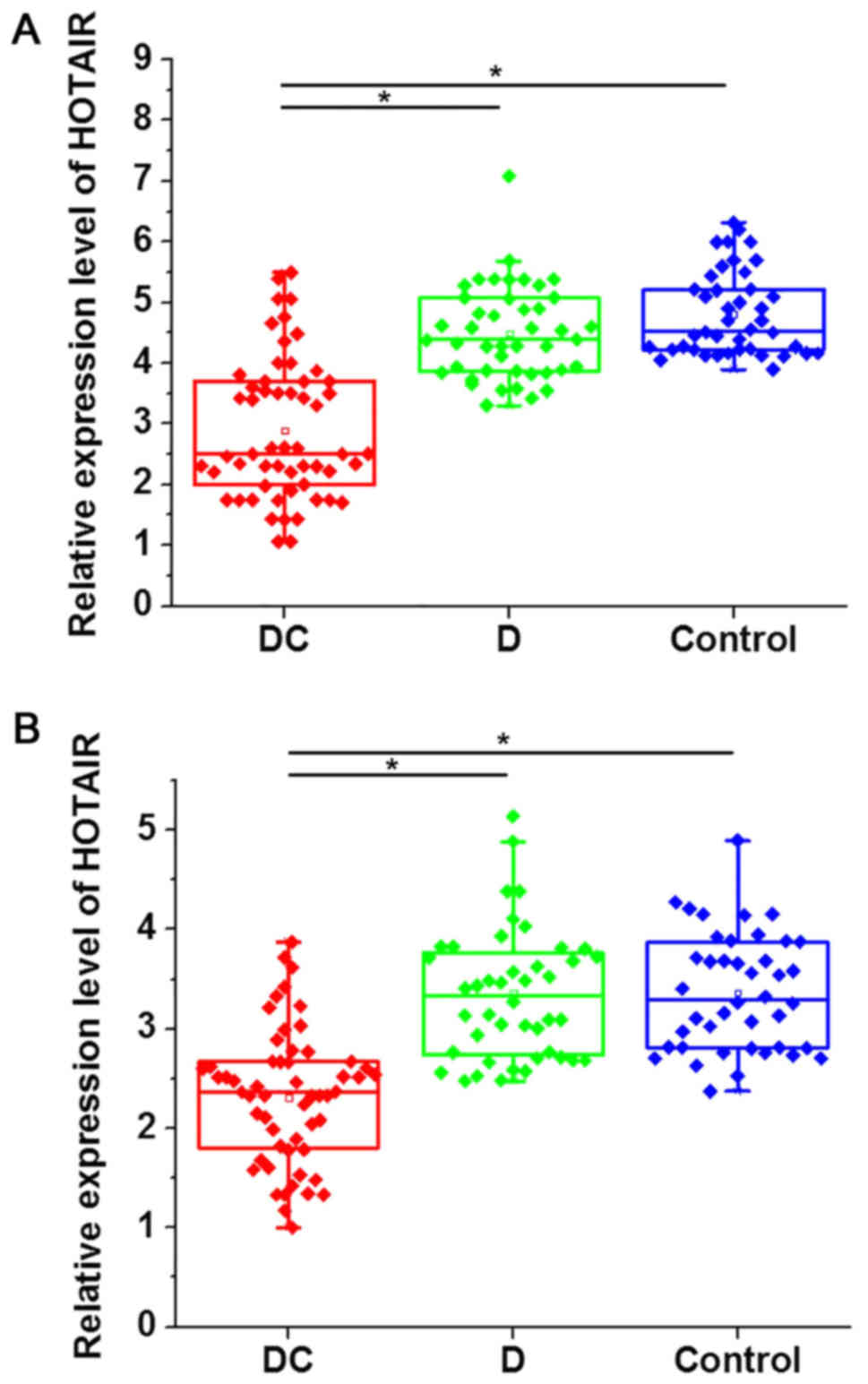
Expression of HOTAIR in myocardial
tissues and serum is specifically downregulated in patients with
diabetic cardiomyopathy
RT-qPCR was performed to detect the expression of
HOTAIR in myocardial tissues and serum of all participants. As
indicated in Fig. 1, the expression
level of HOTAIR in myocardial tissues (Fig. 1A) and serum (Fig. 1B) was significantly downregulated in
patients with diabetic cardiomyopathy compared with patients with
diabetes and healthy controls. However, no significant differences
in HOTAIR expression were identified between diabetic patients
without cardiomyopathy and healthy controls.
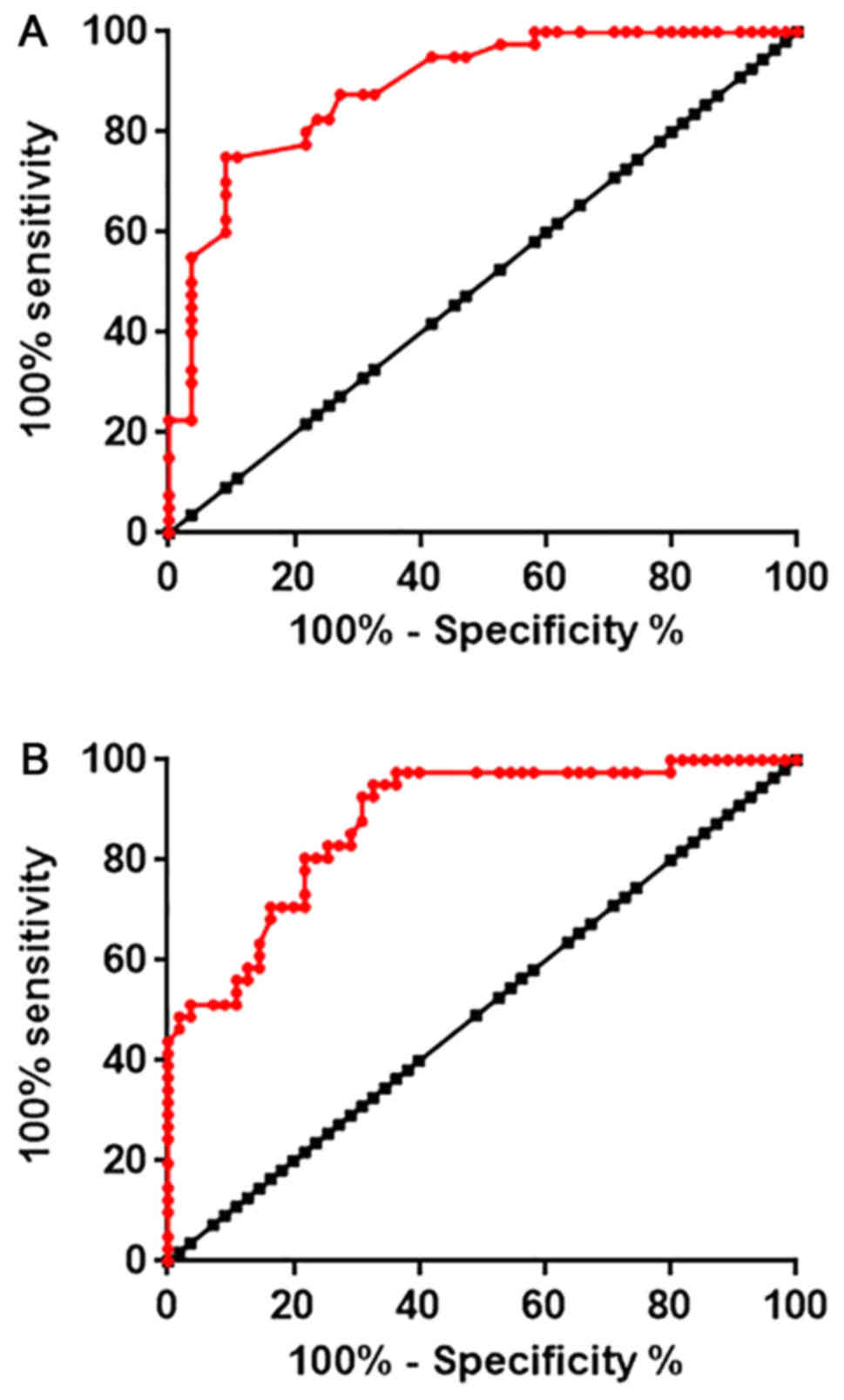
Diagnostic values of HOTAIR expression
for diabetic cardiomyopathy
Receiver operating characteristic (ROC) curve
analysis was performed to evaluate the diagnostic value of
expression level of HOTAIR in myocardial tissues and serum for
diabetic cardiomyopathy. As indicated in Fig. 2A, the area under the curve (AUC) for
the use of HOTAIR expression in myocardial tissues was 0.8880, with
95% confidence interval of 0.8231–0.9526 and standard error of
0.03309 (P<0.0001). In addition, AUC for the use of serum HOTAIR
in the diagnosis of diabetic cardiomyopathy was 0.8780, with 95%
confidence interval of 0.8107–0.9454 and standard error of 0.03436
(P<0.0001; Fig. 2B). Those data
suggested that HOTAIR expression may serve as an effective
diagnostic marker for diabetic cardiomyopathy.
Association between expression level
of HOTAIR and clinicopathological data of patients with diabetic
cardiomyopathy
Patients were divided into a high expression group
and a low expression group according to the median expression level
of HOTAIR in myocardial tissues and serum. A chi-square test was
performed to analyze the association between expression of HOTAIR
and the clinicopathological data of patients with diabetic
cardiomyopathy. As indicated in Tables
I and II, expression of HOTAIR
in myocardial tissues (Table I) and
serum (Table II) of patients with
diabetic cardiomyopathy exhibited no significant associations with
age, sex or smoking and drinking habits, but was significantly
associated with the disease course (P<0.05).
 | Table I.Associations between expression level
of HOTAIR in myocardial tissues and clinicopathological data of
patients with diabetic cardiomyopathy. |
Table I.
Associations between expression level
of HOTAIR in myocardial tissues and clinicopathological data of
patients with diabetic cardiomyopathy.
| Characteristic | Group | Cases | High expression of
HOTAIR | Low expression of
HOTAIR | χ2 | P-value |
|---|
| Sex | Male | 30 | 17 | 13 | 0.63 | 0.43 |
|
| Female | 26 | 11 | 15 |
|
|
| Age (years) | >50 | 29 | 16 | 13 | 0.64 | 0.42 |
|
| <50 | 27 | 12 | 15 |
|
|
| Course of disease
(years) | >2 | 33 | 12 | 21 | 5.98 | 0.02 |
|
| <2 | 23 | 16 | 7 |
|
|
| Smoking | Yes | 25 | 14 | 11 | 0.65 | 0.42 |
|
| No | 31 | 14 | 17 |
|
|
| Drinking | Yes | 28 | 12 | 16 | 1.14 | 0.29 |
|
| No | 28 | 16 | 12 |
|
|
 | Table II.Associations between expression level
of HOTAIR in serum and clinicopathological data of patients with
diabetic cardiomyopathy. |
Table II.
Associations between expression level
of HOTAIR in serum and clinicopathological data of patients with
diabetic cardiomyopathy.
| Characteristic | Group | Cases | High expression of
HOTAIR | Low expression of
HOTAIR | χ2 | P-value |
|---|
| Sex | Male | 30 | 16 | 14 | 0.29 | 0.59 |
|
| Female | 26 | 12 | 14 |
|
|
| Age (years) | >50 | 29 | 17 | 12 | 1.79 | 0.18 |
|
| <50 | 27 | 11 | 16 |
|
|
| Course of disease
(years) | >5 | 33 | 12 | 21 | 5.98 | 0.02 |
|
| <5 | 23 | 16 | 7 |
|
|
| Smoking | Yes | 25 | 14 | 11 | 1.64 | 0.2 |
|
| No | 31 | 14 | 17 |
|
|
| Drinking | Yes | 28 | 11 | 17 | 2.57 | 0.11 |
|
| No | 28 | 17 | 11 |
|
|
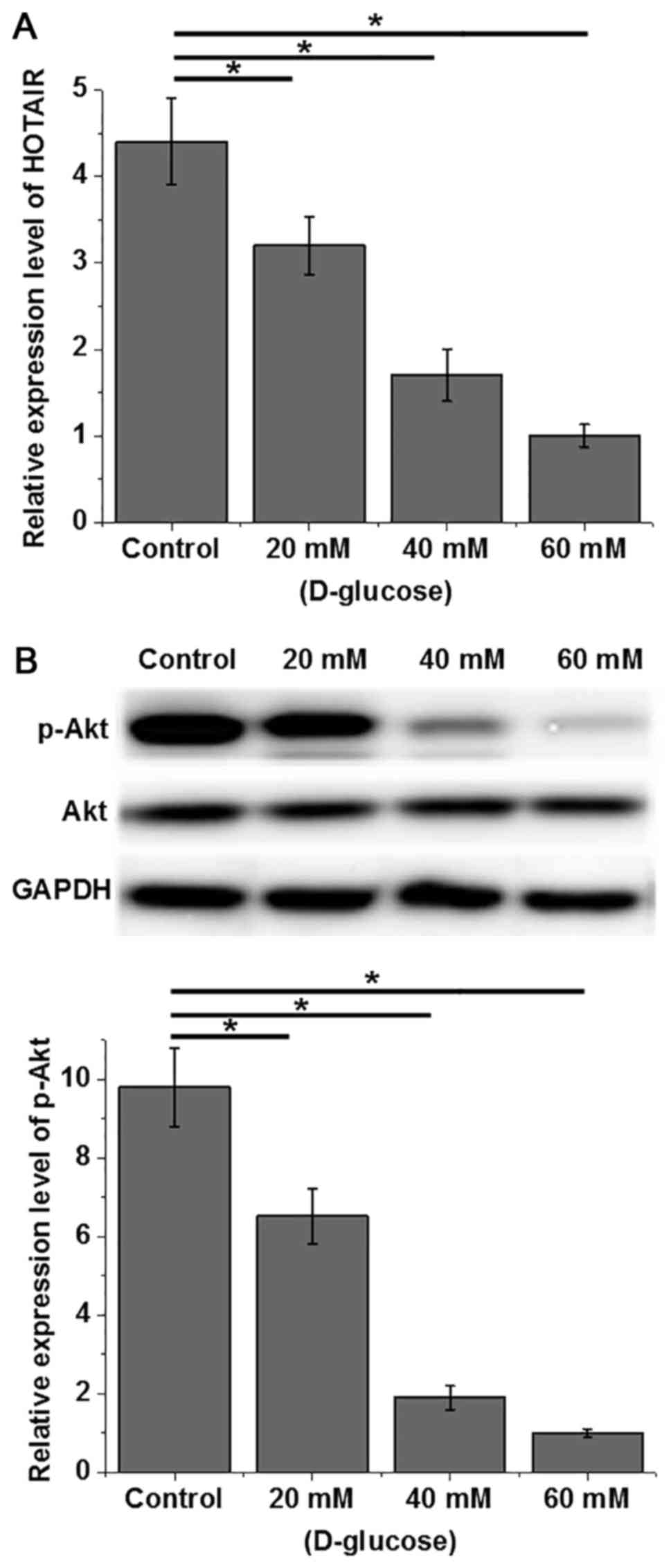
Effects of high glucose on HOTAIR
expression and Akt phosphorylation in human cardiomyocytes
Human cardiomyocytes were treated with different
concentrations of D-glucose (20, 40 and 60 mM) for 48 h, and the
expression of HOTAIR and Akt was detected by RT-qPCR and western
blot analysis, respectively. As indicated in Fig. 3A, D-glucose treatment decreased the
expression level of HOTAIR and expression was reduced with higher
doses of D-glucose in what appeared to be a dose dependent manner
(P<0.05). Although D-glucose exhibited no significant effects on
Akt protein expression, phosphorylation levels of Akt decreased
with increasing concentrations of D-glucose (Fig. 3B; P<0.05). Therefore, a high
glucose environment can lead to the downregulation of HOTAIR
expression and decreased phosphorylation of Akt.
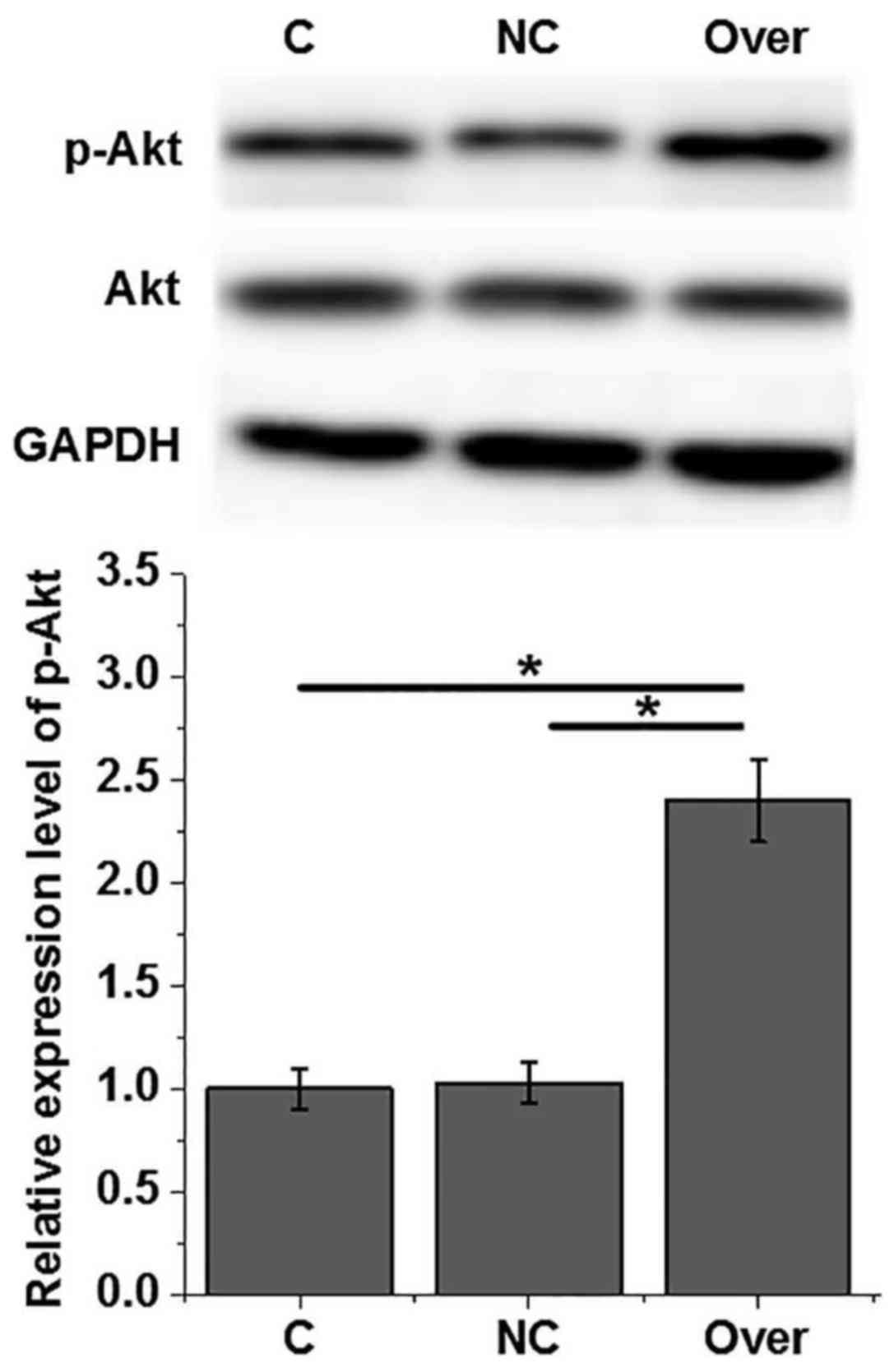
Effects of HOTAIR overexpression and
Akt phosphorylation in human cardiomyocytes
An AC16 cell line with HOTAIR overexpression was
constructed and confirmed by RT-qPCR (data not shown). As indicated
in Fig. 4, HOTAIR overexpression
exerted no significant effects on Akt expression, but significantly
increased the phosphorylation level of Akt (P<0.05). Therefore,
HOTAIR overexpression can upregulate the phosphorylation of Akt in
human cardiomyocytes.
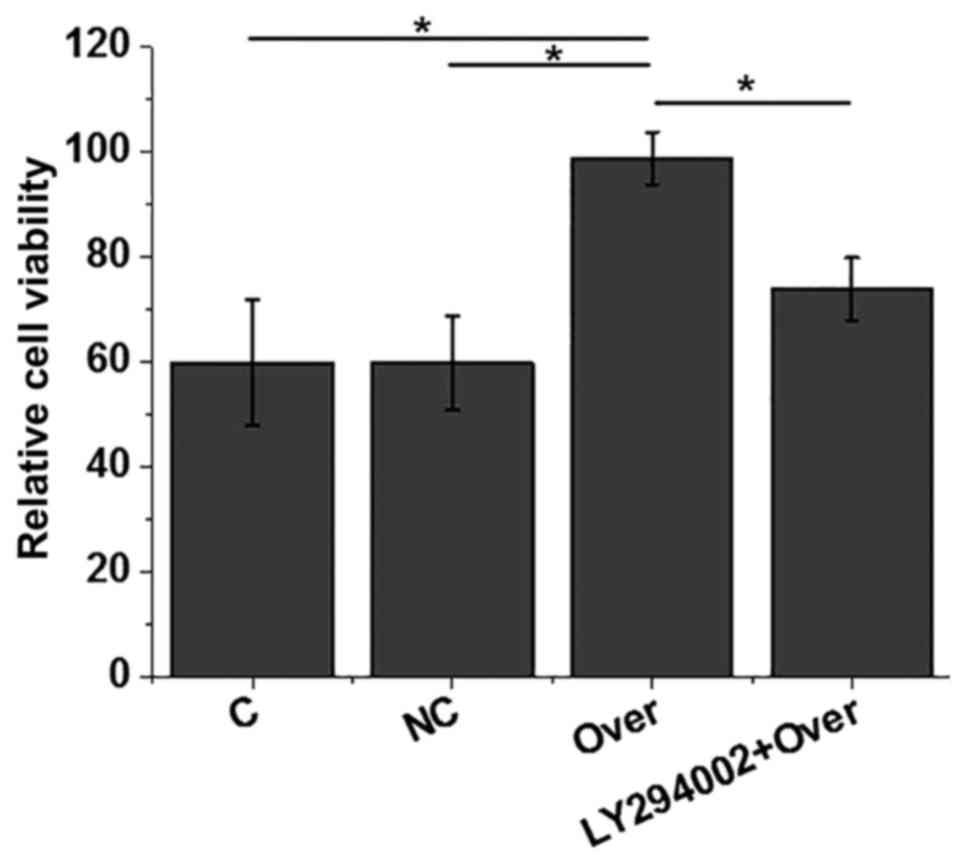
Effects of HOTAIR overexpression on
the viability of AC16 cells under high glucose treatment
AC16 cells were treated with 40 mM D-glucose and
cell viability was measured by MTT assay. As indicated in Fig. 5, compared with control cells without
transfection and negative control cells transfected with empty
vector, HOTAIR overexpression significantly increased cell
viability (P<0.05). However, treatment with PI3K inhibitor
LY294002 (10 µM) significantly reduced the effect of HOTAIR
overexpression on cell viability (P<0.05). Therefore, HOTAIR may
increase the viability of AC16 cells by activating the PI3K
signaling pathway.
Discussion
High blood glucose levels in diabetic patients have
significant effects on the expression of a large set of lncRNAs,
and altered expression of lncRNAs may participate in the
pathogenesis of diabetes-associated complications that promote or
inhibit disease progression (14).
The development of diabetic cardiomyopathy, which is a major
complication in diabetes, is usually accompanied by changes in
expression patterns of certain lncRNAs. It has been reported that
lncRNA myocardial infarction-associated transcript (MIAT)
expression is upregulated in patients with diabetic cardiomyopathy
compared with healthy controls, and upregulated lncRNA MIAT
expression reverses the inhibitory effect of microRNA-22-3p to
promote the development of disease (15). In another study, downregulation of
lncRNA metastasis-associated lung adenocarcinoma transcript-1 was
indicated to improve cardiomyocyte viability, which in turn
inhibited the occurrence of diabetic cardiomyopathy (16). HOTAIR is a well-studied lncRNA with
altered expression in numerous human diseases, particularly in
multiple types of cancer (11).
However, the expression pattern of HOTAIR in diabetes and
diabetes-associated complications has not been well characterized.
In the current study, HOTAIR expression was identified to be
upregulated in patients with diabetic cardiomyopathy but not in
patients with diabetes without cardiomyopathy. These data suggested
that HOTAIR may specifically participate in the development of
cardiomyopathy in patients with diabetes.
Diabetic cardiomyopathy is frequently undiagnosed,
and delayed treatment usually leads to heart failure, which has a
high mortality rate (17).
Therefore, early diagnosis and treatment is critical for the
survival of patients. In the current study, ROC analysis identified
that HOTAIR expression in both myocardial tissues and serum could
be used to accurately distinguish patients with diabetic
cardiomyopathy from healthy controls, indicating that HOTAIR may
serve as a diagnostic marker for diabetic cardiomyopathy. In
addition, detection of serum HOTAIR through blood sampling as a
less invasive procedure may be preferred over myocardial
biopsy.
Expression of lncRNAs is affected by numerous
factors, including aging (18),
alcohol abuse (19) and tobacco
consumption (20). In the current
study, expression of HOTAIR exhibited no significant associations
with patient age or smoking and drinking habits. These data suggest
that HOTAIR may serve as an effective and reliable biomarker for
diabetic cardiomyopathy.
In the current study, treatment with high levels of
glucose significantly reduced the expression level of HOTAIR in
human cardiomyocytes and expression was reduced at higher doses of
D-glucose in what appeared to be a dose dependent manner. High
levels of glucose also inhibited the phosphorylation of Akt. It has
been reported previously that activation of the PI3K/Akt pathway
may improve viability of cardiomyocytes under ischemia conditions
(21), and reduced viability and
increased apoptosis of cardiomyocytes are the main pathological
changes in diabetic cardiomyopathy (22). In addition, it is well established
that HOTAIR exerts its biological effects via interactions with the
PI3K/Akt pathway (23). In the
current study, HOTAIR overexpression significantly promoted the
phosphorylation of Akt, indicating that there was cross-talk
between HOTAIR and the PI3K/Akt pathway in cardiomyocytes. It is
known that HOTAIR is involved in the regulation of viability of
multiple cell types (24,25). In the current study, HOTAIR
overexpression significantly improved viability of cardiomyocytes,
while treatment with PI3K inhibitor significantly reduced this
effect. However, treatment with PI3K inhibitor exhibited no
significant effects on HOTAIR expression (data not shown). These
data suggest that HOTAIR may be involved in diabetic cardiomyopathy
by improving viability of cardiomyocytes via activation of the
PI3K/Akt pathway.
It worth noting that although high levels of glucose
suppressed the expression of HOTAIR in human cardiomyocytes,
patients with diabetes without cardiomyopathy, who would be
expected to have high plasma glucose levels, did not exhibit
significantly downregulated HOTAIR expression compared with healthy
controls. A possible explanation for this is that both heart
lesions and high glucose downregulated HOTAIR, while high blood
glucose itself in patients with diabetes without cardiomyopathy
does not induce significant changes. In addition, in vitro
experiments may not fully mimic in vivo conditions. Further
investigations are required in this area.
In conclusion, HOTAIR expression was specifically
downregulated in patients with diabetic cardiomyopathy. Serum
HOTAIR may serve as a promising biomarker for diabetic
cardiomyopathy. High glucose treatment inhibited HOTAIR expression
and Akt phosphorylation. HOTAIR overexpression promoted Akt
phosphorylation and improved AC16 cell viability. PI3K/Akt
inhibitor treatment reduced this enhancing effect of HOTAIR
overexpression on AC16 cell viability. These results suggest that
lncRNA HOTAIR may improve diabetic cardiomyopathy by improving the
viability of cardiomyocytes through activation of the PI3K/Akt
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KQ and JZ were responsible for the conception and
design of the study, and revised the manuscript. KQ and JZ also
performed the experiments. KQ analyzed and interpreted the data,
and drafted the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The First Hospital of Shanxi
Medical University approved the study and all patients provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus. Provisional report of a WHO Consultation. Diabetic Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Imamura F, O'Connor L, Ye Z, Mursu J,
Hayashino Y, Bhupathiraju SN and Forouhi NG: Consumption of sugar
sweetened beverages, artificially sweetened beverages, and fruit
juice and incidence of type 2 diabetes: Systematic review,
meta-analysis, and estimation of population attributable fraction.
BMJ. 351:h35762015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathan DM: DCCT/EDIC Research Group: The
diabetes control and complications trial/epidemiology of diabetes
interventions and complications study at 30 years: Overview.
Diabetes Care. 37:9–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bugger H and Abel ED: Molecular mechanisms
of diabetic cardiomyopathy. Diabetologia. 57:660–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia G, DeMarco VG and Sowers JR: Insulin
resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat
Rev Endocrinol. 12:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Gen. 15:7–21. 2014. View
Article : Google Scholar
|
|
8
|
Nunes S, Rolo AP, Palmeira CM and Reis F:
Diabetic cardiomyopathy: Focus on oxidative stress, mitochondrial
dysfunction and inflammation. Cardiomyopathies-Types and
Treatments. InTech. 2017. View
Article : Google Scholar
|
|
9
|
Li X, Wang H, Yao B, Xu W, Chen J and Zhou
X: lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by
targeting VDAC1 in diabetic cardiomyopathy. Sci Rep. 6:363402016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang K, Long B, Zhou LY, Liu F, Zhou QY,
Liu CY, Fan YY and Li PF: CARL lncRNA inhibits anoxia-induced
mitochondrial fission and apoptosis in cardiomyocytes by impairing
miR-539-dependent PHB2 downregulation. Nat Commun. 5:35962014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu H, Liu J, Li W, Liu G and Li Z:
LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of
LPS-induced sepsis mice by activating NF-κB pathway. Biochem
Biophys Res Commun. 471:240–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leung A, Amaram V and Natarajan R: Linking
diabetic vascular complications with LncRNAs. Vasc Pharmacol. 2018.
View Article : Google Scholar
|
|
15
|
Zhou X, Zhang W, Jin M, Chen J, Xu W and
Kong X: lncRNA MIAT functions as a competing endogenous RNA to
upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy.
Cell death Dis. 8:29292017. View Article : Google Scholar
|
|
16
|
Zhang M, Gu H, Xu W and Zhou X:
Down-regulation of lncRNA MALAT1 reduces cardiomyocyte apoptosis
and improves left ventricular function in diabetic rats. Int J
cardiol. 203:214–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marcinkiewicz A, Ostrowski S and Drzewoski
J: Can the onset of heart failure be delayed by treating diabetic
cardiomyopathy? Diabetol Metab Syndr. 9:212017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grammatikakis I, Panda AC, Abdelmohsen K
and Gorospe M: Long noncoding RNAs (lncRNAs) and the molecular
hallmarks of aging. Aging (Albany NY). 6:9922014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayfield RD: Emerging roles for ncRNAs in
alcohol use disorders. Alcohol. 60:31–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumor Biol. 36:5523–5528. 2015.
View Article : Google Scholar
|
|
21
|
Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin
Q, Zhu J, Mai L, Shan Z, Yu X, et al: Panax notoginseng saponins
inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway
in cardiomyocytes. J Ethnopharmacol. 137:263–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai L and Kang YJ: Cell death and diabetic
cardiomyopathy. Cardiovasc Toxicol. 3:219–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan J, Dang Y, Liu S, Zhang Y and Zhang G:
LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by
targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumor Biol.
2016. View Article : Google Scholar
|
|
24
|
Qiu JJ, Wang Y, Ding JX, Jin HY, Yang G
and Hua KQ: The long non-coding RNA HOTAIR promotes the
proliferation of serous ovarian cancer cells through the regulation
of cell cycle arrest and apoptosis. Exp Cell Res. 333:238–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Lin C, Yong W, Ye Y and Huang Z:
Calycosin and genistein induce apoptosis by inactivation of
HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells.
Cell Physiol Biochem. 35:722–728. 2015. View Article : Google Scholar : PubMed/NCBI
|