Introduction
Sepsis as a systemic inflammatory response syndrome
(SIRS) caused by an infection can cause damage to the body tissues
and organs themselves and can even be life-threatening (1). Various stress reactions lead to
inflammatory reactions, within which the binding of leukocytes to
vascular endothelial cells induces immune response through
cytokines, selectins (such as E-selectin), and accessory factor
(such as ICAM-1) (2). Sepsis is a
common cause of death in ICU critically ill patients, and in those
cases sepsis is usually caused by different degrees of infection
after major surgery, severe trauma, burns and shock (3). At present, the incidence of sepsis is
approximately 0.3% and the mortality rate is 20–40% (4). The pathophysiology of sepsis is still
unclear, combined with limited clinical means, leading to a poor
prognosis in patients with sepsis.
MicroRNAs have the function of post-transcriptional
regulation and play an important role in many processes such as
secretory metabolism, inflammatory reactions, and tumor formation
(5). Recent studies have revealed
that microRNA-23b can affect cell proliferation, differentiation,
apoptosis, and cell-to-tissue adhesion (6). In addition, studies have suggested that
microRNA-23b can prevent autoimmune diseases (7). Inhibition of the microRNA-23b
expression in tumor tissue can inhibit the proliferation of tumor
cells to a certain extent (8).
MicroRNA can be detected in various types of body fluids and has
high stability (9). Therefore,
microRNAs are generally used as a marker for early disease
diagnosis and prognosis assessment (10). In this study, the expression of
microRNA-23b in peripheral blood of patients with sepsis was
observed to investigate its correlation with levels of leukocytes,
E-selectin and ICAM-1 levels as well as the severity of
disease.
Patients and methods
General information
A total of 87 patients with sepsis was selected from
September 2015 to December 2017 in The Third Xiangya Hospital of
Central South University (Changsha, China). All patients met the
diagnostic criteria of sepsis (11).
In addition, 50 patients with SIRS and 50 normal health people were
included to serve as controls. Patients who met 2 of the following
4 criteria were diagnosed as SIRS: i) heart rate >90 beats/min;
ii) respiratory rate >20 beats/min; iii) total number of white
blood cells (WBC) <4.0×109/l or
>12×109/l; iv) body temperature >38°C or <36°C.
Mean age of subjects in the sepsis, the SIRS, and the control
groups was 55.8±17.6 years, 58.6±15.1 years and 53.8±17.2 years,
respectively. There were 60 males and 27 females in the sepsis
group, 35 males and 15 females in the SIRS group, and 36 males and
14 females in the control group. There was no statistical
difference in age and sex among the three groups. All participants
or family members signed an informed consent. The study was
approved by the Ethics Committee of the Third Xiangya Hospital of
Central South University.
Major reagents and instruments
TRIzol RNA extraction kit (Sangon Biotech Co., Ltd.,
Shanghai, China), cDNA synthesis kit (Sangon Biotech Co., Ltd.),
RT-qPCR kit (Takara Bio, Inc., Otsu, Japan), E-Selectin and ICAM-1
enzyme-linked immunosorbent assay kits (Sangon Biotech Co., Ltd.).
Nucleic acid quantitative detector (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), fluorescence quantitative PCR instrument (ABI,
Foster City, CA, USA). All primers were synthesized by GenScript
Co., Ltd. (Nanjing, China).
Observation of clinical
indicators
Age, sex, primary disease, and arterial blood gas
analysis, inhaled oxygen concentration, WBC, mean arterial
pressure, platelet count, hemoglobin, blood creatinine, urine
volume and GCS within 24 h after onset of patients in the sepsis
group were recorded. The worst-case values of physiological
parameters of these indicators and actual test results were
subjected to sequential organ failure assessment (SOFA) scoring
system (12). Outcomes (survival or
death) at 30 days after onset were recorded.
Reverse transcription-quantitative PCR
(RT-qPCR) to detect the expression of microRNA-23b
After diagnosis, venous blood was collected from
patients in the morning. Blood was centrifuged at 2,100 × g at 4°C
for 10 min to collect serum. Serum samples were stored at −20°C
before use. TRIzol kit was used to extract total RNA from
peripheral blood, and nucleic acid quantification detector was used
to measure RNA concentration. cDNA was synthesized using cDNA
synthesis kit. With U6 as endogenous control, the expression of
microRNA-23b was detected by RT-qPCR. PCR reaction system consisted
of 10 µl of SYBR-Green Master Mix, 0.5 µl of upstream and
downstream primers, 1 µl of cDNA, and 8 µl of ddH2O.
Reaction conditions were: 95°C for 3 min, followed by 40 cycles of
95°C for 30 sec and 58°C for 1 min. Sequences of primers used in
PCR reactions were: 5′-TCTCCCTGGCGTCCTCCCTTCG-3′ (forward) and
5′-CCTTATCAAGAACACCAACCAGT-3′ (reverse) for microRNA-23b;
5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-AACGCTTCACGAATTTGCGT-3′
(reverse) for U6.
MicroRNA-23b stability testing
Peripheral blood collected from 5 participants of
each group was kept as 4°C, and samples were collected at day 0, 1,
3, and 5 respectively. Total RNA was extracted and RNA
concentration was detected by a nucleic acid quantitative detector.
Finally, RT-qPCR was performed to detect the expression of
microRNA-23b and assess its stability.
Enzyme-linked immunosorbent assay
(ELISA) to detect E-selectin and ICAM-1
Peripheral blood of each group (sepsis group and
SIRS collected within 24 h) was collected and centrifuged at 1,680
× g at 4°C for 20 min. Serum was separated and serum E-selectin and
ICAM-1 levels were determined by ELISA according to the
instructions of the kit.
Statistical analysis
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for
statistical analysis. Measurement data were expressed as mean ±
standard deviation. Variance analysis was used for analysis among
multiple groups and the post hoc test was Least Significant
Difference test. t-test was used for comparison between two groups.
Chi-square test was to compare enumeration data. Correlation
analysis was performed using Pearsons correlation analysis.
P<0.05 was considered to indicate a statistically significant
diffe-rence.
Results
MicroRNA-23b stability analysis
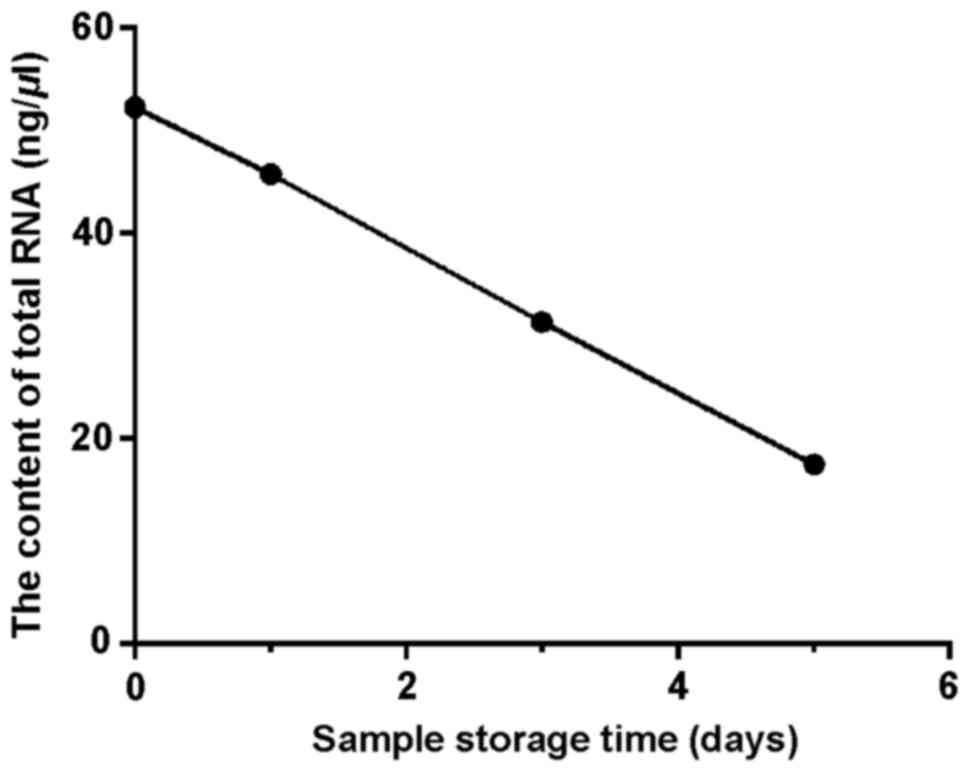
Total RNA concentration decreased from 52.3 to 17.5
ng/µl after 5 days of storage at 4°C, indicating that total RNA in
peripheral blood samples will be gradually degraded over time
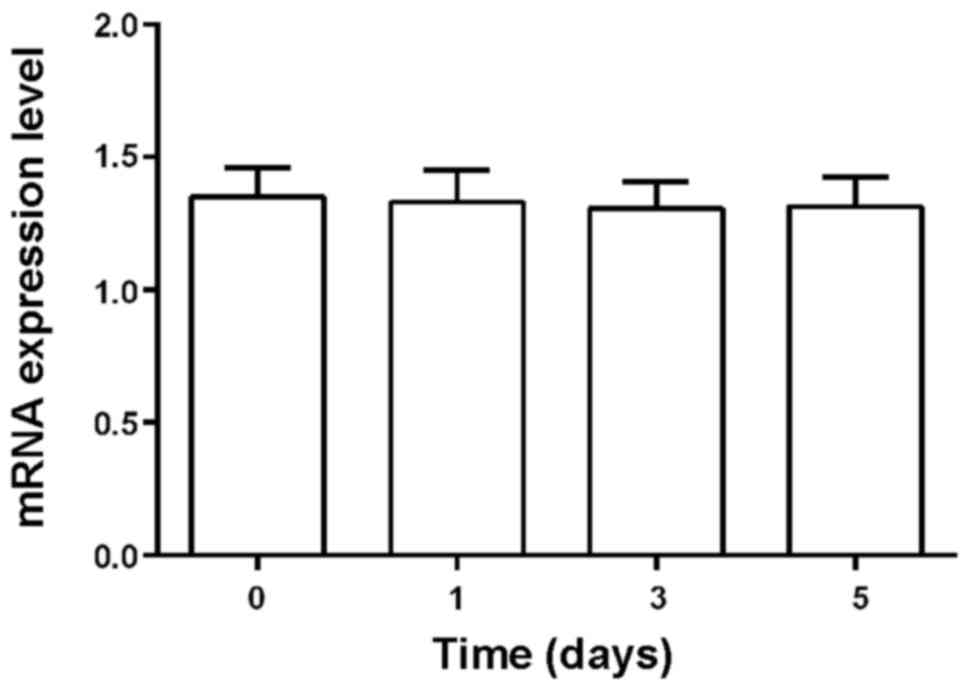
(Fig. 1). However, there was no
significant change in levels of microRNA-23b and endogenous control
U6 in blood samples after storage for 0, 1, 3, and 5 days at 4°C
(Fig. 2).
Comparison of basic clinical data
among groups
Significant differences were found in WBC, mean
arterial pressure, CRP, PCT and blood lactate levels when comparing
the SIRS group or the sepsis group with the control group
(P<0.05). There was no significant difference in these factors
between the SIRS and the sepsis groups (P>0.05). There was no
significant difference in age and sex among the three groups
(P>0.05) (Table I).
 | Table I.Comparison of basic clinical data
among groups. |
Table I.
Comparison of basic clinical data
among groups.
| Clinical data | Control | SIRS | Sepsis | F
(χ2) | P-value |
|---|
| Age (years) | 58.3±6.8 | 60.8±8.7 | 62.4±6.8 | 0.229 | 0.802 |
| Sex (cases) |
|
|
| 0.139 | 0.933 |
| Male | 36 | 35 | 60 |
|
|
|
Female | 14 | 15 | 27 |
|
|
| WBC
(109/l) | 6.15±1.22 |
12.13±2.83a |
12.98±3.61a | 6.578 | 0.031 |
| Mean arterial
pressure (mmHg) | 78.6±7.0 |
52.75±6.75a |
53.25±7.35a | 14.32 | 0.005 |
| CRP (mg/l) | 8.8±1.02 |
78.3±5.05a |
77.3±6.43a | 96.235 | <0.001 |
| PCT (µg/l) | 0.32±0.2 | 9.3±1.8a | 11.9±1.5a | 60.514 | <0.001 |
| Blood lactate
(mmol/l) | 2.0±0.5 | 4.5±0.8a | 4.7±0.6a | 16.296 | 0.004 |
Comparison of levels of microRNA-23b,
WBC, E-selectin and ICAM-1 in peripheral blood among groups
As shown in Table
II, the expression level of microRNA-23b in the sepsis and the
SIRS groups was significantly lower than that in the control group
(P<0.01), while the WBC, E-selectin, and ICAM-1 levels were
significantly increased in the sepsis and SIRS groups compared to
the control group (P<0.05). ICAM-1 level in the sepsis group was
significantly higher than that in the SIRS group (P<0.05), but
there was no significant difference in the microRNA-23b, WBC, and
E-selectin between the sepsis and the SIRS groups (P>0.05).
 | Table II.Comparison of levels of microRNA-23b,
WBC, E selectin and ICAM-1 in peripheral blood among groups. |
Table II.
Comparison of levels of microRNA-23b,
WBC, E selectin and ICAM-1 in peripheral blood among groups.
| Groups | No. of cases | MicroRNA-23b | WBC
(109/l) | E-selectin
(ng/ml) | ICAM-1 (ng/ml) |
|---|
| Control | 50 | 1.08±0.28 | 6.15±1.22 | 51.82±11.86 | 296.37±31.58 |
| SIRS | 50 |
0.21±0.07a |
12.98±2.83a |
129.44±41.32a |
367.39±50.15a |
| Sepsis | 87 |
0.16±0.03a |
12.13±3.61a |
138.35±38.28a |
559.83±53.36a,b |
| F-value |
| 28.607 | 5.535 | 5.863 | 25.662 |
| P-value |
| 0.001 | 0.043 | 0.039 | <0.001 |
Comparison of microRNA-23b,
E-selectin, ICAM-1, WBC and SOFA scores between the survivor and
the death groups in the sepsis group
Mortality rate in the sepsis group was 18.4%
(16/87). The expression level of microRNA-23b in the death group
was significantly lower than that in the survivor group
(P<0.05), and the E-selectin, ICAM-1, and SOFA scores were
significantly higher in the death group than in the survival group
(P<0.05). There was no significant difference in WBC between the
survival and the death groups (Table
III).
 | Table III.Comparison of microRNA-23b,
E-selectin, ICAM-1, WBC and SOFA scores between the survivor and
death groups in the sepsis group. |
Table III.
Comparison of microRNA-23b,
E-selectin, ICAM-1, WBC and SOFA scores between the survivor and
death groups in the sepsis group.
| Groups | No. of cases | MicroRNA-23b | E-selectin | ICAM-1 | WBC | SOFA |
|---|
| Survivor group | 71 | 0.18±0.04 | 112.4±21.7 | 370.25±43.92 | 12.03±2.15 | 10±1 |
| Death group | 16 | 0.05±0.02 | 150.4±27.3 | 612.33±87.52 | 12.08±2.13 | 16±2 |
| t value |
| 11.258 | −11.753 | −9.625 | −19.875 | −10.392 |
| P-value |
| 0.008 | 0.007 | 0.011 | 0.44 | 0.001 |
Correlation analysis
The expression level of microRNA-23b in the sepsis
group was significantly negatively correlated with SOFA score,
serum E-selectin, and ICAM-1 (r= −0.633, −0.585, and −0.439,
respectively, P<0.05), but not WBC (P>0.05). There was no
significant correlation between WBC, E-selectin, ICAM-1 and SOFA
scores (P>0.05) (Table IV).
 | Table IV.Correlation analysis between
microRNA-23b, WBC, E-selectin, ICAM-1, and SOFA scores. |
Table IV.
Correlation analysis between
microRNA-23b, WBC, E-selectin, ICAM-1, and SOFA scores.
|
| MicroRNA-23b | SOFA |
|---|
|
|
|
|
|---|
| Items | r | P-value | r | P-value |
|---|
| WBC | 0.103 | 0.082 | 0.115 | 0.079 |
| E-selectin | −0.585 | 0.008 | 0.206 | 0.065 |
| ICAM-1 | −0.439 | 0.008 | 0.237 | 0.060 |
| SOFA | −0.633 | 0.007 |
|
|
Discussion
Most patients with sepsis have immune dysfunction.
Release of inflammatory transmitters after infection can cause
damage of vascular endothelial cells, which in turn increases
vascular permeability, and further inducing the transition from
SIRS to sepsis shock accompanied by multiple organ dysfunction
(13). Early sepsis is easily
misdiagnosed as SIRS, leading to delayed treatment. Therefore, more
specific markers are required for the diagnosis of sepsis.
MicroRNAs are responsible for the regulation of gene
expression and play an important role in the pathophysiology of
many diseases (14). MicroRNA-125b,
−150, −223, and −146 have been proven to play critical roles in
pathogenesis of sepsis (15).
MicroRNA-23b is a newly discovered microRNA, and studies have shown
that peripheral blood levels of microRNA-23b can reflect the
progression of gastric cancer. With the aggravation of gastric
cancer, the expression level of microRNA-23b is increased (16). Studies have also shown that
microRNA-23b is highly expressed in peripheral blood of patients
with myasthenia gravis (17).
Results of our study showed that the expression of microRNA-23b in
the sepsis group was significantly lower than that in the normal
control group (P<0.01), and the microRNA-23b expression level in
the death group was significantly lower than that in the survivor
group (P<0.05). The data suggest that the development of sepsis
is accompanied by the downregulation of microRNA-23b. Furthermore,
the microRNA-23b expression level was negatively correlated with
SOFA score, suggesting that the expression of microRNA-23b is
related to the severity and prognosis of the disease. However,
there was no significant difference in the expression level of
microRNA-23b between the sepsis and the SIRS groups, so
microRNA-23b could not be used to distinguish between sepsis and
SIRS.
E-selectin is present on the surface of activated
vascular endothelial cells and can bind to leukocytes to promote
the activation of vascular endothelial cells and mediates
inflammatory responses (18).
Nimrichter et al (19) found
that E-selectin can enhance leukocyte aggregation, and Kung et
al (20) suggested that
E-selectin could be used as a marker for severe infections and
bacteremia. Leukocyte aggregation along the vascular wall of lesion
regions is a prerequisite for the adherence of WBC and endothelial
cells and passing through blood vessel wall (21). ICAM-1 is an adhesion molecule with a
very small molecular weight that promotes leukocyte adhesion and
aggravates inflammatory damage (22). Studies have shown that ICAM-1 and
E-selectin are increased in sepsis peripheral blood, and the
content of those two factors in serum can be used to judge the
extent of endothelial damage. Damage of endothelial cells indicates
the development of inflammatory reaction (23). Results of our study showed that
ICAM-1 level in the sepsis group was significantly higher than that
in the control group (P<0.05), and ICAM-1 level in the sepsis
group was significantly higher than that in the SIRS group
(P<0.05). In addition, ICAM-1 level in the death group was
significantly higher than that in the survivor group (P<0.05),
suggesting that the level of ICAM-1 is related to the severity of
sepsis. Bedirli et al (24)
believed that ICAM-1 level is increased in early stages of sepsis,
and high ICAM-1 predicts poor pro-gnosis and high mortality. The
levels of WBC and E-selectin were significantly higher in the
sepsis group than in the control group, but there was no
significant difference between the sepsis and SIRS groups
(P>0.05), suggesting that WBC and E-selectin can only reflect
the presence of inflammation, and cannot be used to distinguish
between sepsis and SIRS. The levels of E-selectin and ICAM-1 in the
death group were significantly higher than those in the survivor
group, suggesting that E-selectin and ICAM-1 levels may be related
to disease severity. E-selectin and ICAM-1 levels have no
significant correlation with SOFA, indicating that E-selectin and
ICAM-1 may not be pro-mising diagnostic and prognostic markers for
sepsis.
In conclusion, the expression level of microRNA-23b
in peripheral blood of patients with sepsis is related to the
manifestation of inflammatory state, and to some extent, it can be
used to judge the severity and prognosis of disease.
Acknowledgements
Not applicable.
Funding
The study was supported by the New Xiangya Talent
Project of Xiangya Hospital of Central South University
(JY201606).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HO drafted the manuscript. HO and XX contributed to
RT-qPCR. YJ and YP contributed to microRNA-23b stability testing.
HO, MY and MG were responsible for ELISA. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Third Xiangya Hospital of Central South University (Changsha,
China). Signed informed consents were obtained from the patients or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caserta S, Kern F, Cohen J, Drage S,
Newbury SF and Llewelyn MJ: Circulating plasma microRNAs can
differentiate human sepsis and systemic inflammatory response
syndrome (SIRS). Sci Rep. 6:280062016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santana H, Sousa Ítalo P, Marques LM,
Figueiredo TB and Silva RAAD: Distinct strains of Staphylococcus
aureus lead to different inflammatory response patterns in a murine
model of intradermal infection. Acta Sci Biol Sci. 38:457–464.
2016. View Article : Google Scholar
|
|
3
|
Suresh PK, Minal J, Rao PS, Ballal K,
Sridevi HB and Padyana M: Volume conductivity and scatter
parameters as an indicator of acute bacterial infections by the
automated haematology analyser. J Clin Diagn Res. 10:EC01–EC03.
2016.
|
|
4
|
Moitra R, Beal DR, Belikoff BG and Remick
DG: Presence of preexisting antibodies mediates survival in sepsis.
Shock. 37:56–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Behbahani GD, Ghahhari NM, Javidi MA,
Molan AF, Feizi N and Babashah S: MicroRNA-mediated
post-transcriptional regulation of epithelial to mesenchymal
transition in cancer. Pathol Oncol Res. 23:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukumoto I, Koshizuka K, Hanazawa T,
Kikkawa N, Matsushita R, Kurozumi A, Kato M, Okato A, Okamoto Y and
Seki N: The tumor-suppressive microRNA-23b/27b cluster regulates
the MET oncogene in oral squamous cell carcinoma. Int J Oncol.
49:1119–1129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enelund L, Nielsen LN and Cirera S:
Evaluation of microRNA stability in plasma and serum from healthy
dogs. Microrna. 6:42–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nassar FJ, Nasr R and Talhouk R: MicroRNAs
as biomarkers for early breast cancer diagnosis, prognosis and
therapy prediction. Pharmacol Ther. 172:34–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chvojka J and Matějovič M: International
guidelines for management of severe sepsis and septic shock 2012 -
comment. Vnitr Lek. 60:59–67. 2014.(In Czech). PubMed/NCBI
|
|
12
|
Minne L, Abu-Hanna A and de Jonge E:
Evaluation of SOFA-based models for predicting mortality in the
ICU: A systematic review. Crit Care. 12:R1612008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roderburg C, Luedde M, Cardenas Vargas D,
Vucur M, Scholten D, Frey N, Koch A, Trautwein C, Tacke F and
Luedde T: Circulating microRNA-150 serum levels predict survival in
patients with critical illness and sepsis. PLoS One. 8:e546122013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hervé M and Ibrahim EC: MicroRNA screening
identifies a link between NOVA1 expression and a low level of IKAP
in familial dysautonomia. Dis Model Mech. 9:899–909. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng HS, Sivachandran N, Lau A, Boudreau
E, Zhao JL, Baltimore D, Delgado-Olguin P, Cybulsky MI and Fish JE:
MicroRNA-146 represses endothelial activation by inhibiting
pro-inflammatory pathways. EMBO Mol Med. 5:1017–1034. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou H, Guo JM, Lou YR, Zhang XJ, Zhong
FD, Jiang Z, Cheng J and Xiao BX: Detection of circulating tumor
cells in peripheral blood from patients with gastric cancer using
microRNA as a marker. J Mol Med (Berl). 88:709–717. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barzago C, Lum J, Cavalcante P, Srinivasan
KG, Faggiani E, Camera G, Bonanno S, Andreetta F, Antozzi C, Baggi
F, et al: A novel infection- and inflammation-associated molecular
signature in peripheral blood of myasthenia gravis patients.
Immunobiology. 221:1227–1236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Velázquez F, Grodecki-Pena A, Knapp A,
Salvador AM, Nevers T, Croce K and Alcaide P: CD43 functions as an
E-Selectin ligand for Th17 cells in vitro and is required for
rolling on the vascular endothelium and Th17 cell recruitment
during inflammation in vivo. J Immunol. 196:1305–1316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nimrichter L, Burdick MM, Aoki K, Laroy W,
Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS,
Tiemeyer M, et al: E-selectin receptors on human leukocytes. Blood.
112:3744–3752. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kung CT, Hsiao SY, Su CM, Tsai TC, Cheng
HH, Tsai NW, Chang WN, Huang CR, Wang HC, Lin WC, et al: Serum
adhesion molecules as predictors of bacteremia in adult severe
sepsis patients at the emergency department. Clin Chim Acta.
421:116–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cahill PA and Redmond EM: Vascular
endothelium - Gatekeeper of vessel health. Atherosclerosis.
248:97–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raffray L, Giry C, Thirapathi Y, Reboux
AH, Jaffar-Bandjee MC and Gasque P: Increased levels of soluble
forms of E-selectin and ICAM-1 adhesion molecules during human
leptospirosis. PLoS One. 12:e01804742017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Figueras-Aloy J, Gómez-López L,
Rodríguez-Miguélez JM, Salvia-Roiges MD, Jordán-García I,
Ferrer-Codina I, Carbonell-Estrany X and Jiménez-González R: Serum
soluble ICAM-1, VCAM-1, L-selectin, and P-selectin levels as
markers of infection and their relation to clinical severity in
neonatal sepsis. Am J Perinatol. 24:331–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bedirli N, Demirtas CY, Akkaya T, Salman
B, Alper M, Bedirli A and Pasaoglu H: Volatile anesthetic
preconditioning attenuated sepsis induced lung inflammation. J Surg
Res. 178:e17–e23. 2012. View Article : Google Scholar : PubMed/NCBI
|