Introduction
With the continuous progression of aging, severe air
pollution and high smoking rate, chronic obstructive pulmonary
disease (COPD) has become a serious health threat in China. In
2013, the prevalence rate of COPD in people >40 years was 7.3%,
making it a leading cause of disability, and it seriously affects
the quality of life of affected patients (1,2). As a
chronic lung disease, COPD comprises an incompletely reversible
airflow limitation and develops progressively. Therefore, the major
purpose of treatment is to relieve symptoms, reduce exacerbation,
and improve lung function and quality of life. The commonly used
medications for COPD include inhaled glucocorticoids,
methylxanthines and bronchodilators (including cholinolytic agents
and β2 receptor agonists); at present, multiple COPD
treatment guidelines recommend combined application of different
types of bronchodilators or application of bronchodilators combined
with inhaled glucocorticoids (3,4).
Tiotropium bromide is a long-acting cholinolytic
bronchodilator. Studies have indicated that tiotropium alone
improves lung function and quality of life, and reduces the risk of
acute attack; in addition, long-term inhalation of tiotropium
bromide and salmeterol/fluticasone reduces the frequency of acute
exacerbation of COPD and the risk of mortality, and improves the
health-associated quality of life of affected patients (5,6).
However, the combined application of tiotropium bromide and
β2 receptor agonist bronchodilators or glucocorticoids
remains controversial (7). Previous
studies assessing inhalation therapy of tiotropium bromide combined
with budesonide/formoterol have provided conflicting results
(8,9). Therefore, the present study aimed to
further evaluate the efficacy and safety of tiotropium bromide
combined with budesonide/formoterol in patients with moderate to
severe COPD.
Patients and methods
Subjects
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Soochow University
(Suzhou, China). A total of 180 patients with moderate to severe
COPD (groups B and C) in the stable phase treated at the First
Affiliated Hospital of Soochow University from January 2014 to
December 2015 were included. Demographic characteristics including
age and sex were collected at the beginning of the study.
The inclusion criteria were as follows: Patients
aged >18 years; patients who were diagnosed with group B and C
COPD in accordance with the Guidelines for the Diagnosis and
Treatment of Chronic Obstructive Pulmonary Disease (revised edition
from 2013), published by the COPD Group of the Chinese Thoracic
Society (3); patients who received
inhalation therapy; patients who did not receive any cholinolytic
drugs within 4 weeks; patients who did not suffer from any acute
attack and did not receive any antibiotic therapy for 4 weeks;
patients who had not received any systemic glucocorticoid therapy
in the past two months; patients who provided written informed
consent. The exclusion criteria were as follows: Patients who were
allergic to tiotropium bromide, budesonide/formoterol inhalation or
any other inhaled ingredients; patients with diseases rendering
them unsuitable for inhalation therapy, including bronchial lung
cancer, dilatation and interstitial lung diseases; patients
suffering from other systemic diseases, including heart, liver,
kidney, hematopoietic and nervous system diseases, glaucoma or
severe prostatic hyperplasia; patients who were considered
unsuitable for participating in the drug trial by the
clinicians.
Grouping and treatment
A total of 180 cases with moderate to severe COPD
were randomly divided into a control group (positive drug controls)
and an intervention group by using a random number table, with 90
cases in each group. Each of the two groups went through a 1-week
washout period, after which they received treatment for 6 months.
The control group received budesonide/formoterol (160/4.5 µg; 1
suction/time, 2 times/day; Symbicort Turbuhaler; AstraZeneca,
Cambridge, UK). Based on the control group treatment, patients in
the intervention group received additional treatment of tiotropium
bromide (1.8 µg inhaled once a day before bed; Spiriva; C.H.
Boehringer Sohn AG & Ko. KG, Ingelheim, Germany). During the
study period, patients were provided with salbutamol inhalation on
demand; antibiotics and antiviral drugs were used in specific cases
if required.
Evaluation indices
The following indices were determined prior to
treatment and after treatment for 3 and 6 months.
Lung function indices included the forced expiratory
volume in 1 second (FEV1), the FEV1/forced
vital capacity (FVC) and the FEV1 as a percentage of the
predicted value (FEV1%pred).
Weight and height were measured for calculating the
body mass index (BMI).
The dyspnea score was evaluated using the modified
Medical Research Council (mMRC) scale (10). The scale included four grades, with a
higher score indicating more serious dyspnea, ranging from 0
points-no breathing difficulties except during strenuous exercise,
to 4 points-dyspnea when leaving the room, dressing or other light
activities. The degrees of dyspnea were scored according to the
patients' own description.
The six-minute walking test (6MWT) was performed by
measuring the longest distance that patients were able to walk
within 6 min while they were walking back and forth on corridor of
20 m in length (11). The test was
performed twice with an interval time of at ≥2 h and the optimum
value was recorded. This test was performed to assess the exercise
tolerance of patients.
Health-associated quality of life was assessed using
St. George's Respiratory Questionnaire (SGRQ) (12). SGRQ consisted of 50 items and three
parts, assessing the influence of symptoms, ability to perform
activities and disease on daily life, with a total score of 100
points. The score was negatively correlated with the health state.
On the day of the pulmonary function test, the questionnaires were
completed by the patients independently, and the researchers
checked whether there was any omission.
During follow-up period, occurrence and time of
acute exacerbation were recorded for the evaluation of acute attack
risk and occurrence of mortality and adverse reactions were
recorded for the evaluation of safety.
Statistical analysis
All data were processed using SPSS 20.0 (IBM Corp.,
Armonk, NY, USA). The measurement data were expressed as the mean ±
standard deviation, and the difference in baseline measurement data
between the two groups were compared with an independent-samples
t-test. The difference test of categorical variables between the
two groups was performed by using the two-tailed χ2 test
or Fisher's exact probability test.
Comparisons of lung function, BMI, mMRC, 6MWT and
SGRQ scores at different time-points, as well as differences in
treatment effects of the two treatment methods at different
time-points (interaction of treatment and time), were analyzed by
repeated-measures analysis of variance (ANOVA). If the overall
difference between the two groups in the repeated-measures ANOVA
was significant, Bonferroni's post-hoc test (significance level set
at 0.05/3=0.0167) was performed for the comparison of detection
values or score at the same time-point. The risk of acute
exacerbation since the start of treatment was compared between the
groups by Kaplan-Meier survival analysis, with significant
differences determined using the log-rank test. The days between
first onset from the start of treatment exhibited a skewed
distribution; therefore, the data were expressed as median (25 and
75% quartile) and the Mann-Whitney U-test was used for assessment
of statistically significant differences. The study was analyzed in
line with the Intent-To-Treat principle; for subjects that dropped
out during the study, analysis was performed according to their
last available data. The level of statistical significance was set
as bilateral α=0.05 (except for Bonferroni's post-hoc test).
Results
General information
During the study period, 1 patient in the
intervention group and 1 in control group dropped out due to of
adverse reactions. The baseline and clinical characteristics of the
patients in the two groups are presented in Table I. There was no significant difference
in age, sex, smoking history, course of disease and degree of COPD
between the two groups, indicating similar baseline and clinical
data.
 | Table I.General information on the patients in
the two groups. |
Table I.
General information on the patients in
the two groups.
| Characteristic | Intervention group
(n=90) | Control group
(n=90) | χ2/t | P-value |
|---|
| Age (years) | 56.7±8.2 | 54.8±7.9 | 1.583 | 0.115 |
| Sex |
|
| 1.693 | 0.193 |
| Male | 67 (74.4) | 59 (65.6) |
|
|
|
Female | 23 (25.6) | 31 (34.4) |
|
|
| Smoking history |
|
| 2.025 | 0.155 |
| No | 13 (14.4) | 7 (7.8) |
|
|
| Yes | 77 (85.6) | 83 (92.2) |
|
|
| Course of disease
(years) | 11.6±5.7 | 10.3±5.1 | 1.612 | 0.109 |
| COPD degree |
|
| 1.105 | 0.293 |
| B | 47 (52.2) | 54 (60.6) |
|
|
| C | 43 (47.8) | 36 (40.0) |
|
|
Co-treatment with tiotropium bromide
significantly enhances the improvement of lung function in COPD
patients receiving budesonide/formoterol
Table II presents
the comparison of lung function between the two groups prior to
treatment, and after treatment for 3 and 6 months. There was no
significant difference in pulmonary function indices between the
two groups prior to treatment. Repeated-measures ANOVA indicated
that over the treatment time, the FEV1,
FEV1/FVC (%) and FEV1%pred exhibited
time-dependent increases in the groups (P=0.034, P=0.026 and
P=0.020, respectively). Over the entire treatment duration, the
differences in the above three indicators between the two groups
were also significant (P=0.030, P=0.042 and P=0.038, respectively),
and the improvement of lung function in the intervention group was
significantly better than that in the control group. The
FEV1 in the intervention group was significantly higher
than that in the control group after treatment for 3 months
(P=0.015) and 6 months (P=0.001); furthermore, at 6 months, the
FEV1/FVC (%, P=0.006) and FEV1%pred (P=0.005)
in the intervention group were significantly higher than those in
the control group.
 | Table II.Pulmonary function evaluation in the
two groups. |
Table II.
Pulmonary function evaluation in the
two groups.
|
|
|
|
| Difference between
groups | Time effect | Group/time
interactiona |
|---|
|
|
|
|
|
|
|
|
|---|
| Pulmonary function
parameter | Prior to
treatment | After 3 months
treatment | After 6 months
treatment | F | P-value | F | P-value | F | P-value |
|---|
| FEV1
(l) |
|
|
| 4.783 | 0.030 | 3.417 | 0.034 | 3.253 | 0.040 |
|
Intervention group | 1.10±0.35 | 1.40±0.42 | 1.46±0.45 |
|
|
|
|
|
|
| Control
group | 1.14±0.39 | 1.25±0.40 | 1.25±0.41 |
|
|
|
|
|
|
| t,
P-valueb | 0.724, 0.470 | 2.453, 0.015 | 3.272, 0.001 |
|
|
|
|
|
|
| FEV1/FVC
(%) |
|
|
| 4.209 | 0.042 | 3.672 | 0.026 | 2.342 | 0.098 |
|
Intervention group | 54.34±9.30 | 58.52±9.79 | 62.01±9.10 |
|
|
|
|
|
|
| Control
group | 53.27±8.67 | 56.02±8.36 | 58.28±8.71 |
|
|
|
|
|
|
| t,
P-valueb | 0.798, 0.426 | 1.842, 0.067 | 2.809, 0.006 |
|
|
|
|
|
|
|
FEV1%pred |
|
|
| 4.357 | 0.038 | 3.944 | 0.020 | 0.571 | 0.565 |
|
Intervention group | 52.32±6.67 | 55.89±7.08 | 59.02±7.24 |
|
|
|
|
|
|
| Control
group | 52.17±7.67 | 53.75±7.15 | 55.93±7.45 |
|
|
|
|
|
|
| t,
P-valueb | 0.140, 0.889 | 2.017, 0.045 | 2.821, 0.005 |
|
|
|
|
|
|
Comparison of BMI, mMRC, 6MWT and SGRQ
scores between the two groups during treatment
Similar to the results regarding pulmonary function,
the BMI (P=0.044), mMRC score (P=0.017), 6MWT score (P=0.021) and
SGRQ score (P=0.024) in the two groups improved significantly
during treatment, suggesting that nutritional status, dyspnea,
exercise endurance and quality of life in the two groups were
significantly improved. The improvements of mMRC, 6MWT and SGRQ
scores in the intervention group were significantly better than
those in the control group (P=0.041, P=0.044 and P=0.034,
respectively), but the difference in the BMI between the two groups
was not statistically significant (P=0.302; Table III).
 | Table III.Comparison of BMI, mMRC, 6MWT and
SGRQ scores in the two groups. |
Table III.
Comparison of BMI, mMRC, 6MWT and
SGRQ scores in the two groups.
|
|
|
|
| Difference between
groups | Time effect | Group/time
interactiona |
|---|
|
|
|
|
|
|
|
|
|---|
| Score | Prior to
treatment | After 3 months
treatment | After 6 months
treatment | F | P-value | F | P-value | F | P-value |
|---|
| BMI
(kg/m2) |
|
|
| 1.072 | 0.302 | 3.142 | 0.044 | 1.282 | 0.279 |
|
Intervention group | 21.2±2.7 | 21.9±2.8 | 22.4±3.2 |
|
|
|
|
|
|
| Control
group | 20.9±2.6 | 21.6±3.0 | 22.0±2.9 |
|
|
|
|
|
|
| mMRC score |
|
|
| 4.233 | 0.041 | 4.110 | 0.017 | 0.469 | 0.626 |
|
Intervention group | 3.02±0.46 | 2.45±0.31 | 1.53±0.26 |
|
|
|
|
|
|
| Control
group | 3.05±0.53 | 2.67±0.36 | 1.85±0.29 |
|
|
|
|
|
|
| t,
P-valueb | 0.406, 0.686 | 4.393, 0.001 | 7.794, 0.001 |
|
|
|
|
|
|
| 6MWT (m) |
|
|
| 4.091 | 0.044 | 3.926 | 0.021 | 2.149 | 0.118 |
|
Intervention group | 283.9±42.65 | 322.8±48.89 | 331.92±49.87 |
|
|
|
|
|
|
| Control
group | 279.5±40.09 | 309.77±47.15 | 314.23±47.29 |
|
|
|
|
|
|
| t,
P-valueb | 0.713, 0.477 | 1.820, 0.070 | 2.442, 0.016 |
|
|
|
|
|
|
| SGRQ score |
|
|
| 4.561 | 0.034 | 3.769 | 0.024 | 1.785 | 0.169 |
|
Intervention group | 59.47±7.15 | 54.56±6.64 | 53.32±5.94 |
|
|
|
|
|
|
| Control
group | 58.64±6.41 | 50.21±6.17 | 48.28±6.05 |
|
|
|
|
|
|
| t,
P-valueb | 0.820, 0.413 | 4.553,
<0.001 | 5.639,
<0.001 |
|
|
|
|
|
|
Effect of treatment on acute
exacerbations
During the study, acute exacerbation in the
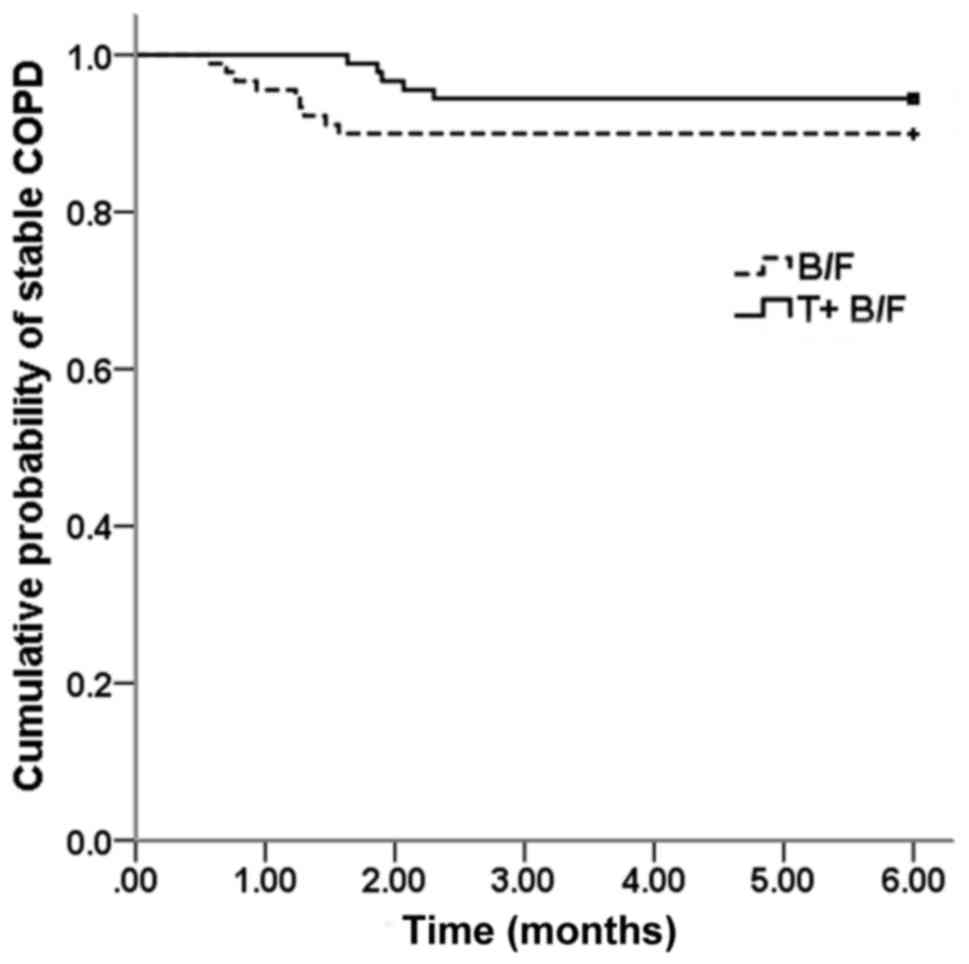
intervention group and the control group occurred in 5 (5.6%) and 9
cases (10%), respectively. Survival analysis indicated that the
difference in the risk of acute exacerbation between the two groups
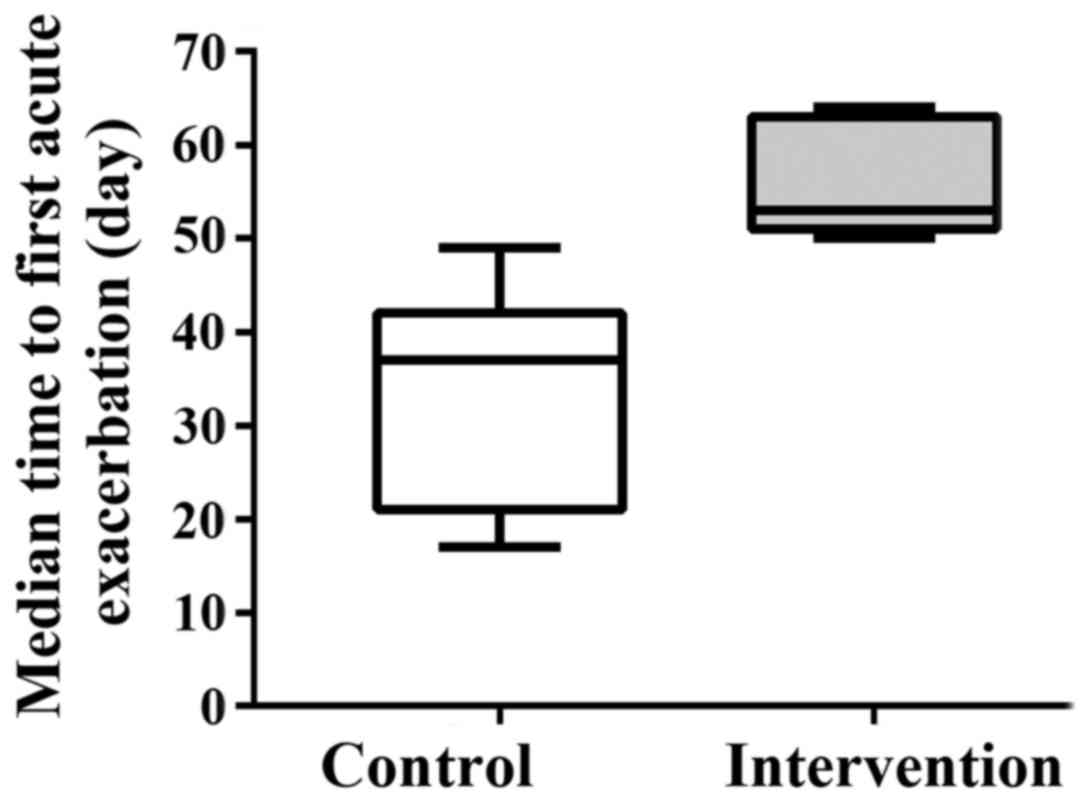
was not significant (log-rank test, P=0.238; Fig. 1). However, the median time to the
first acute exacerbation from the start of the treatment in the
intervention group was 53 days (25% quartile, 50 days; 75%
quartile, 62 days), which was significantly longer than that in the
control group (median, 37 days; 25% quartile, 23 days; 75%
quartile, 39 days; Mann-Whitney U test, P=0.042) (Fig. 2).
Adverse reactions
The incidence of adverse reactions in the
intervention group and control group was 14.4 and 10.0%,
respectively, and there was no significant difference in the rate
of adverse reactions between the two groups. The major adverse
reactions included dry mouth, pharynx discomfort, oral ulcer,
dysuria and sinus arrhythmia (Table
IV). Furthermore, 1 patient in the intervention group and 1
patient in the control group dropped out due to their perceived
lack of a therapeutic effect. No mortalities occurred during
study.
 | Table IV.Adverse reactions in the two
groups. |
Table IV.
Adverse reactions in the two
groups.
| Adverse
reactions | Intervention
(n=90) | Control group
(n=90) | χ2 | P-value |
|---|
| No | 77 (85.6) | 81 (90.0) | 0.829 | 0.363 |
| Yes | 13 (14.4) | 9
(10.0) |
|
|
| Dry mouth | 5
(5.6) | 3
(3.3) |
| 0.720a |
| Pharynx
discomfort | 2
(2.2) | 2
(2.2) |
| 1.000a |
| Oral ulcer | 2
(2.2) | 2
(2.2) |
| 1.000a |
| Dysuria | 2
(2.2) | 2
(2.2) |
| 1.000a |
| Sinus
tachycardia | 2
(2.2) | 0
(0.0) |
| 0.497a |
Discussion
Acute exacerbation of COPD is mainly caused by
airway obstruction, which is triggered by chronic inflammation of
the airways, lung parenchyma and blood vessels, resulting in a
limitation of lung function and serious impairment of the quality
of life of affected patients. However, a study has indicated that
the level of inflammatory markers in patients with COPD in the
stable phase remains higher than that in healthy individuals,
suggesting that inflammation prevails in the stable phase (13). Therefore, the major treatment target
in the stable phase COPD is to control inflammation, relieve
symptoms, improve exercise tolerance and the health status, as well
as to prevent acute exacerbation (14).
Tiotropium bromide is a novel cholinolytic
bronchodilator, which dilates the bronchus through competing with
acetylcholine and combining with the M1 and
M3 receptors; the dissociation time with the
M3 receptor is up to 35 h, resulting in long-lasting
anti-cholinergic effects: After one administration, the dilation of
the bronchus is maintained for ≥24 h. In addition, through
competitive inhibition of the combination of acetylcholine and the
M receptor, it suppresses inflammation and airway remodeling
(15,16). The 4-year large-scale prospective
randomized double-blinded placebo-controlled UPLIFT trial indicated
that compared with placebo, tiotropium bromide continuously and
effectively improved the lung function and quality of life of COPD
patients, and reduced the risk of exacerbation and all-cause
mortality; while it failed to improve the downward trend of the
FEV1, subgroup analysis indicated that the decrease rate
of FEV1 in young patients was significantly improved
(17,18). A systematic evaluation, which
compared tiotropium bromide with placebo, further confirmed these
results (19). In addition, a study
also demonstrated that tiotropium bromide and budesonide/formoterol
had similar effects in the prevention of acute exacerbation
(20).
Formoterol, as a highly selective long-acting
β2 receptor agonist, improves airway obstruction and
pulmonary function of COPD patients, relieves symptoms and
increases the intake of glucocorticoid receptor/liposome complexes
(21). Formoterol and tiotropium
bromide further increase the bronchiectatic effect, but with a
different mechanism (22).
Furthermore, budesonide, an efficient local anti-inflammatory
glucocorticoid, inhibits the production of inflammatory factors and
the activity of inflammatory cells from multiple links, so as to
improve airway inflammation, prevent airway remodeling and increase
the number of cell membrane β2 receptor. Theoretically,
tiotropium bromide, budesonide and formoterol should have
synergistic effects (23). A study
has indicated that compared with tiotropium bromide alone, the
combination of tiotropium bromide and budesonide/formoterol more
effectively improves pulmonary function, mMRC and 6MWT, reduces
acute exacerbation and is also preferable due to its
cost-effectiveness (24). Systematic
reviews have indicated that the combination of the above three
drugs obviously decreased the acute exacerbation of COPD, but their
evaluation indices did not include lung function, nutritional
status and exercise capacity, which ware of great significance in
the evaluation of COPD; furthermore, the analysis suggested that
budesonide and formoterol may be associated with an increased risk
of pneumonia (25,26). Thus, the efficacy and safety of the
combination of the three drugs should be further evaluated.
The results of the present study demonstrated that
the lung function, mMRC, 6MWT and SGRQ scores were significantly
improved in the two groups after treatment, but the improvements in
the intervention group were more significant; however, the
difference in the adverse reaction rates between the two groups was
not significant. These results were similar to those of previous
analogous studies, which indicated that compared with
budesonide/formoterol alone, tiotropium bromide combined with
budesonide/formoterol significantly improved indicators of COPD,
including pulmonary function, quality of life and exercise
capacity, but that adverse reactions were not significantly
different from those in the budesonide/formoterol treatment group
(21,27).
Previous studies have demonstrated that tiotropium
bromide combined with budesonide/formoterol significantly reduced
the risk of acute exacerbation in patients with COPD (28,29). In
the present study, Kaplan-Meier analysis indicated that there was
no significant difference in the risk of acute exacerbations
between the two groups, which may be due to the patients' different
state of COPD among the studies. In one study, COPD was complicated
with respiratory failure in all subjects (29), while the subjects of the present
study were patients with moderate to severe COPD, so the incidence
of acute exacerbations in the short study period was relatively
lower, and the difference in the risk of acute exacerbations
between the two groups was not significant, which was similar to
the results of another two studies on the combined application of
tiotropium bromide with fluticasone/salmeterol (5,30).
However, in the present study, the time to the first acute
exacerbation from the start of the treatment in the intervention
group was significantly longer compared with that in the control
group. Thus, further evaluation is required to elucidate whether
the combination of tiotropium bromide and budesonide/formoterol has
a better efficacy in reducing the risk of acute exacerbation
compared with budesonide/formoterol alone. The nutritional status
in each of the two groups was significantly improved when compared
with that prior to treatment, but the difference between the two
groups was not significant, which was similar to the result of
another study (27); possible
reasons for the insignificant difference between the groups may be
the short study period and the small sample size. In addition, the
improvement of the quality of life in the intervention group was
significantly greater than that in the control group, but according
to a meta-analysis, the difference in the improvement of the SGRQ
score between COPD patients treated with tiotropium bromide
combined with budesonide/formoterol and those with tiotropium
bromide alone was not statistically significant (8).
Of note, the present study had various shortcomings,
including a small sample size, short-term evaluation of the
efficacy and safety, and unavoidable bias from researchers and
patients, as they were not blinded to the treatments. Therefore,
the efficacy and long-term safety of tiotropium bromide combined
with budesonide/formoterol requires further assessment in a
randomized double-blinded controlled trial with a larger sample
size.
In conclusion, the present study comprehensively
evaluated the efficacy of inhalation therapy of tiotropium bromide
combined with budesonide/formoterol in patients with moderate to
severe COPD from the aspects of lung function, nutritional status,
degree of dyspnea and exercise capacity. It was demonstrated that
the combination of tiotropium bromide and budesonide/formoterol is
significantly more efficacious than budesonide/formoterol alone
with regard to various endpoints, including the improvement of lung
function.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DZ was the guarantor for integrity of the study and
contributed to study concepts, experimental studies and data
analysis. CL contributed to study design and statistical analysis.
QG contributed to statistical analysis, provided intellectual
content and revised the manuscript. HX contributed to the
literature research, clinical studies and manuscript preparation.
JJ contributed to manuscript review and data acquisition. The final
version of the manuscript was read and approved by all authors.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Soochow University.
All patients provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
COPD
|
chronic obstructive pulmonary
disease
|
|
FEV1
|
forced expiratory volume in 1
second
|
|
FVC
|
forced vital capacity
|
|
FEV1%pred
|
FEV1 as percentage of
predicted value
|
|
BMI
|
body mass index
|
|
mMRC
|
modified Medical Research Council
|
|
6MWT
|
six-minute walking test
|
|
SGRQ
|
St. George's Respiratory
Questionnaire
|
References
|
1
|
Yin P, Wang H, Vos T, Li Y, Liu S, Liu Y,
Liu J, Wang L, Naghavi M, Murray CJ and Zhou M: A subnational
analysis of mortality and prevalence of COPD in China From 1990 to
2013: Findings from the global burden of disease study 2013. Chest.
150:1269–1280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan WJ, Ran PX and Zhong NS: Prevention
and management of COPD in China: Successes and major challenges.
Lancet Respir Med. 4:428–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
COPD Group of Chinese Thoracic Society:
Guidelines for the diagnosis and treatment of chronic obstructive
pulmonary disease (2013 revised edition), . Chin J Tuberculosis
Respiratory Dis. 4:255–264. 2013.(In Chinese).
|
|
4
|
Tunks M and Miller D: Canadian Thoracic
Society recommendations for management of chronic obstructive
pulmonary disease-2008 update-highlights for primary care. Can
Respir J. 15:2192008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung KS, Park HY, Park SY, Kim SK, Kim YK,
Shim JJ, Moon HS, Lee KH, Yoo JH, Lee SD, et al: Comparison of
tiotropium plus fluticasone propionate/salmeterol with tiotropium
in COPD: A randomized controlled study. Respir Med. 106:382–389.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farne HA and Cates CJ: Long-acting
beta2-agonist in addition to tiotropium versus either tiotropium or
long-acting beta2-agonist alone for chronic obstructive pulmonary
disease. Cochrane Database Syst Rev: CD008989. 2015. View Article : Google Scholar
|
|
7
|
Hodder R: Tiotropium is superior to
salmeterol in reducing frequency of exacerbations: But the effect
of adding tiotropium to the combination of inhaled corticosteroid
and long-acting β(2)-agonist remains unclear. Evid Based Med.
17:93–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng LM, Yu XL, Zhang QH, Peng QM, Zhou ZJ
and Han FD: The Efficacy and Safety of Tiotropium Plus
Budesonide/Formoterol Compared with Tiotropium in Chinese Patients
with Chronic Obstructive Pulmonary Disease: A Meta-analysis. Chin
Zhong Guo Hu Xi Yu Wei Zhong Jian Hu Za Zhi. 2:119–126. 2016.(In
Chinese).
|
|
9
|
Zhang WJ, Li H, Liu XH and Chen J: Study
on the clinical efficacy of combined inhalation of
budesonide/formoterol and tiotropium bromide in the treatment of
moderate and severe COPD. Guide China Med. 36:148–149. 2013.
|
|
10
|
Mahler DA and Wells CK: Evaluation of
clinical methods for rating dyspnea. Chest. 93:580–586. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
ATS Committee on Proficiency Standards for
Clinical Pulmonary Function Laboratories, . ATS statement:
Guidelines for the six-minute walk test. Am J Respir Crit Care Med.
166:111–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones PW, Quirk FH, Baveystock CM and
Littlejohns P: A self-complete measure of health status for chronic
airflow limitation. The St. George's respiratory questionnaire. Am
Rev Respir Dis. 145:1321–1327. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kersul AL, Iglesias A, Rios A, Noguera A,
Forteza A, Serra E, Agusti A and Cosio BG: Molecular mechanisms of
inflammation during exacerbations of chronic obstructive pulmonary
disease. Arch Bronconeumol. 47:176–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YH and Wang C: Breif introduction of
GOLD global strategy for diagnosis, treatment and prevention of
chronic obstructive pulmonary disease (2015 revised edition). Zhong
Guo Yi Xue Qian Yan Za Zhi: Dian Zi Ban. 7:34–39. 2015.(In
Chinese).
|
|
15
|
Bateman ED, Rennard S, Barnes PJ,
Dicpinigaitis PV, Gosens R, Gross NJ, Nadel JA, Pfeifer M, Racké K,
Rabe KF, et al: Alternative mechanisms for tiotropium. Pulm
Pharmacol Ther. 22:533–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toumpanakis D, Loverdos K, Tzouda V,
Vassilakopoulou V, Litsiou E, Magkou C, Karavana V, Pieper M and
Vassilakopoulos T: Tiotropium bromide exerts anti-inflammatory
effects during resistive breathing, an experimental model of severe
airway obstruction. Int J Chron Obstruct Pulmon Dis. 12:2207–2220.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tashkin DP: Impact of tiotropium on the
course of moderate-to-very severe chronic obstructive pulmonary
disease: The UPLIFT trial. Expert Rev Respir Med. 4:279–289. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morice AH, Celli B, Kesten S, Lystig T,
Tashkin D and Decramer M: COPD in young patients: A pre-specified
analysis of the four-year trial of tiotropium (UPLIFT). Respir Med.
104:1659–1667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karner C, Chong J and Poole P: Tiotropium
versus placebo for chronic obstructive pulmonary disease. Cochrane
Database Syst Rev: CD009285. 2012. View Article : Google Scholar
|
|
20
|
Vogelmeier C, Hederer B, Glaab T, Schmidt
H, Rutten-van Molken MP, Beeh KM, Rabe KF and Fabbri LM; POET-COPD
Investigators, : Tiotropium versus salmeterol for the prevention of
exacerbations of COPD. N Engl J Med. 364:1093–1103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye Q, Zhang LB, Fan RM, Li JM, Hao Q and
Zhao LY: Curative effect of the combination of Symbicort Turbuhaler
and Tiotropium Bromide in the treatment of severe stable chronic
obstructive pulmonary disease patients. Zhong Hua Fei Bu Ji Bing Za
Zhi: Dian Zi Ban. 7:45–48. 2014.(In Chinese).
|
|
22
|
Cazzola M and Molimard M: The scientific
rationale for combining long-acting beta2-agonists and muscarinic
antagonists in COPD. Pulm Pharmacol Ther. 23:257–267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peters SP: Tiotropium bromide triple
combination therapy improves lung function and decreases asthma
exacerbations. Evid Based Med. 18:1792013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nielsen R, Kankaanranta H, Bjermer L,
Lange P, Arnetorp S, Hedegaard M, Stenling A and Mittmann N: Cost
effectiveness of adding budesonide/formoterol to tiotropium in COPD
in four Nordic countries. Respir Med. 107:1709–1721. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tricco AC, Strifler L, Veroniki AA, Yazdi
F, Khan PA, Scott A, Ng C, Antony J, Mrklas K, D'Souza J, et al:
Comparative safety and effectiveness of long-acting inhaled agents
for treating chronic obstructive pulmonary disease: A systematic
review and network meta-analysis. BMJ Open. 5:e0091832015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dal Negro RW and Celli BR: Patient related
Outcomes-BODE (PRO-BODE): A composite index incorporating health
utilization resources predicts mortality and economic cost of COPD
in real life. Respir Med. 131:175–178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li HF, Leng J and Huang W: Observation of
curative effect in group C, D COPD pateints treated by inhalation
of tiotropium bromide combined with budesonide/formoterol. J
Kunming Med Univ. 35:62–65. 2014.
|
|
28
|
Lee SD, Xie CM, Yunus F, Itoh Y, Ling X,
Yu WC and Kiatboonsri S: Efficacy and tolerability of
budesonide/formoterol added to tiotropium compared with tiotropium
alone in patients with severe or very severe COPD: A randomized,
multicentre study in East Asia. Respirology. 21:119–127. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Q, Wang M and Du CJ: Observation of
curative effect in pateints with COPD Complicated with chronic
respiratory failure treated by budesonide/formoterol combined with
tiotropium bromide. Chin Health Stand Manag. 6:108–110. 2015.
|
|
30
|
Aaron SD, Vandemheen KL, Fergusson D,
Maltais F, Bourbeau J, Goldstein R, Balter M, O'Donnell D, McIvor
A, Sharma S, et al: Tiotropium in combination with placebo,
salmeterol, or fluticasone-salmeterol for treatment of chronic
obstructive pulmonary disease: A randomized trial. Ann Intern Med.
146:545–555. 2007. View Article : Google Scholar : PubMed/NCBI
|