Introduction
Iron is one of the most important minerals in an
organism, and is crucial for electron transport, the synthesis of
nucleic acids and proteins, and proliferation and differentiation
of cells (1). The majority of iron
in the body iron is found in red blood cells, whereas a lower
amount is incorporated into the structure of myoglobin and enzymes.
Iron absorption and utilization is regulated by a series of
processes involving molecules, including ferroportin and hepcidin
(2). These processes are fine tuned
to maintain the iron balance in an organism. Iron deficiency and
iron overload may lead to different problems in the body. Iron
overload may lead to iron accumulation in different organs and
tissues including the liver, brain and endocrine glands (3). Diseases characterized by iron
accumulation, including hereditary hemochromatosis and thalassemia,
may damage testicular tissue in humans leading to hypogonadism and
decreased fertility (4,5). Iron overload has previously been
demonstrated to induce oxidative damage in the testes in a number
of animal and human studies (6–9). Iron
toxicity results in morphological changes in the seminiferous
tubules, epididymes and sertoli cells (10). Iron toxicity may also damage DNA in
sperm, which means there is a risk that offspring may inherit gene
mutations (11). To date, certain
agents, such as α-tocopherol (12)
or growth hormone/insulin like growth factor-1 (13) have been evaluated in the prevention
of iron induced oxidative stress in the testes.
Glucagon-like peptide-1 (GLP-1) has been
demonstrated to affect the oxidative stress status in several in
vitro (14,15), in vivo (16,17) and
clinical (18,19) studies. GLP-1 and its agonists are
well known to improve glycemic control, decrease food intake,
increase insulin release and increase insulin sensitivity which may
contribute to reduced oxidative stress, but direct effects on
reactive oxygen species (ROS) and antioxidant capacity have also
been suggested to serve a role (20).
Exenatide (active ingredient, exendin-4) is a GLP-1
receptor agonist (GLP-1RA) that is used in the treatment of type 2
diabetes (21). The aim of the
present study to evaluate the effect of exenatide on oxidative
stress parameters and apoptotic markers in the testicular cells of
an iron overload rat model.
Materials and methods
Animals and experimental protocol
The present study was carried out in the Physiology
Laboratory of the Gazi University Medical Faculty (Ankara, Turkey),
and was approved by the Gazi University Ethics Committee of
Experimental Animals. All methods were in accordance with the Guide
for the Care and Use of Laboratory Animals (22).
In the present study, 18 male Wistar Albino rats
weighing between 250 and 300 g and aged 9–10 weeks, raised under
the same environmental conditions, were used. The rats were kept at
20–21°C 50±10% humidity, in a 12-h light/dark cycle and had free
access to food until 2 h prior to the anesthesia procedure. Rats
were randomly divided into the three groups (n=6/group). Rats in
the control group (Group C) received intraperitoneal injections of
saline. Intraperitoneal iron dextran (Cosmofer®; 50
mg/ml; Assos Pharmaceuticals Ilaç, Istanbul, Turkey), was
administered at a dose of 60 mg/kg/day to the second group (Group
Fe), 5 days a week for 4 weeks. The third group (Group Fe + E) was
administered subcutaneous injections of 10 µg/kg exenatide
(Byetta®; Eli Lilly and Company, Indianapolis, IN, USA)
in two divided doses for 4 weeks in addition to intraperitoneal
iron dextran (60 mg/kg/day). All rats were administered
intramuscular ketamine hydrochloride (100 mg/kg; Ketalar;
Parke-Davis; Pfizer, Inc., New York, NY, USA) and xylazine
hydrochloride (Alfazyne, 2%; Ege Vet, Ltd., Izmir, Turkey), and
intracardiac blood samples (≤10 ml) were obtained. The rats were
sacrificed and all rat testes were immediately removed for
immunohistochemical analyses and sera were used for biochemical
experiments.
Immunohistochemical evaluation
Tissues were fixed in 10% formaldehyde for 12 h at
room temperature. Sections (3–4 µm thick) were cut from the fixed
tissue samples, embedded in paraffin blocks and mounted on
poly-L-lysine-coated slides (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The sections were left overnight at 45°C. The sections
were held for 20 min at 75°C, followed by tap fixation and paraffin
extraction. Deparaffinization was performed with a Leica Bond-Max
automatic immunohistochemical/in situ hybridization stainer
(Leica Microsystems GmbH, Wetzlar, Germany). Citrate buffer was
applied for antigen retrieval for 30 min at 75°C and washed with
bond wash solution (Leica Microsystems GmbH). Sections were blocked
with 0.3% hydrogen peroxide for 5 min at room temperature. Sections
were then incubated with primary antibodies against caspase-3
(1:400; p11, C-6; cat. no. sc-271759; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and caspase-8 (1:200; D-8; cat. no. sc-5263;
Santa Cruz Biotechnology, Inc.) for 15 min at room temperature. The
secondary antibodies (Leica Biosystems Newcastle Ltd., Newcastle
Upon Tyne, UK) were incubated with cells for 8 min at room
temperature. The Bond™ Polymer Refine Detection system
(cat. no. DS9800; Leica Biosystems Newcastle Ltd.) was then added
as a horseradish peroxidase polymer (a secondary antibody
substitute) at room temperature for 8 min at room temperature. DAB
(Leica Microsystems GmbH) was applied to the cells for 6 min at
room temperature and the marking became visible. Hematoxylin
counterstaining was also performed at 6 min at room
temperature.
The stained samples were covered with balsam
following washing in water and alcohol and cleared in xylene. The
density and intensity of cytoplasmic caspase-3 and caspase-8
staining were evaluated in seminiferous tubules, stromal cells and
endothelia using a light microscope (Nikon Eclipse E600; Nikon
Corporation, Tokyo, Japan) at a magnification of ×40 and ×100. The
density and intensity were assessed by a pathologist. Staining
intensity was scored as: 0, no staining; 1, mild and 2, intense.
Samples were assessed using the microscope. If >50% of the
seminiferous tubules, stromal and endothelial cells on the section
were stained.
Perls Prussian Blue (NovaUltra; IHC WORLD, LLC.,
Woodstock, MD, USA) was applied to visualize the accumulation of
iron in the tissue. Briefly, the 4-µm sections were deparaffinized
and dehydrated. Solutions of 2% potassium ferrocyanide and 2%
hydrochloride solution were mixed at a ratio of 1:1, and 200 µl was
applied to each section for 20 min at room temperature. Sections
were then washed in tap water for 2–3 min, and fast red was applied
for 5 min at room temperature. Sections were subsequently washed
again in tap water, alcohol and xylene. A mounting medium was added
to the sections, which were then covered with a coverslip. The
concentration of iron accumulation in stromal cells was evaluated
in hematoxylin and eosin staining as follows: 0, no accumulation;
1, mild accumulation; 2, intensive accumulation.
For hematoxylin and eosin staining, slides were kept
in and oven at 72°C for 20 min, deparaffinized in xylene solution
and washed with alcohol three times. Sections were then incubated
in hematoxylin for 4 min at room temperature, washed and exposed to
acid-alcohol and ammonia solutions for a few second. The slides
were then incubated in eosin for 6 min at room temperature, and
then immersed in a descending alcohol series and xylene. Stained
slides were covered with slip, then evaluated with a light
microscope at a magnification of ×100.
Serum superoxide dismutase (SOD)
enzyme activity
Total SOD enzyme activity was measured as previously
described (23), based on measuring
the absorbance increase at 560 nm caused by nitro blue tetrazolium
reduction to diformazan precipitate (NBTH2). One unit of SOD
activity was the amount of enzyme that led to 50% inhibition in the
reduction rate of NBTH2. Data were expressed in units/ml.
Measurement of serum malondialdehyde
(MDA) levels
A thiobarbituric acid (TBA) reactive substances
assay was performed, with a minor modification, as previously
detailed by Van Ye et al (24). The modification was as follows:
proteins were precipitated to remove the adverse effects of protein
residues on the experiment. The sample was mixed with 20% (w/v)
trichloroacetic acid and the precipitate was then centrifuged at
room temperature for 10 min at 1,100 × g. The reaction with TBA at
90–100°C was used to determine the MDA level, as MDA or similar
substances react with TBA and produce a pink pigment (25) that has an absorption maximum of 532
nm (26). To ensure protein
precipitation, the sample is mixed with 4°C 20% (wt/vol)
trichloroacetic acid and the precipitate is then centrifuged for 10
min at 1,100 × g and room temperature to form a pellet. An aliquot
of the supernatant is then placed into an equal volume of 0.6%
(wt/vol) TBA in a boiling water bath for 30 min. Following cooling,
sample and blank absorbance were read at 532 nm and the results
were expressed as nM/ml, based on a graph by our group where
1,1,3,3-tetramethoxypropane was used as the MDA standard (27).
Statistical analysis
SPSS (IBM Corp., Armonk, NY, USA; version 20.0) was
used for statistical analysis. Data were assessed using
Kruskal-Wallis test with a post hoc Bonferroni-adjusted
Mann-Whitney U test. Data are presented as the mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
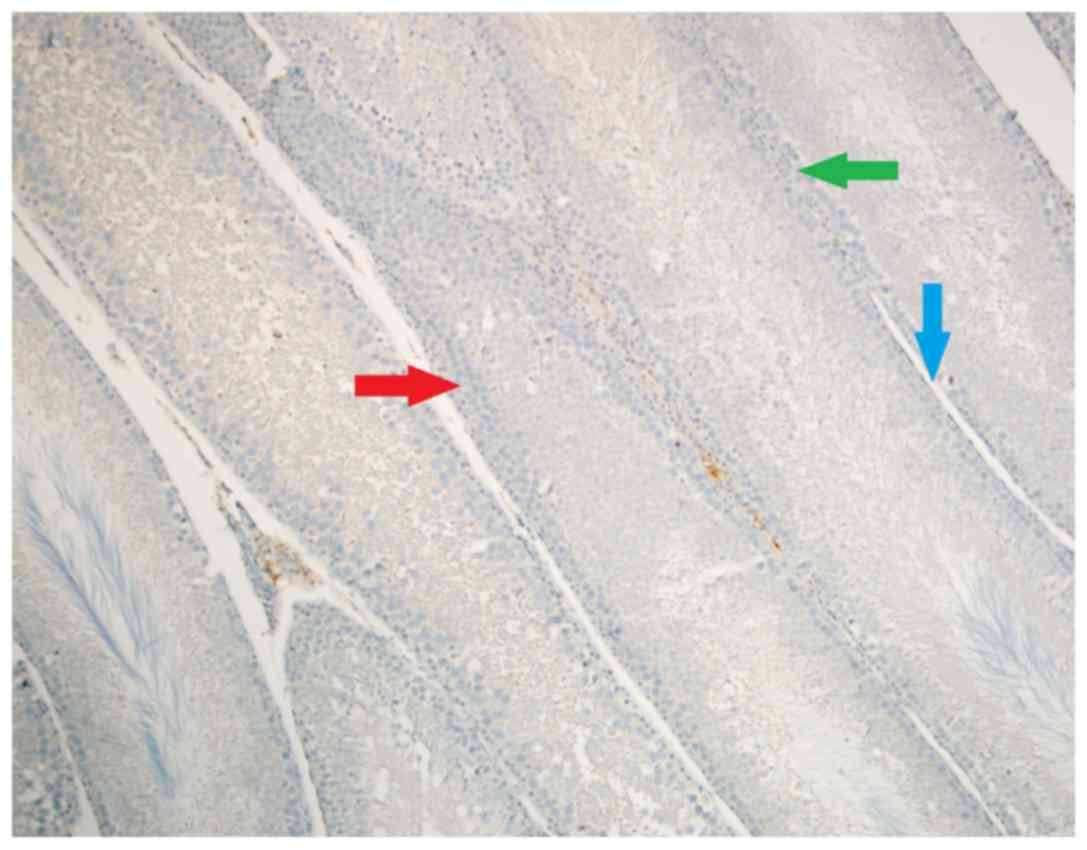
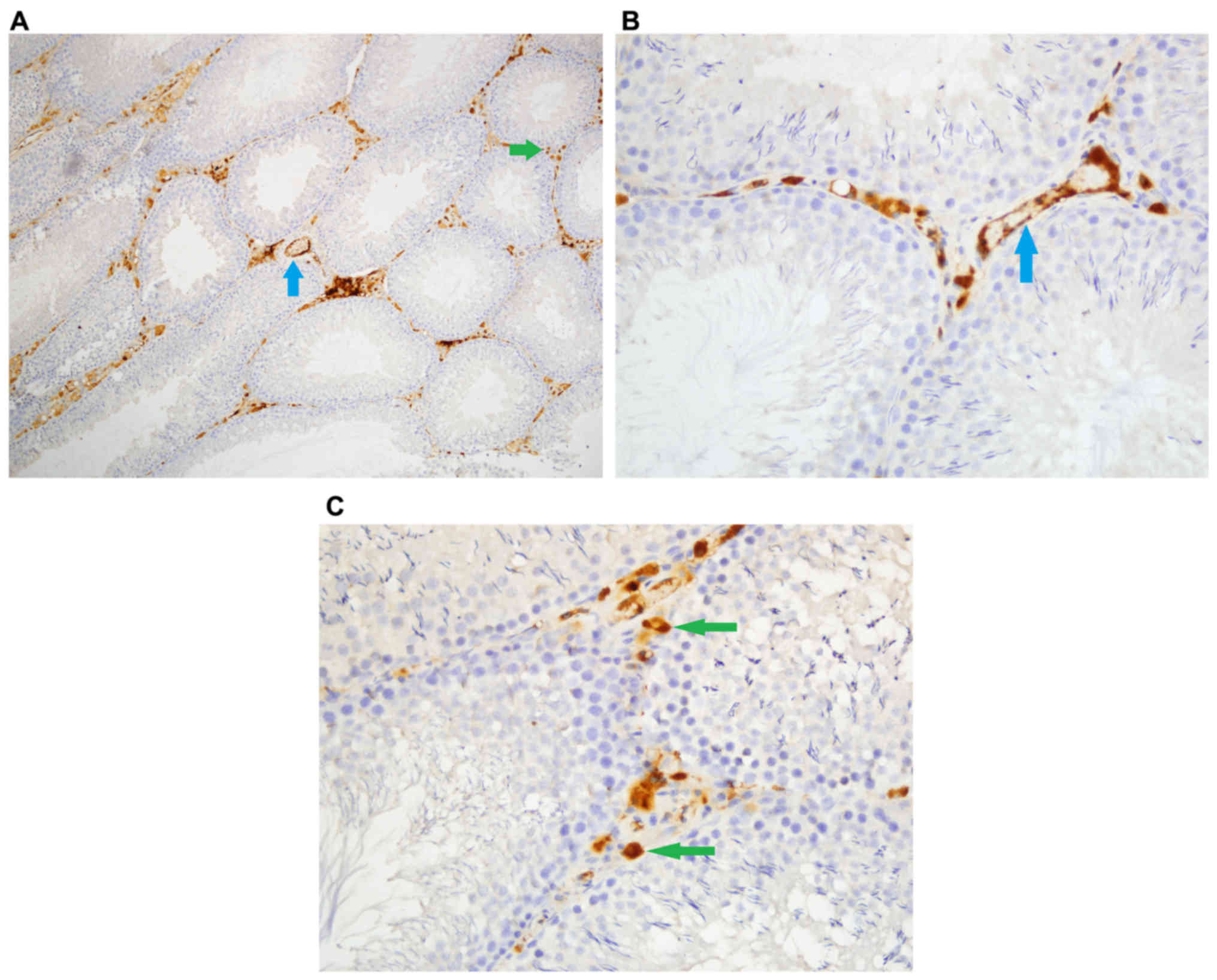
Caspase-8 staining
Density of stromal cell caspase-8 enzyme activity
was significantly different among groups (P<0.05). Stromal cell
caspase-8 staining density was significantly higher in Group Fe and
Group Fe+E when compared with Group C (both P<0.05) (Table I; Figs.
1–3). In addition, stromal cell
caspase-8 staining density was notably higher in Group Fe compared
with Group Fe+E (P<0.05) (Table
I; Figs. 1–3).
 | Table I.Caspase-8 staining in testis
tissue. |
Table I.
Caspase-8 staining in testis
tissue.
| Staining | Group C | Group Fe | Group Fe+E | Kruskal-Wallis
P-value |
|---|
| Seminiferous
tubules | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | – |
| Stromal cells,
density | 0.00±0.00 |
2.00±0.00a |
1.33±0.21a,b | <0.05 |
| Stromal cells,
intensity | 0.00±0.00 |
1.67±0.21a |
1.50±0.22a | <0.05 |
| Endothelial cells,
density | 0.00±0.00 |
1.33±0.21a |
0.67±0.21a,b | <0.05 |
| Endothelial cells,
intensity | 0.00±0.00 |
1.33±0.21a |
0.83±0.31a | <0.05 |
Intensity of caspase-8 staining in stromal cells was
significantly different among groups (P<0.05). Stromal cell
caspase-8 staining intensity was significantly higher in Group Fe
and Group Fe+E when compared with Group C (P< P<0.05)
(Table I; Figs. 1–3).
Caspase-8 staining density in endothelial cells was
significantly different among groups (P<0.05). Caspase-8
staining density in endothelial cells was significantly higher in
Group Fe and Group Fe+E when compared with Group C (both
P<0.05). Caspase-8 staining density in endothelial cells was
significantly lower in Group Fe+E when compared with Group Fe
(P<0.05) (Table I; Figs. 1–3).
Caspase-8 staining intensity in endothelial cells
was significantly higher in Group Fe and Group Fe+E when compared
with Group C (both P<0.05) (Table
I; Figs. 1–3). Caspase-8 enzyme activity in
seminiferous tubules was similar among all groups (Table I; Figs.
1–3).
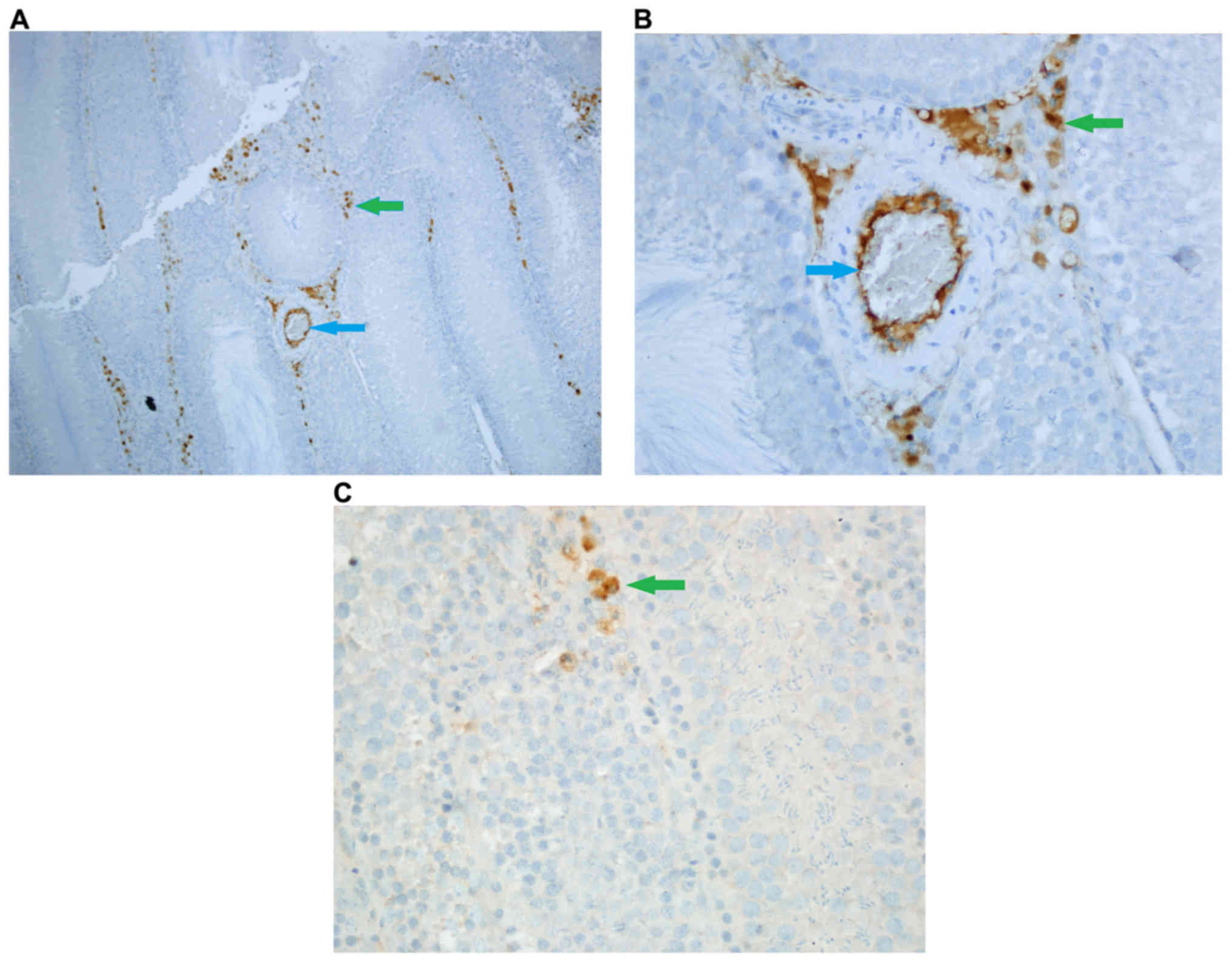
Caspase-3 staining
Density of caspase-3 staining in stromal cells was
significantly different among groups (P<0.05). Stromal cell
caspase-3 staining density was significantly higher in Group Fe and
Group Fe+E when compared with Group C (both P<0.05). Stromal
cell caspase-3 staining density was significantly lower in Group
Fe+E when compared with Group Fe (P<0.05) (Table II; Figs.
4–6).
 | Table II.Caspase-3 staining in testis
tissue. |
Table II.
Caspase-3 staining in testis
tissue.
| Staining | Group C | Group Fe | Group Fe+E | Kruskal-Wallis
P-value |
|---|
| Seminiferous
tubules, density | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | – |
| Seminiferous
tubules, intensity | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | – |
| Stromal cells,
density | 0.00±0.00 |
2.00±0.00a |
1.50±0.22a,b | <0.05 |
| Stromal cells,
intensity | 0.00±0.00 |
2.00±0.00a |
1.33±0.21a,b | <0.05 |
| Endothelial cells,
density | 0.00±0.00 |
2.00±0.00a |
1.50±0.22a,b | <0.05 |
| Endothelial cells,
intensity | 0.00±0.00 |
2.00±0.00a |
1.33±0.21a,b | <0.05 |
Intensity of caspase-3 staining in stromal cells was
significantly different among groups (P<0.05). Stromal cell
caspase-3 staining intensity was significantly higher in Group Fe
and Group Fe+E when compared to Group C (both P<0.05). Stromal
cell caspase-3 staining intensity was significantly lower in Group
Fe+E when compared to Group Fe (P<0.05) (Table II, Figs.
4, 5A-C, and 6A, B).
Caspase-3 staining density in endothelial cells was
significantly different among groups (P<0.05). Caspase-3
staining density in endothelial cells was significantly higher in
Group Fe and Group Fe+E when compared with Group C (both
P<0.05). Caspase-3 staining density in endothelial cells was
significantly lower in Group Fe+E when compared with Group Fe
(P<0.05) (Table II; Figs. 4–6).
Caspase-3 staining intensity in endothelial cells
was significantly different among groups (P<0.05). Caspase-3
staining intensity in endothelial cells was significantly higher in
Group Fe and Group Fe+E when compared with Group C (both
P<0.05). Caspase-3 staining intensity in endothelial cells was
significantly lower in Group Fe+E when compared with Group Fe
(P<0.05) (Table II; Figs. 4–6).
Caspase-3 enzyme activity in seminiferous tubules was similar among
all groups (Table II; Figs. 4–6).
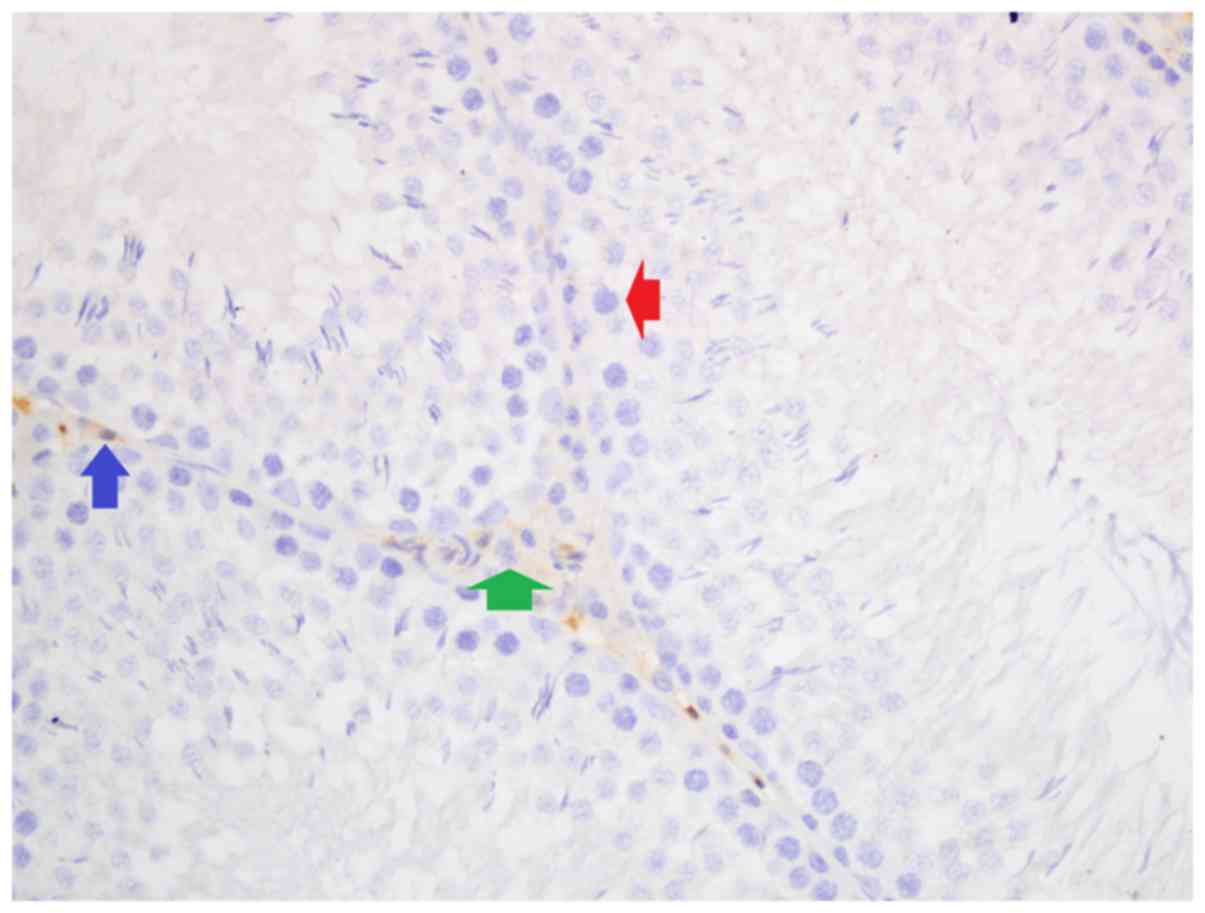
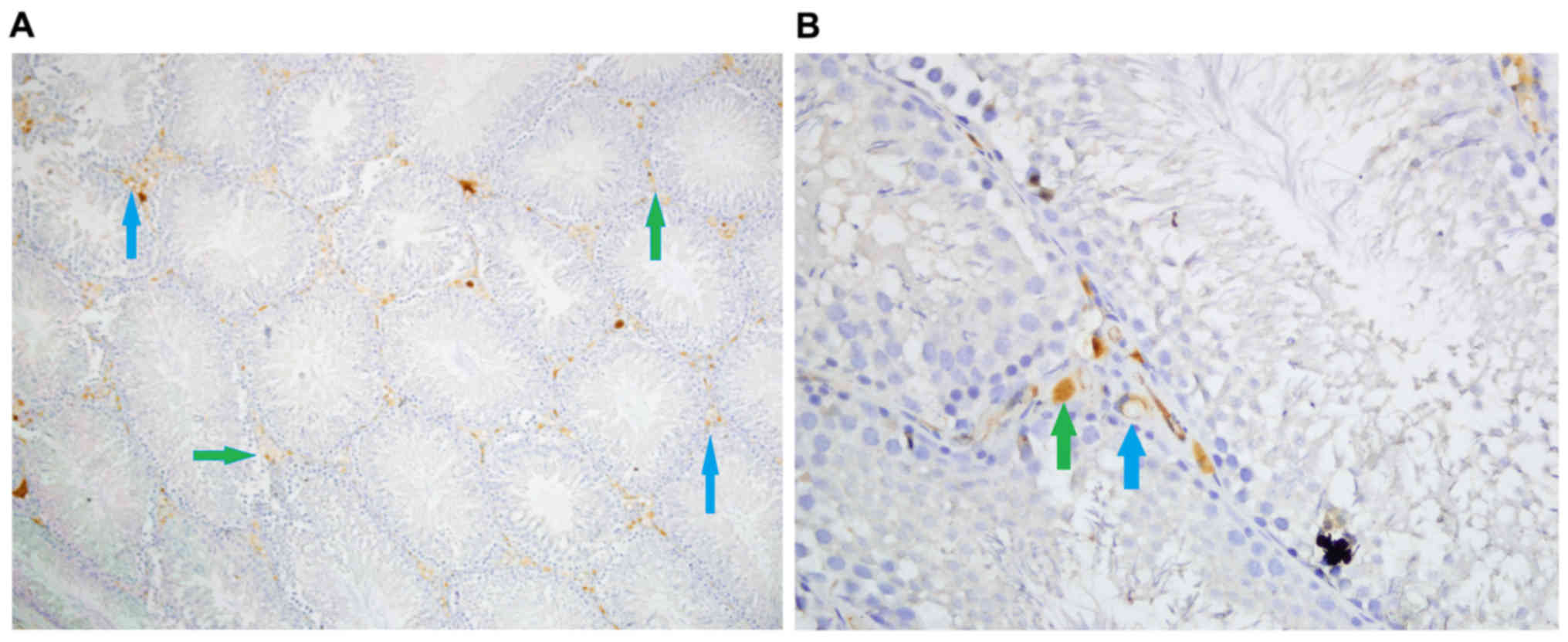
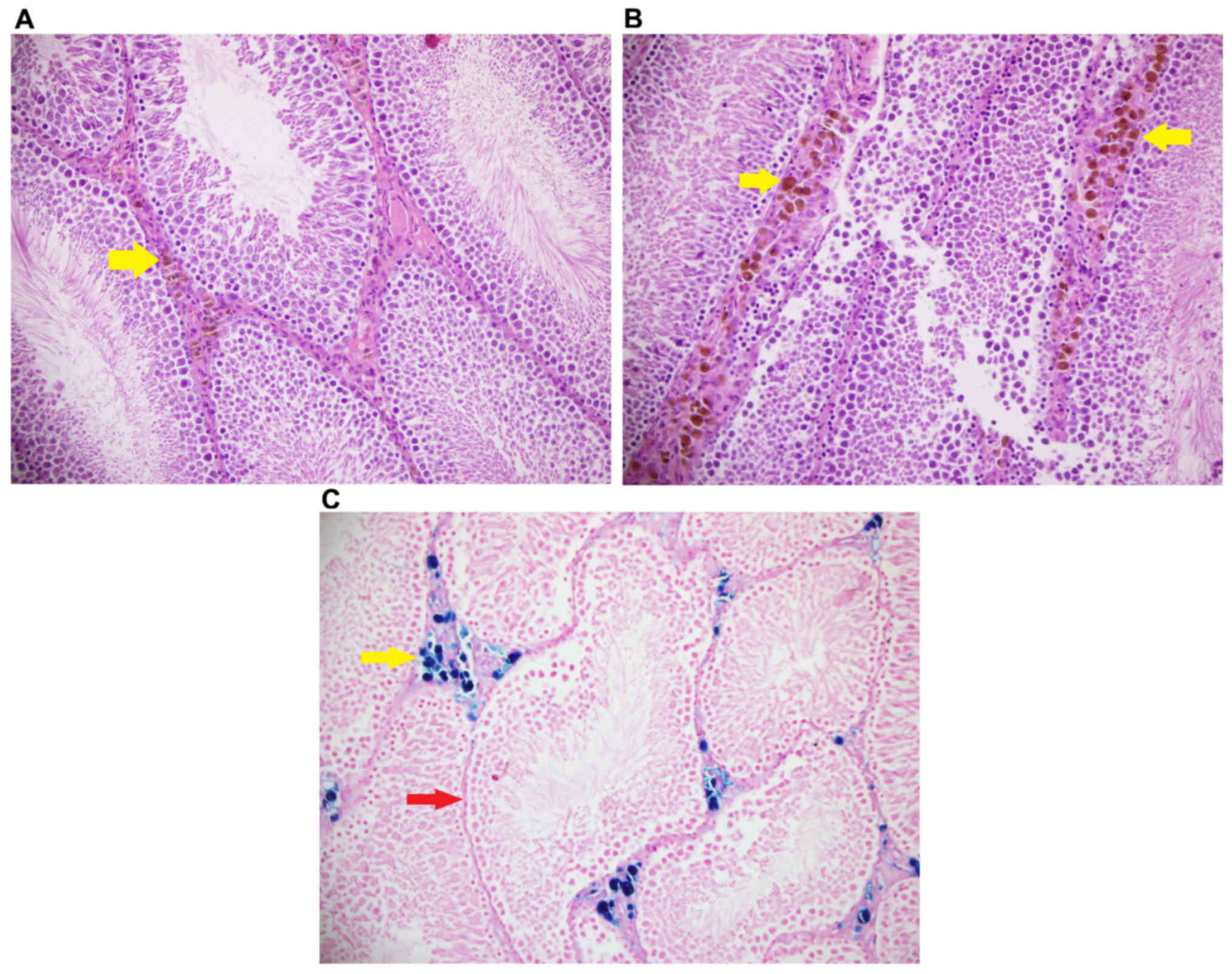
Iron, and hematoxylin and eosin
staining
Iron staining density in stromal cells was
significantly different among groups (P<0.05). Iron staining
intensity in Group Fe and Group Fe+E was significantly higher when
compared with Group C (both P<0.05). Iron staining density in
stromal cells was significantly lower in Group Fe+E when compared
with Group Fe (P<0.05) (Table
III; Figs. 4–6). H&E staining in seminiferous tubules
was not different among groups (Table
III; Fig. 7).
 | Table III.H&E and Iron staining density in
testis tissue. |
Table III.
H&E and Iron staining density in
testis tissue.
| Staining | Group C | Group Fe | Group Fe+E | Kruskal-Wallis
P-value |
|---|
| Seminiferous
tubules, H&E staining density | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | – |
| Stromal cells, iron
staining density | 0.00±0.00 |
2.00±0.00a |
1.50±0.22a,b | <0.05 |
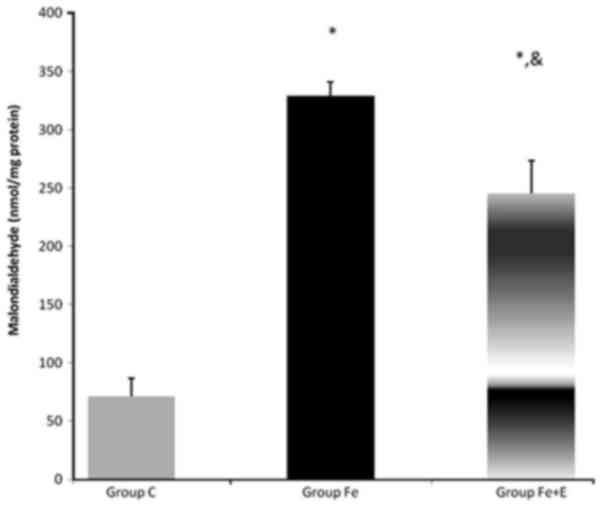
Serum MDA and SOD activity
Serum MDA enzyme activity was significantly
different among groups (P<0.05). MDA enzyme activity was
significantly higher in Group Fe and Group Fe+E when compared with
Group C (both P<0.05). MDA enzyme activity was significantly
lower in Group Fe+E when compared with Group Fe (P<0.05;
Fig. 8).
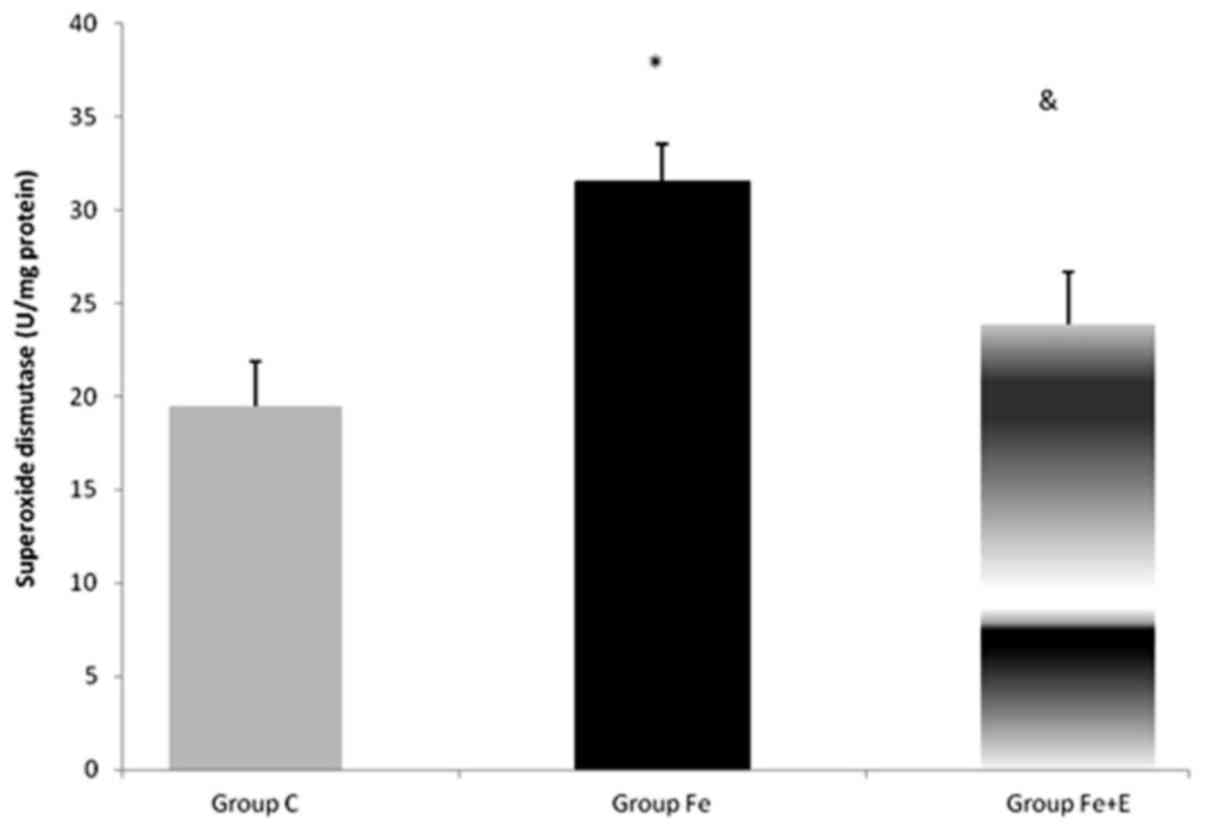
Serum SOD enzyme activity was significantly
different among groups (P<0.05). SOD enzyme activity was
significantly higher in Group Fe when compared with Group C
(P<0.05). SOD enzyme activity was significantly lower in Group
Fe+E when compared with Group Fe (P<0.05; Fig. 9).
Discussion
The beneficial effects of exenatide and exendin-4
include reverting ischemia reperfusion injury (28) and increasing antioxidant enzyme
activity (29). Exenatide has also
been demonstrated to increase sperm motility and quality, and
improve sperm mitochondrial activity and sperm integrity possibly
by reducing the expression of proinflammatory cytokines (30).
Excessive production of reactive oxygen species
causes testicular damage, which can be reverted by administration
of antioxidants (31). Antioxidants
have also been demonstrated to enhance testicular function and
sperm count in rats (32).
Ahangarpour et al (33) created an aging mouse model with D
galactose injections and evaluated the effect of exendin-4 on
age-related changes on the testes. The aforementioned study
demonstrated that the testis weight and volume were decreased as
well as the sperm count. Serum luteinizing hormone (LH) and
follicle stimulating hormone (FSH) levels were increased in the
D-galactose group. Exendin-4 was demonstrated to increase testis
volume and weight. Exendin-4 (1 nmol/kg) also decreased LH and FSH
levels, and increased the serum testosterone level. Exendin-4 also
increased the sperm count in both normal and aging animals. The
authors suggested that exendin-4 administration increased
testicular weight and volume via decreasing free radicals and
increasing antioxidant enzyme activity. In the present study, it
was observed that administering exenatide in an iron overload model
in rats significantly reduced oxidative stress markers in the
testes. This may be associated with the prevention of iron
accumulation, as demonstrated via iron staining, and the
stimulation of antioxidant enzyme activity by exenatide.
Activation of caspases is a crucial step in
apoptosis (34). Caspase-3 is
essential for the terminal or execution pathway of apoptosis, which
results in dismantling of the cell (35), whereas caspase-8 has a role in the
extrinsic pathway of apoptosis (36). In the present study a significant
reduction was observed in caspase-8 and −3 enzyme staining in
testicular stromal and endothelial cells in exenatide injected iron
overloaded rats. This suggests that exenatide may reduce cell death
in testicular tissue. This may be related to a preventive effect of
exenatide against iron accumulation. Further studies are required
to clarify whether exenatide has other contributory effects in the
prevention of testicular cell death. Further studies with terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling are
planned to evaluate the histopathologic changes in detail.
In conclusion, the present study has demonstrated
that exenatide somehow reduces iron accumulation in testis tissue
in an iron overload model in rats. Apoptotic markers caspase-3 and
−8 are lower in iron overload rats that were administered exenatide
at the same time. Oxidative stress markers MDA and SOD enzyme
activities were also lower in exenatide-injected rats. These
findings suggest that exenatide may be protective against iron
accumulation and its harmful effects on testis tissue. Further
studies are required to evaluate how exenatide iron accumulation
reduces oxidative stress and cell death in testis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MA and AKu conceived and designed the study. SY and
SMK wrote the manuscript. NS and AKi analyzed hematoxylin and eosin
staining and immunohistochemistry results. MK analyzed superoxide
dismutase and malondialdehyde activity results. FP and HK reviewed
and edited the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was performed with the approval of
the Gazi University Ethics Committee of Experimental Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lieu PT, Heiskala M, Peterson PA and Yang
Y: The roles of iron in health and disease. Mol Asp Med. 22:1–87.
2001. View Article : Google Scholar
|
|
2
|
Mackenzie EL, Iwasaki K and Tsuji Y:
Intracellular iron transport and storage: From molecular mechanisms
to health implications. Antiox Redox Signal. 10:997–1030. 2008.
View Article : Google Scholar
|
|
3
|
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y and
Kato J: Body iron metabolism and pathophysiology of iron overload.
Int J Hematol. 88:7–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oerter KE, Kamp GA, Munson PJ, Nienhuis
AW, Cassorla FG and Manasco PK: Multiple hormone deficiencies in
children with hemochromatosis. J Clin Endocrinol Metab. 76:357–361.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Safarinejad MR: Evaluation of semen
quality, endocrine profile and hypothalamus-pituitary-testis axis
in male patients with homozygous beta-thalassemia major. J Urol.
179:2327–2332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lucesoli F, Caligiuri M, Roberti MF,
Perazzo JC and Fraga CG: Dose-dependent increase of oxidative
damage in the testes of rats subjected to acute iron overload. Arch
Biochem Biophys. 372:37–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whittaker P, Dunkel VC, Bucci TJ, Kusewitt
DF, Thurman JD, Warbritton A and Wolff GL: Genome-linked toxic
responses to dietary iron overload. Toxicol Pathol. 25:556–564.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen MJ, Peng SS, Lu MY, Yang YL, Jou ST,
Chang HH, Chen SU, Lin DT and Lin KH: Effect of iron overload on
impaired fertility in male patients with transfusion dependent
beta-thalassemia. Pediatr Res. 83:655–661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leichtmann-Bardoogo Y, Cohen LA, Weiss A,
Marohn B, Schubert S, Meinhardt A and Meyron-Holtz EG:
Compartmentalization and regulation of iron metabolism proteins
protect male germ cells from iron overload. Am J Physiol Endocrinol
Metab. 302:E1519–E1530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gunel-Ozcan A, Basar MM, Kisa U and
Ankarali HC: Hereditary haemochromatosis gene (HFE) H63D mutation
shows an association with abnormal sperm motility. Mol Biol Rep.
36:1709–1714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anderson D, Schmid TE and Baumgartner A:
Male-mediated developmental toxicity. Asian J Androl. 16:81–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lucesoli F and Fraga CG: Oxidative stress
in testes of rats subjected to chronic iron intoxication and
alpha-tocopherol supplementation. Toxicology. 132:179–186. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kokoszko A, Dabrowski J, Lewiński A and
Karbownik-Lewińska M: Protective effects of GH and IGF-I against
iron-induced lipid peroxidation in vivo. Exp Toxicol Pathol.
60:453–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakajima S, Numakawa T, Adachi N, Yoon HS,
Odaka H, Ooshima Y and Kunugi H: The inactivation of extracellular
signal-regulated kinase by glucagon-like peptide-1 contributes to
neuroprotection against oxidative stress. Neurosci Lett.
616:105–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oshima N, Onimaru H, Matsubara H, Uchida
T, Watanabe A, Imakiire T, Nishida Y and Kumagai H: Direct effects
of glucose, insulin, GLP-1, and GIP on bulbospinal neurons in the
rostral ventrolateral medulla in neonatal wistar rats.
Neuroscience. 344:74–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Zhang Z, Zhao Y, Jiang N, Qiu J,
Yang Y, Li J, Liang X, Wang X, Tse G, et al: Alogliptin, a
dipeptidyl peptidase-4 inhibitor, alleviates atrial remodeling and
improves mitochondrial function and biogenesis in diabetic rabbits.
J Am Heart Assoc. 15:pii: e0059452017.
|
|
17
|
Li S, Wang X, Zhang J, Li J, Liu X, Ma Y,
Han C, Zhang L and Zheng L: Exenatide ameliorates hepatic steatosis
and attenuates fat mass and FTO gene expression through PI3K
signaling pathway in nonalcoholic fatty liver disease. Braz J Med
Biol Res. 51:e72992018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ceriello A, De Nigris V, Pujadas G, La
Sala L, Bonfigli AR, Testa R, Uccellatore A and Genovese S: The
simultaneous control of hyperglycemia and GLP-1 infusion normalize
endothelial function in type 1 diabetes. Diabetes Res Clin Pract.
114:64–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh YS and Jun HS: Effects of glucagon-like
peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci.
19:pii: E262017. View Article : Google Scholar
|
|
20
|
Petersen KE, Rakipovski G, Raun K and
Lykkesfeldt J: Does glucagon-like peptide-1 ameliorate oxidative
stress in diabetes? Evidence based on experimental and clinical
studies. Curr Diabetes Rev. 12:331–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Knop FK, Brønden A and Vilsbøll T:
Exenatide: Pharmacokinetics, clinical use, and future directions.
Expert Opin Pharmacother. 18:555–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guide for the Care and Use of Laboratory
Animals published by the United States National Institutes of
Health. NIH Publication no. 85-23, revised. 1996.
|
|
23
|
Durak I, Canbolat O, Kavutçu M, Öztürk HS
and Yurtarslani Z: Activities of total, cytoplasmic, and
mitochondrial superoxide dismutase enzymes in sera and pleural
fluids from patients with lung cancer. J Clin Lab Anal. 10:17–20.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Ye TM, Roza AM, Pieper GM, Henderson J
Jr, Johnson CP and Adams MB: Inhibition of intestinal lipid
peroxidation does not minimize morphologic damage. J Surg Res.
55:553–558. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bernheim F, Berheim ML and Wilbur KM: The
reaction between thiobarbituric acid and the oxidation products of
certain lipids. J Biol Chem. 174:257–264. 1948.PubMed/NCBI
|
|
26
|
Yagi K: A simple fluorometric assay for
lipoperoxide in blood plasma. Biochem Med. 15:212–216. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Erbatur ME, Sezen ŞC, Bayraktar AC, Arslan
M, Kavutcu M and Aydın ME: Effects of dexmedetomidine on renal
tissue after lower limb ischemia reperfusion injury in
streptozotocin induced diabetic rats. Libyan J Med. 12:12700212017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaghasiya JD, Sheth NR, Bhalodia YS and
Jivani NP: Exaggerated liver injury induced by renal ischemia
reperfusion in diabetes: Effect of exenatide. Saudi J
Gastroenterol. 16:174–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gezginci-Oktayoglu S, Sacan O, Yanardag R,
Karatug A and Bolkent S: Exendin-4 improves hepatocyte injury by
decreasing proliferation through blocking NGF/TrkA in diabetic
mice. Peptides. 32:223–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang E, Xu F, Liang H, Yan J, Xu H, Li Z,
Wen X and Weng J: GLP-1 receptor agonist exenatide attenuates the
detrimental effects of obesity on inflammatory profile in testis
and sperm quality in mice. Am J Reprod Immunol. 74:457–466. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jervis KM and Robaire B: The effects of
long-term vitamin E treatment on gene expression and oxidative
stress damage in the aging Brown Norway rat epididymis. Biol
Reprod. 71:1088–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki N and Sofikitis N: Protective
effects of antioxidants on testicular functions of varicocelized
rats. Yonago Acta Med. 42:87–94. 1999.
|
|
33
|
Ahangarpour A, Oroojan AA and Heidari H:
Effects of exendin-4 on male reproductive parameters of D-galactose
induced aging mouse model. World J Mens Health. 32:176–183. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feinstein-Rotkopf Y and Arama E: Can't
live without them, can live with them: Roles of caspases during
vital cellular processes. Apoptosis. 14:980–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hikim Sinha AP, Lue Y, Diaz-Romero M, Yen
PH, Wang C and Swerdloff RS: Deciphering the pathways of germ cell
apoptosis in the testis. J Steroid Biochem Mol Biol. 85:175–182.
2003. View Article : Google Scholar : PubMed/NCBI
|