Introduction
Individualized assessment and treatment of chronic
obstructive pulmonary disease (COPD) are important in the
management of this disease. However, the pathogenesis of COPD has
remained to be fully elucidated and requires multiple measures to
assess its severity, including the COPD Assessment test, lung
function test, as well as the determination of the frequency of
acute exacerbations, comorbidities, subtypes and a chest computed
tomography (CT) scan (1–5). In addition, inflammatory cells and
inflammatory mediators are involved in the inflammatory response
and severe disorganization of the lung tissue structure;
particularly metalloproteinases (MMPs) have a key role in the
destruction of airway and lung tissues, as well as in airflow
limitation, associated with COPD (6–8).
Comorbidity is a major factor influencing the mortality of COPD
patients. The COPD-specific comorbidity test (COTE) index has been
utilized by Divo et al (9) to
demonstrate that comorbidities of COPD patients were closely linked
to the fatality rate. The present study aimed to assess the effect
of the severity of emphysema in COPD patients determined by chest
CT on airway inflammation, the level of MMPs in the circulating
blood and a variety of common comorbidities. Such studies in this
field are rare, and the present study is helpful in evaluating the
overall clinical features of COPD patients, while aiding in the
establishment of an index for the individualized treatment of
patients with COPD.
Patients and methods
Study population
The present study was performed at the Petroleum
Clinical Medical College of Hebei Medical University (Langfang,
China). A total of 94 cases that presented at the hospital between
January 2016 and December 2016 were enrolled in the study; of
these, 56 cases were male and 38 were female, and their age ranged
from 48 to 87 years. The mean age of the participants was
71.06±8.99 years. The participants were divided into two groups: A
and B. Group A contained 69 patients (73.4%) who were current and
former smokers, and the remaining 25 patients (26.6%), who had
never smoked, were assigned to group B. The diagnosis of COPD was
based on the criteria established in a study by Celli and MacNee
(10). The present study was
authorized by the Ethics Committee of the Petroleum Clinical
Medical College of Hebei Medical University. All patients who were
enrolled in this study had provided their informed consent and had
been examined for lung function 3 months prior to being
hospitalized. In addition, they had undergone high-resolution CT
(HRCT) of the chest. All 94 patients underwent routine blood
examination; the levels of MMP-9 and its inhibitor, tissue
inhibitor of metalloproteinases-1 (TIMP1) were measured on the
second day after admission. All MMP-9 and TIMP-1 kits were
purchased from Beijing Dongge Biotech Co., Ltd., Beijing, China. A
total of 5 ml of fasting venous blood was collected from all
patients at 6:00-8:00 am using a test tube without pyrogen and
endotoxin to enable sampling over 2 h. Then, blood was centrifuged
at a speed of 1,275 × g for 10 min at 37°C. This separated serum
from the blood. Subsequently, 100 µl serum was packed into several
test tubes. It was then stored at a temperature of −80°C for later
use. According to the Kitaguch method (11) and the score of the low-attenuation
area (LAA), the emphysema of the 94 COPD patients was evaluated,
and based on the results, 28 cases were assigned to group A (LAA,
4–6 points; mild emphysema group), eight cases to group B (LAA, 7–9
points; moderate emphysema group), 40 cases to group C (LAA, ≥10
points; severe emphysema group) and 18 cases to group D (LAA, 0–3
points; non-emphysema group).
Chest HRCT examination
Prior to the examination, all 94 patients were
taught the breath holding method for measuring the maximum end
inspiratory and maximum end expiratory valuesand the examination
was performed using a second generation spiral CT (Siemens AG,
Munich, Germany); subsequently, they were trained to reach their
best condition. The effective tube voltage was 120 kV, the tube
current was auto-mAs, scanning collimation was 128×0.6 mm, the
pitch of screw was 1.45, the rotation time was 0.33 sec, the image
scanning layer thickness was 5 mm, the reconstruction layer
thickness was 1 mm and the scanning ranged from the apex to the
bottom of the lungs. The target scanning parameters were as
follows: Scanning layer thickness, 1 mm; interlamellar spacing, 0.5
mm; a B70f algorithm and 512×512 matrix were used for rebuilding;
the remaining parameters were the same as above.
Assessment of emphysema and bronchial
wall thickness
The measures of emphysema were as follows: Emphysema
was quantitatively measured using the HRCT visual subjective
semi-quantitative method and by processing the thin layer (1 mm) CT
image with Syngo CT Pulmo 3D image processing software (Siemens
Healthcare, Munich, Germany), using an LAA of <-960 HU. The
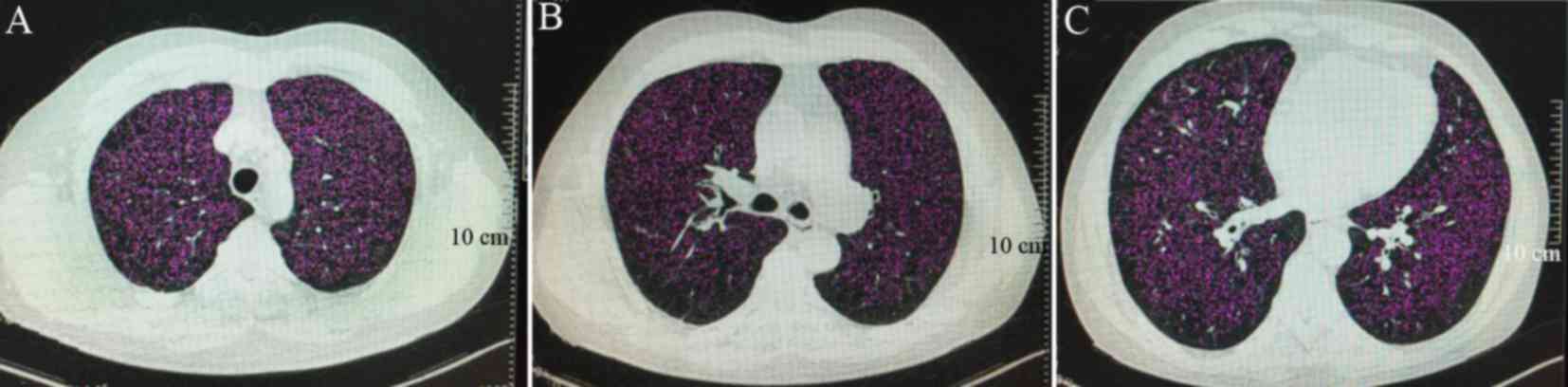
following three anatomical layers were selected for observation, as
presented in Fig. 1: The distance
between the first anatomical layer and the upper margin of the
aortic archwas 1 cm (Fig. 1A); the
second anatomical layer was located 1 cm from the horizontal margin
of the carina (Fig. 1B); and the
other layer contained a distance of 3 cm to theupper margin of the
right diaphragm (Fig. 1C). The LAA
was determined for three lung fields based on the percentage of
each layer in each lung field. The LAA score was calculated for
each layer, as previously described (9): 0 points for LAA <5%, 1 point for
5%≤LAA<25%, 2 points for 25%≤LAA<50%, 3 points for
50%≤LAA<75% and 4 points for LAA>75%. The grading was based
on the sum of the score of the three layers: Grade 0 for 0 points,
grade 1 for 1–3 points, grade 2 for 4–6 points, grade 3 for 7–9
points and grade 4 for 10–12 points.
Following the determination of the cross-section
perpendicular to the fifth bronchial lumen (as confirmed by two
experience radiologists), the scanning images which assessed
bronchial wall enlargement of the right upper lobe were used to
rebuild the apical segmental bronchus with multiplanar reformation.
Bronchial wall thickness (T) and pulmonary artery (PA) diameter in
the same layer were measured using image magnification (×2.5) and
the ratio of airway wall thickness to the pulmonary artery diameter
(T/PA) was calculated. Bronchial wall thickness in 3 layers was
classified as previously described (9) and were as follows: Grade 0 for
T/PA<30%, Grade 1 for 30%≤T/PA<50%, and Grade 2 for
T/PA≥50%.
The COTE index was determined on the basis of the
scoring method designed by Divo et al (9). As presented in Table I, COTE≥4 points was determined in 14
cases and 0≤COTE≤3 points was determined in 80 cases.
 | Table I.Comorbidities and COTE index
score. |
Table I.
Comorbidities and COTE index
score.
| Comorbidities | Risk factor | COTE index |
|---|
| Lung oresophageal
cancer | ≥2 | 6 |
| Pancreatic or breast
cancer |
|
|
| Other tumor
types |
| 2 |
| Anxietyor
depression | 13.76 | 6 |
| Liver Cirrhosis | 1.68 | 2 |
| Atrial
fibrillation | 1.56 | 2 |
| Diabetic
neuropathy | 1.54 | 2 |
| Pulmonary
interstitial fibrosis | 1.51 | 2 |
| Congestive heart
failure | 1.33 | 1 |
| Gastric/duodenal
ulcer | 1.32 | 1 |
| Coronary heart
disease | 1.28 | 1 |
Statistical analysis
Statistical analysis was performed with SPSS 19.0
software (IBM Corp., Armonk, NY, USA). Measurement data were
analyzed by an independent-samples t-test for comparison between
two groups; analysis of variance followed by a least-significant
differences post-hoc test was used for comparison among multiple
groups. Enumeration data were analyzed using the chi-squared test.
Correlations were analyzed by calculation of the Pearson
correlation coefficient. Levene's test was used to determine the
homogeneity of variance among the experimental groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
General characteristics of COPD
patients
The general characteristics of the cohort of COPD
patients, whose major manifestation on the chest CT was emphysema,
are presented in Table II and
Fig. 1. The magenta areas in
Fig. 1 represent the LAA of group C.
The homogeneity of variance test (Levene's test) was used to
analyze the data of each group and the results indicated no
statistically significant difference among all groups (P>0.05).
As presented in Table II, the
percentages of males and patients with a smoking history in the
three emphysema groups (group A, B and C) were significantly higher
than those in the non-emphysema group D (F=7.67 and 5.42,
respectively; P<0.05), and the percentage of patients with a
history of smoking in the severe emphysema group was higher than
that in the mild emphysema group. Within the cohort of COPD
patients, female patients more commonly had no emphysema and no
history of smoking. The proportion of one's full vital capacity of
forced expiratory volume in 1 sec (FEV1% pred) in the non-emphysema
group were significantly higher than those in the emphysema group
(4.33, P<0.05). Furthermore, the level of D-dimer in the
non-emphysema group was significantly lower than that in the mild
and moderate emphysema groups (F=9.38, P<0.05). There was no
statistically significant difference in patient age, neutrophilic
granulocyte percentage (NEUT%), the percentage of lymphocytes,
eosinophilic granulocyte percentage, procalcitonin (PCT), residual
volume/total lung capacity, maximum mean expiratory flow (MMEF)
25–75%, N-terminal pro b-type natriuretic peptide (NT-proBNP),
partial oxygen pressure (PaO2) and TIMP-1 as well as
MMP-9/TIMP-1 among three emphysema groups (P>0.05). In addition,
there was no statistically significant difference in the airway
thickness among the four groups (P>0.05).
 | Table II.Comparison of the general condition in
the 4 groups of patients. |
Table II.
Comparison of the general condition in
the 4 groups of patients.
| Groups | Group A (n=28) | Group B (n=8) | Group C (n=40) | Group D (n=18) | F | P-value |
|---|
| Age | 70.50±7.99 | 70.75±9.74 | 71.75±8.93 | 71.22±9.30 | 0.14 | 0.95 |
| Males | 17 (18.1) | 6
(6.4) | 31 (32.9) | 2
(2.1)a–c | 7.67 | <0.001 |
| Smokers | 19
(67.8) | 7
(87.5)a | 34
(85.0)a | 9
(50.0)a-c | 5.42 | 0.49 |
| NEUT% | 72.85±14.72 | 76.52±12.86 | 75.30±9.96 | 73.81±12.67 | 0.38 | 0.82 |
| LYM% | 18.37±13.11 | 16.62±12.04 | 16.80±8.12 | 18.25±10.25 | 0.29 | 0.92 |
| EO% | 0.84±1.01 | 1.87±3.37 | 0.99±1.86 | 0.98±1.36 | 1.17 | 0.53 |
| PCT | 0.18±0.17 | 0.32±0.19 | 0.48±1.00 | 0.39±0.97 | 0.94 | 0.49 |
| RV/TLC% | 51.83±7.98 | 54.01±5.42 | 51.49±9.10 | 53.33±9.20 | 0.15 | 0.80 |
| MMEF25-75% | 22.59±12.60 | 21.94±15.75 | 25.56±9.83 | 17.26±10.90 | 2.79 | 0.30 |
| FEV1% | 45.78±17.20 | 41.34±15.47 | 39.85±18.42 |
48.28±20.23a | 4.33 | 0.04 |
| NT-proBNP |
2301.57±2309.81 |
1452.09±1387.52 |
1722.99±2170.35 |
2246.94±2616.80 | 0.36 | 0.62 |
| D-dimer | 1.74±1.84 | 1.56±14.64 | 1.34±1.23 |
1.28±1.74a,b | 9.38 | <0.001 |
| PaO2
(mmHg) | 73.46±11.21 | 78.67±7.71 | 71.34±15.95 | 71.09±16.66 | 0.76 | 0.57 |
| MMP-9 (ng/l) | 127.00±10.71 | 128.53±7.07 | 129.30±6.95 | 139.34±11.43 | 0.41 | 0.75 |
| TIMP-1 (ng/l) | 118.43±16.89 | 130.90±18.96 | 118.98±15.76 | 140.20±64.13 | 2.60 | 0.08 |
| MMP-9/TIMP-1 | 0.85±0.11 | 0.84±0.14 | 0.88±0.12 | 0.84±0.16 | 1.64 | 0.58 |
| T/PA | 0.38±0.11 | 0.34±0.08 | 0.36±0.09 | 0.34±0.06 | 0.99 | 0.42 |
Comorbidities and COTE index
The comorbidities, COTE index and glucocorticoid
treatment of the COPD patients in all four groups are presented in
Table III. There was no
statistically significant difference in the number of comorbidities
or the COTE index among the four groups (P>0.05). The number of
COPD patients with pulmonary heart diseasein group A was
significantly lower than that in the other three groups (B, C and
D; P<0.05). Furthermore, the patients in group D were
significantly more likely to have cardiovascular disease as a
comorbidity than those in the other three groups (P<0.05).
Compared with group C, a statistically significant difference in
the number of COPD patients with cardiac insufficiency in groups D
and A was identified (P<0.05). Regarding coronary heart disease
as a comorbidity, the number of affected COPD patients in group A
was significantly higher than that in group C (P<0.05). In terms
of intravenous glucocorticoid infusion, a significantly higher
number of patients received this treatment in groups A, B and C,
compared with those in group D (P<0.05). In addition, there was
no significant difference in the incidence of acute exacerbation
and respiratory failure of patients with COPD among the four groups
(P>0.05; Table III).
 | Table III.Analysis of the comorbidities, COTE
score and hormone intervention in the 4 groups. |
Table III.
Analysis of the comorbidities, COTE
score and hormone intervention in the 4 groups.
| Groups | Group A (n=28) | Group B (n=8) | Group C (n=40) | Group D (n=18) | F | P-value |
|---|
| Number range of
comorbidities | 3.29±1.68 | 1.88±1.36 | 3.00±1.54 | 3.06±1.66 | 1.65 | 0.19 |
| Number range of
patients with pulmonary heart disease | 0.29±0.46 |
0.00±0.00a |
0.15±0.36a,b |
0.11±0.32a,b | 9.15 | <0.001 |
| Number range of
patients with raspiratory failure | 1.29±0.46 | 1.38±0.52 | 1.25±0.44 | 1.22±0.43 | 0.90 | 0.86 |
| Number range of
patients with cardiovascular disease | 0.68±0.48 | 0.38±0.52 | 0.68±0.47 |
0.89±0.32a–c | 19.9 | <0.001 |
| Number range of
patients with coronary heart disease | 0.21±0.42 | 0.25±0.46 |
0.35±0.48a | 0.33±0.49 | 3.34 | 0.06 |
| Number range of
patients with cardiac insufficiency |
0.32±0.48c | 0.25±0.46 | 0.13±0.34 |
0.28±0.46c | 5.16 | 0.04 |
| Number range of
patients with intravenous hormone therapy | 1.07±0.26 | 1.13±0.35 | 1.05±0.22 |
1.00±0.00a–c | 6.21 | 0.02 |
| Number range of
acute exacerbations in the previous year, ≥2 | 1.54±1.04 | 1.13±0.35 | 1.54±1.04 | 1.56±1.19 | 2.63 | 0.11 |
| Range of COTE
score | 2.32±2.42 | 0.50±0.93 | 2.03±2.46 | 1.28±1.26 | 1.03 | 0.21 |
Comorbidities and the level of serum
MMP-9
As presented in Table
IV, there was a statistically significant difference in the
expression of MMP-9 between the COPD patients who exhibited >3
comorbidities or a COTE index of ≥4 points and the patients who
exhibited fewer comorbidities (t=6.40 and 2.53, respectively;
P<0.05). There was a statistically significant difference in the
expression of MMP-9 between the COPD patients with either
cardiovascular disease (hypertension, arrhythmia, coronary heart
disease) or simple coronary heart disease and the patients without
either cardiovascular disease or coronary heart disease (t=3.65 and
2.90, respectively; P<0.05). However, the levels of MMP-9 were
not significantly affected by the presence of pulmonary heart
disease, respiratory failure, cardiac insufficiency or the number
of acute exacerbations in the previous year (P>0.05; Table IV).
 | Table IV.Association of comorbidities and COTE
index with the expression of the MMP-9 and TIMP-1. |
Table IV.
Association of comorbidities and COTE
index with the expression of the MMP-9 and TIMP-1.
| Parameter | N | MMP-9 (ng/l) | TIMP-1 (ng/l) | P-value |
|---|
| Number of
comorbidities |
|
|
|
|
|
0–3 | 55 (58.5%) | 124.34±7.22 | 120.73±21.95 | 0.26 |
|
>3 | 39 (41.5%) |
134.50±8.08a | 128.35±42.75 | <0.001 |
| COTE score |
|
|
|
|
|
0–3 | 80 | 127.59±8.79 | 125.60±34.03 | 0.37 |
| ≥4 | 14 |
134.06±9.04a | 114.15±16.98 | <0.001 |
| Pulmonary heart
disease |
|
|
|
|
| No | 78 (82.9%) | 128.23±8.30 | 124.39±33.49 | 0.44 |
|
Yes | 16 (17.1%) | 130.16±12.38 | 121.48±26.18 | 0.75 |
| Respiratory
failure |
|
|
|
|
| No | 69 (73.4%) | 128.57±9.40 | 124.81±36.90 | 0.98 |
|
Yes | 25 (26.6%) | 128.51±8.29 | 121.37±12.87 | 0.65 |
| Cardiovascular
disease |
|
|
|
|
| No | 29 (30.9%) | 123.76±7.66 | 123.32±25.83 | 0.91 |
|
Yes | 65 (69.1%) |
130.70±8.88a | 124.15±34.93 | <0.001 |
| Coronary heart
disease |
|
|
|
|
| No | 66 (70.2%) | 126.86±8.47 | 126.62±35.87 | 0.21 |
|
Yes | 28 (29.8%) | 132.57±9.33 | 117.46±20.66 | 0.01 |
| Cardiac
insufficiency |
|
|
|
|
| No | 73 (77.7%) | 127.62±8.86 | 124.09±34.58 | 0.92 |
|
Yes | 21 (22.3%) | 131.82±9.27 | 123.23±23.13 | 0.06 |
| Acute exacerbations
in the last year |
|
|
|
|
|
0–1 | 50 (53.2%) | 127.21±9.28 | 129.47±40.26 | 0.55 |
| ≥2 | 44 (46.8%) | 130.09±8.67 | 117.56±18.07 | 0.34 |
Correlation analysis
A correlation analysis between CT types and clinical
parameters indicated that the LAA score was negatively correlated
with the MMEF25-75% and treatment with inhaled bronchodilators
(r=−0.148 and −0.240, respectively; P=0.044 and 0.007); but
positively correlated with the probability of ≥2 acute
exacerbations in the previous year (r=0.211, P=0.001). The LAA
grades were negatively correlated with inhaled bronchodilators
(r=−0.280, P=0.003). The correlation between MMP-9, TIMP-1 or
MMP-9/TIMP-1 and clinical parameters was then assessed. The level
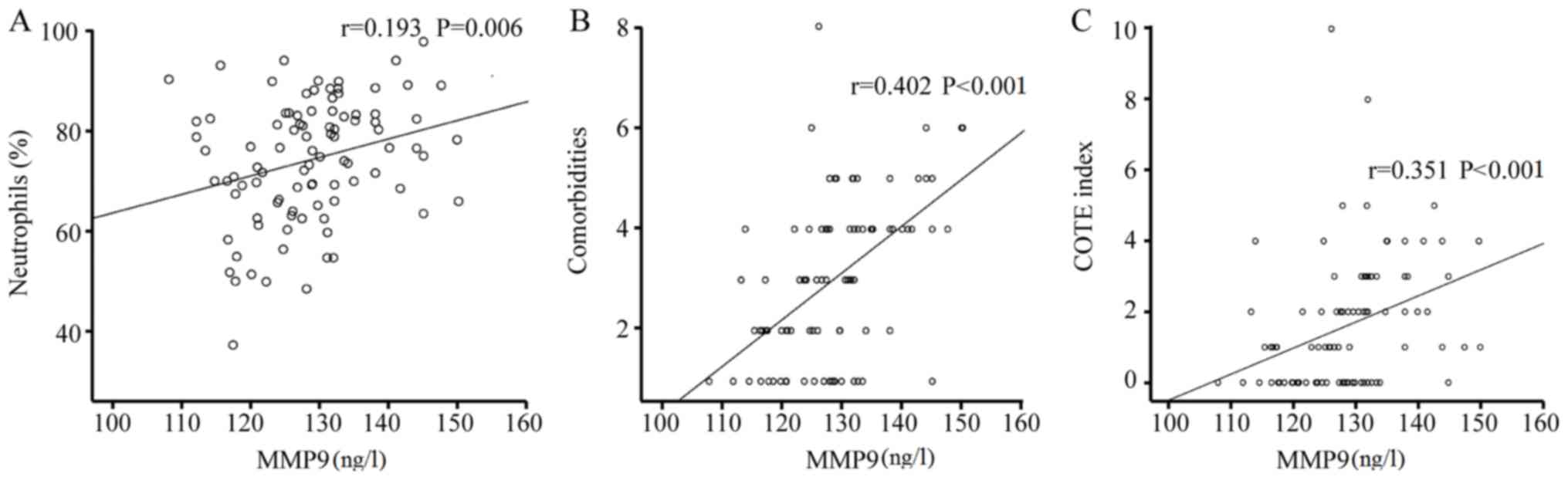
of MMP-9 was positively correlated with the NEUT%, the quantity of
comorbidities, the COTE index and cardiovascular disease as well as
coronary heart disease (r=0.193, 0.402, 0.351, 0.309 and 0.219;
all, P<0.05). The expression of TIMP-1 and PCT were positively
correlated with inhaled bronchodilators (r=0.149 and 0.170; P=0.045
and 0.044, respectively). The MMP-9/TIMP-1 ratio was negatively
correlated with inhaled bronchodilators (r=−0.175, P=0.039).
Further correlation analysis of comorbidities and clinical
parameters revealed that the COTE index was positively correlated
with the NT-proBNP, PCT, application of intravenous hormone therapy
and MMP-9 (r=0.455, 0.166, 0.227, and 0.351; P<0.001, P=0.031,
0.015 and <0.001, respectively). Analysis of the correlation
between the NT-proBNP and clinical parameters then indicated that
the NT-proBNP was positively correlated with the NEUT%, intravenous
hormone therapy, COTE index and the number of comorbidities
(r=0.177, 0.183, 0.264 and 0.410; P=0.012, 0.032, 0.002 and
<0.001, respectively; Fig.
2).
Discussion
The study of COPD is an important aspect in the
field of chronic airway diseases. In the clinic, accurate
evaluation of COPD is helpful for the individualized treatment of
patients. Additional assessments that are of high importance
include the lung function test, recording of clinical symptoms,
acute episodes and symptomatic phenotypes of COPD, determination of
the area of emphysema and the cross-sectional area ratio of the
pulmonary artery to the aorta, the 6-min walking test and the COTE
index. In its essence, COPD is a chronic inflammation in the small
airways with subsequent formation of emphysema (12). The HRCT may be used for macroscopic
identification of the severity of chronic airway inflammation and
emphysema (13,14). The present study aimed to assess the
effect of the destruction of the lung structure on the airway
inflammation, airflow limitation and comorbidities, primarily from
the perspective of the classification of emphysema within the HRCT
of COPD patients; thus, it provides a fundamental theory for
individualized treatment.
In the present study, 94 patients with COPD were
divided into four groups using the Kitaguch method, which was
regardless of whether thickening of the small airway was present,
but according to the LAA score and the severity of emphysema. The
sample size in group B was significantly lower than that in the
other three groups. Thus, it was verified that eachsample size
among the four groups was not significantly different (P>0.05)
by using the homogeneity of variance test, revealing that the
sample size in the four groups was comparable. It was identified
that 80.85% of COPD patients (76/94) exhibited emphysema at various
degrees, while ~20% of patients were free of emphysema. There were
a variety of risk factors, including smoking and air pollution.
These factors may cause small airway stenosis and destruction of
the lung parenchyma (15);
furthermore, they may cause a decrease in the pulmonary elastic
recoil of COPD patients, which leads to emphysema that is invoked
by various inflammatory mediators, including MMP-9 (16–18).
Previous studies have indicated that centrilobular
emphysema is the most common type of emphysema in smoking patients
with COPD, particularly in the right upper lobe (19–21). The
level of peripheral blood leukocytes increased more markedly in
COPD patients who exhibited centrilobular emphysema and panlobular
emphysema than in those with paraseptal emphysema. Furthermore,
smoking was indicated to be a vital factor in increasing the
severity of airway inflammation in patients with centrilobular
emphysema.
The present study indicated that the proportion of
patients with a history or current status of smoking in the
emphysema groups was significantly higher than that in the
non-emphysema group, and the number of smoking patients in the
severe emphysema group was higher than that in the mild emphysema
group, which indicated that the severity of emphysema in COPD
patients was associated with airway inflammation caused by
smoking.
MMP-9 is a proteolytic enzyme, which is dependent on
zinc and calcium ions. It has an important role in the degradation
of extracellular matrix (ECM) and cell membranes, thus causing
enlargement of the alveolar spaces and a diminished elastic recoil
of the alveoli, consequently leading to gas retention; in addition,
MMP-9 is involved in the inflammatory response to airway and lung
remodeling by activating inflammatory factors and destroying the
epithelium or endothelial structure (22). TIMP-1 is an inhibitor of MMP-9; it
inhibits the activation of the MMP-9 by blocking the zymogens
activation of MMPs and specifically binds to zinc ions in the
catalytic active center of MMP-9, consequently preventing the ECM
from excessive degradation. The imbalance of MMP-9/TIMP-1 leads to
airway inflammation and remodeling (2,7,23). In the present study, the expression
of MMP-9 in the COPD patients with mild emphysema was lower than
that in those without emphysema, and there was no statistically
significant difference in the expression of MMP-9 among the three
emphysema groups (P>0.05). The level of TIMP-1 was also
increased in the non-emphysema group compared with that in the
three emphysema groups, but there was no statistically significant
difference (P>0.05). This result was considered to indicate that
MMP-9 has a key role in the pathogenesis of COPD, and in addition,
MMP-9 is known to be involved in the pathogenesis of bronchitis. Of
note, the MMP-9 levels were positively correlated with the
frequency of acute exacerbations, indicating that MMP-9 is an
important inflammatory mediator, which caused airway inflammation
and acute exacerbations. The present study also indicated that the
MMP-9 levels and the MMP-9/TIMP-1 ratio were negatively correlated
with the use of bronchodilators, but that TIMP-1 was positively
correlated with the use of bronchodilators, indicating that a
modest increase of bronchodilators in COPD patients may be
beneficial for controlling airway inflammation and airway
remodeling mediated by MMP-9 and reducing the frequency of acute
exacerbations of COPD.
In the current study, the level of MMP-9 in patients
without emphysema was markedly higher than patients with emphysema
(although this difference was statistically insignificant).
However, the FEV1% pred of the patients with COPD without was
better than those patients with mild emphysema. This indicated that
MMP-9 may be partly involved in the type of airway inflammation and
airway remodeling of patients with COPD.
In the present study, the expression of MMP-9 in the
COPD patients with severe emphysema was obviously higher than that
in the mild emphysema group, suggesting that MMP-9 is not only
involved in the progress of COPD, but that it is also associated
with the severity of emphysema.
A study by Hong et al (24) indicated that among COPD patients, the
small airway wall thickness of non-smoking females was thicker than
that of non-smoking males, whereas the condition of emphysema was
better in female than in male patients. Of note, non-smoking female
patients were more likely to exhibit bronchial wall thickening and
less likely to exhibit emphysema. In the present study, the
proportion of non-smoking andfemale patients in the non-emphysema
group was higher compared with that in the emphysema groups.
A study by Kim et al (25) revealed that patients with the
bronchitis type of COPD exhibited more clinical symptoms and acute
exacerbations; furthermore, the PaO2 in the
non-emphysema group was lower than that in the emphysema group, and
although there was no statistically significant difference, more
clinical symptoms appeared, which was associated with the airway
inflammation, indicating that the repeated outbreak of chronic
airway inflammation was an important source of further clinical
symptoms and acute exacerbations. In addition, compared with that
in the COPD patients who exhibited different degrees of emphysema,
the dosage of intravenous glucocorticoids in the non-emphysema
patients was much lower. A correlation analysis indicated that the
stage of emphysema was negatively correlated with bronchodilators
in patients with COPD. The possible reason was that the airway
stenosis and mucus secretion were more obvious in the non-emphysema
patients who required using more bronchodilators and
mucolytics.
Studies have indicated that comorbidities have a
great influence on the quality of life as well as the frequent
acute exacerbations and death (9,25);
furthermore, different types of comorbidities had significantly
different effects on the relative risk of death, which was much
higher in the COPD patients who exhibited combinations of tumors,
cardiovascular disease, depressive disorders, pulmonary
interstitial fibrosis and cirrhosis. The present study also
suggested that the mortality rate in patients with a COTE index of
≥4 points was 2.2 times higher than that in patients whose COTE
index ranged from 0 to 3 points. The common comorbidities,
including pulmonary heart disease, coronary heart disease, various
types of cardiovascular disease (e.g., hypertension and atrial
fibrillation), as well as various types of respiratory failures and
heart failure, were analyzed in the present study. The level of
MMP-9 was significantly increased in the COPD patients who had
>3 comorbidities and a COTE index of ≥4 points, illustrating
that persistent inflammation in the peripheral blood may be an
important factor for acute attack and the severity of
comorbidieties. Furthermore, it was revealed that in the COPD
patients with various cardiovascular diseases, the number of
non-emphysema patients was greater than the number of emphysema
patients; however, there was no statistically significant
difference among the different severities of emphysema. Previous
studies on COPD patients with atrial fibrillation demonstrated that
recurrent episodes of systemic and local chronic airway
inflammation, as well as continuous increases of inflammatory
mediators in the circulating blood, are important factors leading
to arteriosclerosis and increased cardiovascular events caused by
the dysfunction of vascular endothelium (26–28). In
the present study, the levels of MMP-9 in COPD patients with
cardiovascular disease were higher than those in patients without
cardiovascular disease. A correlation analysis indicated that MMP-9
was positively correlated with the quantity of comorbidities and
the COTE index, and particularly correlated with cardiovascular
disease and coronary heart disease. It also indicated that elevated
MMP-9 in the circulating blood of COPD patients was closely linked
to cardiovascular disease.
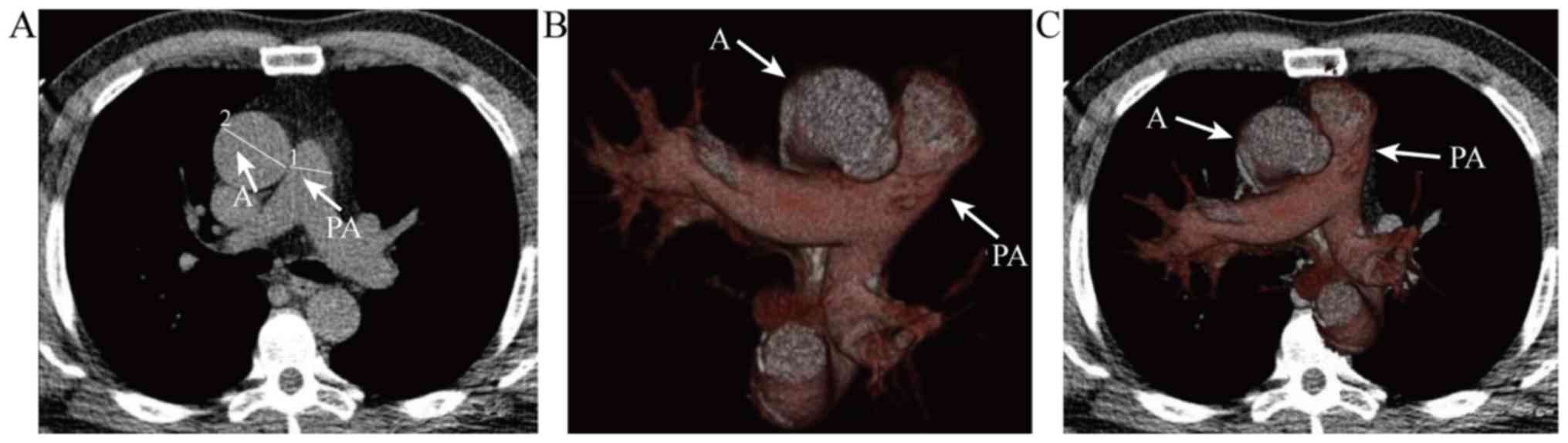
A study by Wells et al (29) reported that pulmonary vascular
disease was also associated with acute exacerbation of COPD. By
using chest CT to measure the pulmonary artery to aorta ratio
(PA:A), as was presented in Fig. 3.
The main pulmonary artery (PA) and aortic (A) diameters at the
level of the bifurcation were used to calculate the PA:A ratio. In
cases where A was not uniform in diameter, two measurements were
taken 90 degrees apart and the larger diameter was used. Panel B
presents the spatial association of PA with A. Panel C is overlaid
on the axial CT image (Fig. 3). The
study revealed that COPD patients with a PA:A of >1 were more
likely to develop severe respiratory and sleep disorders, pulmonary
embolism, hypoxemia and cardiac insufficiency. No statistically
significant difference in the number of COPD patients with
pulmonary heart diseasebetween the non-emphysema and severe
emphysema groups was identified, but there were significantly more
patients who exhibited pulmonary heart diseasein the mild emphysema
group than in the other groups. MMP-9 in the circulating blood was
not correlated with the presence of pulmonary heart disease.
Furthermore, the study indicated that COPD patients with pulmonary
heart disease were not only impacted by MMP-9, but also by other
contributing factors.
The present study was performed only for the
purposes of clinical research for one hospital. Although several
inflammatory factors were involved in the pathogenetic process of
COPD, only MMP-9 and TMP-1 were selected and emphasized in the
current study. Therefore, further studies are required to verify
these results.
In conclusion, MMP-9 is mainly involved in the
pathogenesis of COPD and is correlated with acute exacerbationsand
the number of comorbidities. It was indicated that MMP-9 and the
MMP-9/TIMP-1 ratio could possibly involve in the pathogenesis of
differentemphysematypes of COPD patients and may have a critical
role in various comorbidities of COPD.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to insuffient funding,
but are available from the corresponding author on reasonable
request.
Authors' contributions
ZL designed and implemented the current study, and
wrote the manuscript. FS, J-XL and C-LG collected and sorted cases.
M-MP, JL translated the manuscript and analyzed statistical data.
P-XL advised and instructed the current study.
Ethics approval and consent to
participate
The present study was authorized by the Ethics
Committee of the Petroleum Clinical Medical College of Hebei
Medical University. All patients who were enrolled in this study
had provided their informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests regarding this study.
References
|
1
|
Ostridge K, Williams N, Kim V, Harden S,
Bourne S, Coombs NA, Elkington PT, Estepar RS, Washko G, Staples KJ
and Wilkinson TM: Distinct emphysema subtypes defined by
quantitative CT analysis are associated with specific pulmonary
matrix metalloproteinases. Respir Res. 17:922016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papakonstantinou E, Karakiulakis G,
Batzios S, Savic S, Roth M, Tamm M and Stolz D: Acute exacerbations
of COPD are associated with significant activation of matrix
metalloproteinase 9 irrespectively of airway obstruction, emphysema
and infection. Respir Res. 16:782015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith BM, Austin JH, Newell JD Jr, D'Souza
BM, Rozenshtein A, Hoffman EA, Ahmed F and Barr RG: Pulmonary
emphysema subtypes on computed tomography: The MESA COPD study. Am
J Med. 127:94.e7–e23. 2014. View Article : Google Scholar
|
|
4
|
Foster WL Jr, Pratt PC, Roggli VL, Godwin
JD, Halvorsen RA Jr and Putman CE: Centrilobular emphysema:
CT-pathologic correlation. Radiology. 159:27–32. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hruban RH, Meziane MA, Zerhouni EA, Khouri
NF, Fishman EK, Wheeler PS, Dumler JS and Hutchins GM: High
resolution computed tomography of inflation-fixed lungs.
Pathologic-radiologic correlation of centrilobular emphysema. Am
Rev Respir Dis. 136:935–940. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montaño M, Sansores RH, Becerril C,
Cisneros J, González-Avila G, Sommer B, Ochoa L, Herrera I,
Ramírez-Venegas A and Ramos C: FEV1 inversely correlates with
metalloproteinases 1, 7, 9 and CRP in COPD by biomass smoke
exposure. Respir Res. 15:742014.PubMed/NCBI
|
|
7
|
Grzela K, Litwiniuk M, Zagorska W and
Grzela T: Airway remodeling in chronic obstructive pulmonary
disease and asthma: The role of matrix metalloproteinase-9. Arch
Immunol Ther Exp (Warsz). 64:47–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia TG, Zhao JQ and Liu JH: Serum
inflammatory factor and cytokines in AECOPD. Asian Pac J Trop Med.
7:1005–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Divo M, Cote C, de Torres JP, Casanova C,
Marin JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J,
Hunninghake G, et al: Comorbidities and risk of mortality in
patients with chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 186:155–161. 2002. View Article : Google Scholar
|
|
10
|
Celli BR and MacNee W; ATS/ERS Task Force,
: Standards for the diagnosis and treatment of patients with COPD:
A summary of the ATS/ERS position paper. Eur Respir J. 23:932–946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitaguchi Y, Fujimoto K, Kubo K and Honda
T: Characteristics of COPD phenotypes classified according to the
findings of HRCT. Respir Med. 100:1742–1752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts HR, Wells AU, Milne DG, Rubens MB,
Kolbe J, Cole PJ and Hansell DM: Airflow obstruction in
bronchiectasis: Correlation between computed tomography features
and pulmonary function tests. Thorax. 55:198–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
The definition of emphysema. Report of a
National Heart, Lung, and Blood Institute, Division of Lung
Diseases workshop. Am Rev Respir Dis. 132:182–185. 1985.PubMed/NCBI
|
|
14
|
Hoffman EA, Simon BA and Mclennan G: State
of the art. A structural and functional assessment of the lung via
multidetector-row computed tomography: Phenotyping chronic
obstructive pulmonary disease. Proc Am Thorac Soc. 3:519–532. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh S, Loke YK, Enright PL and Furberg
CD: Mortality associated with tiotropium mist inhaler in patients
with chronic obstructive pulmonary disease: Systematic review and
meta-analysis of randomised controlled trials. BMJ. 342:d32152011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dar KA, Shahid M, Mubeen A, Bhargava R,
Ahmad Z, Ahmad I and Islam N: The role of noninvasive methods in
assessing airway inflammation and structural changes in asthma and
COPD. Monaldi Arch Chest Dis. 77:8–18. 2012.PubMed/NCBI
|
|
17
|
Kwiatkowska S, Noweta K, Zieba M, Nowak D
and Bialasiewicz P: Enhanced exhalation of matrix
metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in
patients with COPD exacerbation: A prospective study. Respiration.
84:231–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linder R, Rönmark E, Pourazar J, Behndig
A, Blomberg A and Lindberg A: Serum metallopteinase-9 is related to
COPD severity and symptoms-cross-sectional data from a population
based cohort-study. Respir Res. 16:282015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson AE Jr and Foraker AG:
Centrerilobolar emphysema and panlobular emphysema: Two different
diseases. Thorax. 28:547–550. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saetta M, Kim WD, Izquierdo JL, Ghezzo H
and Cosio MG: Extent of centriobular and panacinar emphysema in
smokers'lungs: Pathological and mechanical implications. Eur Respir
J. 7:664–671. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawayama T, Kinoshita T, Matsunaga K,
Kobayashi A, Hayamizu T, Johnson M and Hoshino T: Responsiveness of
blood and sputum inflammatory cells in Japanese copd patients,
non-copd smoking controls, and non-copd nonsmoking controls. Int J
Chron Obstruct Pulmon Dis. 11:295–303. 2016.PubMed/NCBI
|
|
22
|
Segura-Valdezl L, Pardo A, Gaxiola M, Uhal
BD, Becerril C and Selman M: Upregulation of gelatinases A and B,
collagenases 1 and 2, and increased parenchymal cell death in COPD.
Chest. 117:684–694. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linder R, Rönmark E, Pourazar J, Behndig
A, Blomberg A and Lindberg A: Serum metalloproteinase-9 is related
to COPD severity and symptoms-cross-sectional data from a
population based cohort-study. Respir Res. 16:282015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong Y, Ji W, An S, Han SS, Lee SJ and Kim
WJ: Sex differences of COPD phenotypes in nonsmoking patiens. Int J
Chron Obstruct Pulmon Dis. 11:1657–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim V, Han MK, Vance GB, Make BJ, Newell
JD, Hokanson JE, Hersh CP, Stinson D, Silverman EK and Criner GJ;
COPDGene Investigators, : The chronic bronchitic phenotype of COPD:
An analysis of the COPDGene study. Chest. 140:626–633. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith MC and Wrobel JP: Epidemiology and
clinical impact of major comorbidities in patients with COPD. Int J
Chron Obstruct Pulmon Dis. 9:871–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Selvarajah S, Todd I, Tighe PJ, John M,
Bolton CE, Harrison T and Fairclough LC: Multiple Circulating
cytokines are coelevated in chronic obstrictive pulmonary disease.
Mediators Inflamm. 2016:36048422016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eickhoff P, Valipour A, Kiss D, Schreder
M, Cekici L, Geyer K, Kohansal R and Burghuber OC: Determinants of
systemic vascular function in patients wity stable chronic
obstructive pulmonary disease. Am J Respir Crit Care Med.
178:1211–1218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wells JM, Washko GR, Han MK, Abbas N, Nath
H, Mamary AJ, Regan E, Bailey WC, Martinez FJ, Westfall E, et al:
Pulmonary arterial enlargement and acute exacerbations of COPD. N
Engl Med. 367:913–921. 2012. View Article : Google Scholar
|