Introduction
Approximately 28% of pregnant women experience
ectopic pregnancy during childbirth (1), of which intra-abdominal hemorrhage is
the most common complication of ectopic pregnancy (2). According to the statistics report by
Riaz et al (3), the
occurrence of massive bleeding in patients with ectopic pregnancy
was as high as 69.5%. Abdominal bleeding will lead to difficult
births, fetal death and suffocation, and serious bleeding will even
threaten life (4). For patients with
massive bleeding in pregnancy, blood transfusions are given in
clinical practice, and generally can have a good rescue effect on
patients with acute ischemia (5).
However, patients with massive bleeding are prone to adverse
reactions, and Magee et al (6) reported that approximately 70% of
patients with pregnancy-induced bleeding occurred varying degrees
of adverse reactions. Therefore, how to effectively avoid and
reduce adverse reactions in patients has become an important
clinical topic. In recent years, multiple studies (7–9) have
demonstrated that monitoring blood coagulation is of great clinical
significance for patients undergoing transfusion and can
effectively determine the patient's blood flow. For patients with
massive bleeding during pregnancy undergoing blood transfusion
therapy, whether detecting the coagulation function also has high
value has not been studied. Therefore, this retrospective
comparative analysis of adverse reactions and no adverse reactions
in patients with massive bleeding in pregnancy before and after
blood transfusion was carried out in order to provide effective and
reliable reference for future clinical response to such
patients.
Patients and methods
Patient information
A total of 627 pregnant women admitted to Zibo First
Hospital (Zibo, China) from July 2014 to March 2017 were
retrospectively analyzed. Patients were aged 22–35 years, mean age
27.34±5.43 years. In the selected patients bleeding during
pregnancy with transfusion in Zibo First Hospital, 369 patients had
adverse reactions after blood transfusions (hypotension,
tachycardia, urticaria and postoperative infection) and were
selected as the experimental group. Another 258 patients without
adverse reactions were selected as the control group.
Inclusion and exclusion criteria
Inclusion criteria were: All pregnant patients
admitted for childbirth in Zibo First Hospital; have complete case
details, aged between 22–35 years. Exclusion criteria were:
Suffering from other blood diseases, suffering from other immune
diseases, suffering from other heart and brain and other organ
diseases, physical disability, surgical tolerance of patients,
preoperative chemotherapy. The study was approved by the Ethics
Committee of Zibo First Hospital. Patients who participated in this
research had complete clinical data. Signed informed consents were
obtained from the patients or the guardians.
Methods
Criteria for determining massive bleeding were with
reference to 2013 guidelines for the diagnosis of ectopic pregnancy
(10): Blood loss >1,000 ml.
Patients with massive bleeding in pregnancy was treated with strict
reference to the guidelines for the treatment for blood
transfusions in 2013 (11), and the
blood transfusion standards are shown in Table I. After each infusion of 4–6 U red
blood cells or 400–600 ml of blood, the patient's coagulation
function was tested, so that coagulation of patients was maintained
at normal conditions, and the patient was given intravenous
injection of 1 gr calcium gluconate. From the two groups of
patients 4 ml venous blood was drawn 8 h before and after blood
transfusion, one sample for blood tests (using the Beckman DHS
hematology analyzer, Beckman Coulter, Inc., Brea, CA, USA) and one
sample for coagulation tests after centrifugation at 3,250 × g for
5 min at 4°C.
 | Table I.Blood transfusion standards. |
Table I.
Blood transfusion standards.
| Blood loss of
patients | Blood transfusion
content |
|---|
| HB <70 g/l | Red blood cell
suspension |
| PT | Fresh frozen |
| APTT >1.5 times
FIB | plasma or
cryoprecipitate |
| PLT
<50×109/l | Apheresis
platelets |
Observation indicator
Blood routine observation index: Red blood cell
count (RBC), white blood cell count (WBC), platelet count (PLT),
HB, hematocrit (HCT). Coagulation function indicators: Thrombin
time (TT), prothrombin time (PT), activated partial thromboplastin
time (APTT), fibrinogen (FIB).
Statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk,
NY, USA) was used to analyze and process the data. The enumeration
data are expressed as rate. Chi-square test was used to compare
between groups. Measurement data are expressed as mean ± standard
deviation, and t-test was used to compare between groups. Paired
t-test was used for comparison between before and after treatment.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basic information of the patients
The clinical data of two groups of patients were
compared. There was no significant difference between the two
groups in age, pregnancy time, body weight, smoking, drinking,
exercise habits and education level (P>0.05) (Table II).
 | Table II.Comparison of clinical data between
two groups (n, %). |
Table II.
Comparison of clinical data between
two groups (n, %).
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Experimental
(n=369) | Control (n=258) | t/χ2 | P-value |
|---|
| Age (years) | 26.83±4.69 | 26.94±5.09 | 0.28 | 0.780 |
| Pregnancy time
(weeks) | 12.35±1.82 | 12.62±2.04 | 1.74 | 0.083 |
| Weight (kg) |
|
| 0.02 | 0.897 |
|
<60 | 134 (36.31) | 95 (36.82) |
|
|
| ≥60 | 235 (63.69) | 163 (63.18) |
|
|
| Smoking habits |
|
| 0.08 | 0.775 |
| Yes | 25 (6.78) | 16 (6.20) |
|
|
| No | 344 (93.22) | 242 (93.80) |
|
|
| Drinking habits |
|
| 0.27 | 0.604 |
| Yes | 39 (10.57) | 24 (9.30) |
|
|
| No | 330 (89.43) | 234 (90.70) |
|
|
| Exercise habits |
|
| 0.10 | 0.748 |
| Yes | 127 (34.42) | 92 (35.66) |
|
|
| No | 242 (65.58) | 166 (64.34) |
|
|
| Degree of
education |
|
| 0.15 | 0.696 |
| <High
school | 126 (34.15) | 92 (35.66) |
|
|
| ≥High
school | 243 (65.85) | 166 (64.34) |
|
|
Blood test results before and after
blood transfusion
There was no significant difference in RBC, WBC,
PLT, HB, HCT between the blood transfusion group and the control
group before the blood transfusion (P>0.05). The RBC in the
experimental group after transfusion was 3.74±0.69
×1012/l, which was significantly lower than that in the
control group 4.84±0.51 ×1012/l (P<0.001). The WBC in
the experimental group was 6.56±2.08 ×109/l, which was
significantly lower than that in the control group 7.64±1.89
×109/l (P<0.001). The levels of PLT, HB and HCT were
102.23±10.62 ×109/l, 78.63±7.05 g/l and 0.34±0.08
respectively, which were also lower than those in the control group
135.22±14.53 ×109/l, 91.36±9.56 g/l and 0.45±0.06
respectively (P<0.001) (Tables
III and IV).
 | Table III.Blood test results of two groups
before transfusion. |
Table III.
Blood test results of two groups
before transfusion.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Experimental
(n=369) | Control (n=258) | t | P-value |
|---|
| RBC
(×1012/l) | 2.63±0.57 | 2.65±0.55 | 0.44 | 0.661 |
| WBC
(×109/l) | 4.86±1.37 | 5.01±1.53 | 1.29 | 0.199 |
| PLT
(×109/l) | 48.60±6.52 | 49.34±7.06 | 1.35 | 0.177 |
| HB (g/l) | 45.36±12.26 | 44.67±13.07 | 0.67 | 0.500 |
| HCT | 0.26±0.06 | 0.26±0.03 | 0.0 | >0.999 |
 | Table IV.Blood test results of two groups after
transfusion. |
Table IV.
Blood test results of two groups after
transfusion.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Experimental
(n=369) | Control (n=258) | t | P-value |
|---|
| RBC
(×1012/l) | 3.74±0.69 | 4.84±0.51 | 21.78 | <0.001 |
| WBC
(×109/l) | 6.56±2.08 | 7.64±1.89 | 6.64 | <0.001 |
| PLT
(×109/l) | 102.23±10.62 | 135.22±14.53 | 32.84 | <0.001 |
| HB (g/l) | 78.63±7.05 | 91.36±9.56 | 19.19 | <0.001 |
| HCT | 0.34±0.08 | 0.45±0.06 | 18.71 | <0.001 |
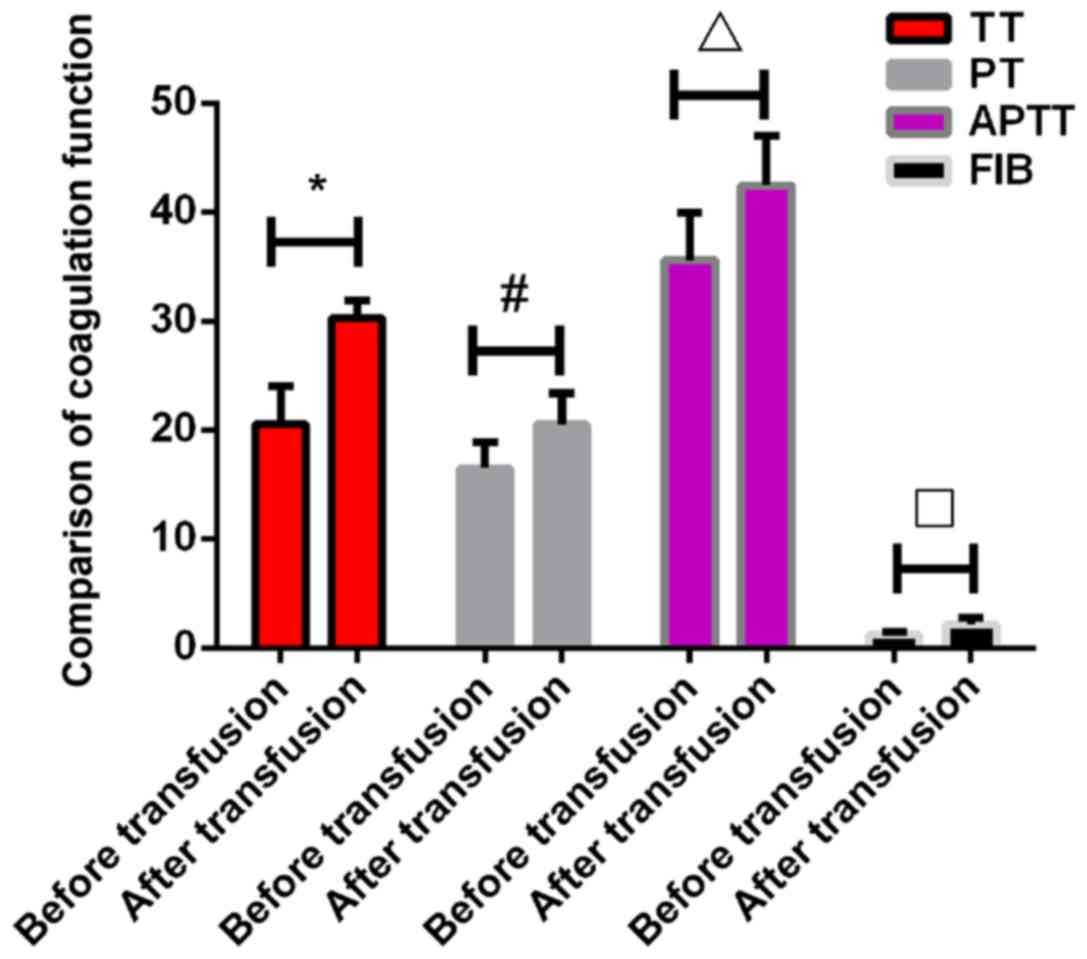
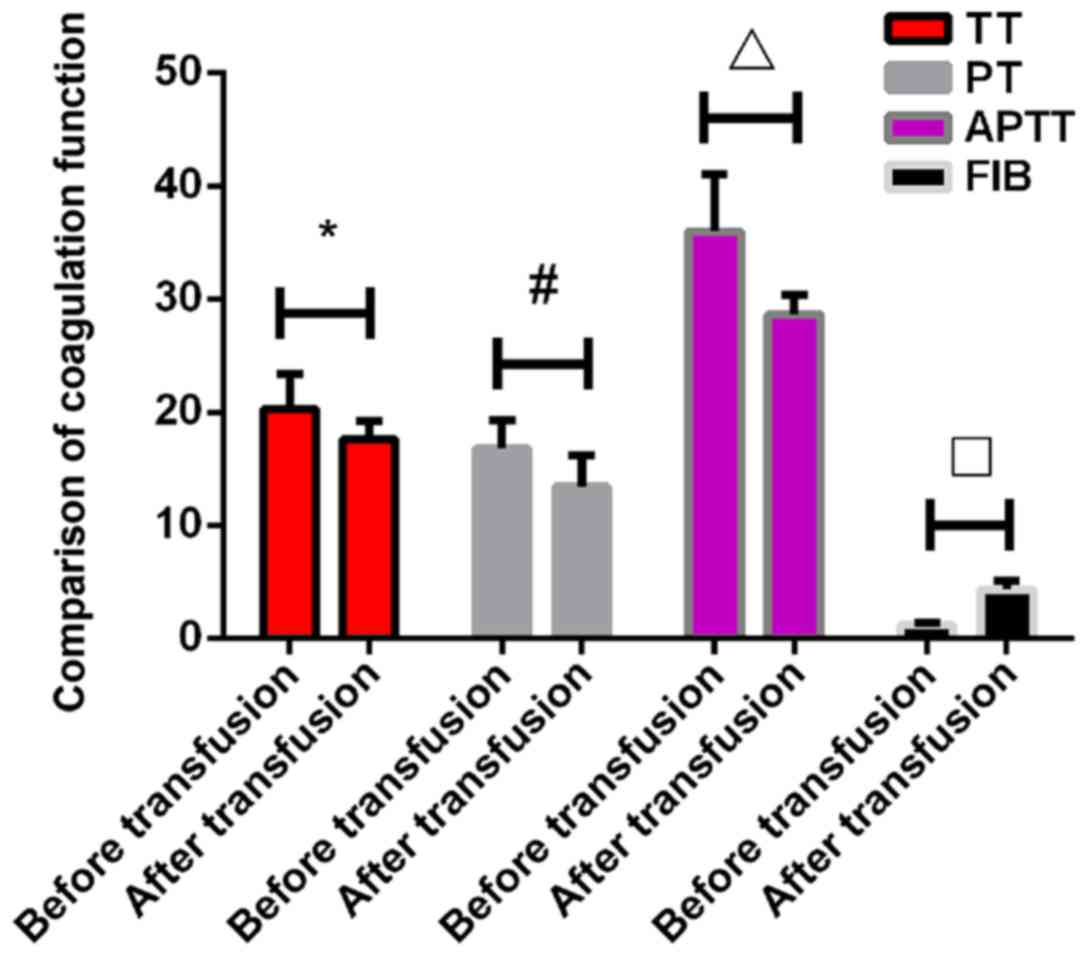
Blood coagulation test results before
and after transfusion
There were no significant differences in TT, PT,
APTT and FIB before transfusion between the two groups (P>0.05).
The TT in the experimental group after transfusion was 30.26±1.6
sec, which was significantly higher than that in control group
17.65±1.53 sec (P<0.001). The PT in the experimental group after
transfusion was 20.53±2.83 sec, which was also significantly higher
than that in the control group 13.42±2.82 sec (P<0.001). The
APTT in the experimental group after transfusion was 42.49±4.56
sec, which was significantly higher than that in the control group
28.65±1.69 sec (P<0.001). The FIB in the experimental group
after transfusion was 2.15±0.62 g/l, which was significantly lower
than that in the control group 4.32±0.82 g/l (P<0.005). All
indexes of coagulation function in the experimental group were
significantly increased after transfusion compared with those
before transfusion (P<0.05). However, the levels of TT, PT and
APTT in the control group were significantly lower than those
before the transfusion (P<0.05), while the levels of FIB in the
control group were significantly higher than those before
transfusion (P<0.05) (Tables V
and VI; Figs. 1 and 2).
 | Table V.Coagulation tests results in two
groups before blood transfusion. |
Table V.
Coagulation tests results in two
groups before blood transfusion.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Experimental
(n=369) | Control
(n=258) | t | P-value |
|---|
| TT (sec) | 20.53±3.52 | 20.27±3.08 | 0.96 | 0.339 |
| PT (sec) | 16.49±2.36 | 16.82±2.51 | 1.68 | 0.094 |
| APTT (sec) | 36.59±4.35 | 35.98±5.04 | 1.62 | 0.106 |
| FIB (g/l) | 1.20±0.25 | 1.19±0.18 | 0.55 | 0.582 |
 | Table VI.Coagulation tests results in two
groups after blood transfusion. |
Table VI.
Coagulation tests results in two
groups after blood transfusion.
|
| Groups |
|
|
|---|
|
|
|
|
|
|---|
| Items | Experimental
(n=369) | Control
(n=258) | t | P-value |
|---|
| TT (sec) | 30.26±1.62 | 17.65±1.53 | 98.12 | <0.001 |
| PT (sec) | 20.53±2.83 | 13.42±2.82 | 31.00 | <0.001 |
| APTT (sec) | 42.49±4.56 | 28.65±1.69 | 46.56 | <0.001 |
| FIB (g/l) | 2.15±0.62 | 4.32±0.82 | 37.71 | <0.001 |
Discussion
Massive bleeding in ectopic pregnancy patients leads
to critical condition, lack of blood circulation, decreased red
blood cell oxygen carrying capacity, decreased blood perfusion
which causes a serious threat to the patients and the fetuses
(12,13). In clinical practice, these patients
are mainly treated with blood transfusion for emergency rescue, and
the purpose of which is to supplement the patient's blood volume
and blood coagulation factors, and to improve the patient's
hypoxia-induced necrosis caused by ischemia (14,15).
Transfusion therapy can not only strive for more treatment time,
but also provide more effective protection for the fetus of
patients (16). However, patients
occur with different degrees of adverse reactions after massive
bleeding, and the adverse reactions not only may make the disease
become more complex, severe adverse reactions will directly result
in premature birth and stillbirth (17,18). Our
study found that the detection of coagulation in pregnant patients
can be used as an indicator of adverse reactions in patients and
can be implemented in the clinic.
The results of this study showed that coagulation
disorders were common in patients with massive bleeding during the
course of transfusion therapy. The blood parameters before and
after transfusion were improved in both groups, but the improvement
in the control group was significantly better than that in the
experimental group, suggesting that adverse reactions of bleeding
can affect the recovery of blood condition in pregnant patients.
After transfusion, the indexes of coagulation function in the
experimental group were significantly increased, while the TT, PT
and APTT in the control group showed a downward trend and the FIB
increased. Adverse reactions affect the patient's coagulation
function, adverse reactions in the experimental group of patients
resulting in poor effects of transfusion therapy. For patients with
ectopic pregnancy, due to massive bleeding caused by rupture of
fallopian tube, and pregnancy also speeds up the rate of blood
coagulation, causing diffuse blood clotting, it will not only lead
to coagulation disorders in patients, but also may lead to rupture
of fetal membranes in patients and cause injury to the fetus
(19).
By detecting the difference of coagulation function
before and after transfusion, the improvement of coagulation
function in the experimental group was significantly worse than
that in the control group. Although the FIB in the experimental
group also increased significantly compared with that before
treatment, the increase was not as good as that in the control
group. The reason for this is that FIB is a glycoprotein involved
in the coagulation of the patient. When the patient's ability to
synthesize protein is weakened, the FIB will show a downward trend
(20,21). Therefore, for patients with adverse
reactions, their protein synthesis ability has been suppressed,
resulting in non-significant improvement of FIB protein after
transfusion. However, the increase of PT, TT and APTT showed that
the decrease of coagulation function in the experimental group
aggravated the possibility of bleeding in patients.
In this study, due to limited conditions, there are
still deficiencies, such as the study population, relatively simple
testing instruments, and we will be performing follow-up survey for
a longer period and continuing to improve our experiment in the
future, in order to achieve the best experimental results.
In conclusion, coagulation test can be used as an
indicator of adverse reactions in patients with massive bleeding in
pregnancy after transcortical treatment, providing reference and
guidance for clinical diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FK designed the study, wrote the manuscript and
treated patients. YL was responsible for blood routine observation
index. XL collected and analyzed basic information on patients. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zibo First Hospital (Zibo, China). Patients who participated in
this research had complete clinical data. Signed informed consents
were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perkins KM, Boulet SL, Kissin DM and
Jamieson DJ; National ART Surveillance (NASS) Group, : Risk of
ectopic pregnancy associated with assisted reproductive technology
in the United States, 2001–2011. Obstet Gynecol. 125:70–78. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang C, Huang R, Wei LN and Jia L:
Frozen-thawed day 5 blastocyst transfer is associated with a lower
risk of ectopic pregnancy than day 3 transfer and fresh transfer.
Fertil Steril. 103:655–661, e3. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riaz RM, Williams TR, Craig BM and Myers
DT: Cesarean scar ectopic pregnancy: Imaging features, current
treatment options, and clinical outcomes. Abdom Imaging.
40:2589–2599. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taran FA, Kagan KO, Hubner M, Hoopmann M,
Wallwiener D and Brucker S: The diagnosis and treatment of ectopic
pregnancy. Dtsch Arztebl Int. 112:693–703. 2015.PubMed/NCBI
|
|
5
|
Kelly RJ, Höchsmann B, Szer J,
Kulasekararaj A, de Guibert S, Röth A, Weitz IC, Armstrong E,
Risitano AM, Patriquin CJ, et al: Eculizumab in pregnant patients
with paroxysmal nocturnal hemoglobinuria. N Engl J Med.
373:1032–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magee LA, von Dadelszen P, Rey E, Ross S,
Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, et
al: Less-tight versus tight control of hypertension in pregnancy. N
Engl J Med. 372:407–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wafaisade A, Wyen H, Mutschler M,
Lendemans S, Bouillon B, Flohe S, Paffrath T, Maegele M, Tjardes T
and Probst C; Sektion NIS der DGU, : Current practice in
coagulation and transfusion therapy in multiple trauma patients: A
German nation-wide online survey. Unfallchirurg. 118:1033–1040.
2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nystrup KB, Stensballe J, Bøttger M,
Johansson PI and Ostrowski SR: Transfusion therapy in paediatric
trauma patients: A review of the literature. Scand J Trauma Resusc
Emerg Med. 23:212015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karkouti K, McCluskey SA, Callum J,
Freedman J, Selby R, Timoumi T, Roy D and Rao V: Evaluation of a
novel transfusion algorithm employing point-of-care coagulation
assays in cardiac surgery: A retrospective cohort study with
interrupted time-series analysis. Anesthesiology. 122:560–570.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alkatout I, Honemeyer U, Strauss A,
Tinelli A, Malvasi A, Jonat W, Mettler L and Schollmeyer T:
Clinical diagnosis and treatment of ectopic pregnancy. Obstet
Gynecol Surv. 68:571–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heal JM and Blumberg N: Optimizing
platelet transfusion therapy. Blood Rev. 18:149–165. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detterich JA, Kato RM, Rabai M, Meiselman
HJ, Coates TD and Wood JC: Chronic transfusion therapy improves but
does not normalize systemic and pulmonary vasculopathy in sickle
cell disease. Blood. 126:703–710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beverung LM, Strouse JJ, Hulbert ML,
Neville K, Liem RI, Inusa B, Fuh B, King A, Meier ER, Casella J, et
al: SIT trial investigators: Health-related quality of life in
children with sickle cell anemia: Impact of blood transfusion
therapy. Am J Hematol. 90:139–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gutfraind A and Meyers LA: Evaluating
large-scale blood transfusion therapy for the current Ebola
epidemic in Liberia. J Infect Dis. 211:1262–1267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marfin AA and Price TH: Granulocyte
transfusion therapy. J Intensive Care Med. 30:79–88. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou ST and Fasano RM: Management of
patients with sickle cell disease using transfusion therapy:
Guidelines and complications. Hematol Oncol Clin North Am.
30:591–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taher AT, Radwan A and Viprakasit V: When
to consider transfusion therapy for patients with
non-transfusion-dependent thalassaemia. Vox Sang. 108:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colebunders RL and Cannon RO: Large-scale
convalescent blood and plasma transfusion therapy for Ebola virus
disease. J Infect Dis. 211:1208–1210. 2015.PubMed/NCBI
|
|
19
|
Berry J, Davey M, Hon MS and Behrens R: A
5-year experience of the changing management of ectopic pregnancy.
J Obstet Gynaecol. 36:631–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu CH, Gong P and Yang J: Relationship
between circulating tumor cells and coagulation factors in primary
lung cancer patients. Zhonghua Zhong Liu Za Zhi. 38:368–371.
2016.(In Chinese). PubMed/NCBI
|
|
21
|
Onelöv L, Gustafsson E, Grönlund E,
Andersson H, Hellberg G, Järnberg I, Schurow S, Söderblom L and
Antovic JP: Autoverification of routine coagulation assays in a
multi-center laboratory. Scand J Clin Lab Invest. 76:500–502. 2016.
View Article : Google Scholar : PubMed/NCBI
|