Introduction
Retinoblastoma (RB) is the most common type of
primary intraocular malignant tumor in pediatric patients that has
an occult onset and a high degree of malignancy (1). Without treatment, affected pediatric
patients generally die within 1–2 years (2). Invasion and metastasis are the leading
causes of death in pediatric patients with RB, making the disease a
complex process that involves multiple genes, factors and
procedures (3,4). In recent years, enucleation has been
excluded from the treatment options for RB, and different
treatments are adopted according to the different stages of the
tumor, aiming to retain the eyes and their functions (5). Chemoreduction, mainly systemic
chemotherapy, has become the first-line approach for the clinical
treatment of RB (6,7). It greatly improved the survival rate of
the patients without affecting the structure and function of
eyeballs. However, failure of RB treatment remains common in
clinical practice due to two reasons. First, tumor cells may
develop resistance to drugs (8);
furthermore, continued use of chemotherapeutic drugs may induce
bone marrow suppression and secondary leukemia, increasing the
mortality risk of the patients (9).
Therefore, it is of great clinical significance to develop a safer
and more effective adjuvant therapy for the treatment of patients
with RB.
MicroRNAs (miRNAs or miRs) are non-coding small RNA
molecules of 18–22 nucleotides in length that regulate mRNA
translation by binding with the 3′-untranslated region (UTR) of
their target mRNAs (10,11). miRNA molecules widely exist in
eukaryotes and participate in multiple pathological processes. It
has been reported that a variety of miRNA molecules act as
oncogenes or tumor-suppressor genes in RB (12). For instance, miR-21 promotes the
occurrence and development of RB by activating phosphatase and
tensin homologue (PTEN)/phosphoinositide-3 kinase (PI3K)/AKT
signaling pathway, and miR-21 inhibitor blocks the function of
miR-21 (13). Bai et al
(14) reported that miR-125b
promotes the proliferation and inhibits the apoptosis of RB cells
by targeting DNA damage-regulated autophagy modulator 2 gene
expression. Zhang et al (15)
reported that miR-125a-5p enhances the proliferation of RB cells by
activating taffazin-epithelial growth factor receptor signaling
pathway.
miR-106b is a member of the miR-106-25 family, which
also includes miR-25 and miR-93 (16,17). The
expression of miR-106b in gastric cancer tissues is significantly
higher than that in adjacent normal tissue, and abnormal expression
of miR-106b is observed in the peripheral blood of gastric cancer
patients, suggesting that miR-106b has an important role in the
occurrence and development of gastric cancer (18). Dai et al (19) discovered that miR-106b promotes the
invasion and migration of esophageal squamous cancer cells in a
Kazakh population by downregulating the expression of Smad7. Li
et al (20) reported that
miR-106b increases the proliferation, invasion and migration of
breast cancer cells by regulating the expression of α-(1,3)-fucosyltransferase. These studies suggest
that miR-106b has varied functions and mechanisms of action in
different types of tumor. However, the expression and mechanism of
action of miR-106b in RB has remained to be elucidated. The present
study investigated the function of miR-106b in the proliferation,
invasion and migration of RB cells, and aimed to elucidate the
underlying mechanisms.
Patients and methods
Patients
A total of 56 patients (34 males and 22 females; age
range, 2 months-11 years; mean age, 35.5±8.5 months) who received
treatment for RB at Jining First People's Hospital between December
2013 and December 2015 were enrolled in the present study. No
patients received any chemotherapy or other treatments prior to
sampling. Additionally, patients with chronic, genetic or
autoimmune diseases were excluded from the present study. Among the
patients, 36 had RB in one eye and 20 had RB in both eyes.
According to the degrees of optic nerve invasion, the patients were
divided into four groups according to tumor necrosis metastasis
staging system: N0, RB did not invade optic nerves (n=9); N1, RB
invaded optic papilla, but did not exceed sieve plate (n=6); N2, RB
passed through sieve plate, but did not invade the end of optic
nerves (n=25); and N3, RB cells were identified at the end of optic
nerves (n=16) (21). Depending on
whether RB cells were arranged into a Flexner-Winterstein rose
ring, RB tissues were stratified into a high-differentiation group
(n=39 cases) and a poor-differentiation group (n=17). Furthermore,
normal retinal tissues were obtained from the adjacent tissues of
RB tissues from 20 randomly selected patients. All procedures were
approved by the Ethics Committee of Jining No. 1 People's Hospital.
Written informed consent was obtained from parents or guardians of
all pediatric patients.
Cell line, culture and
transfection
The RB cell line WERI-Rb-1 (Cell Bank of the Chinese
Academy of Sciences, Shanghai, China) was cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (BD Biosciences,
Franklin Lakes, NJ, USA), 100 IU/ml penicillin and 100 IU/ml
streptomycin at 37°C in an atmosphere with 5% CO2 and
70% humidity. The cells were passaged once every four days, and
those in the logarithmic growth phase were collected for
experiments. On the day before transfection, the cells
(3×105) were seeded onto 24-well plates containing
RPMI-1640 without fetal bovine serum or antibiotics. The cells were
divided into negative control (NC) and miR-106b mimics groups. When
reaching 70% confluency, 1.5 µl miR-negative control (NC), miR-106b
mimics or miR-106b inhibitor (20 pmol/µl; RiboBio, Guangzhou,
China) and 1 µl liposome (Lipofectamine 2000; Thermo Fisher
Scientific, Inc. Waltham, MA, USA) were mixed with 50 µl OptiMem
medium (Thermo Fisher Scientific, Inc.), respectively, in
individual Eppendorf tubes. After standing still for 5 min, the
mixtures in the two Eppendorf tubes were mixed and kept at room
temperature for 20 min, followed by addition into each culture
well. After 6 h of incubation, the medium was replaced with fresh
RPMI-1640 medium supplemented with 10% fetal bovine serum, and the
cells were cultured under normal conditions prior to use. For RNA
interference or overexpression of the zinc finger and BTB domain
containing 4 (ZBTB4) gene, WERI-Rb-1 cells were transfected with
lentiviral vectors LV-puro-shR-ZBTB4 and LV-puro-ZBTB4,
respectively (HanBio, Shanghai, China) with a multiplicity of
infection of 20. An empty vector was used as a negative control.
After incubation for 72 h, the cells were harvested for further
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RB and control tissues (100 mg) were ground into
powder in liquid nitrogen and mixed with 1 ml TRIzol (Thermo Fisher
Scientific, Inc.) for lysis. Total RNA was then extracted using the
phenol chloroform method. The purity of the RNA was determined via
the absorbance at 260 vs. 280 nm using ultraviolet
spectrophotometry (Nanodrop ND2000; Thermo Fisher Scientific,
Inc.). The complementary (c)DNA was obtained by reverse
transcription from 1 µg RNA and stored at −20°C. Reverse
transcription of mRNA was performed using the PrimeScript™ II First
Strand cDNA Synthesis kit (Takara, Dalian, China), and reverse
transcription of miRNA was performed using the One Step PrimeScript
miRNA cDNA Synthesis kit (Takara).
The expression of miR-106b was determined using the
SYBR PrimeScript RT-PCR Kit (Takara, Dalian, China), using U6 as an
internal reference. The reaction system (20 µl) contained 10 µl
real-time quantitative PCR mix, 0.5 µl upstream primer (miR-106b;
5′-TAAAGTGCTGACAGTGCAGAT-3′), 0.5 µl downstream universal primer
(provided by the kit), 2 µl cDNA and 7 µl double-distilled
(dd)H2O. The reaction protocol was as follows: Initial
denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
1 min and 60°C for 30 sec (iQ5; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The 2−ΔΔCq method (22) was used to calculate the relative
expression of miR-106b against the internal reference. Each sample
was tested in triplicate for PCR amplification.
The SYBR Green RT-qPCR kit (Kapa Biosystems,
Wilmington, MA, USA) was used to detect mRNA expression of ZBTB4,
using GAPDH as an internal reference. The reaction system (20 µl)
was composed of 10 µl SYBR EX Taq-Mix, 0.5 µl upstream primer
(ZBTB4, 5′-TCCCTTTTGCACTGAGGCTT-3′; GAPDH,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′), 0.5 µl downstream primer (ZBTB4,
5′-AGAAGGGACTTGAAGCAGCC-3′; GAPDH, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′),
5 µl cDNA and 4 µl ddH2O. The PCR conditions were as
follows: Initial denaturation at 95°C for 10 min; 40 cycles of
denaturation at 95°C for 1 min, annealing at 60°C for 40 sec and
elongation at 72°C for 30 sec; and final elongation at 72°C for 1
min (iQ5; Bio-Rad Laboratories, Inc.). The 2−ΔΔCq method
was used to calculate the relative expression of ZBTB4 mRNA against
GAPDH. Each sample was tested in triplicate.
Cell Counting kit 8 (CCK-8) assay
Cells were seeded at 2,000/well in 96-well plates
for transfection. At 48 h after transfection, the cells were
subjected to the CCK-8 assay for the detection of proliferation. At
0, 24 and 48 h, the medium was discarded and the cells were washed
with PBS twice, followed by addition of 20 µl CCK-8 reaction
reagent (5 g/l) diluted in medium. On the last day, 150 µl CCK-8
reaction solution was added and the cells were incubated at 37°C
for 2 h. The absorbance of each well was measured at 490 nm for
plotting the cell proliferation curves. Each group was tested in 3
replicate wells and the values were averaged. Each experiment was
performed in triplicate.
Transwell assay
For the invasion assay, Matrigel was thawed at 4°C
overnight and diluted at 1:2 with serum-free RPMI-1640 medium. The
mixture (50 µl) was evenly smeared into the upper chambers of a
Transwell insert (pore size, 0.8 µm; EMD Millipore, Billerica, MA,
USA) and incubated at 37°C for 1 h to solidify. For both the
invasion and migration assay, 1×105 cells from each
group were then seeded into the upper chamber in 200 µl serum-free
RPMI-1640 medium. In addition, 500 µl RPMI-1640 medium supplemented
with 10% fetal bovine serum was added into the lower chamber. After
24 h, the cells in the upper chambers were wiped off. After fixing
of the membranes with 4% formaldehyde for 10 min, the membrane was
stained at room temperature for 2 min using the Giemsa method for
microscopic observation of 5 random fields (magnification, ×200).
The number of cells that had transgressed through the membrane was
calculated for the evaluation of cell invasion and migration
ability. All procedures were performed on ice with pipetting tips
being cooled at 4°C.
Western blot analysis
Tissues or cells were ground into powder in liquid
nitrogen and 100 mg of the powder was mixed with 100 µl pre-cooled
radioimmunoprecipitation assay lysis buffer containing 1%
phenylmethylsulfonyl fluoride for lysis overnight at 4°C. The
mixture was then centrifuged at 12,000 × g and 4°C for 10 min. The
supernatant was used to determine the protein concentration by the
bicinchoninic acid protein concentration determination kit (cat.
no. RTP7102; Real-Times Biotechnology Co., Ltd., Beijing, China).
Protein samples (50 µg) were then mixed with 5X SDS loading buffer
prior to denaturation in a boiling water bath for 10 min.
Subsequently, the samples (10 µl) were subjected to 10% SDS-PAGE at
100 V. The resolved proteins were transferred to polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc.) on ice (250 mA, 1
h) and blocked with 50 g/l skimmed milk at room temperature for 1
h. The membranes were then incubated with rabbit anti-human ZBTB4
(1:1,000 dilution; cat. no. ab106554; Abcam, Cambridge, UK) and
GAPDH (1:4,000 dilution; cat. no. ab9485; Abcam) polyclonal primary
antibodies at 4°C overnight. After extensive washing with PBS
containing Tween-20 (PBST) for 5 times for 5 min each, the
membranes were incubated with polyclonal goat anti-rabbit
horseradish peroxidase-conjugated secondary antibody (1:4,000
dilution; cat. no. ab6721; Abcam) for 1 h at room temperature prior
to washing with PBST as above. The membrane was then developed with
an enhanced chemiluminescence detection kit (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for imaging. Image lab v3.0 software
(Bio-Rad Laboratories, Inc.) was used to acquire and analyze
imaging signals. The relative expression of ZBTB4 protein was
calculated against GAPDH.
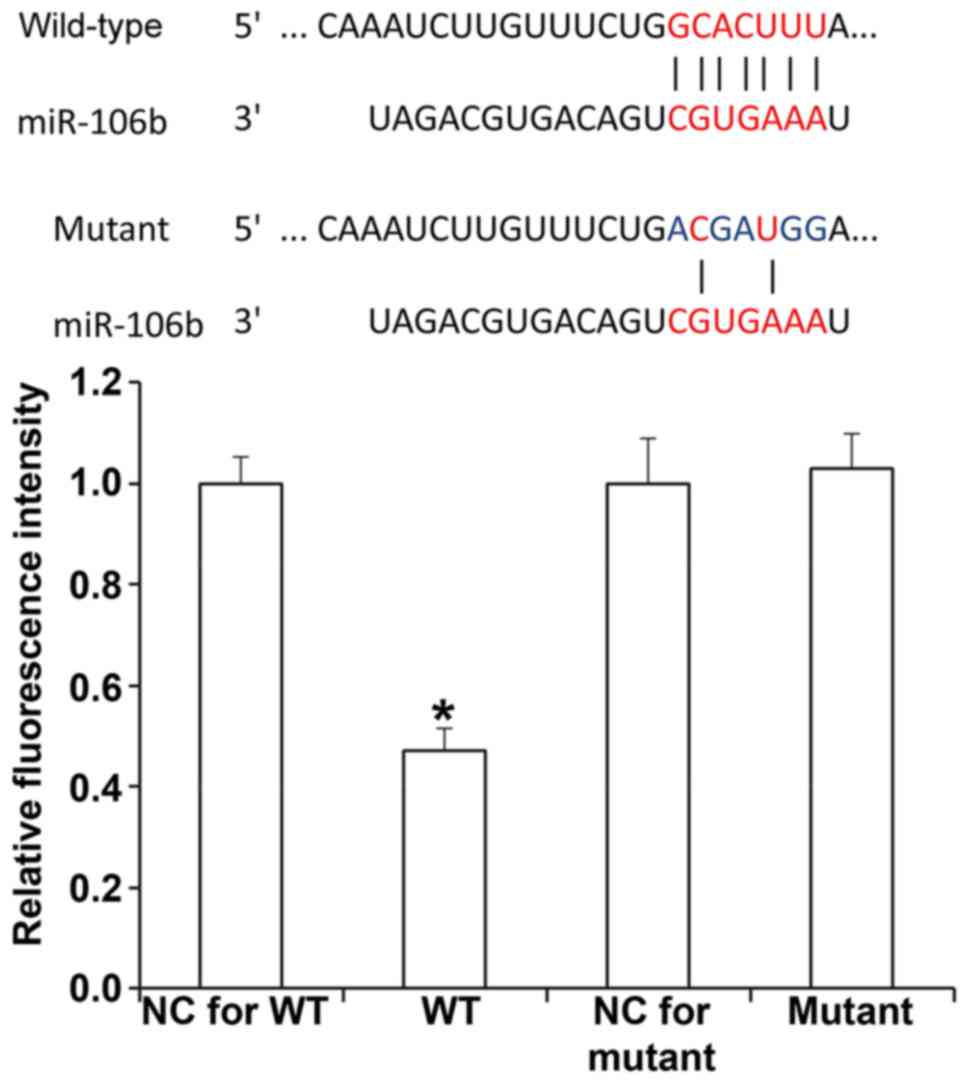
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool for the
study of the functions of miRNAs. To understand the regulatory
mechanisms of miR-106b in RB, miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org), PiTa
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PICTAR (http://pictar.mdc-berlin.de/) were used to predict
mRNAs that could be targeted by miR-106b, and identified that
miR-106b was able to potentially regulate ZBTB4. According to the
results of the bioinformatics analysis, wild-type (WT) and mutant
seed regions of miR-106b in the 3′-UTR of the ZBTB4 gene were
chemically synthesized, Spe-1 and HindIII restriction sites were
added, and the sequences were cloned into pMIR-REPORT luciferase
reporter plasmids (Ambion; Thermo Fisher Scientific, Inc.).
Plasmids (0.5 µg) with WT or mutant 3′-UTR DNA sequences were
co-transfected with miR-106b mimics (100 nM; Sangon Biotech) into
293T cells. After cultivation for 24 h, the cells were lysed using
a dual luciferase reporter assay kit (Promega Corp., Madison, WI,
USA) according to the manufacturer's protocols, and the
fluorescence intensity was measured usinga GloMax 20/20 luminometer
(Promega Corp.). Using Renilla fluorescence activity as an internal
reference, the fluorescence values of each group of cells were
measured.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (IBM Corp., Armonk, NY, USA). Measurement data were
expressed as the mean ± standard deviation. Differences between two
groups were compared using Student's t-test. Multigroup comparisons
were made using one-way analysis of variance. In the case of
homogeneity of variance, the Least Significant Difference and the
Student-Newman-Keuls methods were used; in the case of
heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was
used. P<0.05 was considered to indicate a statistically
significant difference.
Results
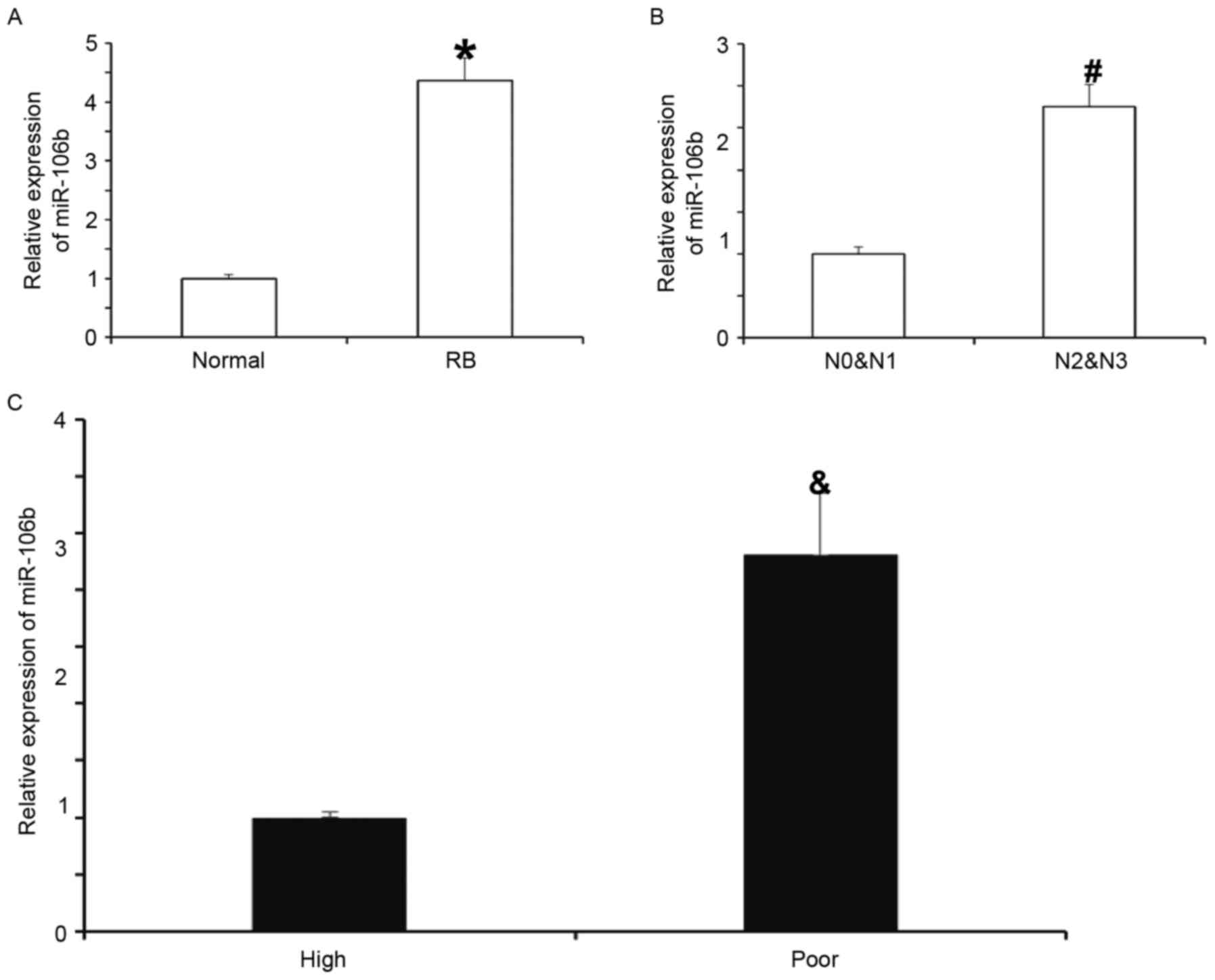
Expression of miR-106b in RB tissues
is elevated and closely associated with the disease severity
To measure the expression of miR-106b in RB tissues,
RT-qPCR was performed. The results indicated that miR-106b levels
in RB tissues were significantly higher than those in normal
tissues (P<0.05; Fig. 1A). In
addition, the levels of miR-106b in the N2 and N3 groups were
significantly higher than those in the N0 and N1 groups (P<0.05;
Fig. 1B). Furthermore, miR-106b
expression in the poor-differentiation group was significantly
higher than that in the high-differentiation group (P<0.05;
Fig. 1C). These results suggest that
the expression of miR-106b in RB tissues is elevated and closely
associated with the severity of the disease.
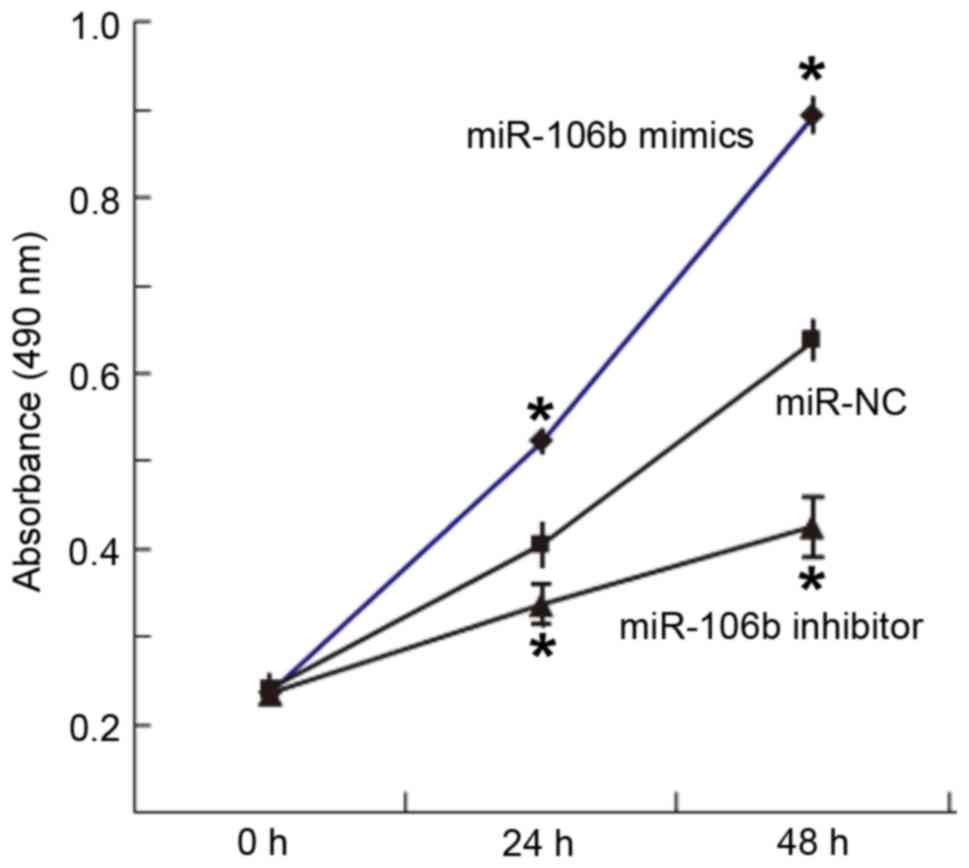
Overexpression of miR-106b increases
but inhibition of miR-106b expression decreases the proliferation
ability of WERI-Rb-1 cells
To determine the proliferation of WERI-Rb-1 cells, a
CCK-8 assay was used. The results indicated that the absorbance of
cells transfected with miR-106b mimics was significantly higher
than that in the miR-NC group at 24 and 48 h (P<0.05), while the
absorbance of cells transfected with miR-106b inhibitor was
significantly lower than that in the miR-NC group at 24 and 48 h
(P<0.05; Fig. 2). This result
indicated that overexpression of miR-106b increases but inhibition
of miR-106b expression decreases the proliferation ability of
WERI-Rb-1 cells.
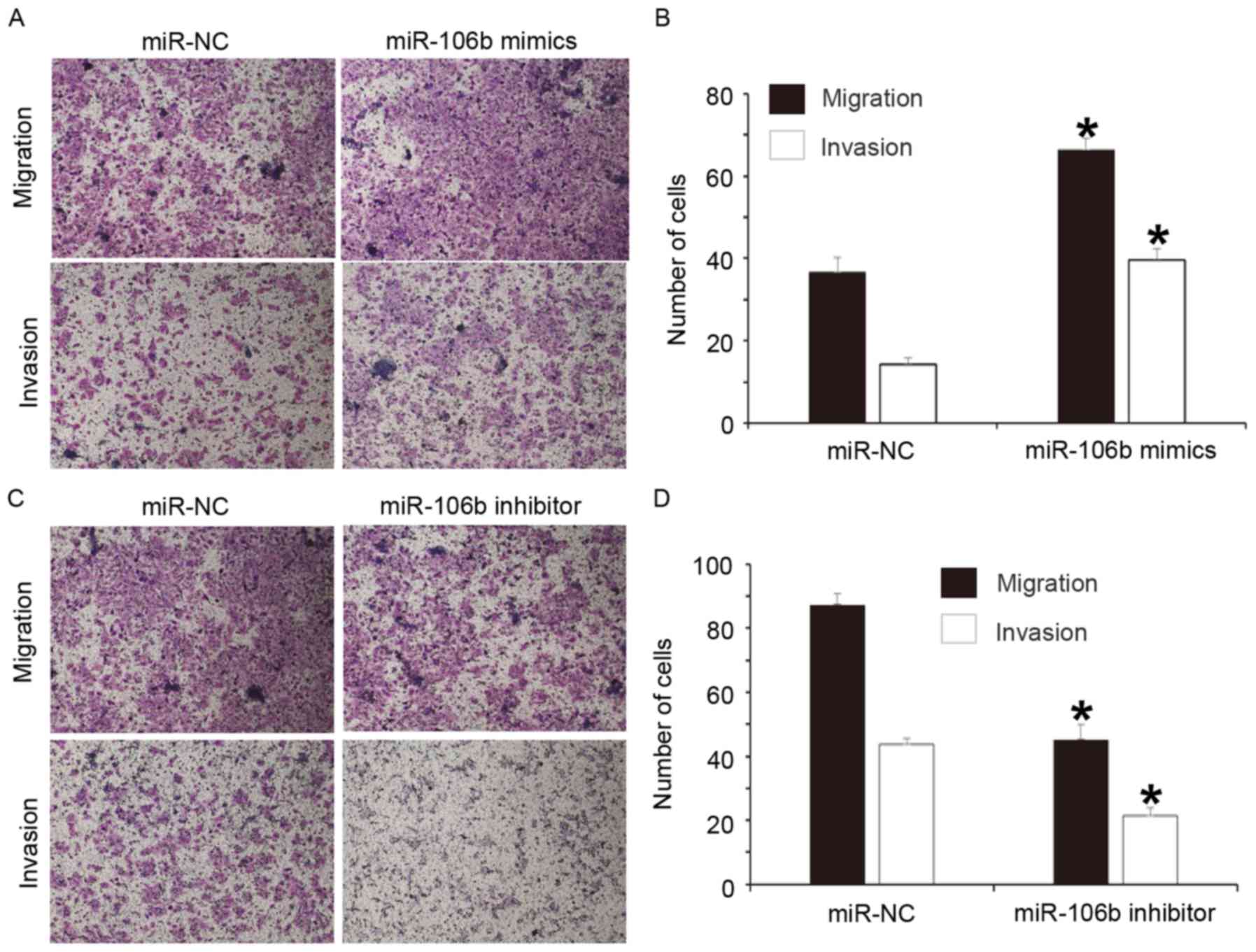
Overexpression of miR-106b increases
but inhibition of miR-106b expression decreases the migration and
invasion abilities of WERI-Rb-1 cells
To study the effect of miR-106b on the migration and
invasion of WERI-Rb-1 cells, a Transwell assay was performed. The
results indicated that the numbers of cells in the miR-106b mimics
group that transgressed through the Transwell membrane in the
migration and invasion assays were significantly higher than those
in the miR-NC group (P<0.05 for both; Fig. 3A and B). In addition, the numbers of
cells in the miR-106b inhibitor group that transgressed through the
Transwell membrane in the migration and invasion assays were
significantly reduced compared with those in the miR-NC group
(P<0.05 for both; Fig. 3C and D).
These results suggest that overexpression of miR-106b increases but
inhibition of miR-106b expression decreases the migration and
invasion abilities of WERI-Rb-1 cells.
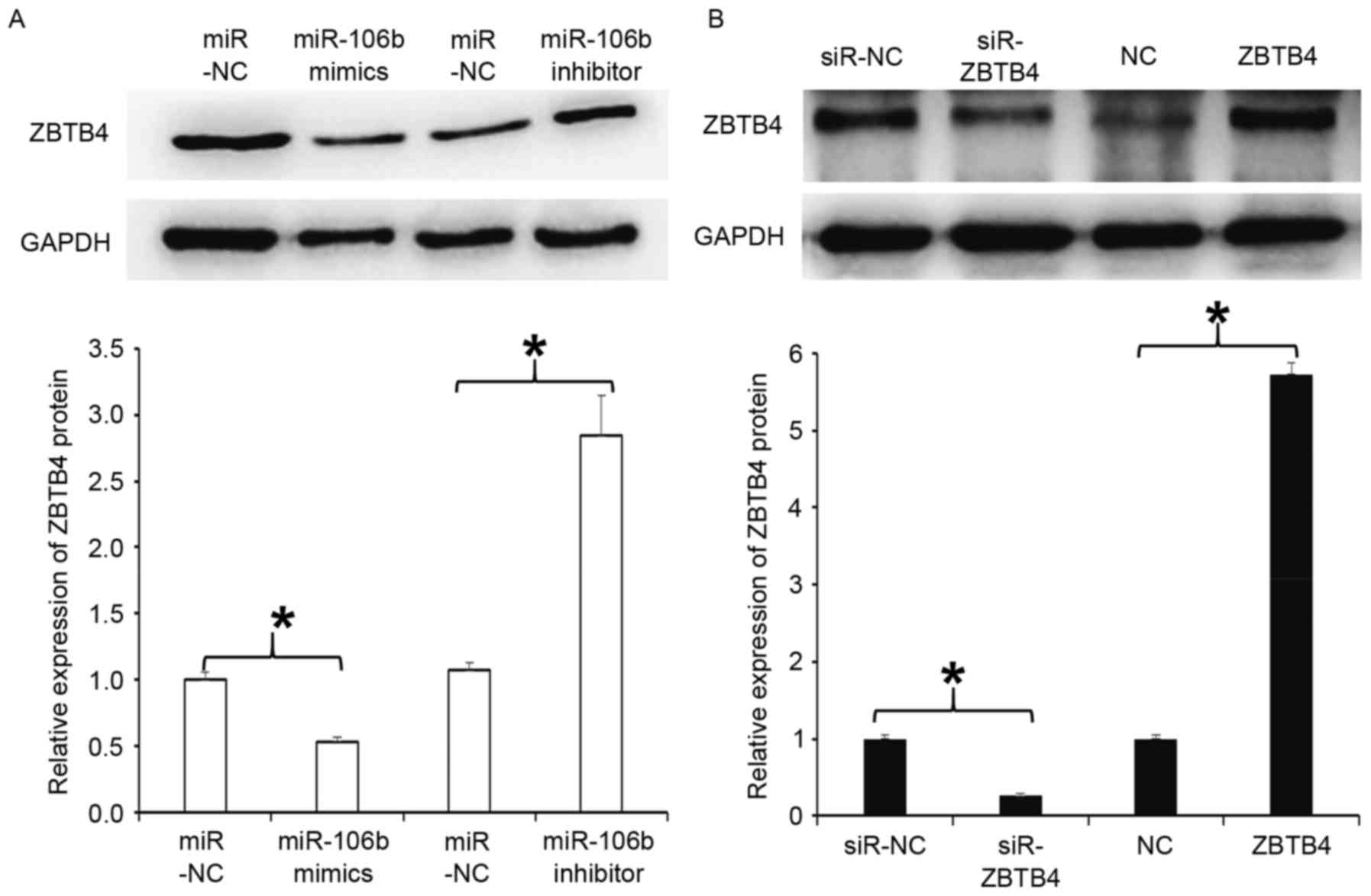
Overexpression of miR-106b decreases
but inhibition of miR-106b expression increases ZBTB4 protein
expression in WERI-Rb-1 cells
To examine the expression of ZBTB4 protein, western
blot analysis was employed. The results indicated that ZBTB4
expression in cells transfected with miR-106b mimics was
significantly reduced compared with that in the respective miR-NC
group (P<0.05), but that in cells transfected with miR-106b
inhibitor was significantly increased (P<0.05; Fig. 4A). In addition, WERI-Rb-1 cells with
silencing of ZBTB4 by transfection with lentiviral vector had a
significantly reduced expression of ZBTB4 protein compared with
that in the respective miR-NC group (P<0.05), while cells with
overexpression of ZBTB4 by transfection with lentiviral vector had
a significantly enhanced expression of ZBTB4 protein compared with
that in the NC group (P<0.05; Fig.
4B). The results indicate that overexpression of miR-106b
decreases but inhibition of miR-106b expression increases ZBTB4
protein expression in WERI-Rb-1 cells.
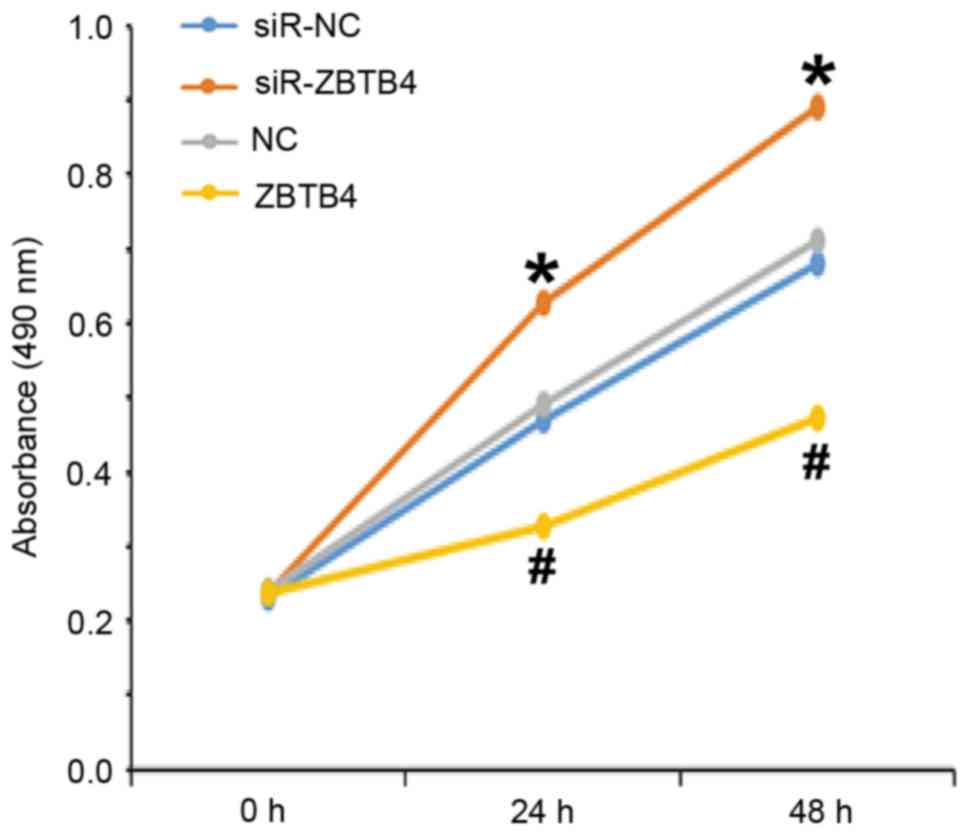
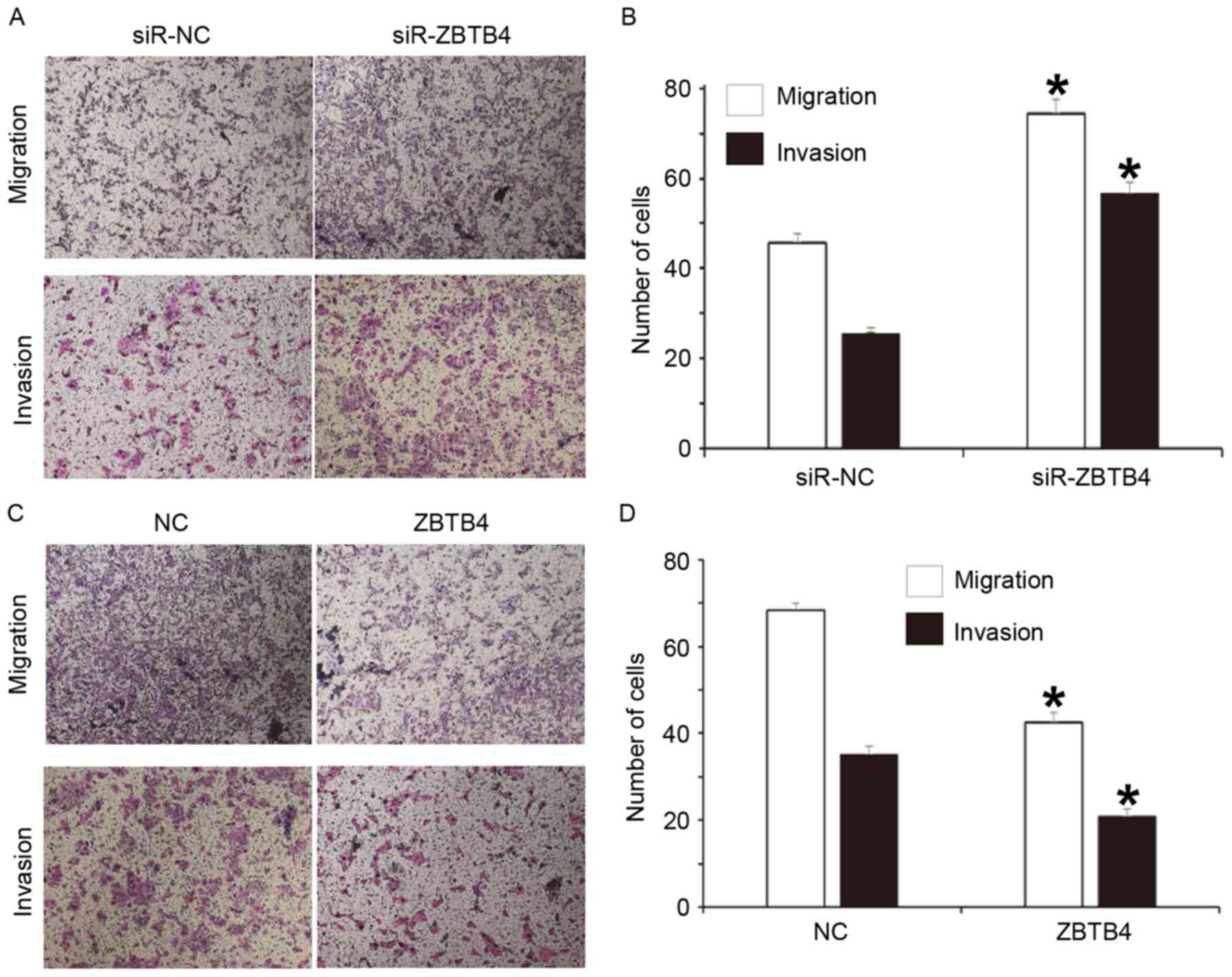
Overexpression of ZBTB4 reduces but
inhibition of ZBTB4 expression promotes the proliferation,
migration and invasion of WERI-Rb-1 cells
To assess the effect of ZBTB4 protein expression on
the biological function of WERI-Rb-1 cells, a CCK-8 assay and
Transwell assay were performed on cells with silenced expression or
overexpression of ZBTB4. The results indicated that the absorbance
of cells transfected with siR-ZBTB4 was significantly higher than
that of cells transfected with siR-NC at 24 and 48 h (P<0.05),
while the absorbance of cells in the ZBTB4 group was significantly
lower than that of cells transfected with NC at 24 and 48 h
(Fig. 5). The Transwell assay
indicated that the numbers of cells in the siR-ZBTB4 group that
transgressed through the Transwell membrane in the migration and
invasion assays were significantly higher than those in the siR-NC
group (P<0.05 for both; Fig. 6A and
B). In addition, the numbers of cells in the ZBTB4 group that
transgressed through the Transwell membrane in the migration and
invasion assays were significantly reduced compared with those in
the NC group (P<0.05 for both; Fig.
6C and D). These results suggest that overexpression of ZBTB4
reduces but inhibition of ZBTB4 expression promotes the
proliferation, migration and invasion of WERI-Rb-1 cells.
miR-106b inhibits the expression of
ZBTB4 by binding with the 3′-UTR of ZBTB4 mRNA
To understand whether miR-106b directly targets
ZBTB4, a dual luciferase reporter assay was performed. The results
demonstrated that transfection with miR-106b mimics and
pMIR-REPORT-wild-type ZBTB4 led to significantly reduced
fluorescence intensity compared with that in the negative control
miR-transfected sample (P<0.05), while transfection with
miR-106b mimics and pMIR-REPORT-mutant ZBTB4 resulted in similar
fluorescence intensity compared with that in the negative control
miR-transfected sample (P>0.05; Fig.
7). This result indicates that miR-106b regulates the
expression of ZBTB4 by binding with the 3′-UTR of ZBTB4 mRNA.
Discussion
Certain miRNAs have important roles in the
occurrence and development of tumors. Members of the miR-106b-25
family display abnormal expression in multiple tumor tissues and
peripheral blood, participating in the regulation of proliferation,
apoptosis, invasion and metastasis of tumors (23). For instance, the expression of the
miR-106b-25 cluster is elevated in recurrent acute myeloid leukemia
in pediatric patients caused by mixed lineage leukemia gene
rearrangement, suggesting that miR-106b-25 is closely associated
with the recurrence of acute myeloid leukemia (24). Zheng et al (25) discovered that miR-106b induces
radiotherapy resistance of colon cancer cells by regulating the
PTEN/PI3K/AKT signaling pathway and p21 expression. It has been
reported that miR-106b-25 cluster expression is elevated in gastric
cancer tissues and peripheral blood of patients, and promotes the
proliferation, invasion and metastasis of gastric cancer cells
(23). In the present study, it was
discovered that miR-106b expression is significantly increased in
RB tissues, and patients at N2 and N3 stages had higher miR-106b
levels than patients at N0 and N1 stages, suggesting that miR-106b
expression is associated with the invasion and metastasis of RB.
Regarding the degree of differentiation, miR-106b expression in the
poor-differentiation group was significantly higher than that in
the high-differentiation group, indicating that miR-106b expression
is associated with RB cell differentiation. At the cellular level,
silencing of miR-106b expression inhibited the proliferation,
migration and invasion of WERI-Rb-1 cells, while overexpression of
miR-106b had the opposite effect, suggesting that miR-106b acts as
an oncogene in RB.
The ZBTB protein family is a class of proteins that
have a BTB domain at the N-terminus and multiple zinc finger
domains at the C-terminus (26,27).
Most members of the ZBTB family have a transcription factor
function. ZBTB4, a member of the ZBTB family comprising 1,013 amino
acids, has 1 BTB domain at N-terminus and 6
C2H2-type zinc finger structures at the
C-terminus. ZBTB4 is a phosphorylation substrate for
homeodomain-interacting protein kinase 2, and its phosphorylation
induces the degradation of ZBTB4 protein and DNA injury (28). It was also reported that ZBTB4
directly binds to the promoter of p21 with the assistance of
ZBTB17, inhibits p21 transcription, prevents cell cycle arrest via
p21 after DNA injury and induces apoptosis (29). The bioinformatics study performed in
the present study suggested that ZBTB4 mRNA is one of the potential
targets of miR-106b. Western blot analysis and the dual luciferase
reporter assay demonstrated that miR-106b directly binds to the
3′-UTR of ZBTB4 and reduces the expression of ZBTB4 protein. By
contrast, miR-106b inhibitor increased the expression of ZBTB4
protein in WERI-Rb-1 cells, suggesting that ZBTB4 is a target gene
of miR-106b. Further loss- and gain-of-function experiments
indicated that overexpression of ZBTB4 inhibits the proliferation,
migration and invasion of WERI-Rb-1 cells, while silencing of ZBTB4
expression reduces the proliferation, migration and invasion of
WERI-Rb-1 cells, indicating that ZBTB4 has a tumour suppressor
function in WERI-Rb-1 cells. At present, there are few reports on
the effect of miR-106b in RB. Similar with breast cancer and
cerebral tumors (30,31), miR-106b promotes the occurrence and
development of RB. More importantly, the present study demonstrated
that miR-106b regulates the biological activities of RB via ZBTB4
gene and elucidates its underlying mechanism. In conclusion,
miR-106b promotes the proliferation, migration and invasion of RB
cells by inhibiting ZBTB4 expression. It is a potential therapeutic
target and biomarker for RB.
Acknowledgements
The authors would like to thank Jining No. 1
People's Hospital (Jining, China) for the provision of facilities.
The authors would also like to thank Dr Shuyin Sun and Dr Jie Feng
of the Department of Ophthalmology, Jining No. 1 People's Hospital
for reading the manuscript and providing valuable suggestions.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WB, YW and XM designed the study. WB and YW
performed the experiments, and WB, YW and XM analyzed the data. All
authors interpreted the results, and produced and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of Jining No. 1 People's Hospital. Written informed consent was
obtained from parents or guardians of all pediatric patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soliman SE, Dimaras H, Khetan V, Gardiner
JA, Chan HS, Héon E and Gallie BL: Prenatal versus postnatal
screening for familial retinoblastoma. Ophthalmology.
123:2610–2617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Natalino RJ, Antoneli CB, Ribeiro KC,
Campos AH and Soares FA: Immunohistochemistry of apoptosis-related
proteins in retinoblastoma. Pathol Res Pract. 212:1144–1150. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta AK, Jones M, Prelog K, Bui J, Zhu J,
Ng A and Dalla-Pozza L: Pineal cysts-A benign association with
familial retinoblastoma. Pediatr Hematol Oncol. 33:408–414. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian T, Ji XD, Zhang Q, Peng J and Zhao
PQ: A delayed diagnosis of unsuspected retinoblastoma in an in
vitro fertilisation infant with retinopathy of prematurity. Int J
Ophthalmol. 9:1361–1363. 2016.PubMed/NCBI
|
|
5
|
Silva BB, Sapienza L, Castro DG, Ferreira
DDV, Leão CR, Neves DFGDS, Silva B, Aiza A, Scintini AC, Pellizzon
C and Regalin M: eye plaque brachytherapy for retinoblastoma-A
uni-institutional retrospective analysis of 40 eyes in 38 patients
treated from 2001 to 2014. Int J Radiat Oncol Biol Phys.
96:E5542016. View Article : Google Scholar
|
|
6
|
Assayag F, Nicolas A, Vacher S, Dehainault
C, Bieche I, Meseure D, Aerts I, Cassoux N, Houdayer C, Doz F and
Decaudin D: Combination of carboplatin and bevacizumab is an
efficient therapeutic approach in retinoblastoma patient-derived
xenografts. Invest Ophthalmol Vis Sci. 57:4916–4926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shehata HH, Ghalia Abou AH, Elsayed EK,
Said Ahmed AM and Mahmoud SS: Clinical significance of high levels
of survivin and transforming growth factor beta-1 proteins in
aqueous humor and serum of retinoblastoma patients. J AAPOS.
20:444.e441–444.e449. 2016. View Article : Google Scholar
|
|
8
|
Singh U, Malik MA, Goswami S, Shukla S and
Kaur J: Epigenetic regulation of human retinoblastoma. Tumour Biol.
37:14427–14441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malorni L, Piazza S, Ciani Y, Guarducci C,
Bonechi M, Biagioni C, Hart CD, Verardo R, Di Leo A and Migliaccio
I: A gene expression signature of Retinoblastoma loss-of-function
is a predictive biomarker of resistance to palbociclib in breast
cancer cell lines and is prognostic in patients with ER positive
early breast cancer. Oncotarget. 7:68012–68022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin D and Lee H: Prioritizing
cancer-related microRNAs by integrating microRNA and mRNA datasets.
Sci Rep. 6:353502016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lima TI, Araujo HN, Menezes ES, Sponton
CH, Araújo MB, Bomfim LH, Queiroz AL, Passos MA, Sousa E TA,
Hirabara SM, et al: Role of microRNAs on the regulation of
mitochondrial biogenesis and insulin signaling in skeletal muscle.
J Cell Physiol. 232:958–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Carvalho IN, de Freitas RM and Vargas
FR: Translating microRNAs into biomarkers: What is new for
pediatric cancer? Med Oncol. 33:492016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gui F, Hong Z, You Z, Wu H and Zhang Y:
MiR-21 inhibitor suppressed the progression of retinoblastoma via
the modulation of PTEN/PI3K/AKT pathway. Cell Biol Int.
40:1294–1302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai S, Tian B, Li A, Yao Q, Zhang G and Li
F: MicroRNA-125b promotes tumor growth and suppresses apoptosis by
targeting DRAM2 in retinoblastoma. Eye (Lond). 30:1630–1638. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Xue C, Zhu X, Zhu X, Xian H and
Huang Z: Suppression of microRNA-125a-5p upregulates the TAZ-EGFR
signaling pathway and promotes retinoblastoma proliferation. Cell
Signal. 28:850–860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng Q, Jin C, Chen W, Xia F, Wang Q, Fan
F, Du J, Guo Y, Lin C, Yang K, et al: Downregulation of serum
miR-17 and miR-106b levels in gastric cancer and benign gastric
diseases. Chin J Cancer Res. 26:711–716. 2014.PubMed/NCBI
|
|
17
|
Xu X, Liu Z, Wang J, Ling Q, Xie H, Guo H,
Wei X, Zhou L and Zheng S: miRNA profiles in livers with different
mass deficits after partial hepatectomy and miR-106b~25 cluster
accelerating hepatocyte proliferation in rats. Sci Rep.
6:312672016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu D, Shin HS, Lee YS and Lee YC: miR-106b
modulates cancer stem cell characteristics through TGF-β/Smad
signaling in CD44-positive gastric cancer cells. Lab Invest.
94:1370–1381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai F, Liu T, Zheng S, Liu Q, Yang C, Zhou
J, Chen Y, Sheyhidin I and Lu X: MiR-106b promotes migration and
invasion through enhancing EMT via downregulation of Smad 7 in
Kazakh's esophageal squamous cell carcinoma. Tumour Biol.
37:14595–14604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Liu Y, Miao Y, Zhao L, Zhou H and
Jia L: MicroRNA-106b targets FUT6 to promote cell migration,
invasion, and proliferation in human breast cancer. IUBMB Life.
68:764–775. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishaq SM, Kehar SI, Zafar S and Hasan SF:
Correlation of CD24 expression with histological grading and TNM
staging of retinoblastoma. Pak J Med Sci. 32:160–164.
2016.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Wang W, Li F, Zhang H and Liu J:
MicroRNA-106b~25 expressions in tumor tissues and plasma of
patients with gastric cancers. Med Oncol. 31:2432014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verboon LJ, Obulkasim A, de Rooij JD,
Katsman-Kuipers JE, Sonneveld E, Baruchel A, Trka J, Reinhardt D,
Pieters R, Cloos J, et al: MicroRNA-106b~25 cluster is upregulated
in relapsed MLL-rearranged pediatric acute myeloid leukemia.
Oncotarget. 7:48412–48422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng L, Zhang Y, Liu Y, Zhou M, Lu Y,
Yuan L, Zhang C, Hong M, Wang S and Li X: MiR-106b induces cell
radioresistance via the PTEN/PI3K/AKT pathways and p21 in
colorectal cancer. J Transl Med. 13:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim K, Chadalapaka G, Pathi SS, Jin UH,
Lee JS, Park YY, Cho SG, Chintharlapalli S and Safe S: Induction of
the transcriptional repressor ZBTB4 in prostate cancer cells by
drug-induced targeting of microRNA-17-92/106b-25 clusters. Mol
Cancer Ther. 11:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang WS, Chadalapaka G, Cho SG, Lee SO,
Jin UH, Jutooru I, Choi K, Leung YK, Ho SM, Safe S and Kim K: The
transcriptional repressor ZBTB4 regulates EZH2 through a
MicroRNA-ZBTB4-specificity protein signaling axis. Neoplasia.
16:1059–1069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim K, Chadalapaka G, Lee SO, Yamada D,
Sastre-Garau X, Defossez PA, Park YY, Lee JS and Safe S:
Identification of oncogenic microRNA-17-92/ZBTB4/specificity
protein axis in breast cancer. Oncogene. 31:1034–1044. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamada D, Pérez-Torrado R, Filion G, Caly
M, Jammart B, Devignot V, Sasai N, Ravassard P, Mallet J,
Sastre-Garau X, et al: The human protein kinase HIPK2
phosphorylates and downregulates the methyl-binding transcription
factor ZBTB4. Oncogene. 28:2535–2544. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guarnieri AL, Towers CG, Drasin DJ,
Oliphant MUJ, Andrysik Z, Hotz TJ, Vartuli RL, Linklater ES, Pandey
A, Khanal S, et al: The miR-106b-25 cluster mediates breast tumor
initiation through activation of NOTCH1 via direct repression of
NEDD4L. Oncogene. Apr 17–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gruszka R and Zakrzewska M: The oncogenic
relevance of miR-17-92 cluster and its paralogous miR-106b-25 and
miR-106a-363 clusters in brain tumors. Int J Mol Sci. 19:pii:
E8792018. View Article : Google Scholar
|