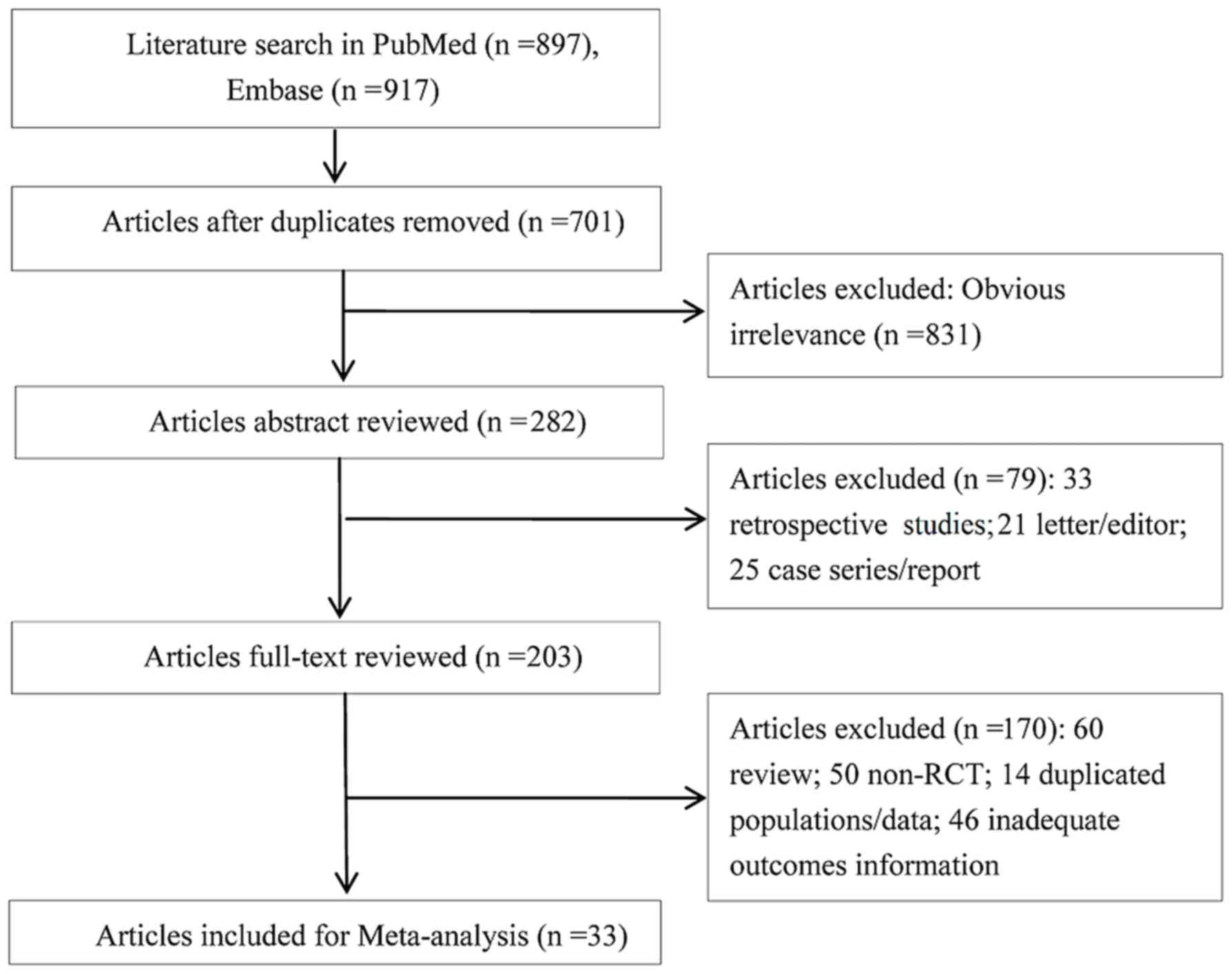
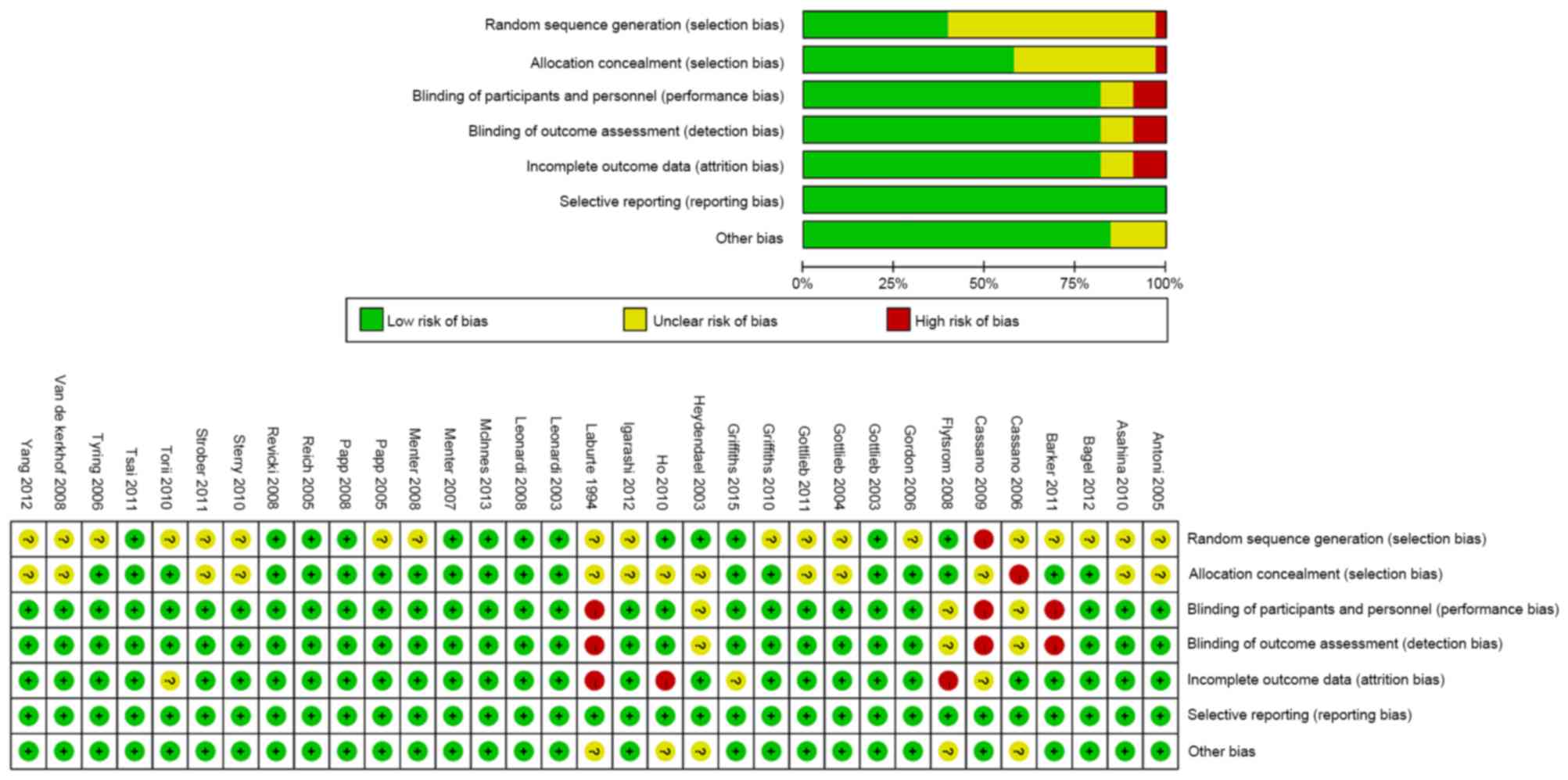
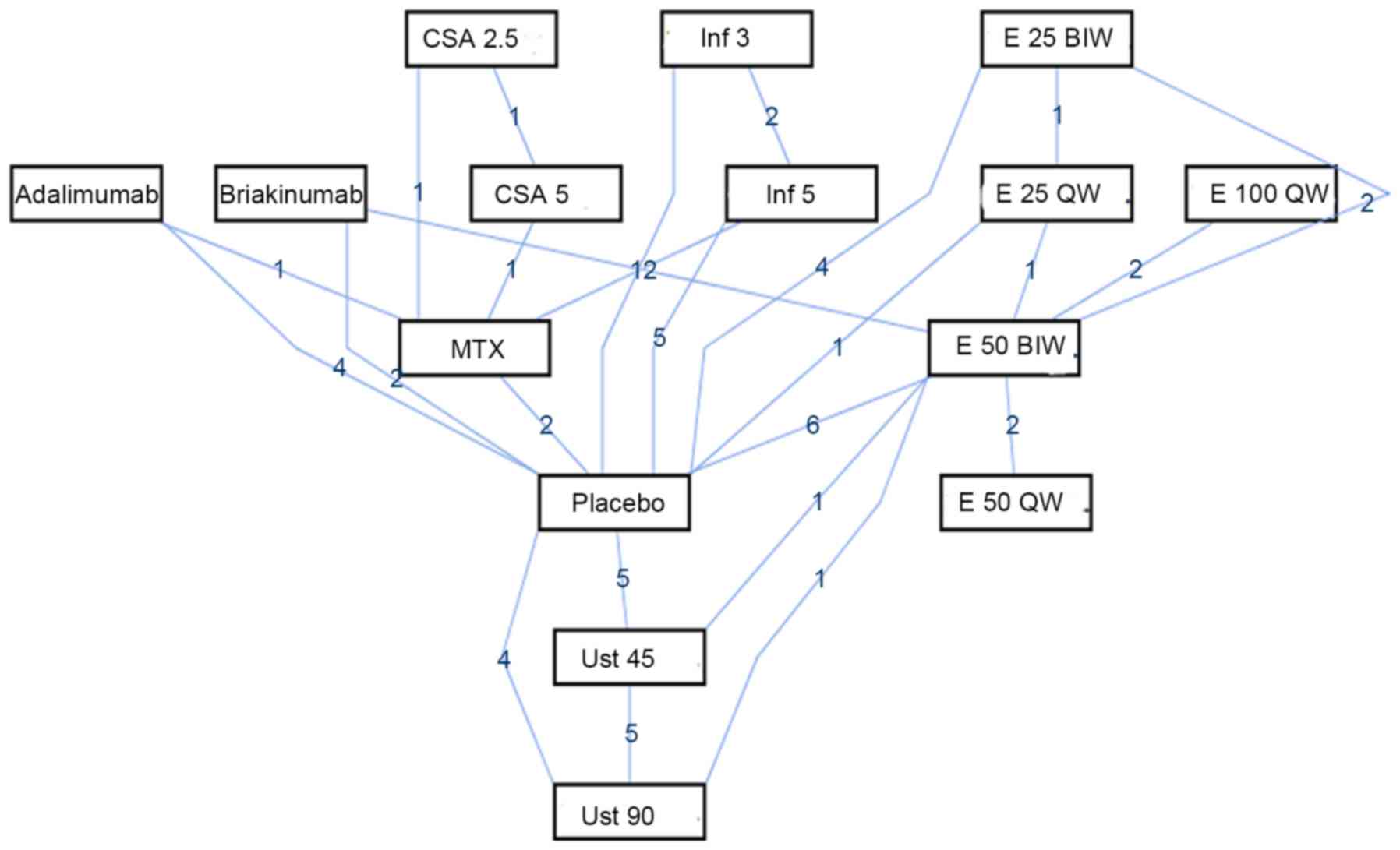
|
1
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM; Identification and Management of Psoriasis and
Associated ComorbidiTy (IMPACT) project team, . Global epidemiology
of psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Menter A, Gottlieb A, Feldman SR, Van
Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA,
Korman NJ, et al: Guidelines of care for the management of
psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis
and guidelines of care for the treatment of psoriasis with
biologics. J Am Acad Dermatol. 58:826–850. 2008.
|
|
3
|
Griffiths CE and Barker JN: Pathogenesis
and clinical features of psoriasis. Lancet. 370:263–271. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antoni CE, Kavanaugh A, Kirkham B, Tutuncu
Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E,
Gladman D, et al: Sustained benefits of infliximab therapy for
dermatologic and articular manifestations of psoriatic arthritis:
Results from the infliximab multinational psoriatic arthritis
controlled trial (IMPACT). Arthritis Rheum. 52:1227–1236. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahina A, Nakagawa H, Etoh T and Ohtsuki
M; Adalimumab M04-688 Study Group, . Adalimumab in Japanese
patients with moderate to severe chronic plaque psoriasis: Efficacy
and safety results from a Phase II/III randomized controlled study.
J Dermatol. 37:299–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffiths C, Sterry W, Brock F, Dilleen M,
Stefanidis D, Germain JM and Mallbris L: Pattern of response in
patients with moderate-to-severe psoriasis treated with etanercept.
Br J Dermatol. 172:230–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sterry W, Ortonne JP, Kirkham B, Brocq O,
Robertson D, Pedersen RD, Estojak J, Molta CT and Freundlich B:
Comparison of two etanercept regimens for treatment of psoriasis
and psoriatic arthritis: PRESTA randomised double blind multicentre
trial. BMJ. 340:c1472010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strober BE, Crowley JJ, Yamauchi PS, Olds
M and Williams DA: Efficacy and safety results from a phase III,
randomized controlled trial comparing the safety and efficacy of
briakinumab with etanercept and placebo in patients with moderate
to severe chronic plaque psoriasis. Br J Dermatol. 165:661–668.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagel J, Lynde C, Tyring S, Kricorian G,
Shi Y and Klekotka P: Moderate to severe plaque psoriasis with
scalp involvement: A randomized, double-blind, placebo-controlled
study of etanercept. J Am Acad Dermatol. 67:86–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schäfer I, Hacker J, Rustenbach SJ, Radtke
M, Franzke N and Augustin M: Concordance of the psoriasis area and
severity index (PASI) and patient-reported outcomes in psoriasis
treatment. Eur J Dermatol. 20:62–67. 2010.PubMed/NCBI
|
|
11
|
Mrowietz U, Kragballe K, Reich K, Spuls P,
Griffiths CE, Nast A, Franke J, Antoniou C, Arenberger P, Balieva
F, et al: Definition of treatment goals for moderate to severe
psoriasis: A European consensus. Arch Dermatol Res. 303:1–10. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dias S, Welton NJ, Caldwell DM and Ades
AE: Checking consistency in mixed treatment comparison
meta-analysis. Stat Med. 29:932–944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barker J, Hoffmann M, Wozel G, Ortonne JP,
Zheng H, van Hoogstraten H and Reich K: Efficacy and safety of
infliximab vs. methotrexate in patients with moderate-to-severe
plaque psoriasis: Results of an open-label, active-controlled,
randomized trial (RESTORE1). Br J Dermatol. 165:1109–1117.
2011.
|
|
15
|
Cassano N, Miracapillo A, Coviello C,
Loconsole F, Bellino M and Vena GA: Treatment of psoriasis vulgaris
with the two-compound product calcipotriol/betamethasone
dipropionate followed by different formulations of calcipotriol.
Clin Drug Investig. 26:227–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flytstrom I, Stenberg B, Svensson A and
Bergbrant IM: Methotrexate vs. ciclosporin in psoriasis:
effectiveness, quality of life and safety. A randomized controlled
trial. Br J Dermatol. 158:116–121. 2008.
|
|
17
|
Gordon KB, Langley RG, Leonardi C, Toth D,
Menter MA, Kang S, Heffernan M, Miller B, Hamlin R, Lim L, et al:
Clinical response to adalimumab treatment in patients with moderate
to severe psoriasis: Double-blind, randomized controlled trial and
open-label extension study. J Am Acad Dermatol. 55:598–606. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gottlieb AB, Evans R, Li S, Dooley LT,
Guzzo CA, Baker D, Bala M, Marano CW and Menter A: Infliximab
induction therapy for patients with severe plaque-type psoriasis: A
randomized, double-blind, placebo-controlled trial. J Am Acad
Dermatol. 51:534–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gottlieb AB, Leonardi C, Kerdel F, Mehlis
S, Olds M and Williams DA: Efficacy and safety of briakinumab vs.
etanercept and placebo in patients with moderate to severe chronic
plaque psoriasis. Br J Dermatol. 165:652–660. 2011.
|
|
20
|
Gottlieb AB, Matheson RT, Lowe N, Krueger
GG, Kang S, Goffe BS, Gaspari AA, Ling M, Weinstein GD, Nayak A, et
al: A randomized trial of etanercept as monotherapy for psoriasis.
Arch Dermatol. 139:1627–1632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Griffiths CE: Comparing biological
therapies in psoriasis: Implications for clinical practice. J Eur
Acad Dermatol Venereol. 24 (Suppl 6):S10–S14. 2010. View Article : Google Scholar
|
|
22
|
Heydendael VM, Spuls PI, Opmeer BC, de
Borgie CA, Reitsma JB, Goldschmidt WF, Bossuyt PM, Bos JD and de
Rie MA: Methotrexate versus cyclosporine in moderate-to-severe
chronic plaque psoriasis. N Engl J Med. 349:658–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho SG, Yeung CK and Chan HH: Methotrexate
versus traditional Chinese medicine in psoriasis: A randomized,
placebo-controlled trial to determine efficacy, safety and quality
of life. Clin Exp Dermatol. 35:717–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Igarashi A, Kato T, Kato M, Song M and
Nakagawa H; Japanese Ustekinumab Study Group, . Efficacy and safety
of ustekinumab in Japanese patients with moderate-to-severe
plaque-type psoriasis: Long-term results from a phase 2/3 clinical
trial. J Dermatol. 39:242–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laburte C, Grossman R, Abi-Rached J,
Abeywickrama K and Dubertret L: Efficacy and safety of oral
cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic
severe plaque psoriasis. Br J Dermatol. 130:366–375. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leonardi CL, Kimball AB, Papp KA, Yeilding
N, Guzzo C, Wang Y, Li S, Dooley LT and Gordon KB; PHOENIX 1 study
investigators, . Efficacy and safety of ustekinumab, a human
interleukin-12/23 monoclonal antibody, in patients with psoriasis:
76-week results from a randomised, double-blind, placebo-controlled
trial (PHOENIX 1). Lancet. 371:1665–1674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leonardi CL, Powers JL, Matheson RT, Goffe
BS, Zitnik R, Wang A and Gottlieb AB; Etanercept Psoriasis Study
Group, . Etanercept as monotherapy in patients with psoriasis. N
Engl J Med. 349:2014–2022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McInnes IB, Kavanaugh A, Gottlieb AB, Puig
L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM,
et al: Efficacy and safety of ustekinumab in patients with active
psoriatic arthritis: 1 year results of the phase 3, multicentre,
double-blind, placebo-controlled PSUMMIT 1 trial. Lancet.
382:780–789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menter A, Feldman SR, Weinstein GD, Papp
K, Evans R, Guzzo C, Li S, Dooley LT, Arnold C and Gottlieb AB: A
randomized comparison of continuous vs. intermittent infliximab
maintenance regimens over 1 year in the treatment of
moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 56(31):
e1–e15. 2007.PubMed/NCBI
|
|
30
|
Menter A, Tyring SK, Gordon K, Kimball AB,
Leonardi CL, Langley RG, Strober BE, Kaul M, Gu Y, Okun M and Papp
K: Adalimumab therapy for moderate to severe psoriasis: A
randomized, controlled phase III trial. J Am Acad Dermatol.
58:106–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Papp KA, Langley RG, Lebwohl M, Krueger
GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, et al:
Efficacy and safety of ustekinumab, a human interleukin-12/23
monoclonal antibody, in patients with psoriasis: 52-week results
from a randomised, double-blind, placebo-controlled trial (PHOENIX
2). Lancet. 371:1675–1684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papp KA, Tyring S, Lahfa M, Prinz J,
Griffiths CE, Nakanishi AM, Zitnik R, van de Kerkhof PC and Melvin
L; Etanercept Psoriasis Study Group, . A global phase III
randomized controlled trial of etanercept in psoriasis: Safety,
efficacy and effect of dose reduction. Br J Dermatol.
152:1304–1312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reich K, Nestle FO, Papp K, Ortonne JP,
Evans R, Guzzo C, Li S, Dooley LT and Griffiths CE: EXPRESS study
investigators: Infliximab induction and maintenance therapy for
moderate-to-severe psoriasis: A phase III, multicentre,
double-blind trial. Lancet. 366:1367–1374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Revicki D, Willian MK, Saurat JH, Papp KA,
Ortonne JP, Sexton C and Camez A: Impact of adalimumab treatment on
health-related quality of life and other patient-reported outcomes:
Results from a 16-week randomized controlled trial in patients with
moderate to severe plaque psoriasis. Br J Dermatol. 158:549–557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Torii H and Nakagawa H; Japanese
Infliximab Study investigators, . Infliximab monotherapy in
Japanese patients with moderate-to-severe plaque psoriasis and
psoriatic arthritis. A randomized, double-blind, placebo-controlled
multicenter trial. J Dermatol Sci. 59:40–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsai TF, Ho JC, Song M, Szapary P, Guzzo
C, Shen YK, Li S, Kim KJ, Kim TY, Choi JH, et al: Efficacy and
safety of ustekinumab for the treatment of moderate-to-severe
psoriasis: A phase III, randomized, placebo-controlled trial in
Taiwanese and Korean patients (PEARL). J Dermatol Sci. 63:154–163.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tyring S, Gottlieb A, Papp K, Gordon K,
Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, et al:
Etanercept and clinical outcomes, fatigue, and depression in
psoriasis: Double-blind placebo-controlled randomised phase III
trial. Lancet. 367:29–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van de Kerkhof PC, Segaert S, Lahfa M,
Luger TA, Karolyi Z, Kaszuba A, Leigheb G, Camacho FM, Forsea D,
Zang C, et al: Once weekly administration of etanercept 50 mg is
efficacious and well tolerated in patients with moderate-to-severe
plaque psoriasis: A randomized controlled trial with open-label
extension. Br J Dermatol. 159:1177–1185. 2008.PubMed/NCBI
|
|
39
|
Yang HZ, Wang K, Jin HZ, Gao TW, Xiao SX,
Xu JH, Wang BX, Zhang FR, Li CY, Liu XM, et al: Infliximab
monotherapy for Chinese patients with moderate to severe plaque
psoriasis: A randomized, double-blind, placebo-controlled
multicenter trial. Chin Med J (Engl). 125:1845–1851.
2012.PubMed/NCBI
|
|
40
|
Cassano N, Loconsole F, Miracapillo A,
Travaglini M, Digiuseppe MD, Congedo M, Galluccio A, Buquicchio R,
Mastrandrea V, Filieri M, et al: Treatment of psoriasis with
different dosage regimens of etanercept: Preliminary results from
the Tαranta plastic study group. Int J Immunopathol Pharmacol.
23:797–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mussi A, Bonifati C, Carducci M, D'Agosto
G, Pimpinelli F, D'Urso D, D'Auria L, Fazio M and Ameglio F: Serum
TNF-alpha levels correlate with disease severity and are reduced by
effective therapy in plaque-type psoriasis. J Biol Regul Homeost
Agents. 11:115–118. 1997.PubMed/NCBI
|
|
42
|
Cooper C, Shafran S, Greenbloom S, Enns R,
Farley J, Hilzenrat N, Williams K, Elkashab M, Abadir N and Neuman
M: Single-dose infliximab in hepatitis C genotype 1 treatment-naive
patients with high serum tumour necrosis factor-alpha does not
influence the efficacy of pegylated interferon alpha-2b/ribavirin
therapy. Can J Gastroenterol Hepatol. 28:35–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bedini C, Nasorri F, Girolomoni G, Pità Od
and Cavani A: Antitumour necrosis factor-alpha chimeric antibody
(infliximab) inhibits activation of skin-homing CD4+ and CD8+ T
lymphocytes and impairs dendritic cell function. Br J Dermatol.
157:249–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gottlieb AB, Chamian F, Masud S, Cardinale
I, Abello MV, Lowes MA, Chen F, Magliocco M and Krueger JG: TNF
inhibition rapidly down-regulates multiple proinflammatory pathways
in psoriasis plaques. J Immunol. 175:2721–2729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steenholdt C, Svenson M, Bendtzen K,
Thomsen OØ, Brynskov J and Ainsworth MA: Severe infusion reactions
to infliximab: aetiology, immunogenicity and risk factors in
patients with inflammatory bowel disease. Aliment Pharmacol Ther.
34:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Van Valkenhoef G, Tervonen T, Zwinkels T,
de Brock B and Hillege H: ADDIS: A decision support system for
evidence-based medicine. Decis Support Syst. 55:459–475. 2013.
View Article : Google Scholar
|