Introduction
Chronic kidney disease (CKD) has become a
significant public health problem. In 2014, the United States
National Center for Chronic Disease Prevention and Health Promotion
reported an overall prevalence rate of CKD in adults of 10%,
suggesting that >20 million Americans have CKD (1). In China, the prevalence was estimated
to be 10.8% of the adult population in 2012, including ~119.5
million individuals with CKD (2).
Secondary hyperparathyroidism (SHPT) is a common chronic
complication of CKD, particularly in dialysis patients, with a
prevalence rate of 28–54.5% (3,4). SHPT
can result in fluctuating parathyroid hormone (PTH) levels that are
refractory to treatment and in disordered mineral metabolism that
increases the risk of cardiovascular disease, fractures and
mortality (5,6).
An increasing number of options are currently
available for the treatment of SHPT (7). Treatment with conventional medications,
including calcium-based phosphate binders, non-calcium-based
phosphate binders and vitamin D analogues, may cause hypercalcemia
and hyperphosphatemia, potentially accelerating vascular
calcification (3). Previously, two
novel medications, cinacalcet and paricalcitol, have been
increasingly prescribed for the treatment of SHPT. Cinacalcet was
the first calcimimetic agent approved by the United States Food and
Drug Administration for treating dialysis patients with SHPT and it
has been demonstrated to reduce PTH levels and improve bone mineral
metabolism without increasing all-cause mortality or adverse
cardiovascular outcomes (8,9). Several randomized controlled trials
(RCTs) have suggested that the use of cinacalcet plus vitamin D
analogues can achieve a better treatment effect in patients with
SHPT compared with conventional therapy, in terms of controlling
PTH levels, serum calcium and phosphate levels (10,11).
Paricalcitol is a third-generation selective vitamin D analogue,
with a higher affinity for the parathyroid glands compared with the
gastrointestinal tract and thus, it can effectively reduce PTH
levels (12,13). However, it remains unclear as to
which therapeutic regimen is most efficacious in controlling PTH
levels with the most favorable side effect profile.
Bayesian network analyses are an extension of
traditional meta-analysis that integrate direct and indirect
evidence, and enable to concurrently compare and indirectly
estimate the relative efficacy of several agents in the presence of
inadequate data from direct head-to-head RCTs (14). A Bayesian network analysis was
conducted to evaluate the relative efficacy and relatively common
side effects of cinacalcet, paricalcitol and cinacalcet plus
low-dose vitamin D analogues therapeutic regimens in dialysis
patients with SHPT.
Materials and methods
Search strategy and selection
criteria
A systematic review according to preferred reporting
items for systematic reviews and meta-analyses guidelines (15) was performed in the present study. The
search included PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), the Cochrane
Library (https://www.cochranelibrary.com/), Embase (https://www.embase.com/), China Biology Medicine disc
(http://sdd.sxsrsc.com/), Wanfang database
(http://www.wanfangdata.com/) and the
China National Knowledge Infrastructure (http://global.cnki.net/new/index.html) using the
Population Intervention Comparison Outcome Study design (PICOS)
strategy with advanced queries and with Medical Subject Headings
(MeSH) search terms from inception to December 10th 2017 without
language restrictions. Only patients with CKD on dialysis and SHPT
that were treated with cinacalcet (Sensipar®),
paricalcitol (Zemplar®) or cinacalcet plus vitamin D
analogues, were included. Reference lists of review articles,
meta-analyses and original studies were evaluated in order to
determine further eligible trials.
PICOS criteria
Selection using PICOS was based on the following:
Population, dialysis-dependent patients with CKD and SHPT;
intervention, paricalcitol, cinacalcet or cinacalcet with a vitamin
D analogues therapy regimens; comparator, placebo or conventional
therapy; outcomes, abnormal PTH levels, hypocalcemia and/or nausea;
and study design, RCTs.
MeSH search terms
Search terms included: Secondary
hyperparathyroidism, dialysis, paricalcitol, cinacalcet and
randomized controlled trials.
Study inclusion criteria
The following inclusion criteria were applied in the
selection of eligible studies: i) RCTs; and ii) adult patients (≥18
years old) receiving dialysis for >3 months. Patients with
parathyroidectomy or kidney transplantation were excluded.
Data extraction and quality
assessment
Data were extracted from primary studies by two
independent researchers, including article information (Jadad
score, first author, publication year, geographic region) and
participant characteristics (sample size, mean age, gender,
duration of intervention). The five-point Jadad scale (16) was used to assess the methodological
quality of studies, including randomization, blinding and
withdrawals and dropouts. A score of ≤2 points was defined as low
quality, while a score of ≥3 points was ranked as high quality.
Two reviewers (HY and PY) performed the title,
abstract, content review and quality assessment of each trial and
every comparison. Data extraction was performed by the same
reviewers (HY and PY). Disagreements were resolved by discussion
with a third researcher (YK) to reach consensus. All data were
entered into Aggregate Data Drug Information System (ADDIS) 1.16.5
software (17) by one reviewer (ZZ)
and was verified by a second reviewer (ZL).
Data analysis
Primary outcomes in this study included the rate of
attaining normal PTH levels following treatment. Stata 14.0
(StataCorp LP, College Station, TX, USA) was used to assess
consistency and inconsistency with the Bayesian method and to
explore discrepancies among studies and differences among direct
and indirect comparisons. Inconsistency was evaluated using the
Higgins model. Basic network diagrams and comparison-adjusted
funnel plots were also prepared with Stata 14.0 and ‘network meta’
orders.
Pair-wise meta-analysis of the same interventions
was conducted using ADDIS with a random effects model. The GEADE
approach was applied for rating the quality of evidence obtained
for every comparison (18). Bayesian
network analyses were performed using ADDIS with consistency and
inconsistency models. For PTH analysis, 4 chains, including 20,000
burn-ins, 50,000 simulation iterations, 10,000 inference samples
and a thinning interval of 10 for each chain were applied. To
attain a good convergence property for symptoms of nausea and
hypocalcaemia, 4 chains, including 20,000 burn-ins, 40,0000
simulation iterations and 160,000 inference samples with a thinning
interval of 10 were used. A potential scale reduction factor
parameter (PSRF) was assessed using the Brooks-Gelman-Rubin method
to show convergence of the model. PSRF values <1.2 were
acceptable and the closer to 1, the better the convergence effect.
A sensitivity analysis was performed that included the trial with
outcomes defined as ≥30% reduction in PTH using ADDIS software with
the consistency random effects model.
The method named ‘the effective sample size’ was
applied to calculate the effective sample size for indirect
evidence in the present publication (19). It was used as an approach to measure
the degree of power and precision from an indirect comparison to
consider the collection of trials included in each comparison as
one clinical trial.
Results
Literature selection and study
characteristics
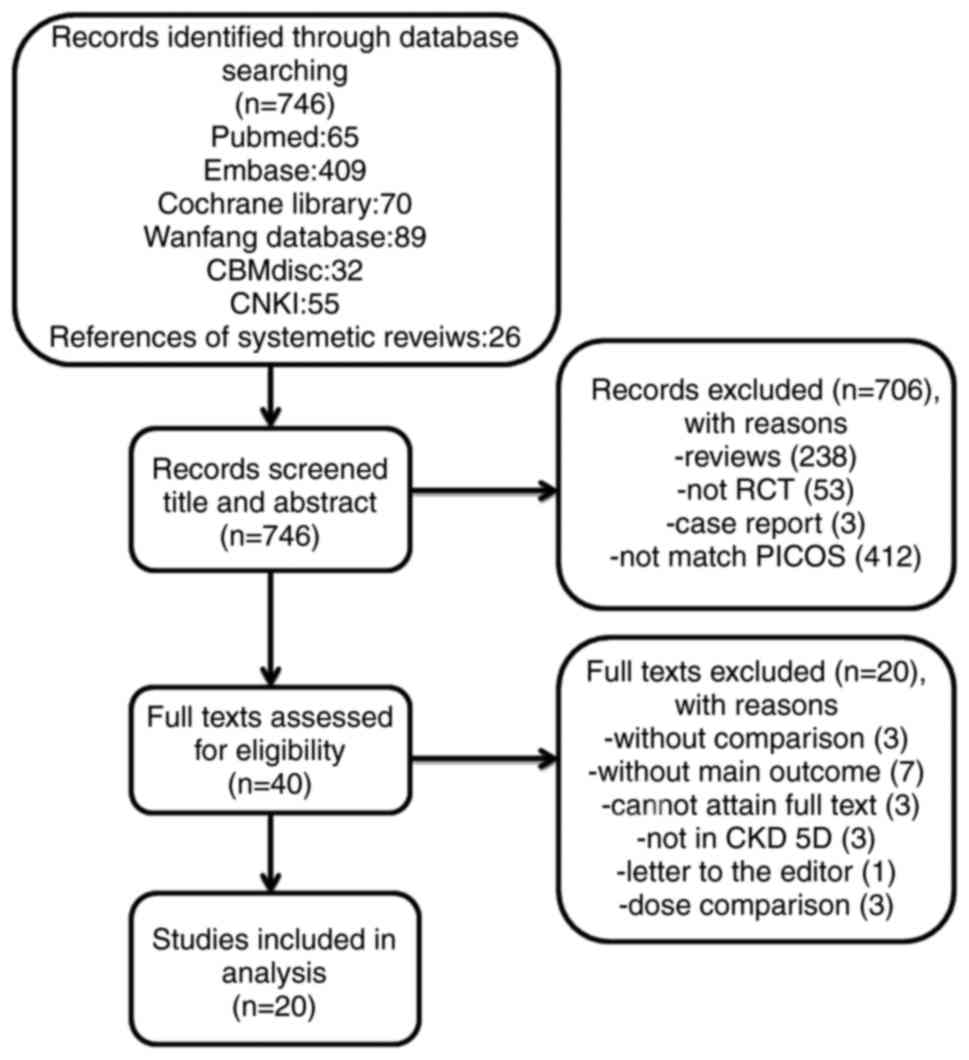
A total of 720 articles were identified in the
initial literature query and 26 further articles were identified
through screening references of various relevant systematic
reviews. Following screening titles and abstracts, 40 articles
eligible for PICOS analysis remained. Out of these, a total of
5,390 dialysis patients from 20 RCTs that met the inclusion
criteria and were entered into the analysis (6,10,11,13,20–35)
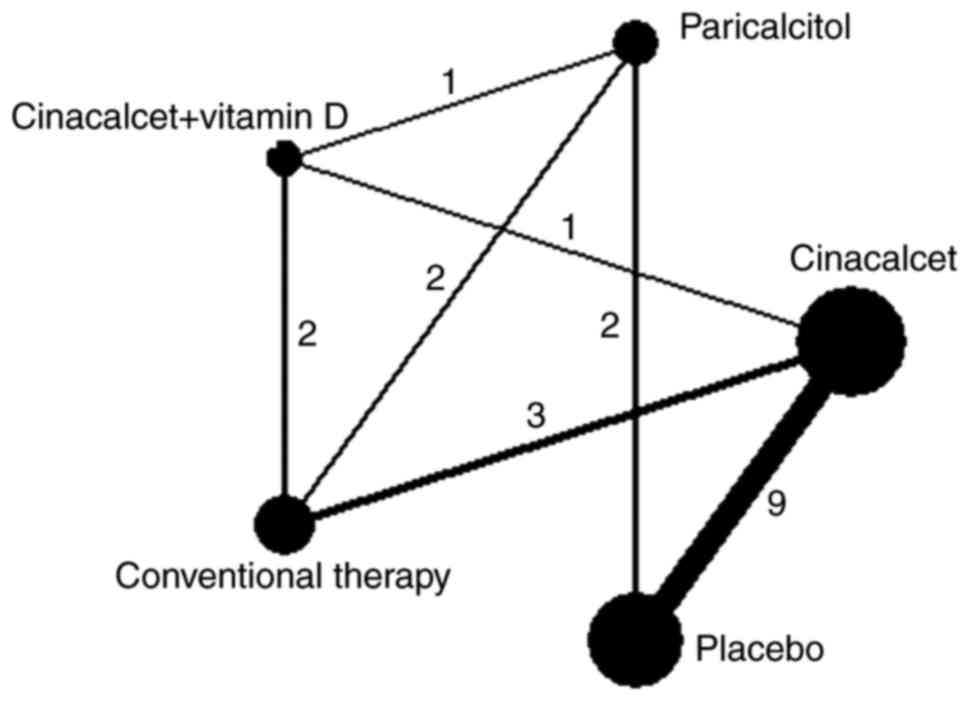
The selection procedure is summarized in Fig. 1. Eligible comparisons for the primary
outcome are presented in Fig. 2 and
a summary of the characteristics of included studies is presented
in Tables I and II.
 | Table I.Basic characteristics of included
studies. |
Table I.
Basic characteristics of included
studies.
|
|
|
| Age (years) | Gender
(male/female) | Duration of
dialysis (month) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year | Jadad score | Country (no.
centers) | T | C | T | C | T | C | Refs. |
|---|
| Moe et al,
2005 | 4 | North America,
Europe and Australia (182) | NS | NS | 407/258 | 295/176 | NS | NS | (8) |
| Fishbane et
al, 2008 | 2 | USA (42) |
57.7±14.9b |
59.0±12.4b | 52/35 | 45/41 |
46.3±36.4b |
46.8±44.1b | (10) |
| Urena-Torres et
al, 2013 | 3 | France |
57.9±13.6b |
57±14.6b | 83/70 | 95/56 |
87.5±30.5b |
84.2±33.4b | (11) |
| Martin et
al, 1998 | 3 | USA
(multicenter) | 54±14b | 54±16b | 21/19 | 19/19 | NS | NS | (13) |
| Block et al,
2004 | 4 | North America (63),
Europe and Australia (62) | 54±14b | 55±15b | 226/145 | 229/141 | 72±63b | 72±68b | (20) |
| El-Shafey et
al, 2011 | 2 | Egypt (5) |
51.5±12.7b |
51.81±14.96b | 27/28 | 14/13 |
48.1±25.1b |
44.1±21.4b | (21) |
| Fukagawa et
al, 2008 | 5 | Japan (29) |
54.7±11.0b |
55.7±11.7b | 40/32 | 37/34 |
170.4±93.7b |
173.3±76b | (22) |
| Hansen et
al, 2011 | 2 | Denmark |
63.5±15.3b |
49.2±3.8b | 28/17 | 27/14 | 38
(3–236)a | 36
(3–262)a | (23) |
| Ketteler et
al, 2012 | 3 | 12 countries
worldwide (89) | NS | NS | 87/81 | 47/53 | NS | NS | (24) |
| Lindberg et
al, 2003 | 4 | USA (23), Canada
(2) |
52.7±16.4b |
48.8±15.6b | 24/15 | 22/17 |
60.3±58.3b |
69.7±53.9b | (25) |
| Lindberg et
al, 2005 | 4 | USA, Canada,
Australia |
51.8±14b |
53.5±13.9b | 181/113 | 64/37 |
56.4±53.1b |
63.6±65b | (26) |
| Martin et
al, 2005 | 5 | USA and Canada
(65) | 53±14b | 54±15b | 123/82 | 123/82 | 67±56b | 62±55b | (27) |
| Messa et al,
2008 | 2 | Europe (111) |
58.5±14.5b |
58.3±14.5b | 224/144 | 117/67 |
64.1±72.1b |
69.4±73.6b | (28) |
| Quarles et
al, 2003 | 4 | USA (17) |
49.6±8.5b |
47.9±14.2b | 27/9 | 17/18 |
71.3±54.3b |
71.1±66.2b | (29) |
| Ross et al,
2008 | 4 | USA
(multicenter) |
57.0±1.62b |
56.4±2.5b | 37/24 | 22/5 | NS | NS | (30) |
| Sprague et
al, 2003 | 3 | USA, Netherlands,
Spain, Switzerland (27) |
56.7±15.5b |
56.6±14.3b | 70/60 | 80/53 | NS | NS | (31) |
| Sterrett et
al, 2007 | 5 | USA (47), Europe
(10), Australia (5), Canada (4) |
51.6±13.4b |
52.9±15.2b | 54/45 | 71/40 | 72±63b | 66±67b | (32) |
| Wetmore et
al, 2015 | 2 | USA, Russia,
Canada, Australia (58) | 53
(21–81)a | 55
(22–86)a | 93/62 | 95/62 | 32.9a (4.3–216.7) | 38.1
(4.5–308.2)a | (33) |
| Mei et al,
2016 | 3 | China (12) |
50.02±11.17b |
50.12±11.34b | 68/50 | 68/48 |
92.4±53.9b |
89.7±55.1b | (34) |
| Han et al,
2015 | 2 | China |
58.9±19.8b |
68.8±4.4b | 28/22 | 29/21 |
51.3±17.9b |
58.9±19.8b | (35) |
 | Table II.Treatment and outcome characteristics
of included studies. |
Table II.
Treatment and outcome characteristics
of included studies.
|
| Interventions |
|
| At target | Nausea | Calcium
abnormity |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | T | C | Dialysis modality
(duration in weeks) | Target PTH
level | T (n) | C (n) | T (n) | C (n) | T (n) | C (n) | Refs. |
|---|
| Moe et al,
2005 | 30–180 mg/day
cinacalcet | Placebo | HD and PD (26) | 100–250 pg/ml | 307 | 42 | 170 | 78 | NS | NS | (8) |
| Fishbane et
al, 2008 | Cinacalcet with
paricalcitol or doxercalciferol (2 µg or 1 µg, TIW) | 2 µg paricalcitol,
1 µg doxercalciferol (TIW) | HD (33) | ≥30% decrease | 38 | 20 | 9 | 0 | 6 | 0 | (10) |
| Urena-Torres et
al, 2013 | Cinacalcet plus
calcitriol, 0.25 µg paricalcitol 1 µg or alfacalcidol 0.25 µg per
day | Phosphate binders,
vitamin D sterols | HD (52) | ≥30% decrease | 96 | 57 | 30 | 15 | 25 | 1 | (11) |
| Martin et
al, 1998 | 0.04 µg/kg TIW
paricalcitol | Placebo | HD (12) | ≥30% decrease | 27 | 3 | NS | NS | NS | NS | (13) |
| Block et al,
2004 | 30–180 mg/day
cinacalcet | Placebo | HD (26) | ≥30% decrease | 239 | 42 | 119 | 70 | 19 | 3 | (20) |
| El-Shafey et
al, 2011 | 30–180 mg/day
cinacalcet | Alfacalcidol
TIW | HD (36) | 150–300 pg/ml | 30 | 5 | 7 | 1 | 6 | 0 | (21) |
| Fukagawa et
al, 2008 | 25–100 mg/day
cinacalcet | Placebo | HD (14) | PTH ≤250 pg/ml | 37 | 2 | 26 | 14 | 4 | 2 | (22) |
| Hansen et
al, 2011 | 18.1 µg/week
paricalcitol | 5.3 µg/week
alfacalcidol | HD (16) | ≥30% decrease | 39 | 31 | NS | NS | 24 | 21 | (23) |
| Ketteler et
al, 2012 | (Dose unavailable)
Paricalcitol | Cinacalcet plus 1.0
µg doxercalciferol TIW or oral alfacalcidol 0.25 ug/day | HD (28) | 150–300 pg/ml | 62 | 39 | NS | NS | 6 | 52 | (24) |
| Lindberg et
al, 2003 | 20–50 mg/day
cinacalcet | Placebo | HD (18) | ≥30% decrease | 14 | 3 | 8 | 12 | 3 | 0 | (25) |
| Lindberg et
al, 2005 | 30–180 mg/day
cinacalcet | Placebo | HDPD (26) | ≥30% decrease | 187 | 13 | 86 | 22 | 14 | 1 | (26) |
| Martin et
al, 2005 | 30–180 mg/day
cinacalcet | Placebo | HD (26) | ≥30% decrease | 125 | 23 | NS | NS | NS | NS | (27) |
| Messa et al,
2008 | 30–180 mg/day
cinacalcet | Phosphate binders
and vitamin D sterols | HD and PD (26) | PTH ≤300 pg/ml | 261 | 40 | 32 | 3 | NS | NS | (28) |
| Quarles et
al, 2003 | 25–100 mg/day
cinacalcet | Placebo | HD (18) | ≥30% decrease | 19 | 8 | NS | NS | NS | NS | (29) |
| Ross et al,
2008 | Paricalcitol | Placebo | HD, 62; PD, 26
(12) | ≥30% decrease | 53 | 3 | NS | NS | 1 | 0 | (30) |
| Sprague et
al, 2003 | 0.12 µg/kg
paricalcitol | 0.03 µg/kg
paricalcitol | HD (32) | ≥50% decrease | 81 | 72 | NS | NS | 30 | 39 | (31) |
| Sterrett et
al, 2007 | 30–180 mg/day
cinacalcet | Placebo | HD (52) | ≥30% decrease | 61 | 16 | 13 | 6 | 11 | 2 | (32) |
| Wetmore et
al, 2015 | 30–180 mg/day
cinacalcet | 0.5 mg calcitriol
TIW | HD (40–52) | ≥30% decrease | 66 | 53 | NS | NS | 27 | 0 | (33) |
| Mei et al,
2016 | 25–100 mg/day
cinacalcet | Placebo | HD | PTH ≤250 pg/ml | 30 | 4 | 34 | 3 | 9 | 2 | (34) |
| Han et al,
2015 | 25–75 mg/day
cinacalcet plus calcitriol 25 mg TIW | 25–75 mg/day
cinacalcet | HD (12) | 25–75 pg/ml | 47 | 41 | NS | NS | NS | NS | (35) |
In the current study, the following treatment
regimens were considered for analysis: i) Cinacalcet; ii)
paricalcitol; iii) cinacalcet plus low-dose active vitamin D
analogues; iv) conventional therapy, including phosphate binders
and/or vitamin D analogues; and v) placebo.
In the present analysis, 3,552 patients were males
(65.7%). Among the 20 trials, 14 (70.0%) (6,11,13,20,22,24–27,29–32,34)
had detailed records on withdrawals and dropouts and 14 (70.0%)
(6,11,13,20,22,24–27,29–32,34) had
Jadad scores ≥3 points. A total of 17 (85.0%) (6,10,13,20–22,24–34)
trials were reported as multicenter studies and 10 (50.0%)
(6,20,24–28,31–33) were
multinational.
Consistency and inconsistency of the
network analysis
Consistency testing of the network analysis was
performed using ‘network meta’ orders in Stata 14.0. The results
suggested low heterogeneity among the data sets, with a standard
deviation for estimating the heterogeneity between studies of 0.41
(P>0.05; data not shown). The method was further applied to test
for inconsistency using the Higgins model, exhibiting no evidence
for inconsistency, with P>0.05 (data not shown). As a
consequence, the Bayesian network analysis was conducted with the
consistency random effect models.
Meta-analysis results
Table III indicated
that treatment strategies with cinacalcet and paricalcitol had
significantly higher rates of controlling PTH levels compared with
the placebo, with odds ratios (ORs) of 11.48 (95% confidence
interval CI, 9.20–14.67) and 35.24 (95% CI, 13.7–93.11),
respectively, and low heterogeneity was observed
(I2=12.0 and 0.62%). Cinacalcet plus low-dose active
vitamin D analogues and paricalcitol had better effects in
controlling PTH compared with conventional therapy with ORs of 1.51
(95% CI, 0.95–2.41) and 2.7 (95% CI, 1.85–3.95), respectively, with
little heterogeneity (I2=0.0% for both). In addition,
cinacalcet had significantly higher rates of controlling PTH levels
compared with conventional therapy, with OR 3.97 (95% CI,
1.07–14.70), but high heterogeneity was observed
(I2=93.6%).
 | Table III.Parathyroid hormone response rates
and efficacy from meta-analyses of direct comparisons between
treatments. |
Table III.
Parathyroid hormone response rates
and efficacy from meta-analyses of direct comparisons between
treatments.
|
|
|
| Response rate |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Treatment
group | Studies (n) | Patients (n) | Treatment | Control | OR (95% CI) | I2
(%) |
|---|
| Paricalcitol
vs. |
|
|
|
|
|
|
|
Conventional therapy | 2 | 343 | 120/172 | 103/171 | 1.51
(0.95–2.41) | 0 |
| Placebo | 2 | 166 | 80/101 | 6/65 | 35.24
(13.7–93.11) | 0.62 |
| Cinacalcet vs. |
|
|
|
|
|
|
| Conventional
therapy | 3 | 946 | 357/578 | 98/368 | 3.97
(1.07–14.70) | 93.6 |
| Placebo | 9 | 3,765 | 1,265/2,115 | 185/1,650 | 11.48
(9.20–14.67) | 12 |
| Cinacalcet +
vitamin D analogues vs. |
|
|
|
|
|
|
| Conventional
therapy | 2 | 477 | 134/240 | 77/237 | 2.7
(1.85–3.95) | 0 |
In addition, a novel GRADE approach was applied for
rating the quality of evidence obtained for every comparison
(18). The comparison of
paricalcitol vs. placebo exhibited a high quality of evidence.
Three other pairs exhibited moderate qualities (cinacalcet vs.
placebo, cinacalcet + vitamin D analogues vs. paricalcitol and
paricalcitol vs. conventional therapy; Table IV).
 | Table IV.Estimated effects and quality ratings
based on parathyroid hormone levels for treatment comparisons. |
Table IV.
Estimated effects and quality ratings
based on parathyroid hormone levels for treatment comparisons.
|
| Direct
evidence | Indirect
evidence | Network
meta-analysis |
|---|
|
|
|
|
|
|---|
| Treatment
group | OR (95% CI) | Quality | OR (95% CI) | Quality | OR (95% CI) | Quality |
|---|
| Cinacalcet vs. |
|
Cinacalcet + vitamin D
analogues | 1.32 (−0.57,
3.38) | Low | −0.20 (−1.33,
0.96) | Low | 0.17 (−0.80,
1.22) | Low |
|
Conventional therapy | −1.37 (−2.15,
−0.62) | Low | 0.44 (−0.85,
1.82) | Low | −0.90 (−1.63,
−0.12) | Low |
|
Placebo | −2.44 (−2.95,
−1.92) | Moderate | −3.81 (−5.66,
−1.95) | Moderate | −2.54 (−3.05,
−2.01) | Moderate |
| Paricalcitol
vs. |
|
Cinacalcet + vitamin D
analogues | 0.77 (−0.76,
2.31) | Moderate | −0.06 (−1.33,
1.28) | Moderate | 0.31 (−0.70,
1.30) | Moderate |
|
Conventional therapy | −0.56 (−1.65,
0.45) | Moderate | −2.23 (−3.36,
−1.17) | Moderate | −1.37 (−2.27,
−0.53) | Moderate |
|
Placebo | −3.72 (−5.15,
−2.45) | High | −2.40 (−3.69,
−1.12) | High | −3.00 (−4.05,
−2.09) | High |
| Cinacalcet +
vitamin D analogues vs. |
| Conventional
therapy | −0.98 (−2.11,
0.11) | Low | −1.23 (−2.83,
0.17) | Low | −1.07 (−1.96,
−0.23) | Low |
Bayesian network analysis results
All 20 included trials reported the number of
patients reaching normal PTH levels following treatment (6,10,11,13,20–35).
As presented in Table V, treatment
with paricalcitol, cinacalcet and cinacalcet plus vitamin D
analogues improved clinical outcomes for normalized serum PTH
compared with conventional therapy or the placebo with regards to
consistency and inconsistency models.
 | Table V.Bayesian network analysis of
parathyroid hormone levels. |
Table V.
Bayesian network analysis of
parathyroid hormone levels.
| A. Consistency
model |
|---|
| Treatment
group | Cinacalcet | Cinacalcet +
vitamin D analogues | Paricalcitol | Placebo | Conventional
therapy |
|---|
| Cinacalcet | – | 1.19
(0.47–3.31) | 1.62
(0.65–4.39) | 0.08
(0.05–0.13) | 0.41
(0.20–0.88) |
| Cinacalcet +
vitamin D analogues | 0.84
(0.30–2.15) | – | 1.37
(0.51–3.65) | 0.07
(0.02–0.18) | 0.34
(0.14–0.78) |
| Paricalcitol | 0.62
(0.23–1.54) | 0.73
(0.27–1.97) | – | 0.05
(0.02–0.12) | 0.25
(0.10–0.57) |
| Placebo | 12.56
(7.44–21.10) | 14.89
(5.48–43.53) | 20.32
(8.18–56.63) | – | 5.09
(2.25–12.12) |
| Conventional
therapy | 2.47
(1.14–5.04) | 2.91
(1.28–6.95) | 3.99
(1.76–9.93) | 0.20
(0.08–0.44) | – |
|
| B. Inconsistency
model |
|
| Treatment
group |
Cinacalcet | Cinacalcet +
vitamin D analogues |
Paricalcitol | Placebo | Conventional
therapy |
|
| Cinacalcet | – | 2.02
(0.53–11.03) | 2.94
(0.66–20.96) | 0.09
(0.05–0.14) | 0.29
(0.14–0.64) |
| Cinacalcet +
vitamin D analogues | 0.50
(0.09–1.89) | – | 1.50
(0.49–4.58) | 0.04
(0.01–0.19) | 0.38
(0.16–0.89) |
| Paricalcitol | 0.34
(0.05–1.52) | 0.67
(0.22–2.02) | – | 0.03
(0.01–0.09) | 0.43
(0.15–1.16) |
| Placebo | 11.67
(7.31–18.54) | 23.60
(5.23–115.12) | 35.54
(11.23–126.85) | – | 14.95
(3.35–80.01) |
| Conventional
therapy | 3.44
(1.57–7.03) | 2.64
(1.13–6.18) | 2.34
(0.86–6.79) | 0.07
(0.01–0.30) | – |
Furthermore, based on the Bayesian probability
framework, the primary outcome was ranked as paricalcitol >
cinacalcet plus low-dose active vitamin D analogues > cinacalcet
> conventional treatment > placebo. As presented in Table VI, paricalcitol had the highest
probability of being the most effective therapy (68%), followed by
cinacalcet plus low-dose active vitamin D (45% probability for rank
2) and cinacalcet (59% probability for rank 3).
 | Table VI.Ranking of treatment based on
parathyroid hormone levels. |
Table VI.
Ranking of treatment based on
parathyroid hormone levels.
|
| Rank |
|---|
|
|
|
|---|
| Treatment | 1 | 2 | 3 | 4 | 5 |
|---|
| Cinacalcet | 0.1 | 0.31 | 0.59 | 0.01 | 0 |
| Cinacalcet +
vitamin D analogues | 0.22 | 0.45 | 0.32 | 0.01 | 0 |
| Paricalcitol | 0.68 | 0.24 | 0.08 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 | 1 |
| Conventional
therapy | 0 | 0 | 0.02 | 0.98 | 0 |
Regarding the occurrence of nausea, a frequent side
effect of cinacalcet, 11 studies were included (6,10,11,20–22,25,26,28,32).
The ranks of the incidence of nausea were cinacalcet (74%) >
placebo (44%) > cinacalcet plus low-dose active vitamin D
analogues (52%) > conventional treatment (96%; data not
shown).
Hypocalcaemia was reported in 10 studies (10–11,20–22,25,26,32–34). The
ranks of incidence of hypocalcaemia were cinacalcet (100%) >
cinacalcet plus low-dose active vitamin D analogues (99%) >
placebo (99%) > conventional treatment (100%; data not
shown).
Sensitivity analysis
As trial outcomes differed, with ≥30% reduction in
PTH in certain cases (10,11,13,20,23,25–27,29,30,32,33) and
numerical or guideline-based targets (6,21,22,24,28,31,34,35)
in others, a sensitivity analysis was performed that solely
included trials with the outcome defined as ≥30% reduction in PTH.
The result suggested that paricalcitol had the highest probability
of being the most effective therapy (85%). However, cinacalcet
(54%) obtained second place while cinacalcet plus low-dose active
vitamin D analogues (52%) ranked third (data not shown).
Publication bias
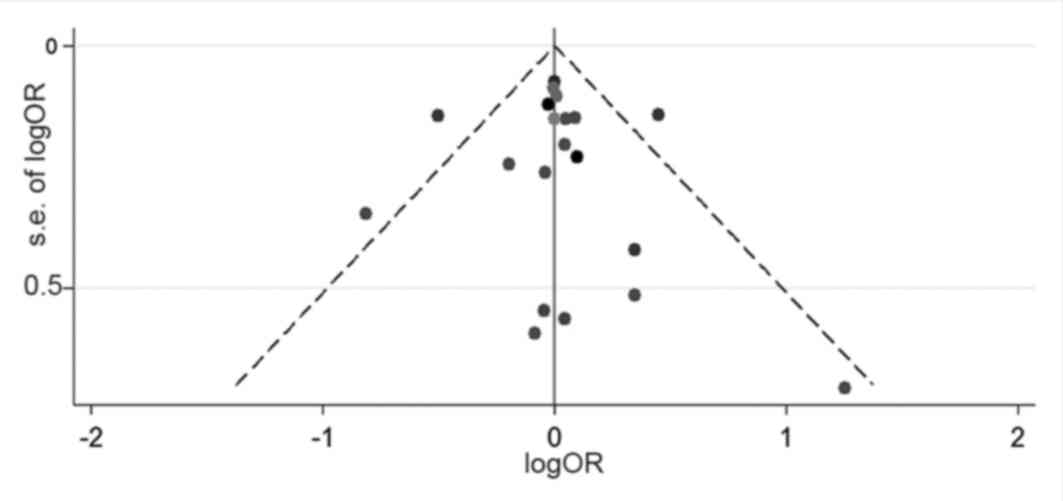
A funnel plot analysis was performed to assess
publication bias. The results presented in Fig. 3 exhibited little publication
bias.
Sample size
A method called effective sample size was applied to
calculate the effective sample size for indirect evidence in the
present publication (19). The
results suggested this number was 112 in the comparison of
conventional treatment and placebo and 312 in the comparison of
paricalcitol and conventional treatment (data not shown). These
values were below the number of patients included in the analysis
performed in the present study.
Discussion
Previous meta-analysis suggested that all treatment
strategies of cinacalcet, paricalcitol and cinacalcet plus vitamin
D analogues were effective at controlling PTH levels among
dialysis-dependent patients with CKD (9,36).
However, due to inadequate data from direct head-to-head RCTs, the
best strategy cannot be identified. Thus, a Bayesian network
analysis was performed in the present study to take advantage of
direct evidence to indirectly estimate the relative efficacy. The
analysis revealed that among the treatment strategies, paricalcitol
was the best therapeutic approach for patients with CKD and SHPT in
the dialysis stage, followed by cinacalcet plus low-dose active
vitamin D, cinacalcet, conventional therapy and placebo. It was
further revealed that treatment with cinacalcet plus low-dose
active vitamin D analogues may reduce the incidence of nausea and
hypocalcaemia compared with cinacalcet alone.
The results demonstrated that both cinacelcet and
paricalcitol are more efficient at controling iPTH than
conventional therapy, which is consistent with findings from
previous meta-analyses (9,36,37). As
a calcimimetic agent, cinacalcet acts on vitamin D and
Ca2+-sensing receptors of the parathyroid glands to
suppress PTH secretion and to reduce PTH serum levels (22,38,39).
Treatment with cinacalcet produces a greater proportion of
patients, who achieved the Kidney Disease Outcomes Quality
Initiative (KDOQI) target (target, a PTH of 150–300 pg/ml;
OR=10.75; 95% CI, 6.65–17.37) when compared with conventional
therapy (9). Rat models of SHPT
demonstrated that cinacalcet attenuates the progression of
parathyroid hyperplasia by reducing the number of parathyroid cells
and decreasing the weight of the parathyroid (40). Yamada et al (41) reported that cinacalcet therapy
combined with vitamin D analogues significantly decreased the
volume of the parathyroid gland and PTH serum levels in
hemodialysis patients with SHPT. These findings support the
application of combination therapy to enable patients to achieve
KDOQI targets (OR=3.51; 95% CI, 2.38–5.17) (37). Paricalcitol was further reported to
be superior to conventional therapy in terms of decreasing serum
PTH levels (36). Paricalcitol is a
tissue-selective vitamin D sterol with an increased affinity for
the parathyroid glands compared with the intestine (42). Thus, it can differentially regulate
PTH secretion from parathyroid glands and absorption of calcium and
phosphate by the gastrointestinal tract.
The present analysis suggested that treatment
strategies with cinacalcet, cinacalcet plus low-dose active vitamin
D analogues and paricalcitol were superior to conventional therapy
in dialysis patients with SHPT. In addition, consistent with
previous observations (25,43), it was revealed that paricalcitol may
be the most effective drug for controlling PTH and serum calcium.
Previously, a retrospective cohort study suggested that patients
receiving paricalcitol had a 74% lower parathyroidectomy incidence
rates compared with patients receiving cinacalcet (43). Even with adjustment for
comorbidities, gender, therapy time and other risk factors, the
risk of parathyroidectomy was increased in the cinacalcet group
compared with the paricalcitol group (43). Collectively, the data from the
current study and the literature suggested that paricalcitol may
provide marked benefits for treating SHPT in dialysis patients with
advanced CKD.
Based on the results of the Bayesian network
analysis, insufficient data was obtained to suggest that cinacalcet
plus low-dose vitamin D analogues provided increased control of PTH
levels compared with cinacalcet alone. However, with lower a
probability for developing nausea or hypocalcaemia, cinacalcet plus
vitamin D analogues may be a more suitable treatment, improving
hypocalcaemia and/or hyperphosphatemia side effects caused by
cinacalcet (40,44). The combination of cinacalcet plus
low-dose vitamin D therapy further allows reducing the dosage of
cinacalcet, resulting in decreased occurrence of nausea (9).
There are several limitations to the present network
analysis. Due to the absence of head-to-head RCTs on paricalcitol
and cinacalcet plus vitamin D analogues in dialysis patients with
SHPT, future studies are required for further elucidation. Only two
trials included peritoneal dialysis patients and therefore the
determined outcomes may be more relevant for hemodialysis patients.
Calcium and phosphate burden, and vascular calcification effects of
the treatments in patients with SHPT were not sufficiently
considered. Finally, included studies applied varying treatment
dosages, which may affect the drawn conclusions.
In conclusion, all three therapeutic treatment
options were efficacious in the treatment of dialysis patients with
advanced CKD and SHPT. To maintain stable control of serum PTH
levels with potentially fewer side effects, including nausea and
hypocalcaemia, cinacalcet with low-dose vitamin D analogues was
determined to be a safe and efficacious option. Among the
therapeutic regimens studied, paricalcitol may offer the best
profile for efficacy with the lowest rates of side effects.
Acknowledgements
The authors would like the thank Professor Huiyao
Lan of the Li Ka Shing Institute of Health Sciences in Chinese
University of Hong Kong, who provided assistance in the language
preparation of the manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HY and PY conceived and designed the study. HY, PY
and YK collected and abstracted the data. HY, ZZ, AH and ZL
undertook the statistical analysis. HY, AH, ZL and ZZ drafted the
manuscript. All authors had access to the data and critically
revised the manuscript for important intellectual content. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CKD
|
chronic kidney disease
|
|
SHPT
|
secondary hyperparathyroidism
|
|
PTH
|
parathyroid hormone
|
|
RCT
|
randomized controlled trial
|
References
|
1
|
National Center for Chronic Disease
Prevention and Health Promotion. CS250738-A: National Chronic
Kidney Disease Fact Sheet. 2014, https://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf
|
|
2
|
Zhang L, Wang F, Wang L, Wang W, Liu B,
Liu J, Chen M, He Q, Liao Y, Yu X, et al: Prevalence of chronic
kidney disease in China: A cross-sectional survey. Lancet.
379:815–822. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douthat WG, Castellano M, Berenguer L,
Guzmán MA, de Arteaga J, Chiurchiu CR, Massari PU, Garay G, Capra R
and de La Fuente JL: High prevalence of secondary
hyperparathyroidism in chronic kidney disease patients on dialysis
in argentina. Nefroloqia. 33:657–666. 2013.(In English,
Spanish).
|
|
4
|
Jeloka T, Mali M, Jhamnani A, Konde S and
Jadhav V: Are we overconcerned about secondary hyperparathyroidism
and underestimating the more common secondary hypoparathyroidism in
our dialysis patients? J Assoc Physicians India. 60:102–105.
2012.PubMed/NCBI
|
|
5
|
Block GA, Hulbert-Shearon TE, Levin NW and
Port FK: Association of serum phosphorus and calcium × phosphate
product with mortality risk in chronic hemodialysis patients: A
national study. Am J Kidney Dis. 31:607–617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goodman WG, Goldin J, Kuizon BD, Yoon C,
Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, et al:
Coronary-artery calcification in young adults with end-stage renal
disease who are undergoing dialysis. N Engl J Med. 342:1478–1483.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenzoni V, Trieste L and Turchetti G:
The cost-effectiveness of drug therapies to treat secondary
hyperparathyroidism in renal failure: A focus on evidence regarding
paricalcitol and cinacalcet. Expert Rev Pharmacoecon Outcomes Res.
15:622–624. 2015. View Article : Google Scholar
|
|
8
|
Moe SM, Chertow GM, Coburn JW, Quarles LD,
Goodman WG, Block GA, Drueke TB, Cunningham J, Sherrard DJ, McCary
LC, et al: Achieving NKF-K/DOQI bone metabolism and disease
treatment goals with cinacalcet HCl. Kidney Int. 67:760–771. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Li M, You L, Li H, Ni L, Gu Y,
Hao C and Chen J: Effects and safety of calcimimetics in end stage
renal disease patients with secondary hyperparathyroidism: A
meta-analysis. PLoS One. 7:e480702012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fishbane S, Shapiro WB, Corry DB, Vicks
SL, Roppolo M, Rappaport K, Ling X, Goodman WG, Turner S and
Charytan C: Cinacalcet HCl and concurrent low-dose vitamin D
improves treatment of secondary hyperparathyroidism in dialysis
patients compared with vitamin D alone: The ACHIEVE study results.
Clin J Am Soc Nephrol. 3:1718–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urena-Torres P, Bridges I, Christiano C,
Cournoyer SH, Cooper K, Farouk M, Kopyt NP, Rodriguez M, Zehnder D
and Covic A: Efficacy of cinacalcet with low-dose vitamin D in
incident haemodialysis subjects with secondary hyperparathyroidism.
Nephrol Dial Transplant. 28:1241–1254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coyne D, Acharya M, Qiu P, Abboud H,
Batlle D, Rosansky S, Fadem S, Levine B, Williams L, Andress DL, et
al: Paricalcitol capsule for the treatment of secondary
hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis.
47:263–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin KJ, Gonzalez EA, Gellens M, Hamm
LL, Abboud H and Lindberg J: 19-Nor-1-alpha-25-dihydroxyvitamin D2
(Paricalcitol) safely and effectively reduces the levels of intact
parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol.
9:1427–1432. 1998.PubMed/NCBI
|
|
14
|
Alegre MM, Weyant MJ, Bennett DT, Yu JA,
Ramsden MK, Elnaggar A, Robison RA and O'Neill KL: Serum detection
of thymidine kinase 1 as a means of early detection of lung cancer.
Anticancer Res. 34:2145–2151. 2014.PubMed/NCBI
|
|
15
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: Checklist and explanations. Ann Intern Med.
162:777–784. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valkenhoef G van, Tervonen T, Zwinkels T,
Brock B de and Hillege H: ADDIS: A decision support system for
evidence-based medicine. Decision Support Systems. 55:459–575.
2013. View Article : Google Scholar
|
|
18
|
Puhan MA, Schünemann HJ, Murad MH, Li T,
Brignardello-Petersen R, Singh JA, Kessels AG and Guyatt GH; GRADE
Working Group, . A GRADE working group approach for rating the
quality of treatment effect estimates from network meta-analysis.
BMJ. 349:g56302014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thorlund K and Mills EJ: Sample size and
power considerations in network meta-analysis. Syst Rev. 1:412012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Block GA, Martin KJ, de Francisco AL,
Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa A,
Messa P, et al: Cinacalcet for secondary hyperparathyroidism in
patients receiving hemodialysis. New Engl J Med. 350:1516–1525.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Shafey EM, Alsahow AE, Alsaran K, Sabry
AA and Atia M: Cinacalcet hydrochloride therapy for secondary
hyperparathyroidism in hemodialysis patients. Ther Apher Dial.
15:547–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fukagawa M, Yumita S, Akizawa T, Uchida E,
Tsukamoto Y, Iwasaki M and Koshikawa S; KRN1493 study group, .
Cinacalcet (KRN1493) effectively decreases the serum intact PTH
level with favorable control of the serum phosphorus and calcium
levels in Japanese dialysis patients. Nephrol Dial Transplant.
23:328–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansen D, Rasmussen K, Danielsen H,
Meyer-Hofmann H, Bacevicius E, Lauridsen TG, Madsen JK, Tougaard
BG, Marckmann P, Thye-Roenn P, et al: No difference between
alfacalcidol and paricalcitol in the treatment of secondary
hyperparathyroidism in hemodialysis patients: A randomized
crossover trial. Kidney Int. 80:841–850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ketteler M, Martin KJ, Wolf M, Amdahl M,
Cozzolino M, Goldsmith D, Sharma A, Marx S and Khan S: Paricalcitol
versus cinacalcet plus low-dose vitamin D therapy for the treatment
of secondary hyperparathyroidism in patients receiving
haemodialysis: Results of the IMPACT SHPT study. Nephrol Dial
Transplant. 27:3270–3278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindberg JS, Moe SM, Goodman WG, Coburn
JW, Sprague SM, Liu W, Blaisdell PW, Brenner RM, Turner SA and
Martin KJ: The calcimimetic AMG 073 reduces parathyroid hormone and
calcium × phosphorus in secondary hyperparathyroidism. Kidney Int.
63:248–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindberg JS, Culleton B, Wong G, Borah MF,
Clark RV, Shapiro WB, Roger SD, Husserl FE, Klassen PS, Guo MD, et
al: Cinacalcet HCl, an oral calcimimetic agent for the treatment of
secondary hyperparathyroidism in hemodialysis and peritoneal
dialysis: A randomized, double-blind, multicenter study. J Am Soc
Nephrol. 16:800–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin KJ, Jüppner H, Sherrard DJ, Goodman
WG, Kaplan MR, Nassar G, Campbell P, Curzi M, Charytan C, McCary
LC, et al: First- and second-generation immunometric PTH assays
during treatment of hyperparathyroidism with cinacalcet HCl. Kidney
Int. 68:1236–1243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Messa P, Macário F, Yaqoob M, Bouman K,
Braun J, von Albertini B, Brink H, Maduell F, Graf H, Frazão JM, et
al: The OPTIMA study: Assessing a new cinacalcet (Sensipar/Mimpara)
treatment algorithm for secondary hyperparathyroidism. Clin J Am
Soc Nephrol. 3:36–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quarles LD, Sherrard DJ, Adler S, Rosansky
SJ, McCary LC, Liu W, Turner SA and Bushinsky DA: The calcimimetic
AMG 073 as a potential treatment for secondary hyperparathyroidism
of end-stage renal disease. J Am Soc Nephrol. 14:575–583. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ross EA, Tian J, Abboud H, Hippensteel R,
Melnick JZ, Pradhan RS, Williams LA, Hamm LL and Sprague SM: Oral
paricalcitol for the treatment of secondary hyperparathyroidism in
patients on hemodialysis or peritoneal dialysis. Am J Nephrol.
28:97–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sprague SM, Llach F, Amdahl M, Taccetta C
and Batlle D: Paricalcitol versus calcitriol in the treatment of
secondary hyperparathyroidism. Kidney Int. 63:1483–1490. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sterrett JR, Strom J, Stummvoll HK, Bahner
U, Disney A, Soroka SD, Corpier C, Arruda JA, Schwanauer LE,
Klassen PS, et al: Cinacalcet HCI (Sensipar/Mimpara) is an
effective chronic therapy for hemodialysis patients with secondary
hyperparathyroidism. Clin Nephrol. 68:10–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wetmore JB, Gurevich K, Sprague S, Da Roza
G, Buerkert J, Reiner M, Goodman W and Cooper K: A randomized trial
of cinacalcet versus Vitamin D analogs as monotherapy in secondary
hyperparathyroidism (PARADIGM). Clin J Am Soc Nephrol.
10:1031–1040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mei C, Chen N, Ding X, Yu X, Wang L, Qian
J, Wang M, Jiang G, Li X, Hou F, et al: Efficacy and safety of
Cinacalcet on secondary hyperparathyroidism in Chinese chronic
kidney disease patients receiving hemodialysis. Hemodial Int.
20:589–600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han YY, Wang T, Wenyu Z and Wenxiu C:
Clinical observation of calcitriol combined with cinacalcet in
hemodialysis patients with secondary hyperparathyroidism. Drug
Clinic. 30:1451–1454. 2015.
|
|
36
|
Cai P, Tang X, Qin W, Ji L and Li Z:
Comparison between paricalcitol and active non-selective vitamin D
receptor activator for secondary hyperparathyroidism in chronic
kidney disease: A systematic review and meta-analysis of randomized
controlled trials. Int Urol Nephrol. 48:571–584. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Shao L, Zhou H, Jiang W, Zhang W and
Xu Y: The efficacy of cinacalcet combined with conventional therapy
on bone and mineral metabolism in dialysis patients with secondary
hyperparathyroidism: A meta-analysis. Endocrine. 43:68–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cunningham J, Danese M, Olson K, Klassen P
and Chertow GM: Effects of the calcimimetic cinacalcet HCl on
cardiovascular disease, fracture, and health-related quality of
life in secondary hyperparathyroidism. Kidney Int. 68:1793–1800.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
EVOLVE Trial Investigators, . Chertow GM,
Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog
CA, Kubo Y, London GM, Mahaffey KW, et al: Effect of cinacalcet on
cardiovascular disease in patients undergoing dialysis. N Engl J
Med. 367:2482–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colloton M, Shatzen E, Miller G,
Stehman-Breen C, Wada M, Lacey D and Martin D: Cinacalcet HCl
attenuates parathyroid hyperplasia in a rat model of secondary
hyperparathyroidism. Kidney Int. 67:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yamada S, Tokumoto M, Taniguchi M,
Toyonaga J, Suehiro T, Eriguchi R, Fujimi S, Ooboshi H, Kitazono T
and Tsuruya K: Two years of cinacalcet hydrochloride treatment
decreased parathyroid gland volume and serum parathyroid hormone
level in hemodialysis patients with advanced secondary
hyperparathyroidism. Ther Apher Dial. 19:367–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slatopolsky E, Finch J, Ritter C and
Takahashi F: Effects of 19-nor-1,25(OH)2D2, a new analogue of
calcitriol, on secondary hyperparathyroidism in uremic rats. Am J
Kidney Dis. 32 Suppl 2:S40–S47. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schumock GT, Walton SM, Lee TA, Marx SE,
Audhya P and Andress DL: Comparative effectiveness of paricalcitol
versus cinacalcet for secondary hyperparathyroidism in patients
receiving hemodialysis. Nephron Clin Pract. 117:c151–c159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zittermann A and Koerfer R: Protective and
toxic effects of vitamin D on vascular calcification: Clinical
implications. Mol Aspects Med. 29:423–432. 2008. View Article : Google Scholar : PubMed/NCBI
|