Introduction
At present, cholangiocarcinoma (CCA) is the most
prevalent bile duct malignancy in the clinic and is a fatal
malignancy originating from biliary epithelial cells (1,2). In
recent decades, the incidence of CCA has increased (3,4). Surgery
is the preferred treatment for CCA, but it is also carries risks
and there are numerous complications following surgery (5). Therefore, postoperative care and
rehabilitation training for patients with postoperative CCA is very
important. Currently, the prognosis of patients with CCA remains
unsatisfactory, with a 5-year survival rate of ~5% (6). In addition, CCA is generally resistant
to chemotherapeutic drugs, and is prone to relapse and metastasis
(7). Therefore, it is of great
clinical significance to identify highly effective therapeutic
drugs with minimal side effects and to clarify the mechanism of
action, in order to improve the therapeutic effect and prolong the
survival of patients with CCA.
Propofol (2, 6-diisopropylphenyl), an alkyl acid, is
a fast-acting intravenous anesthetic. It is a widely used drug for
induction and maintenance of anesthesia in the clinic (8). Propofol injection has the
characteristics of rapid distribution (half-life of 2–4 min) and
rapid elimination (half-life of 30–60 min) (9). Numerous studies have demonstrated the
superiority of propofol over volatile agents, because propofol does
not suppress the immune system in a cancerous environment (10–14). In
recent decades, numerous studies have demonstrated that propofol
has a variety of other effects, including possible anti-cancer
actions (15–18). However, to the best of our knowledge,
the effect and mechanism of propofol on CCA cells remains unclear.
Therefore, the current study aimed to investigate the effect and
molecular mechanism of propofol on CCA cancer in vitro.
Materials and methods
Materials
The human CCA cell line QBC939 was obtained from
American Type Culture Collection (Manassas, VA, USA). Dulbecco's
modified Eagle medium (DMEM) and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). All primary antibodies [anti-β-actin (cat. no. 4970),
anti-CyclinE (cat. no. 20808), anti-Bax (cat. no. 5023), anti-Bcl-2
(cat. no. 4223), anti-Wnt3a (cat. no. 2721), anti-Snail1 (cat. no.
3879), anti-c-myc (cat. no. 13987) and anti-β-catenin (cat. no.
8480)] were acquired from Cell Signaling Technology, Inc. (Danvers,
MA, USA).
Cell culture and treatment
Human CCA QBC939 cells were cultured in DMEM
supplemented with 10% FBS and 1% penicillin/streptomycin. The cells
were incubated in a humidified incubator at 37°C with 5%
CO2. Propofol was dissolved in dimethyl sulfoxide (DMSO)
and stored at −20°C. QBC939 cells were treated with various
concentrations of propofol (0, 1, 5 or 10 µg/ml) for 24, 48 or 72 h
at 37°C (19,20). Then, the cells were subjected to
assays as described below.
MTT assay
Cells were collected at logarithmic phase,
inoculated in a 96-well plate with 1×104 cells/well and
incubated at 37°C with 5% CO2 for 12 h. Then, the cells
were treated with various concentrations of propofol (0, 1, 5 or 10
µg/ml) for 24, 48 or 72 h at 37°C. Subsequently, MTT (20 µl; 5
mg/ml) was added to each well, and the cells were incubated for a
further 4 h. The formazan crystals were dissolved in 150 µl DMSO
and stirred slowly for 10 min. The optical density (OD) of each
sample was determined at the wavelength of 570 mm with an
immunoassay analyzer. The cell inhibition rate=(1-OD value of
treatment group/OD value of control) ×100%. All experiments were
performed in triplicate and repeated three times.
Western blotting
QBC939 cells were treated with various
concentrations of propofol (0, 1, 5 or 10 µg/ml) for 48 h at 37°C.
Then the cells were washed with PBS three times and lysed on ice in
radioimmunoprecipitation buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology, Nanjing, China) with 1 mM PMSF for 30
min at 4°C. Protein was collected and stored −20°C. Bicinchoninic
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) was used
to detect protein concentration. Equal amounts of protein (30
µg/lane) were separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. Then, the membrane was blocked
at room temperature for 2 h with 5% skimmed milk in PBS with 0.1%
Tween-20 (PBST) and incubated with primary antibodies (β-actin,
CyclinE, Bcl-2, Bax, Wnt3a, β-catenin, Snail1 and c-myc; all
1:1,000; Cell Signaling Technology, Inc.) overnight at 4°C. The
following day, the membrane was washed four times in 1X PBST (10
min/wash) and incubated with anti-rabbit immunoglobulin G
horseradish peroxidase-coupled secondary antibodies (cat. no. 7074;
1:1,000; Cell Signaling Technology, Inc.) for 2 h at room
temperature. Proteins were detected using SignalFire™
Plus ECL Reagent (cat. no. 12630; Cell Signaling Technology, Inc.)
and imaged. β-actin was used as an internal control.
Transwell assay
To investigate the effects of propofol on QBC939
cell migration and invasion, a 24-well Transwell plate (8-µm pore
size) was used. The chamber inserts were coated with or without 200
mg/ml of BD Matrigel™ Matrix (BD Biosciences, Franklin
Lakes, NJ, USA) for the invasion and migration assay, respectively.
Logarithmic phase QBC939 cells were inoculated into 6-well plates
(1×104 cells/well) and placed in a constant temperature
incubator for routine culture. When the cells reached 70–80%
confluence, they were treated with various concentrations of
propofol for 48 h. Then, 100 µl DMEM containing 10% FBS was added
to the upper chamber for 1 h. Subsequently, the cells were digested
with 0.25% trypsin and resuspended in DMEM to prepare a single cell
suspension. The cell density was adjusted to 106
cells/ml. DMEM (0.5 ml) containing 10% FBS was added to the lower
chamber, and 100 µl cell suspension was added to the upper chamber
of each insert. The plates were cultured at 37°C with 5%
CO2 for 24 h. Then, cells that had not migrated or
invaded from the upper chamber to the lower chamber were gently
wiped away with a clean cotton swab. The cells on the lower chamber
were stained with 0.5 ml 0.1% crystal violet at room temperature
for 20 min. Five fields of view were observed for each chamber by a
light microscope and the mean value was calculated.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from QBC939 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The RNA concentration was
detected using NanoDrop 2000 (Thermo Fisher Scientific, Inc.).
Total RNA was reverse transcribed into cDNAs using the PrimeScript
RT Reagent kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. qPCR was performed using QuantiFast SYBR
Green PCR kit (Qiagen, Inc., Valencia, CA, USA) and a CFX Connect
Real-Time system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Amplification conditions were as follows: 10 min at 95°C, followed
by 35 cycles of 15 sec at 95°C and 40 sec at 55°C. The primer
sequences used for RT-qPCR were listed in Table I. Relative gene expression was
analyzed using the 2−ΔΔCq method (21).
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
|
| Primer (5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| Bcl-2 |
TTGGATCAGGGAGTTGGAAG |
TGTCCCTACCAACCAGAAGG |
| Bax |
CGTCCACCAAGAAGCTGAGCG |
CGTCCACCAAGAAGCTGAGCG |
| Cyclin E |
AGCCAGCCTTGGGACAATAAT |
GAGCCTCTGGATGGTGCAAT |
| Wnt3a |
GGCTCCTCTCGGATACCTCT |
GGGCATGATCTCCACGTAG |
| β-catenin |
AACAGGGTCTGGGACATTAGTC |
CGAAAGCCAATCAAACACAAAC |
| Snail1 |
GGTTCTTCTGCGCTACTGCTG |
GTCGTAGGGCTGCTGGAAGG |
| c-myc |
CATCAGCACAACTACGCAGC |
GCTGGTGCATTTTCGGTTGT |
| GAPDH |
CTTTGGTATCGTGGAAGGACTC |
GTAGAGGCAGGGATGATGTTCT |
Flow cytometry analysis
QBC939 cells were collected in logarithmic growth
phase, and the cell suspension density was adjusted and inoculated
into 6-well plates at 1×105 cells/well. Subsequently,
cells were treated with various concentrations of propofol for 48
h. Then, the cells were fixed with 70% methanol at −20°C overnight,
washed with PBS twice, stained with propidium iodide (PI; cat. no.
4087; Cell Signaling Technology, Inc.) and incubated at 4°C for 30
min in the dark. Flow cytometry was performed to detect cell-cycle
distribution. For detection of cell apoptosis, cells were stained
with Annexin V and PI for 15 min at room temperature in the dark
prior to flow cytometry. Data were analyzed using WinMDI (version
2.5; Purdue University Cytometry Laboratories, West Lafayette, IN,
USA).
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. All experiments were repeated three times.
Differences between multiple groups were compared by one-way
analysis of variance followed by Tukey's test, and differences
between two groups were compared by Student's t-test. GraphPad
Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA) was
used to perform statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Propofol inhibits QBC939 cell
proliferation
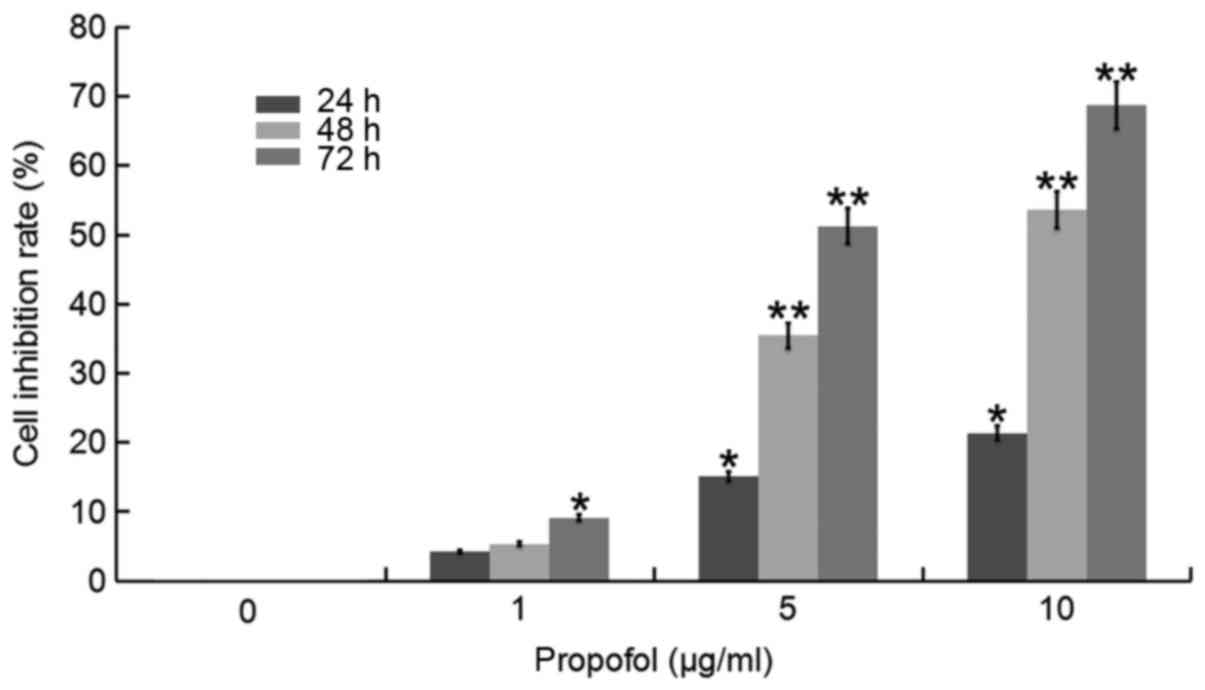
In order to study the anti-tumor effect of propofol
on human CCA cells, an MTT assay was performed. Following treatment
with various concentrations of propofol for 24, 48 or 72 h,
respectively, inhibition of proliferation appeared to increase as
concentration of propofol in QBC939 cells and the duration of the
incubation increased (Fig. 1).
Propofol inhibits migration and
invasion capacity in QBC939 cells
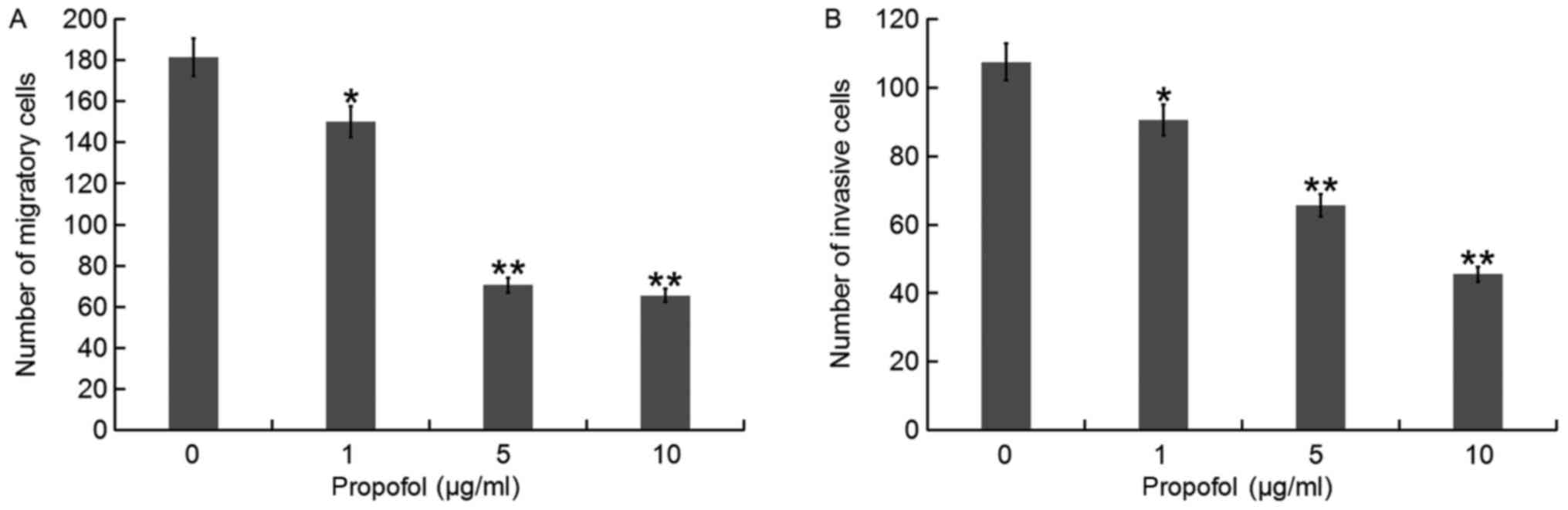
The effects of propofol on migration and invasion of
QBC939 cells were examined by Transwell assay. The results
indicated that migration and invasion of cells decreased gradually
with increased concentrations of propofol compared with the control
(Fig. 2). Doses of 1, 5 and 10 µg/ml
significantly decreased migration and invasion compared with the 0
µg/ml group.
Propofol induces QBC939 cell
apoptosis
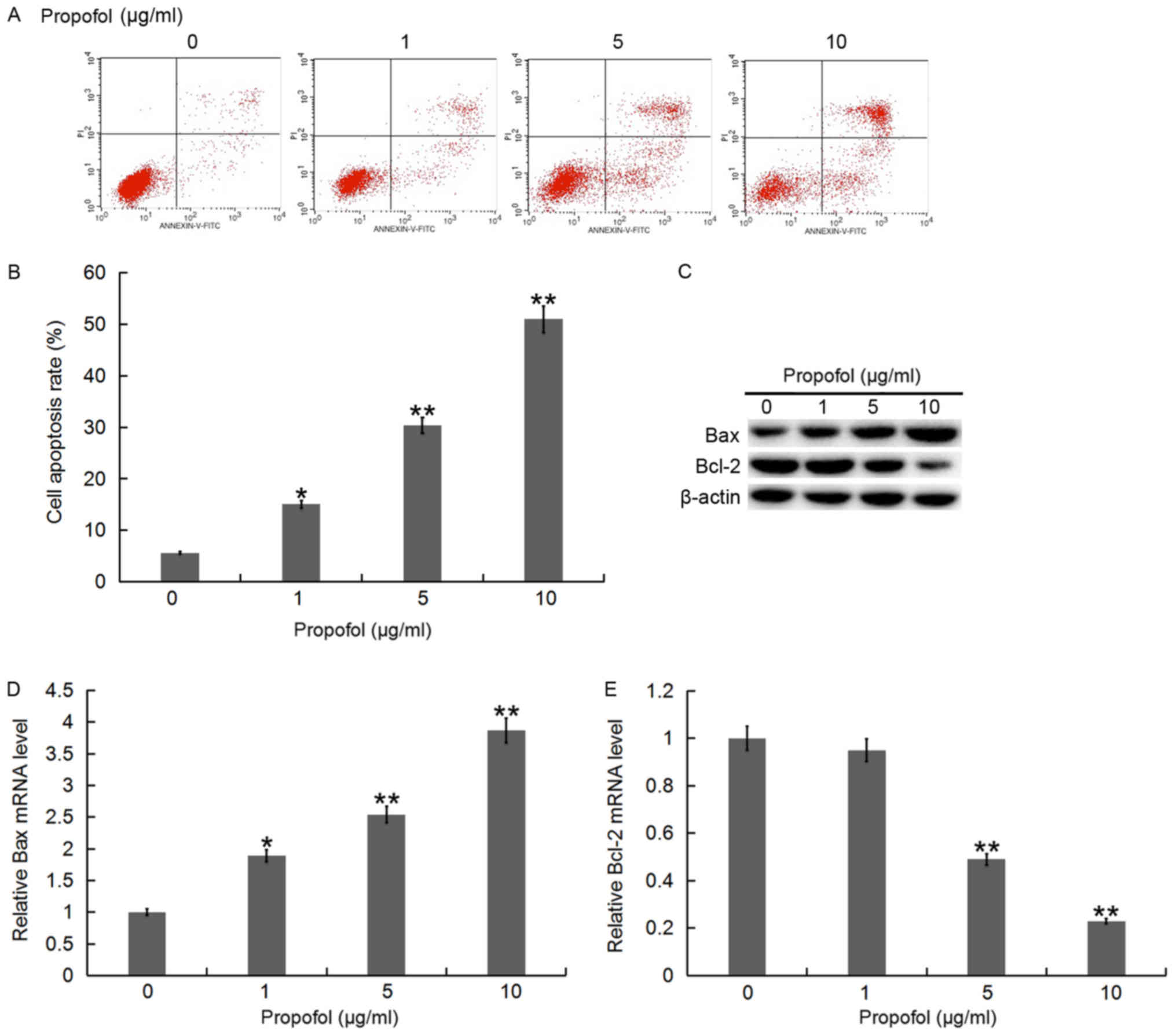
To investigate whether propofol inhibited the
proliferation of QBC939 cells via inducing apoptosis, flow
cytometry was performed to detect cell apoptosis. Following
treatment with various concentrations of propofol for 48 h, the
apoptosis rate of QBC939 cells appeared to increase as
concentration of propofol increased (Fig. 3A and B). Furthermore, RT-qPCR and
western blot analysis were conducted to determine the expression
levels of apoptosis-associated genes, Bax and Bcl-2. The results
indicated that with an increase of propofol concentration, the Bax
mRNA and protein level in QBC939 cells gradually increased, while
the mRNA and protein level of Bcl-2 decreased (Fig. 3C-E).
Propofol induces cell-cycle arrest in
QBC939 cells
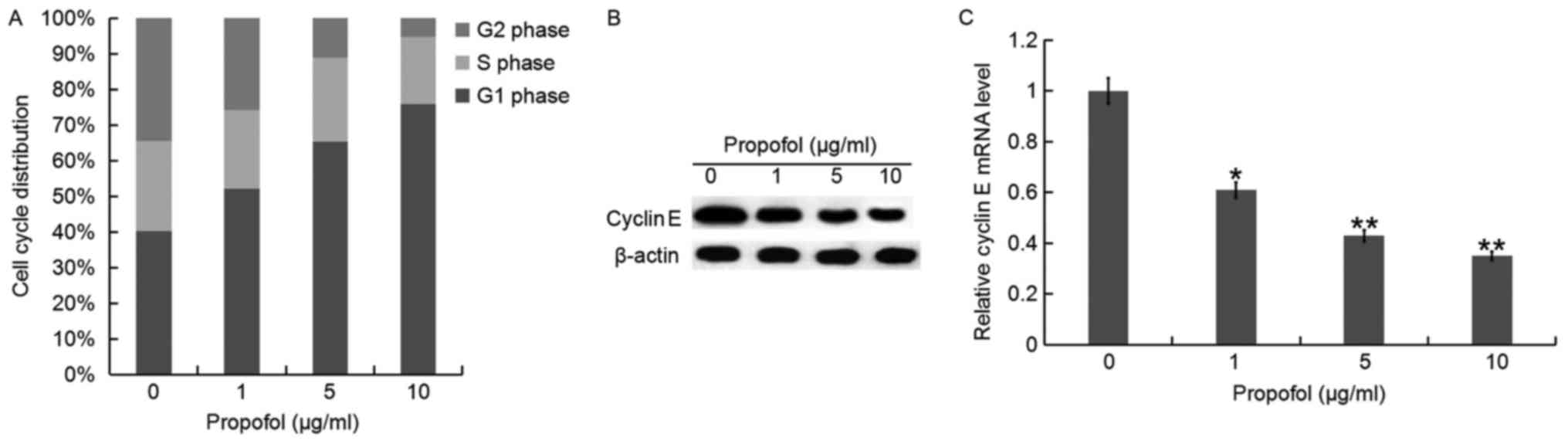
To demonstrate the possible mechanism of
propofol-induced cell growth inhibition in QBC939 cells, cell cycle
progression was also analyzed. QBC939 cells were treated with
different concentrations of propofol for 48 h and then the cell
cycle distribution was analyzed using flow cytometry (data not
shown). The results indicated that the percentage of G1 phase of
QBC939 cells appeared to increase as concentration of propofol
increased (Fig. 4A). In addition,
the mRNA and protein expression of CyclinE, an important regulator
of G1 and S phases in the cell cycle, was detected. It was
identified that the expression of CyclinE was gradually decreased
at the mRNA and protein level in QBC939 cells as propofol
concentration increased compared with the control (Fig. 4B and C).
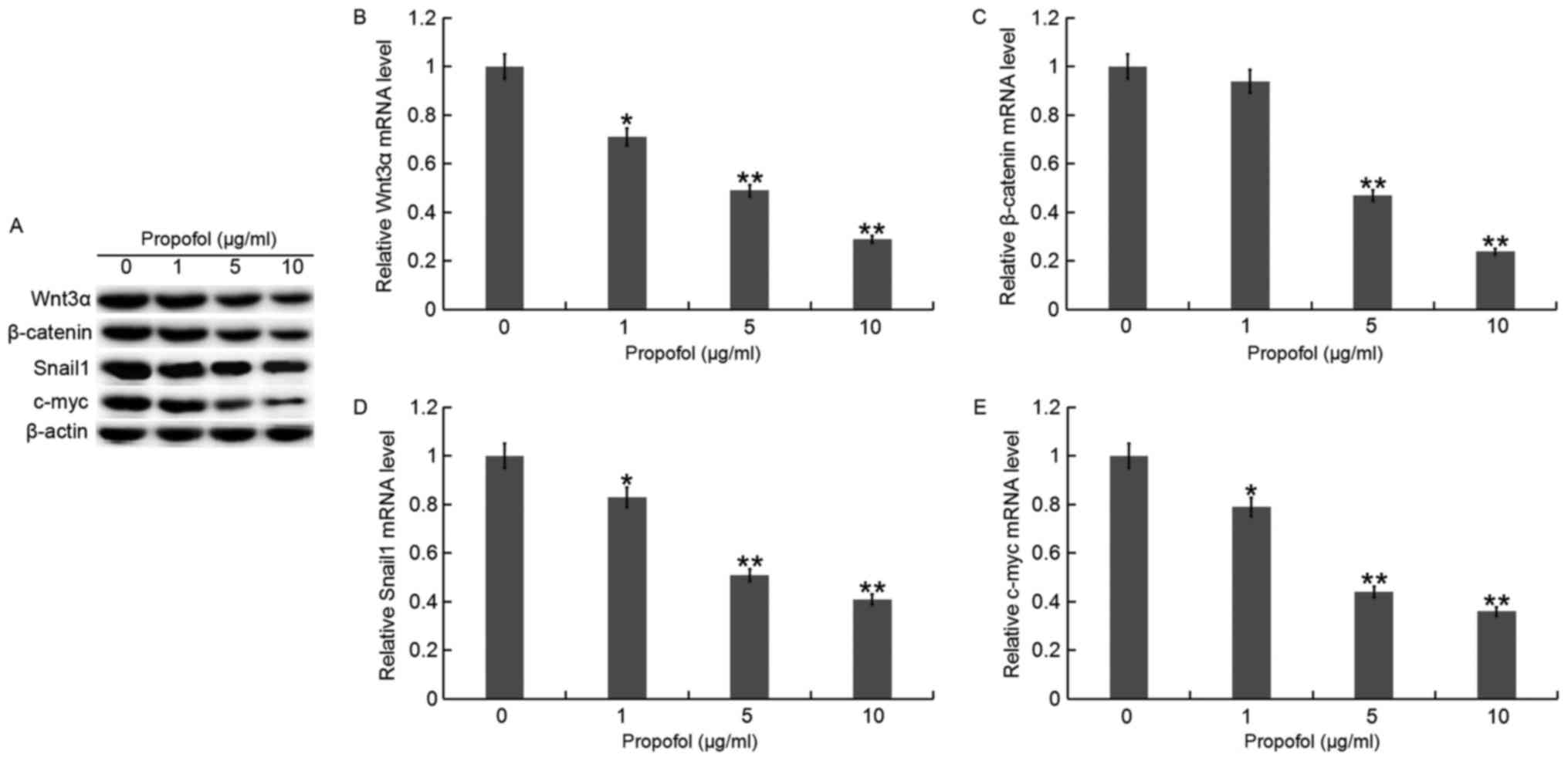
Propofol inhibits the Wnt/β-catenin
signaling pathway
QBC939 cells were treated with various
concentrations of propofol for 48 h, then the Wnt/β-catenin
signaling pathway was analyzed. RT-qPCR and western blot assays
indicated that the expression of Wnt3α, β-catenin, Snaill and c-myc
was gradually decreased at both the protein (Fig. 5A) and mRNA (Fig. 5B-E) level in QBC939 cells as propofol
concentration increased compared with the control.
Discussion
CCA is a rapidly growing and lethal cancer that is
usually incurable unless the primary tumor and any metastases are
removed completely by surgery (22).
As surgical treatment is prone to complications and seriously
affects the quality of life of patients, in the postoperative
period, it is necessary to strengthen the rehabilitation of
patients with CCA from psychological and dietary perspectives
(23,24). Following CCA surgery, patients should
follow their doctor's advice and regularly attend check-ups at
their hospital, participate in light physical activity and avoid
prolonged periods of sitting or low activity to facilitate the
recovery of body function (25).
However, the majority of CCA patients are diagnosed at an advanced
stage, when surgery is not possible (26). Therefore, CCA treatment is still a
major clinical challenge, and exploring new and effective drugs and
strategies for the treatment of CCA is of great clinical
significance.
Propofol is one of many anesthetics and has been
extensively studied. Previous studies have suggested that it has an
anti-cancer effect in addition to its anesthetic effect. Research
has demonstrated that propofol could inhibit cancer cell
proliferation, migration and invasion, and thus exert anti-tumor
function (27–30). Previous studies have identified that
propofol could inhibit the development of numerous cancer types,
including pancreatic (31), breast
(32), colon (33), gastric (34) and cervical (35) cancer. Zhang et al (36) demonstrated that propofol promotes the
proliferation and invasion of gallbladder cancer cells by
activating Nrf2. By contrast, Liu et al (37) indicated that propofol inhibits the
proliferation and invasion of pancreatic cells by modulating the
microRNA-21/Slug signaling pathway. However, there are few reports
on the role of propofol in CCA, and the precise mechanism is still
unknown. Therefore, the aim of the current study was to explore the
effect of propofol on CCA QBC939 cells and its mechanism of
action.
In the current study, an MTT assay demonstrated that
as concentration of propofol and treatment time increased, the cell
inhibition rate of QBC939 cells increased. Next, QBC939 cells were
treated with different concentrations of propofol, and a Transwell
assay was used to detect cell migration and invasion. Compared with
the control group, experimental groups exhibited a significant
decrease in the number of migratory and invasive cells. Therefore,
these results indicated that propofol significantly inhibits the
proliferation, invasion and migration of QBC939 cells, providing a
theoretical basis for using propofol as a therapeutic drug for
CCA.
Furthermore, flow cytometry was performed to analyze
the apoptosis of QBC939 cells, and it was identified that the
apoptosis rate of QBC939 cells appeared to increase as
concentration of propofol increased. The effect of propofol on
apoptosis-associated proteins in QBC939 cells was evaluated by
RT-qPCR and western blot assays. The results suggested that
propofol inhibited the expression of the anti-apoptotic protein
Bcl-2 and promoted the expression of the pro-apoptotic protein Bax.
Another important finding in the current study was that propofol
arrested cell cycle in the G1 phase, and it may be associated with
a decrease in the protein level of CyclinE.
In recent decades, it has been reported that the
Wnt/β-catenin signaling pathway is closely associated with the
occurrence of cancer. The Wnt/β-catenin signaling pathway plays an
important regulatory role in the proliferation, survival and
metastasis of tumor cells (38,39). To
further explore the molecular mechanism of propofol-induced
apoptosis in human CCA QBC939 cells, the effect of propofol on
certain genes (Wnt3α, β-catenin, Snaill and c-myc) in the
Wnt/β-catenin signaling pathway was examined. It was identified
that as the concentration of propofol increased, the expression of
Wnt3α, β-catenin, Snaill and c-myc gradually decreased. In future
experiments, the association between propofol and this pathway will
be studied in greater detail.
In summary, the current results indicate that
propofol inhibits the proliferation, migration and invasion of
QBC939 cells, and induces apoptosis and cell cycle arrest. Its
mechanism of action may be associated with the Wnt/β-catenin
signaling pathway. This could provide a target and experimental
basis for clinical treatment of CCA. However, the current study is
a preliminary study of the effects of propofol on CCA and more
detailed research in this area is required. Future studies will aim
to investigate the effects of propofol on other CCA cell lines and
study the molecular mechanisms of propofol in depth.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ collaborated to design the study. MZ and SW
assessed and analyzed the data. All authors, including CW,
collaborated to interpret the results and develop the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blechacz B and Gores GJ:
Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and
treatment. Hepatology. 48:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shaib YH, Davila JA, Mcglynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: A true increase? J Hepatol. 40:472–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1998. Ca Cancer J Clin. 48:6–29. 2010.
View Article : Google Scholar
|
|
5
|
Wang Q, Du T and Lu C: Perioperative blood
transfusion and the clinical outcomes of patients undergoing
cholangiocarcinoma surgery: A systematic review and meta-analysis.
Eur J Gastroenterol Hepatol. 28:1233–1240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan D, Huang S, Berger E, Liu L, Gross N,
Heinzmann F, Ringelhan M, Connor TO, Stadler M, Meister M, et al:
Kupffer cell-derived Tnf triggers cholangiocellular tumorigenesis
through JNK due to chronic mitochondrial dysfunction and ROS.
Cancer Cell. 31:771–789. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahnemai-Azar AA, Weisbrod AB, Dillhoff M,
Schmidt C and Pawlik TM: Intrahepatic cholangiocarcinoma: Current
management and emerging therapies. Expert Rev Gastroenterol
Hepatol. 11:439–449. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Wu Q, Sun P, Zhao Y, Zhu M and
Miao C: Propofol disrupts aerobic glycolysis in colorectal cancer
cells via inactivation of the NMDAR-CAMKII-ERK Pathway. Cell
Physiol Biochem. 46:492–504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cockshott ID, Briggs LP, Douglas EJ and
White M: Pharmacokinetics of propofol in female patients. Studies
using single bolus injections. Br J Anaesth. 59:1103–1110. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buckley A, McQuaid S, Johnson P and Buggy
DJ: Effect of anaesthetic technique on the natural killer cell
anti-tumour activity of serum from women undergoing breast cancer
surgery: A pilot study. Br J Anaesth. 113:56–62. 2014. View Article : Google Scholar
|
|
11
|
Zhang T, Fan Y, Liu K and Wang Y: Effects
of different general anaesthetic techniques on immune responses in
patients undergoing surgery for tongue cancer. Anaesth Intensive
Care. 42:220–227. 2014.PubMed/NCBI
|
|
12
|
Jaura AI, Flood G, Gallagher HC and Buggy
DJ: Differential effects of serum from patients administered
distinct anaesthetic techniques on apoptosis in breast cancer cells
in vitro: A pilot study. Br J Anaesth. 113:63–67. 2014. View Article : Google Scholar
|
|
13
|
Ecimovic P, McHugh B, Murray D, Doran P
and Buggy DJ: Effects of sevoflurane on breast cancer cell function
in vitro. Anticancer Res. 33:4255–4260. 2013.PubMed/NCBI
|
|
14
|
Kurosawa S and Kato M: Anesthetics, immune
cells, and immune responses. J Anesth. 22:263–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui RA, Zerouga M, Wu M, Wu M,
Castillo A, Harvey K, Zaloga GP and Stillwell W: Anticancer
properties of propofol-docosahexaenoate and
propofol-eicosapentaenoate on breast cancer cells. Breast Cancer
Res. 7:R645–R654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu KC, Yang ST, Hsia TC, Yang JS, Chiou
SM, Lu CC, Wu RS and Chung JG: Suppression of cell invasion and
migration by propofol are involved in down-regulating matrix
metalloproteinase-2 and p38 MAPK signaling in A549 human lung
adenocarcinoma epithelial cells. Anticancer Res. 32:4833–4842.
2012.PubMed/NCBI
|
|
17
|
Qian J, Shen S, Chen W and Chen N:
Propofol reversed Hypoxia-induced docetaxel resistance in prostate
cancer cells by preventing epithelial-Mesenchymal transition by
inhibiting hypoxia-Inducible factor 1α. Biomed Res Int.
2018:41742322018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Zhang L, Han Y, Jiang Z and Wang Q:
Propofol reduces MMPs expression by inhibiting NF-kB activity in
human MDA-MB-231 cells. Biomed Pharmacother. 66:52–56. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng C, Song L, Wang J, Li D, Liu Y and
Cui X: Propofol induces proliferation partially via downregulation
of p53 protein and promotes migration via activation of the Nrf2
pathway in human breast cancer cell line MDA-MB-231. Oncol Rep.
37:841–848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang X, Teng Y, Yang H and Ma J: Propofol
inhibits invasion and growth of ovarian cancer cells via regulating
miR-9/NF-κB signal. Braz J Med Biol Res. 49:e57172016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2011.
View Article : Google Scholar
|
|
22
|
Liu XF, Tang K, Sui LL and Xu G:
Cholangiocarcinoma: Present status and molecular aspects of
diagnosis. Onco Res. 22:177–183. 2014. View Article : Google Scholar
|
|
23
|
El Chafic AH, Dewitt J, Leblanc JK, El
Hajj II, Cote G, House MG, Sherman S, McHenry L, Pitt HA, Johnson
C, et al: Impact of preoperative endoscopic ultrasound-guided fine
needle aspiration on postoperative recurrence and survival in
cholangiocarcinoma patients. Endoscopy. 45:883–889. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong S, Zheng B, Wang J, Chi J, Tong Y,
Xia L, Xu N, Zhang J, Kong X, Gu J and Xia Q: Transarterial
chemoembolization: A favorable postoperative management to improve
prognosis of hepatitis B virus-associated intrahepatic
cholangiocarcinoma after surgical resection. Int J Biol Sci.
13:1234–1241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kovalenko YA, Vishnevsky VA, Chzhao AV and
Zharikov YO: New criteria of radical surgery and long-term outcomes
of hilar cholangiocarcinomamanagement (Russian). Khirurgiia (Mosk).
4–11. 2018.PubMed/NCBI
|
|
26
|
Yao D, Kunam VK and Li X: A review of the
clinical diagnosis and therapy of cholangiocarcinoma. J Int Med
Res. 42:3–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng F, Ouyang M, Wang X, Yao X, Chen Y,
Tao T, Sun X, Xu L, Tang J and Zhao L: Differential role of
intravenous anesthetics in colorectal cancer progression:
Implications for clinical application. Oncotarget. 7:77087–77095.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ecimovic P, Murray D, Doran P and Buggy
DJ: Propofol and bupivacaine in breast cancer cell function in
vitro-role of the NET1 gene. Anticancer Res. 34:1321–1331.
2014.PubMed/NCBI
|
|
29
|
Peng Z and Zhang Y: Propofol inhibits
proliferation and accelerates apoptosis of human gastric cancer
cells by regulation of microRNA-451 and MMP-2 expression. Genet Mol
Res. 15:2016. View Article : Google Scholar
|
|
30
|
Mammoto T, Mukai M, Mammoto A, Yamanaka Y,
Hayashi Y, Mashimo T, Kishi Y and Nakamura H: Intravenous
anaesthetic, propofol inhibits invasion of cancer cells. Cancer
Lett. 184:165–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Wu Q, You L, Chen S, Zhu M and
Miao C: Propofol attenuates pancreatic cancer malignant potential
via inhibition of NMDA receptor. Eur J Pharmacol. 795:150–159.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Lu P, Chen L, Yang SJ, Shen HY, Yu
DD, Zhang XH, Zhong SL, Zhao JH and Tang JH: Perioperative
propofol-paravertebral anesthesia decreases the metastasis and
progression of breast cancer. Tumour Biol. 36:8259–8266. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu YJ, Li SY, Cheng Q, Chen WK, Wang SL,
Ren Y and Miao CH: Effects of anaesthesia on proliferation,
invasion and apoptosis of LoVo colon cancer cells in vitro.
Anaesthesia. 71:147–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang ZT, Gong hY, Zheng F, Liu DJ and Yue
XQ: Propofol suppresses proliferation and invasion of gastric
cancer cells via downregulation of microRNA-221 expression. Genet
Mol Res. 14:8117–8124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang D, Zhou XH, Zhang J, Zhou YX, Ying
J, Wu GQ and Qian JH: Propofol promotes cell apoptosis via
inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem
Biophys Res Commun. 468:561–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Wang N, Zhou S, Ye W, Jing G and
Zshang M: Propofol induces proliferation and invasion of
gallbladder cancer cells through activation of Nrf2. J Exp Clin
Cancer Res. 31:662012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Z, Zhang J, Hong G, Quan J, Zhang L
and Yu M: Propofol inhibits growth and invasion of pancreatic
cancer cells through regulation of the miR-21/Slug signaling
pathway. Am J Transl Res. 8:4120–4133. 2016.PubMed/NCBI
|
|
38
|
Guo Q, Shen S, Liao M, Lian P and Wang X:
NGX6 inhibits cell invasion and adhesion through suppression of
Wnt/β-catenin signal pathway in colon cancer. Acta Biochim Biophys
Sin (Shanghai). 42:450–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mohammed MK, Shao C, Wang J, Wei Q, Wang
X, Collier Z, Tang S, Liu H, Zhang F, Huang J, et al: Wnt/β-catenin
signaling plays an ever-expanding role in stem cell self-renewal,
tumorigenesis and cancer chemoresistance. Genes Dis. 3:11–40. 2016.
View Article : Google Scholar : PubMed/NCBI
|