Introduction
Herpes simplex (HS) is a kind of infectious mucosa
and skin disease caused by herpes simplex virus (HSV) and oral
infection is mainly caused by HSV-1 (1,2). HS is a
worldwide health problem. It has been reported that there are
approximately 150 million people who suffer from recurrent HS
worldwide, and antibodies against HSV have been found in the serum
of 90% research subjects, which suggested that these subjects used
to or are still infected with HSV (3,4).
Micro ribonucleic acid (miR) is widely expressed in
eukaryotic organisms and it regulates cell proliferation and
apoptosis, participates in multiple pathophysiological processes
and regulates cell differentiation (5,6).
Substantial research has shown that miR-138 can inhibit the
expression of infected cell protein 0 (ICP0) (4,7,8). ICP0 is a protein with strong functions.
It is encoded by virus and can activate the expression of virus β
and gene γ in turn. Besides, ICP0 also plays a vital role in
activating the virus during the transition from latent period to
lytic multiplication period (9,10).
Therefore, the significance of miR-138 expression in
HSV was studied in this experiment in which HSV rat models were
established, miR-138 expression in serum was detected and miR-138
expression in 293T cells infected by HSV was regulated.
Materials and methods
Research objects
Specific pathogen-free (SPF) Sprague-Dawley (SD)
rats were purchased from the Laboratory Animal Center of Jilin
University [certification number: SCXK(JI)2008-0004] and fed with
LAD0011 feed (Trophic Animal Feed High-Tech Co., Ltd., Nantong,
China). These SD rats, aged 7–8 weeks, weighing 200–240 g, were
kept at temperature of 21±1.5°C and humidity of 40–60%, with
natural lighting and free access to food and water. The rearing
cages and water bottles were changed twice a week.
The study was approved by the Ethics Committee of
The Second Qilu Hospital of Shandong University (Jinan, China).
Establishment of SD rat models
The titre of Macaque HSV-1 (Shanghai Y-J Biological
Technology Co., Ltd., Shanghai, China) was adjusted to TCID50
10−5 before use. Hela cells were used to produce and
detect the virus. After all rats were injected with pentobarbital
sodium (50 mg/kg) by intraperitoneal injection for satisfactory
general anesthesia, an operation was performed to make an incision
of ‘pound sign’ on the surface of the right eye cornea of each rat
with a 1 G sterile needle under a microscope (Olympus Corporation,
Tokyo, Japan). Then 10 µl viral suspension (virus concentration of
TCID50 10−5) was dropped into the right eye cornea of
each rat in the observation group and remained for 15 sec, and
normal saline was dropped into the right eye of each rat in the
control group. The eyelids of all the rats were massaged for fast
absorption, and lincomycin hydrochloride and erythrocin were used
to decrease inflammation after operation. Six days later, all rats
with HSV were diagnosed via the method reported by Heiligenhaus
et al (11).
Virus infection of 293T cells
The construction and package of viral vectors were
performed by Hunan Auragene Bioscience Corporation Inc. (Changsha,
China). After the linearization of viral vectors via DNA Pac I
enzyme, 293T cells (cat. no. CRL-11268; Bio-protocol LLC,
Sunnyvale, CA, USA) were transfected using liposomes method (Xiamen
Yanke Biotechnology Co., Ltd.). After 24 h of cultivation in
Dulbecco's modified Eagles medium (DMEM), polybrene was added to
accelerate infection efficiency of the virus. Then the medium was
replaced with fresh media containing miR-138 mimics (Group A) and
miR-138 complementary oligonucleotide inhibitor (Group B),
respectively, and cultivated for 2 weeks (replaced every 24 h).
RNA extraction
The total RNA in serum and cells was extracted using
TRIzol reagents and the operation was conducted in accordance with
the instructions provided by Invitrogen; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). The concentration and purity of the
extracted RNA were analyzed with an ultraviolet spectrophotometer
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the
completeness of RNA was analyzed by 3% agarose gel
electrophoresis.
Synthesis of complementary DNA
(cDNA)
TaqMan® MicroRNA reverse transcription
kit [Thermo Fisher Scientific (China) Co., Ltd., Shanghai, China]
was used and the synthesis of cDNA via reverse transcription was
conducted in accordance with the instructions. Reaction conditions
are as follows: 37°C for 45 min and 95°C for 5 min. The reaction
product was preserved at −20°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The 25 µl reaction system was: pre-degeneration at
95°C for 5 min, degeneration at 95°C for 30 sec, annealing at 60°C
for 45 sec and extension at 72°C for 3 min, a total of 35 cycles,
followed by extension at 72°C for 5 min. ABI Prism 7900PCR
instrument was used in PCR. miR-138, forward,
5′-GTATTGACTAGATTAATCACTGT-3′ and reverse, 5′-CTCGCTTCGGCAGCACA-3′;
ICP0 mRNA, forward, 5′-TCTCGAACAGTTCCGTGTCCGT-3′ and reverse,
5′-TCTCCGCATCACCACAGAAG-3′. U6 (Shanghai Meixuan Biological Science
and Technology Ltd., Shanghai, China) was used as an internal
reference for reaction: Forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-TCTCCGCATCACCACAGAAG-3′. The experiment was repeated
for all the samples in 3 wells and the results were analyzed by
2−ΔΔCq method (12).
Observation indexes
The peripheral blood of rats in the observation and
control groups were collected by cutting the tail at three
different time-points (n=15), respectively: at the same day of
diagnosis with HS (day 1), at 7 days (day 7) and 14 days (day 14)
after diagnosis with HS. After completion, the rats were
anesthetized by intraperitoneal injection of pentobarbital sodium
(100 mg/kg, Shanghai Rongbai Biological Technology Co., Ltd.) and
sacrificed by cervical dislocation. The expression levels of
miR-138 in serum and the expression levels of miR-138 and ICP0 mRNA
in cells in the two groups were detected via RT-qPCR, and the
expression levels of ICP0 (mouse anti-rat HSV1 ICP0 monoclonal
antibody; 1:3,000, cat. no. ab6513; Abcam, Cambridge, MA, USA)
protein were detected via enzyme-linked immunosorbent assay
(ELISA).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (AsiaAnalytics Formerly SPSS China) was used.
Chi-square test was used for the comparison of rates. Measurement
data were presented as (mean ± SD), the non-parametric K-S test was
used for the comparison between the two groups and t-test was used
for the intra-group comparison at different time-points. The
correlation of miR-138 with ICP0 mRNA was analyzed by logistic
regression analysis. P<0.05 suggested that the difference was
statistically significant.
Results
The success rate of model establishment was 56.25%.
Forty-five SD rat models with HS were established successfully in
this study, 7 rats died and 28 rat models did not qualify. There
were 26 male and 19 female SD rats in the observation group with a
mean age of 8.2±0.5 weeks, a mean length of 17.9±1.1 cm and a mean
weight of 221.4±15.3 g. There were 23 male and 17 female SD rats in
the control group with a mean age of 8.5±0.7 weeks, a mean length
of 18.2±1.4 cm and a mean weight of 215.4±13.2 g. The rats in both
groups were given free access to food, water and natural lighting.
No differences were found in the sex, age and weight of rats
between the two groups (P>0.05) (Table I).
 | Table I.General data of rats between the two
groups. |
Table I.
General data of rats between the two
groups.
| Variables | Observation group
(n=45) | Control group
(n=40) | t/χ2
test | P-value |
|---|
| Age (weeks) | 8.2±0.5 | 8.5±0.7 | 2.64 | 0.785 |
| Sex
(male/female) | 26/19 | 23/17 | 1.69 | 0.693 |
| Length (cm) | 17.9±1.1 | 18.2±1.4 | 2.13 | 0.712 |
| Weight (g) | 221.4±15.3 | 215.4±13.2 | 4.11 | 0.842 |
RT-PCR amplification results of
miR-138 in rat serum and 293T cells
RT-PCR amplification of miR-138 and ICP0 mRNA in rat
serum and in 293T cells were conducted. The statistical analysis on
the detection results showed that differences were found in the
expression level of miR-138 at the same period between the two
groups (P=0.035, P=0.041), and the expression level in rat serum in
the observation group was significantly lower than that in the
control group. The rats were sacrificed at different time-points.
There was no obvious change in the expression level of miR-138 in
rat serum in the control group, while the expression level of
miR-138 in rat serum in the observation group was gradually
decreased over time. The expression level of miR-138 in rat serum
at day 14 was significantly lower than that at day 1. Then, the
expression level of miR-138 in 293T cells was analyzed and the
result showed that the expression level of miR-138 in Group A was
significantly higher than that in Group B (P<0.05) (Table II).
 | Table II.Analysis on the expression levels of
miR-138 in rat serum and 293T cells. |
Table II.
Analysis on the expression levels of
miR-138 in rat serum and 293T cells.
| Variables |
| Observation
group | Control group | t-test | P-value |
|---|
| Rat serum | d1 (n=15) | 1.729 | 2.617 | 3.44 | 0.035 |
|
| d7 (n=15) | 1.233 | 2.543 | 8.75 | 0.011 |
|
| d14 (n=15) | 0.475a | 2.596 | 14.96 | 0.001 |
|
|
| Group A | Group B |
|
|
| 293T cells |
| 2.135 | 0.032 | 15.44 | 0.001 |
RT-PCR amplification results of ICPO
mRNA in 293T cells
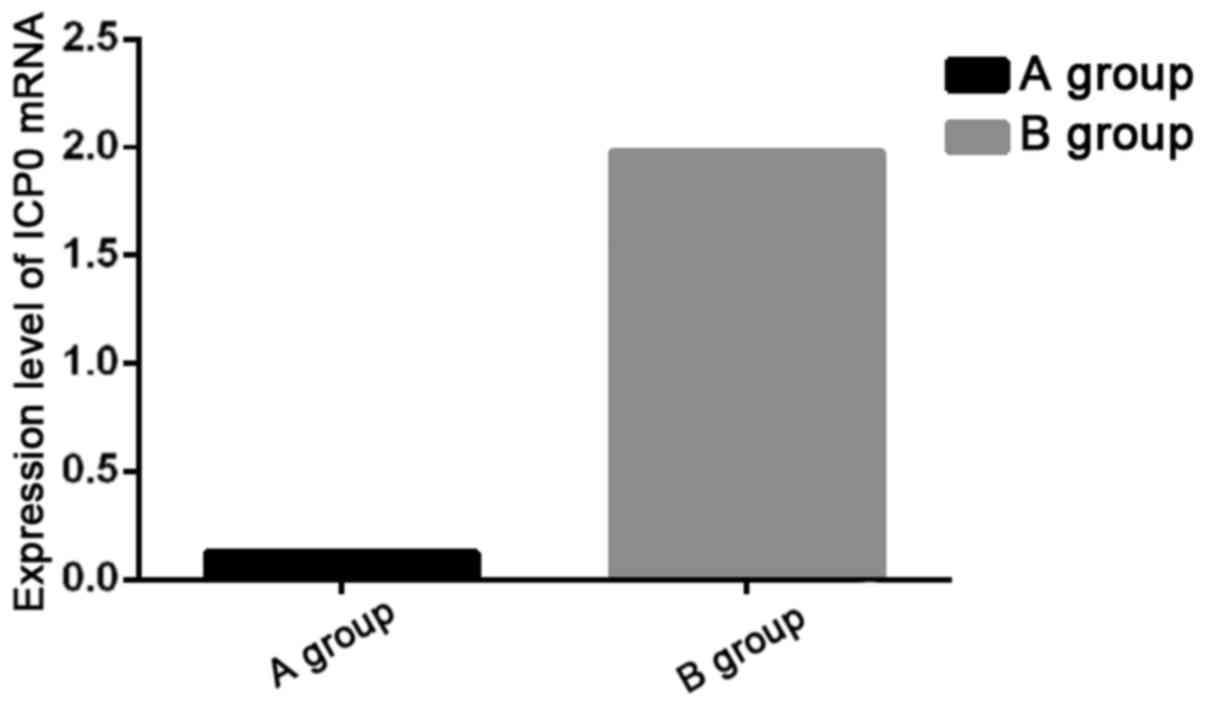
RT-PCR amplification of ICP0 mRNA in 293T cells were
conducted, and the statistical analysis on the detection results
showed that the expression level of ICP0 mRNA in Group A was
significantly lower than that in Group B (P<0.05) (Fig. 1).
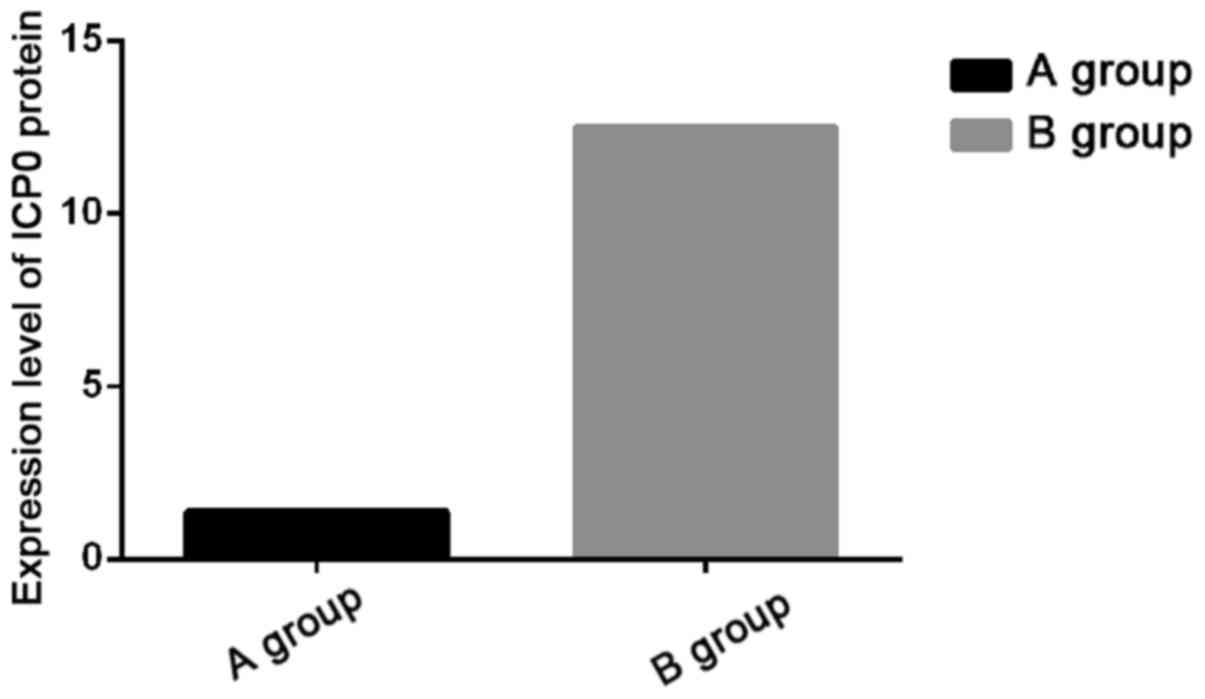
ELISA detection results of ICP0 in
293T cells
The expression levels of ICP0 protein in 293T cells
were detected by ELISA, and the statistical analysis on the
detection results showed that the expression level of ICP0 protein
in Group A was significantly lower than that in Group B (P<0.05)
(Fig. 2).
Logistic regression analysis
The correlation of miR-138 with ICP0 mRNA was
analyzed by logistic regression analysis, and the result showed
that the expression level of miR-138 was negatively correlated with
that of ICP0 mRNA (r=−0.667, P=0.006).
Discussion
HSV has a very high infection rate in the world.
According to statistics, 90% of the individuals have had a history
of HSV (3). When the immunity in
human body is reduced, the expression of latent HSV gene starts to
reduplicate and enter into lytic proliferation period, resulting in
severe epithelial herpes (13,14). A
lot of research has been conducted on HSV from the perspective of
molecular biology (15,16). Research has shown that miR-138 can
inhibit the expression of ICP0 (4,7,8), and ICP0 plays a vital role in HSV.
Therefore, the expression level of miR-138 was investigated to
study the value of its expression.
A total of 45 rat models with HS were established in
this study. After the models were confirmed to be established
successfully, the rats were sacrificed by cutting tail at day 1, 7
and 14, and then the serum was collected. The RT-PCR amplification
results of miR-138 in the rat serum showed that the expression
level of miR-138 in the serum was gradually decreased with the
extension of the course of disease in HS rats, and the expression
level of miR-138 at day 14 was decreased to 0.475 which had a
significant difference from that at day 1 (1.729) (P<0.05).
miR-138 is a neuron-specific microRNA. It was (17) found that the decrease in the
expression of miR-138 can make patients with latent HSV enter into
lytic proliferation period and thus, increase the mortality rate of
patients. Guo and Steitz (18) also
reported in their study that miR-138 can inhibit the expression of
ICP0 protein and produce a longer latent period of HSV-1, which is
similar to the results in this study. However, as this study was
based on animal models and rats died artificially, the change in
the mortality rate of patients with HSV and the influence of
miR-138 on the latent period of HSV-1 cannot be explored in this
study. Yet, they will be further clarified in our future
studies.
The change in the expression levels of ICP0 was also
investigated in this study through the construction of 293T cells
infected by HSV-1 and the regulation of the expression levels of
miR-138. The results showed that the expression levels of miR-138
were successfully regulated via miR-138 mimics and miR-138
complementary oligonucleotide inhibitor, and significant difference
was found between the two groups. The detection results of ICP0
indicated that the expression levels of ICP0 mRNA and ICP0 protein
in 293T cells after the upregulation of miR-138 via miR-138 mimics
were significantly lower than those after the downregulation of
miR-138 via inhibitor, which indicated that miR-138 can regulate
the expression of ICP0 in cells. Moreover, the logistic regression
analysis found that miR-138 was negatively correlated with ICP0.
Therefore, it can be speculated that the expression level of
miR-138 in patients with latent HSV-1 will be decreased with the
extension of latent period. In addition, with the stimulation of
environment and the decline in immune function, HSV-1 latent period
will transform into lytic multiplication period and thus result in
HSV with symptoms such as herpes. The results of Boutell and
Everett (9) indicated that ICP0 is a
protein essential for the re-activation of HSV-1, and the study of
Pare and Sullivan (17) also
demonstrated that the expression level of ICP0 was significantly
decreased by miR-138 and this influence is specific. This result
can be further verified in the future through high-throughput
sequencing analysis on patients with HS.
The present study was based on experimental animal
models and cell strain, so the ethical problem was avoided.
However, the mRNA used in this study had been preserved at −20°C,
which may influence the activity of miR-138 and ICP0 mRNA.
Therefore, the experimental results in this study need to be
verified by more experiments and conclusions also need to be
supported by large sample data.
In conclusion, the increase in the expression level
of miR-138 in patients with HSV can inhibit the expression of ICPO
and thus, it prevents the duplication of HSV-1. Therefore, the
expression level of miR-138 may be used as a potential therapeutic
target for oral HS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY drafted this manuscript and performed PCR. JS
contributed to the establishment of SD rat models. KY helped with
virus infection of 293T cells. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Qilu Hospital of Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Armangue T, Leypoldt F, Málaga I,
Raspall-Chaure M, Marti I, Nichter C, Pugh J, Vicente-Rasoamalala
M, Lafuente-Hidalgo M, Macaya A, et al: Herpes simplex virus
encephalitis is a trigger of brain autoimmunity. Ann Neurol.
75:317–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cockle JV, Ilett E, Brüningrichardson A,
Scott K, Picton S, Short S and Melcher A: OP03 oncolytic herpes
simplex virus inhibits paediatric high grade glioma and diffuse
intrinsic pontine glioma migration and invasion; Mechanism and
potential for clinical application. Neuro-Oncology.
17:viii16.3–viii16. 2015. View Article : Google Scholar
|
|
3
|
Looker KJ, Magaret AS, Turner KM,
Vickerman P, Gottlieb SL and Newman LM: Correction: Global
estimates of prevalent and incident herpes simplex virus type 2
infections in 2012. PLoS One. 10:e01286152015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan D, Flores O, Umbach JL, Pesola JM,
Bentley P, Rosato PC, Leib DA, Cullen BR and Coen DM: A
neuron-specific host microRNA targets herpes simplex virus-1 ICP0
expression and promotes latency. Cell Host Microbe. 15:446–456.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lingam S, Beta M, Dendukuri D and
Krishnakumar S: A focus on microfluidics and nanotechnology
approaches for the ultra sensitive detection of microRNA. MicroRNA.
3:18–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coleman JR, Papamichail D, Skiena S,
Futcher B, Wimmer E and Mueller S: Virus attenuation by
genome-scale changes in codon pair bias. Science. 320:1784–1787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma S, Hussain S, Soni K, Singhal P,
Tripathi R, Ramachandran VG, Sharma S, Das S, Pillai B and
Bharadwaj M: Novel MicroRNA signatures in HPV-mediated cervical
carcinogenesis in Indian women. Tumour Biol. 37:4585–4595. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boutell C and Everett RD: Regulation of
alphaherpesvirus infections by the ICP0 family of proteins. J Gen
Virol. 94:465–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orzalli MH, Broekema NM and Knipe DM:
Relative contributions of herpes simplex virus 1 ICP0 and VHS to
loss of cellular IFI16 vary in different human cell types. J Virol.
90:8351–8359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heiligenhaus A, Li HF, Yang Y, Wasmuth S,
Steuhl KP and Bauer D: Transplantation of amniotic membrane in
murine herpes stromal keratitis modulates matrix metalloproteinases
in the cornea. Invest Ophthalmol Vis Sci. 46:4079–4085. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hacohen Y, Deiva K, Pettingill P, Waters
P, Siddiqui A, Chretien P, Menson E, Lin JP, Tardieu M, Vincent A,
et al: N-methyl-D-aspartate receptor antibodies in post-herpes
simplex virus encephalitis neurological relapse. Mov Disord.
29:90–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradley H, Markowitz LE, Gibson T and
McQuillan GM: Seroprevalence of herpes simplex virus types 1 and 2
- United States, 1999-2010. J Infect Dis. 209:325–333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohammad SS, Sinclair K, Pillai S, Merheb
V, Aumann TD, Gill D, Dale RC and Brilot F: Herpes simplex
encephalitis relapse with chorea is associated with autoantibodies
to N-Methyl-D-aspartate receptor or dopamine-2 receptor. Mov
Disord. 29:117–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orzalli MH, Broekema NM, Diner BA, Hancks
DC, Elde NC, Cristea IM and Knipe DM: cGAS-mediated stabilization
of IFI16 promotes innate signaling during herpes simplex virus
infection. Proc Natl Acad Sci USA. 112:E1773–E1781. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pare JM and Sullivan CS: A host MicroRNA
brokers truce with HSV-1. Cell Host Microbe. 15:395–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo YE and Steitz JA: Virus meets host
microRNA: The destroyer, the booster, the hijacker. Mol Cell Biol.
34:3780–3787. 2014. View Article : Google Scholar : PubMed/NCBI
|