Introduction
In the present, cardiovascular diseases are the
leading cause of death worldwide, with an estimated 17,7 million
deaths per year of which 7.4 million are attributed to coronary
artery disease (CAD) alone (1).
Between 18 and 91 per 100,000 inhabitants benefit from coronary
artery bypass grafting (CABG) in Europe (2) and, according to both European (3) and American (4) Societies' guidelines, CABG is associated
with an increase in quality of life and survival in patients with
unprotected left main (or equivalent) and multi-vessel disease;
however, the ideal grafting technique has not been established.
Early CABG interventions were performed almost
entirely using saphenous vein grafts (SVG) with a late attrition
rate of 2–5% per year after surgery related to the intrinsic
pathologic changes (5). Compared
with veins, internal thoracic arteries (ITA) have an extremely low
attrition rate with very good long-term patency rates (96.4% over
15 years) (6). The use of an ITA
graft results in a mean survival of 4.4 years longer than CABG with
veins alone over a 20-year follow-up period (7), and bilateral ITA [left internal
thoracic artery (LITA) and right internal thoracic artery (RITA)]
use offers even better survival rates than single ITA grafts
(8).
Most patients with CAD have a multi-vessel disease
and require revascularisation of more than two coronary arteries.
The pursuit of additional grafts and anastomosis techniques to
achieve complete revascularisation (5–6 distal anastomoses) has not
proved to be an easy task (9,10). One
option is the radial artery (RA), which supplies a long graft with
a calibre superior to ITA but is prone to spasm and intimal
hyperplasia in case of inadequate harvesting and preparation due to
its muscular structure, leading to its abandonment in the early
years of CABG (11). Improved
harvesting and preparation finally made RA a viable option as a
graft superior to SVG in terms of long-term patency (91.8% vs.
86.4%) (12,13). Various teams have proposed
alternative grafts (right gastroepiploic artery, inferior
epigastric artery, splenic artery, ulnar artery, subcapsular
artery, left gastric artery and lateral circumflex femoral artery),
but their long-term reliability has not yet been confirmed
(14).
Complete revascularisation usually has to be
accomplished using two to three grafts with the best-proven patency
rates. In these circumstances, sequential, composite, Y-grafts,
T-grafts, and combinations are required to bypass all
stenosis/occlusions with a limited number of grafts. Buxton
described several graft configurations to be used (15) in total arterial revascularisation,
but safety, efficacy, and long-term patency have not been assessed
for the majority of these configurations (16–18).
The purpose of the current study was to identify
surgical factors associated with long-term graft patency.
Patients and methods
Patient population, surgical
technique, and postoperative treatment
Demographic, clinical, echocardiographic, and
angiographic data on patients undergoing CABG at the Cardiovascular
Diseases Institute in Iasi, Romania, have been retrospectively
collected and introduced into a database since 2000 together with
intraoperative parameters (extracorporeal circulation type and
time, aortic cross clamp time, CABG technique, number and type of
grafts, associated procedures) and postoperative data (intensive
care unit parameters, complications, and within 30 days mortality).
The CABG technique varied according to the international trend at
the time of surgery from total venous to total arterial
revascularisation and from one graft-one anastomosis to composite
and sequential grafting. All interventions were performed by the
same experienced primary surgeon (>7,000 cardiac surgery
interventions performed) using cardiopulmonary bypass (CPB) and
continuous sutures with Prolene 8-0 for graft anastomosis. No
intraoperative flow measurements were performed.
Long-term postoperative treatment consisted of beta
blockers, statins, and enteric-coated aspirin in all cases.
Patients who benefited from a RA graft received a calcium channel
blocker (Amlodipine) for the first 3 months to prevent spasm.
Treatment was adjusted according to the blood pressure, left
ventricular ejection fraction (LVEF), and comorbidities.
Patient follow-up
In 2016, the status of all 394 patients who received
surgery between 2000 and 2006 and were discharged from the hospital
was verified through the National Health Insurance House database,
and there were 269 identified survivors (68.27%). All survivors
were recalled for an ambulatory examination and coronary computed
tomography angiography (CCTA) evaluation of graft patency by
invitation letter submitted to the last known address or by
telephonic contact. A total of 159 patients responded, and the CCTA
assessment of graft patency (interpretable images) was performed in
127 patients who had received surgery over 10 years earlier and who
consented to and presented no contraindications for the
examination. The 127 patients were evaluated after a mean
postoperative interval of 139.78±36.64 months and represented the
study group of the current article. No redo CABG or percutaneous
transluminal coronary angioplasty (PTCA) after CABG were performed
in the current study group.
CCTA evaluation
All CCTA evaluations were performed using a 2nd
generation 2*128-slices dual source multi-detector CT (MDCT)
scanner (Siemens Somatom Definition Flash; Siemens Healthineers,
Erlangen, Germany). Optimal reconstructions at different percentage
times of an R-R interval were performed (0.75 mm slice thickness)
and submitted to the Syngo.via workstation (Siemens Healthineers)
for image analysis (graft type and status, type of proximal and
distal anastomosis, anastomosis angle).
Image analysis
All CT examinations were evaluated twice by the same
radiologist for the following parameters: graft type and status
(confronted with the operative protocol), proximal and distal
anastomosis type (confronted with the operative protocol),
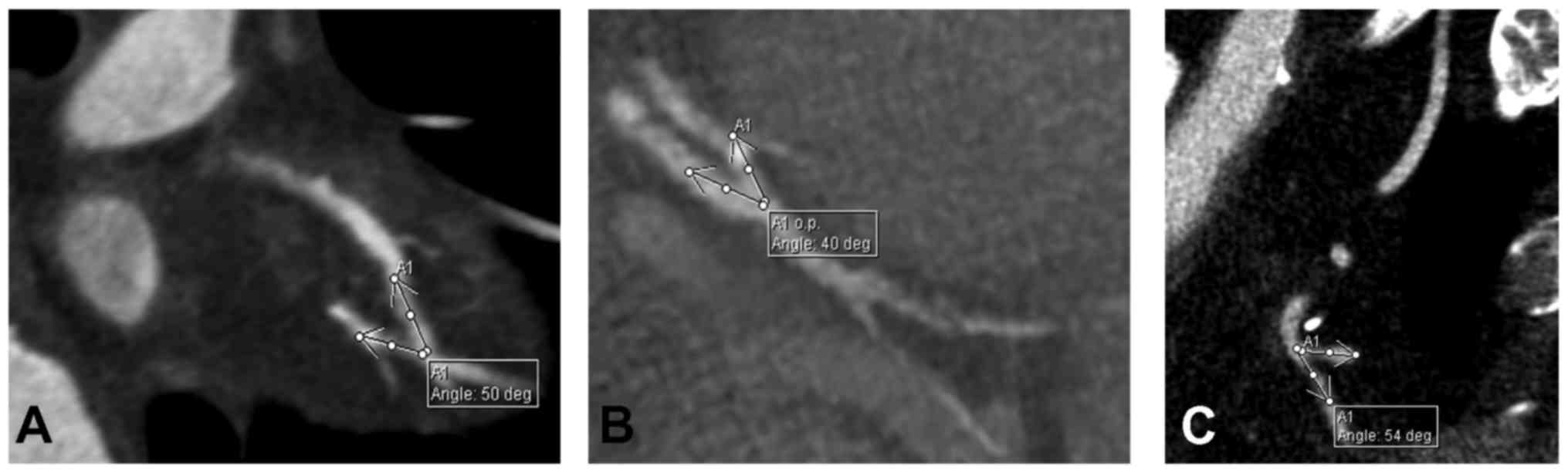
anastomosis angle (Y anastomosis angle measured between the
branches of the Y, distal end-to-side and side-to-side anastomosis
angle measured between the native vessel and the graft) (Fig. 1). All angles were measured on
multiplanar reconstructions (MPR) with the two vessels (graft and
native coronary artery) in the same plane.
Statistical analysis
Continuous variables are expressed as mean ±
standard deviation (SD) and categorical variables as percentages.
Groups were compared using the chi-square test for categorical
variables, and the Student t test and Wilcoxon-Mann-Whitney U test
for continuous variables on the basis of their distribution.
Normality was verified using the Kolmogorov-Smirnov and the
Shapiro-Wilk W tests. Logistic regression was employed for testing
the association between categorical and continuous variables
previously identified as affecting the outcome (graft patency) at
univariable analysis with P<0.05. A receiver operating
characteristic (ROC) curve was used for identifying discrimination
threshold (cut-off) value for continuous variables. Statistical
analyses were performed using SPSS Statistics 24 for Mac OS X (IBM,
Corp., Armonk, NY, USA).
The study was approved by the Ethics Committee of
the ‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania) and by the Ethics Committee of the ‘Prof. Dr. George I.M.
Georgescu’ Cardiovascular Diseases Institute (Iasi, Romania).
Results
Baseline characteristics
The preoperative, operative, and postoperative data
of the 127 patients are summarised in Tables I–III.
 | Table I.Preoperative data. |
Table I.
Preoperative data.
| Parameter | Value (127
patients) | Percentage (%) |
|---|
| Mean age (years) ±
SD | 67.54±8.84 | – |
| ≤65 years | 44 | 34.65 |
| >65 years | 83 | 65.35 |
| Sex, female | 19 | 14.96 |
| Family history | 41 | 32.28 |
| Smoking | 49 | 38.58 |
| Diabetes
mellitus | 28 | 22.05 |
| Dyslipidaemia | 97 | 76.38 |
| MAD | 19 | 14.96 |
| AHT | 77 | 60.63 |
| COPD | 8 | 6.30 |
| NYHA II heart
failure | 18 | 14.17 |
| NYHA III–IV heart
failure | 24 | 18.90 |
| Prior AMI | 65 | 51.18 |
| Arrhythmias | 23 | 18.11 |
| Mean LVEF (%) | 53.81±10.77 | – |
| Number of affected
coronary arteries | 2.86±1.24 | – |
| Diffuse
disease | 29 | 22.83 |
| Three vessel
disease | 71 | 55.91 |
 | Table III.Postoperative data (initial 30
days). |
Table III.
Postoperative data (initial 30
days).
| Parameter | Value (127
patients) | Percentage |
|---|
| Reintervention for
haemorrhage or sternal dehiscence | 10 | 7.87 |
| Acute renal
failure | 2 | 1.57 |
| Arrhythmia | 31 | 24.41 |
| Neurological
complications | 2 | 1.57 |
| Deep sternal wound
infection | 2 | 1.57 |
| Other infections
(urinary tract, pneumonia) | 3 | 2.36 |
| Digestive
complications | 4 | 3.15 |
| (ileus,
Clostridium difficile infection) |
|
|
Graft patency assessment
The 127 patients presented a total number of 340
grafts (2.68 grafts/patient) and 399 distal anastomoses (3.14
anastomoses/patient), 220 (55.14%) performed using arterial grafts
(122 LITA, 53 RA, 45 RITA) and 179 (44.86%) using SVG. Overall
graft patency at 10 to 16 years after the surgical intervention was
of 90.16% for LITA (12 occluded grafts), 75.55% for RITA (11
occluded grafts), 79.25% for RA (11 occluded grafts), and 74.3% for
SVG (46 occluded grafts) with variations according to coronary
territory (Table IV).
 | Table IV.Graft patency according to coronary
territory. |
Table IV.
Graft patency according to coronary
territory.
| Variable | Right coronary
territory (%) | Circumflex artery
territory (%) | Anterolateral
territory (%) |
|---|
| Right internal
thoracic | 3 occluded grafts
out of | 7 occluded grafts
out of 23 (30.43) | 1 occluded graft
out of |
| artery graft | 8 (37.5) |
| 14 (7.14) |
| Radial artery
graft | 6 occluded grafts
out of | 4 occluded grafts
out of 16 (25) | 1 occluded graft
out of |
|
| 31 (19.35) |
| 6 (16.67) |
| Saphenous vein
graft | 24 occluded grafts
out of | 11 occluded grafts
out of 63 (17.46) | 11 occluded graft
out of |
|
| 68 (35.29) |
| 48 (22.92) |
The effect of proximal anastomosis
type
In case of RITA and RA, the authors analysed graft
patency according to proximal anastomosis type (in
situ graft, composite graft with Y/T anastomosis, and graft
anastomosed to the ascending aorta). All saphenous vein grafts were
anastomosed proximally to the ascending aorta, and LITA was used
in situ in all cases except one so both grafts were excluded
from this analysis.
RITA was used in situ in 8 cases (17.78%), as
a composite graft anastomosed Y/T to LITA in 36 cases (80%), and as
a free graft anastomosed to the ascending aorta in 1 case (2.22%).
The occlusion rate was of 3/8 (37.5%) for in situ RITA and
8/3936 (22.22%) for composite grafting. The free graft anastomosed
to the ascending aorta was patent. Chi-square test showed no
association of RITA patency with proximal anastomosis type
(P=0.501).
RA was used as a free graft anastomosed to the
ascending aorta in 40 cases (75.47%) with an occlusion rate of 6/40
(15%) and as a composite graft anastomosed Y/T to LITA in 13 cases
(25.43%) with an occlusion rate of 5/13 (38.46%). The Pearson
chi-square test proved a significant association of graft patency
with proximal anastomosis type (χ2=10.932, P=0.001)
(Table V). Logistic regression
showed a 0.110 OR (P=0.002) for RA anastomosed to the ascending
aorta, thus certifying a protective effect against graft failure
(Table VI).
 | Table V.Association of RA graft patency with
proximal anastomosis type. |
Table V.
Association of RA graft patency with
proximal anastomosis type.
| Variable | Occluded (%) | Patent (%) | Pearson
chi-square | P-value |
|---|
| RA-Aorta | 6 (15) | 34 (85) | 10.932 | 0.001 |
| RA-LITA | 5 (38.46) | 8 (61.54) |
|
|
 | Table VI.Prognostic value of the proximal
anastomosis type (RA-aorta). |
Table VI.
Prognostic value of the proximal
anastomosis type (RA-aorta).
|
|
|
|
|
|
| 95% CI for
EXP(B) |
|---|
|
|
|
|
|
|
|
|
|---|
| B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper |
|---|
| Proximal
anastomosis |
|
|
|
|
|
|
|
|
−2.205 | 0.722 | 9.327 | 1 | 0.002 | 0.110 | 0.027 | 0.454 |
| Constant |
|
|
|
|
|
|
|
|
0.470 | 0.570 | 0.680 | 1 | 0.410 | 1.600 |
|
|
The effect of Y/T anastomosis
angle
The mean angle for Y/T anastomoses with both
grafts patent was 47.21° compared with 56° for anastomoses with
occlusion of the free arterial graft (RA or RITA). The
Wilcoxon-Mann-Whitney U test identified a significant difference
between the anastomosis angle of patent vs. occluded grafts
(P=0.015), a smaller angle being registered in case of patent
anastomosis (Table VII) (Fig. 2). The authors did not identify a high
sensitivity and specificity threshold angle with prognostic value
in affirming the risk of occlusion for Y-anastomoses.
 | Table VII.Proximal and distal anastomosis angle
comparison between occluded and patent grafts. |
Table VII.
Proximal and distal anastomosis angle
comparison between occluded and patent grafts.
| Variable | Occluded | Patent | P-value |
|---|
| Y/T anastomosis
angle | 56±27.22° | 47.21±25.05° | 0.015 |
| Side-to-side
anastomosis (sequential grafting) | 53.97±23.54° | 48.60±21.14° | 0.005 |
| End-to-side
anastomosis (sequential grafting) | 90.80±11.27° | 65.12±26.04° | 0.002 |
| End-to-side (single
distal anastomosis) | 44.94±19.38° | 39.46±18.71° | 0.034 |
The effect of distal anastomosis type
and angle
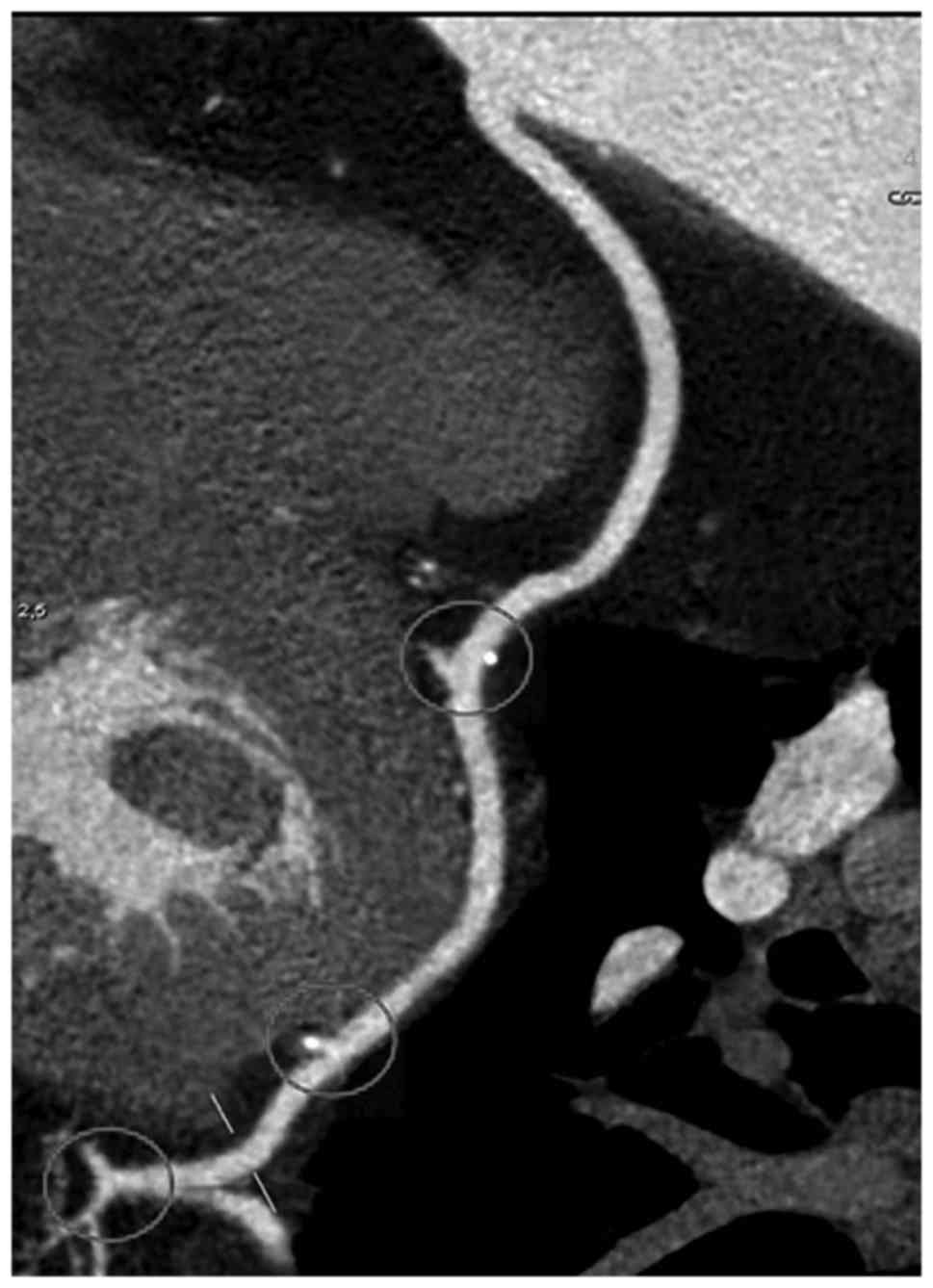
In case of distal anastomosis, 31 were
side-to-side performed with 27 sequential grafts, the rest of 368
being end-to-side (Fig. 3). Of the
27 sequential grafts, 9 were venous (occlusion of 2 end-to-side
anastomoses and 0/11 side-to-side anastomoses) and 18 arterial
(occlusion of 7 end-to-side anastomoses and 9/20 side-to-side
anastomoses). The small number of cases did not allow statistical
interpretation, but one can note a higher patency rate of venous
grafts with sequential anastomoses.
Concerning the anastomosis angle in sequential
grafting, the angle was of 48.60° for patent side-to-side
anastomoses compared with 53.97° for occluded ones. Similarly, the
anastomosis angle was smaller for patent end-to-side anastomoses
(65.12°) compared with occluded ones (90.80°) (P=0.002) (Table VII). The analysis was performed
irrespective to graft type.
In case of single distal anastomosis (end-to-side),
arterial grafts proved to be sensitive to the anastomosis angle
with a mean value of 39.46°±21.97° for patent grafts and
44.94°±29.52° for occluded ones (P=0.034) (Table VII). For venous grafts, the
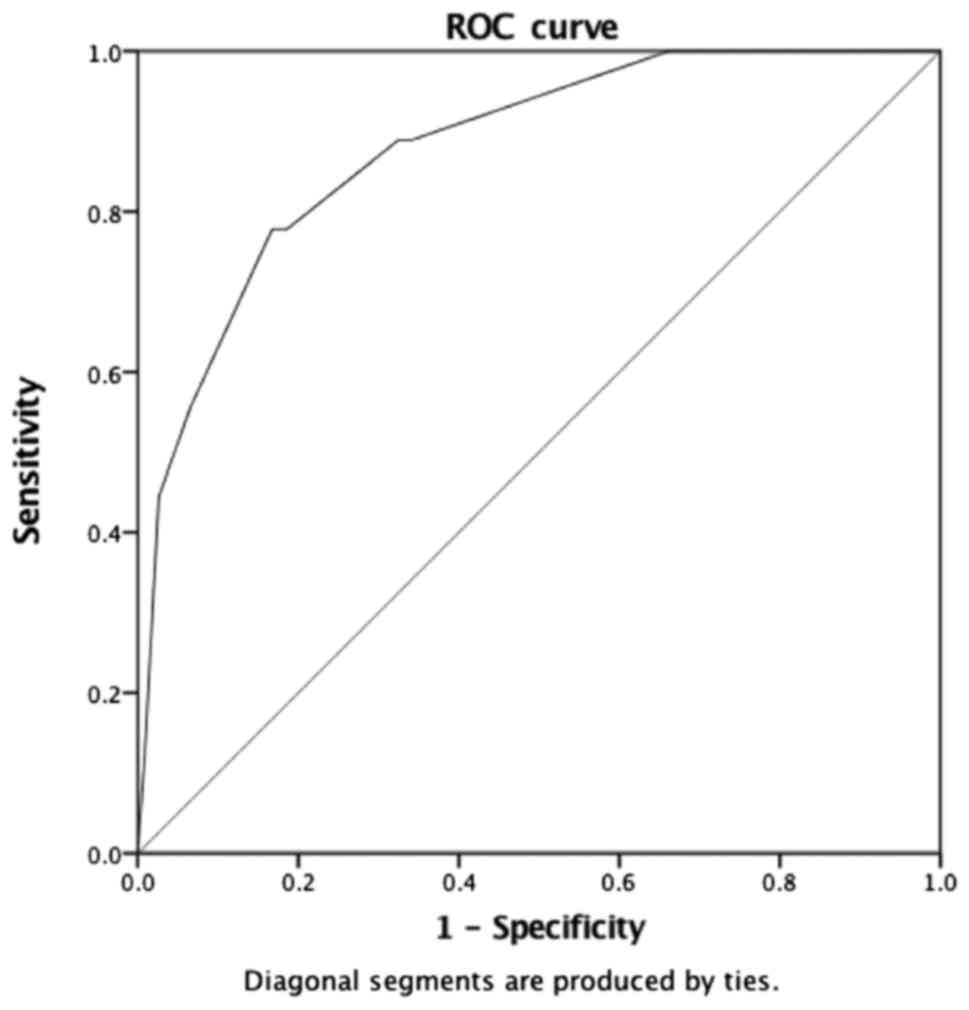
difference was not statistically significant. Using the area under
the curve (AUC) of ROC, an angle of 60° was determined as a
threshold value with an 80% sensitivity and specificity in
affirming arterial graft occlusion (Fig.
4). Logistic regression identified an occlusion OR of 5.149 for
arterial grafts in case of distal anastomosis angle of
60° or greater (P<0.001) (Table VIII).
 | Table VIII.Prognostic value of a distal
anastomosis angle ≥60°. |
Table VIII.
Prognostic value of a distal
anastomosis angle ≥60°.
|
|
|
|
|
|
| 95% CI for
EXP(B) |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| B | SE | Wald | Df | Sig. | Exp(B) | Lower | Upper | Occluded
anastomoses |
|---|
| Angle |
|
|
|
|
|
|
|
|
|
1.639 | 0.444 | 13.630 | 1 | <0.01 | 5.149 | 2.157 | 12.292 | 14 (8.38%) for
<60° |
| Constant |
|
|
|
|
|
|
|
|
|
−2.367 | 0.302 | 61.476 | 1 | <0.01 | 0.094 |
|
| 6 (17.14%) for
≥60° |
Discussion
Over the decades, graft patency has been assessed
for individual grafts alone and not for CABG as an entity per
se, while the dilemma of designing an optimal grafting
technique bringing considerable haemodynamic improvement and graft
patency remained unsolved despite the increase in worldwide
research. A literature review allowed us to classify factors
influencing graft patency into morphological (vessel type, graft
length, and calibre) (11,15), pathophysiological (competitive flow
through the native coronary artery, graft degenerative changes)
(15), and surgical (technical
expertise, graft harvesting and preparation, grafting design, and
anastomosis technique) categories (16,17).
This single-centre study analysed graft patency according to
surgical technique, namely proximal and distal anastomosis type and
angle.
Proximal anastomosis
We found a higher patency rate for RITA used as a
composite graft for the anterolateral or CX territory (78.38%)
compared with in situ RITA graft for the RCA territory
(62.5%) and for RA anastomosed to the ascending aorta (85%)
compared with composite grafting with LITA (61.54%).
A number of studies (18–22)
analysed the patency of in situ and free pedicled ITAs. Dion
et al prefer an in situ pedicled ITA each time the
local anatomy, the topography, and number of lesions allow it, the
physiological response being predictable in terms of flow and
calibre according to their study (23). They evaluated ITA grafts irrespective
of type and target coronary territory at 7.5 years from surgery and
found a patency rate of 96.3% for in situ ITAs compared with
86.5% for free grafts anastomosed to the ascending aorta or used
for Y/T anastomosis.
Fukui, Calafiore, and Tatoulis consider, based on
angiographic results obtained at 12 months, 17.5 months, and 41,5
months postoperatively, that there are no differences in patency
rates and prognosis between free ITAs with composite anastomosis
and in situ ITAs on one hand and between pedicled and
skeletonised grafts on the other hand (19–21). In
our study, in situ RITA was used exclusively for the RCA
territory and free ITAs as composite grafts for the anterolateral
or CX territory, thus introducing another variable in the equation,
the coronary territory. In 2012, Mukherjee et al performed a
meta-analysis on 226 articles to compare the outcome of RITA and
SVG used to revascularise the right coronary territory and
concluded that SVG may offer a superior patency rates when used to
revascularise RCA compared with RITA (23). The poor outcome of RITA grafted to
the right coronary territory could be explained by graft stretching
in cases of left ventricular dilation, different haemodynamic
conditions, and runoff.
In our group, RA was used as a free graft with a
higher patency rate when anastomosed to the ascending aorta vs.
composite grafting. Similar results were previously reported in
2009 by Jung et al, who analysed 893 patients that benefited
from RA grafts, 451 with direct RA to aortic anastomosis (group I)
and 422 with RA composite grafting with the LITA (group II). The
patency rate was significantly higher in group I than in group II
in the early postoperative period (98.3% vs. 94.5%), at 1 year
(93.8 vs. 90.5%), 2 years (90.5% vs. 85.3%), and 5 years (74.3% vs.
65.2%). Jung et al attribute the difference to a higher
drive pressure and flow afforded by the direct aortic connection,
thus preventing occlusion (24).
In case of composite grafting, the long-term patency
was conditioned by the anastomosis angle, with smaller values in
cases of patent free graft (47.21°) compared with occluded free
graft (56°). No in vivo study analysing Y anastomosis angle
was identified in the literature. Experimental research performed
by Song et al using three-dimensional simulation of a Y
anastomosis by computational fluid dynamics support our results by
demonstrating that the more acute is the angle of anastomosis, the
smaller are the energy loss and the back flow into the graft
(25).
Distal anastomosis
Compared to venous grafts, arterial grafts also
proved sensitive to distal anastomosis angle, with a mean value of
39.46° for patent grafts vs. 44.94° for occluded ones and a 5.149
occlusion OR in cases of distal anastomosis angle 60° or greater
for grafts with single end-to-side anastomosis. The first efforts
to imagine an optimal distal anastomosis technique aimed to improve
local haemodynamics by changing the anastomosis geometry, as tissue
remodelling is influenced by the end-to-side anastomosis angle, a
factor conditioning flow fields and wall shear stress (WSS)
(26). Staalsen et al
demonstrated using polyurethane grafts that no flow disturbances
were detected at the toe and one diameter downstream with an
anastomosis angle of 15°, and a zone of recirculation extending
from the toe to one diameter downstream was identified in the 45°
and 90° anastomoses. An anastomosis angle of 30° is generally
associated with a uniform flow and a smooth transition between
graft flow and target vessel flow (26).
Similar results were obtained for sequential
anastomosis, with an angle of 48.60° for patent side-to-side
anastomoses compared with 53.97° for occluded and 65.12° for patent
end-to-side anastomoses vs. 90.80° for occluded irrespective of
graft type. One can notice wider anastomoses angles for distal
end-to-side anastomoses of sequential grafts compared with grafts
with single distal anastomosis. The final anastomosis of
side-to-side grafts is usually performed with posterolateral
arteries on the diaphragmatic surface of the heart or with the
posterior descending artery, thus explaining a wider angle compared
with grafts with single distal anastomosis performed with diagonal
or marginal arteries. In cases of sequential anastomoses,
Frauenfelder et al (27) and
Fei et al (28) demonstrated
that wider anastomotic angles generate higher WSS values and flow
oscillation around the toe and along the bed of the target vessel.
These studies consider the graft and target vessel as forming a
planar configuration, which is far from reality, but similar
results were obtained by Sherwin et al (29), who investigated the influence of
out-of-plane geometry and found that the anastomosis bed was most
affected by flow oscillation generated by wider anastomoses angles
yielding to intimal hyperplasia and long-term occlusion.
Bonert et al (30) compared the patency of side-to-side
with end-to-side anastomoses and concluded that the parallel form
of side-to-side anastomosis contributes to maintaining graft
patency in side-to-side anastomoses by diminishing the haemodynamic
risk and spatial gradients. In cases of end-to-side anastomosis,
the haemodynamic forces act perpendicular to the target vessel wall
and trigger important variations of WSS (1.5 Pa) and formation of
stagnation and flow separation zones (29). In our study, the limited number of
sequential anastomoses did not allow a statistically significant
comparison of side-to-side to end-to-side anastomoses, but venous
grafts proved to have a higher patency with side-to-side
anastomoses compared with arterial ones.
Although limited by the small sample and reduced
number of cases for each graft configuration, our study is the
first to quantify potential factors conditioning graft patency on
CCTA images, as there is a paucity of in vivo studies
examining morphological, pathophysiological, and surgical factors
that influence long-term graft patency. Morphological factors
(e.g., graft length, target vessel calibre, graft-to-host diameter
ratio, degree of stenosis) were analysed together with surgical
factors and will be the subject of a different article. Currently,
the majority of the research in the field is carried on in in
vitro or computerised models that cannot take into account all
possible graft configurations. Tremblay et al (31) performed the only study up to date
that uses CCTA to identify morphological and morphometrical factors
associated with a saphenous vein bridge (SVB) dysfunction. They
analysed SVB length, SVB segment lengths, SVB lumen sectional
areas, anastomostic angulations and intrinsic SVB angulations on a
group of 40 patients and concluded that CT could be used to
identify quantitative graft parameters associated with graft
dysfunction. Our study extends this idea and identifies
morphological and morphometrical factors associated with long-term
graft patency according to graft type, grafted territory,
anastomosis type and angle; factors that could lead to adjustment
of the surgical technique to increase long-term patency rates and
reduce the risk of major adverse cardiac events. Bias was
controlled as much as possible by analysing data according to the
aforementioned factors, all cases being operated by the same
surgical team. The current research is to be considered a pilot
study and will be further extended by systematic recall of patients
who received surgery more than 10 years ago for a specific analysis
focused on target vessels, especially the RCA.
In conclusion, surgical technique is an important
factor conditioning long-term graft patency. A small anastomosis
angle, both for proximal Y and distal anastomoses, is associated to
a higher long-term patency of the free graft. RA grafts registered
higher patency rates when anastomosed to the ascending aorta
compared with composite grafting with LITA, whereas in situ
RITA anastomosed to the right coronary territory is associated with
a lower patency rate compared with free RITA used to revascularise
the anterolateral or CX territory as part of composite
grafting.
Acknowledgements
Not applicable.
Funding
The scientific research was financed by the ‘Grigore
T. Popa’ University of Medicine and Pharmacy under the contract no.
29031/28.12.2016.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
GT was the primary surgeon who designed the study
and revised the manuscript. ROC evaluated and quantified graft
patency, performed the statistical analysis and participated in
writing the manuscript. DBI digitized patient data, contributed to
the study design, offered ethics counselling and assisted with
literature research. CF was a major contributor in writing the
manuscript, participated in collecting patient data, counselled
statistical analysis and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committees of
the ‘Prof. Dr. George I.M. Georgescu’ Cardiovascular Institute and
‘Grigore T. Popa’ University of Medicine and Pharmacy. All patients
provided written informed consent prior to their inclusion in the
study.
Patient consent for publication
Written informed consent for the publication of any
associated data and accompanying images was obtained from all
patients included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eurostat-Cardiovascular diseases
statistics. http://ec.europa.eu/eurostat/statistics-explained/index.php/Cardiovascular_diseases_statisticsAugust
31–2017
|
|
2
|
Eurostat-Surgical operations and
procedures statistics. http://ec.europa.eu/eurostat/statistics-explained/index.php/Surgical_operations_and_procedures_statisticsAugust
31–2017
|
|
3
|
Authors/Task Force members, . Windecker S,
Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm
C, Head SJ, et al: 2014 ESC/EACTS guidelines on myocardial
revascularization: The Task Force on Myocardial Revascularization
of the European Society of Cardiology (ESC) and the European
Association for Cardio-Thoracic Surgery (EACTS) Developed with the
special contribution of the European Association of Percutaneous
Cardiovascular Interventions (EAPCI). Eur Heart J. 35:2541–2619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coronary Revascularization Writing Group,
. Patel FR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA;
Technical Panel, ; Masoudi FA, Dehmer GJ, Patel MR, et al:
ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria
for coronary revascularization focused update: A report of the
American College of Cardiology Foundation Appropriate Use Criteria
Task Force, Society for Cardiovascular Angiography and
Interventions, Society of Thoracic Surgeons, American Association
for Thoracic Surgery, American Heart Association, American Society
of Nuclear Cardiology, and the Society of Cardiovascular Computed
Tomography. J Thorac Cardiovasc Surg. 143:780–803. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sabik JF III: Understanding saphenous vein
graft patency. Circulation. 124:273–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatoulis J, Buxton BF and Fuller JA:
Patencies of 2127 arterial to coronary conduits over 15 years. Ann
Thorac Surg. 77:93–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cameron AA, Green GE, Brogno DA and
Thornton J: Internal thoracic artery grafts: 20-year clinical
follow-up. J Am Coll Cardiol. 25:188–192. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taggart DP, D'Amico R and Altman DG:
Effect of arterial revascularisation on survival: A systematic
review of studies comparing bilateral and single internal mammary
arteries. Lancet. 358:870–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Georghiou GP, Vidne BA and Dunning J: Does
the radial artery provide better long-term patency than the
saphenous vein? Interact Cardiovasc Thorac Surg. 4:304–310. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petrovic I, Nezic D, Peric M, Milojevic P,
Djokic O, Kosevic D, Tasic N, Djukanovic B and Otasevic P: Radial
artery vs saphenous vein graft used as the second conduit for
surgical myocardial revascularization: Long-term clinical
follow-up. J Cardiothorac Surg. 10:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aydin S, Aydin S, Eren Nesimi M, Sahin I,
Yilmaz M, Kalayci M and Gungor O: The cardiovascular system and the
biochemistry of grafts used in heart surgery. Springerplus.
2:6122013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buxton BF and Hayward PA: The art of
arterial revascularization-total arterial revascularization in
patients with triple vessel coronary artery disease. Ann
Cardiothorac Surg. 2:543–551. 2013.PubMed/NCBI
|
|
13
|
Drouin A, Noiseux N, Chartrand-Lefebvre C,
Soulez G, Mansour S, Tremblay JA, Basile F, Prieto I and Stevens
LM: Composite versus conventional coronary artery bypass grafting
strategy for the anterolateral territory: Study protocol for a
randomized controlled trial. Trials. 14:2702013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohira S, Doi K, Okawa K, Dohi M, Yamamoto
T, Kawajiri H and Yaku H: Safety and efficacy of sequential left
internal thoracic artery grafting to left circumflex area. Ann
Thorac Surg. 102:766–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porto I, Gaudino M, De Maria GL, Di Vito
L, Vergallo R, Bruno P, Bonalumi G, Prati F, Bolognese L, Crea F
and Massetti M: Long-term morphofunctional remodeling of internal
thoracic artery grafts: A frequency-domain optical coherence
tomography study. Biomed Eng Online. 6:269–276. 2013.
|
|
16
|
Ghista DN and Kabinejadian F: Coronary
artery bypass grafting hemodynamics and anastomosis design: A
biomedical engineering review. Biomed Eng Online. 12:1292013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Xie B, Gu C, Gao M, Zhang F, Wang J,
Dai L and Yu Y: Distal end side-to-side anastomoses of sequential
vein graft to small target coronary arteries improve intraoperative
graft flow. BMC Cardiovasc Disord. 14:652014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dion R, Glineur D, Derouck D, Verhelst R,
Noirhomme P, El Khoury G, Degrave E and Hanet C: Long-term clinical
and angiographic follow-up of sequential internal thoracic artery
grafting. Eur J Cardiothorac Surg. 17:407–714. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calafiore AM, Contini M, Vitolla G, Di
Mauro M, Mazzei V, Teodori G and Di Giammarco G: Bilateral internal
thoracic artery grafting: Long-term clinical and angiographic
results of in situ versus Y grafts. J Thorac Cardiovasc Surg.
120:990–996. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tatoulis J, Buxton BF and Fuller JA:
Patencies of 2127 arterial to coronary conduits over 15 years. Ann
Thorac Surg. 77:93–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukui S, Fukuda H, Toda K, Yoshitatsu M,
Funatsu T, Masai T and Miyamoto Y: Remodeling of the radial artery
anastomosed to the internal thoracic artery as a composite straight
graft. J Thorac Cardiovasc Surg. 134:1136–1142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taggart DP: Current status of arterial
grafts for coronary artery bypass grafting. Ann Cardiothorac Surg.
2:427–430. 2013.PubMed/NCBI
|
|
23
|
Mukherjee D, Cheriyan J, Kourliouros A and
Athanasiou T: Does the right internal thoracic artery or saphenous
vein graft offer superior revascularization of the right coronary
artery? Interact Cardiovasc Thorac Surg. 15:244–247. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung SH, Song H, Choo SJ, Je HG, Chung CH,
Kang JW and Lee JW: Comparison of radial artery patency according
to proximal anastomosis site: Direct aorta to radial artery
anastomosis is superior to radial artery composite grafting. J
Thorac Cardiovasc Surg. 138:76–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song MH, Sato M and Ueda Y:
Three-dimensional simulation of coronary artery bypass grafting
with the use of computational fluid dynamics. Surg Today.
30:993–998. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Staalsen NH, Ulrich M, Winther J, Pedersen
EM, How T and Nygaard H: The anastomosis angle does change the flow
fields at vascular end-to-side anastomoses in vivo. J Vasc Surg.
21:460–471. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frauenfelder T, Boutsianis E, Schertler T,
Husmann L, Leschka S, Poulikakos D, Marincek B and Alkadhi H: Flow
and wall shear stress in end-to-side and side-to-side anastomosis
of venous coronary artery bypass grafts. Biomed Eng Online.
6:352007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fei DY, Thomas JD and Rittgers SE: The
effect of angle and flow rate upon hemodynamics in distal vascular
graft anastomoses: A numerical model study. J Biomech Eng.
116:331–336. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sherwin SJ, Doorly DJ, Franke P and Peiró
J: Unsteady near wall residence times and shear exposure in model
distal arterial bypass grafts. Biorheology. 39:365–371.
2002.PubMed/NCBI
|
|
30
|
Bonert M, Myers JG, Fremes S, Williams J
and Ethier CR: A numerical study of blood flow in coronary artery
bypass graft side-to-side anastomoses. Ann Biomed Eng. 30:599–611.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tremblay JA, Stevens LM, Chandonnet M,
Soulez G, Basile F, Prieto I, Noiseux N and Chartrand-Lefebvre C: A
morphometric 3D model of coronary artery bypass graft dysfunction
with multidetector computed tomography. Clin Imaging. 39:1006–1011.
2015. View Article : Google Scholar : PubMed/NCBI
|