Introduction
Acute pancreatitis (AP) is an acute abdominal
disease caused by pancreatic enzyme activation through a variety of
triggers, followed by a pancreatic local inflammatory response as
the major clinical feature. In an estimate of 10–20% of patients
with AP, the condition deteriorates rapidly and develops into
persistent organ failure, further evolving into severe acute
pancreatitis (SAP) (1,2). In the early phases of the disease, the
gastrointestinal tract is one of the major targets, with an
overwhelming release of inflammatory cytokines and exudation of
fluids, leading to a sharp decline of the effective circulatory
blood volume, thereby affecting the permeability of the colonic
mucosal epithelium and water metabolism. Clinical studies have
reported that the mortality rate of SAP within 14 days was as high
as 50% and that effective early intervention had a significant
impact on the prognosis of SAP (3,4). Chen
et al (5) demonstrated that
fluid resuscitation via the rectum improved organ function and
decreased the serum levels of inflammatory factors.
Aquaporins (AQPs) are integral membrane proteins
that belong to the major intrinsic protein family. AQPs constitute
channels in the cell membrane, which control the transportation of
water and restrict the movement of ions or other micromolecules in
and out of the cells (6,7). AQPs, including AQP-1, −3, −7, −8 and
−11, are abundantly expressed in the human colon tissue, and have a
vital role in digestive disturbances, including diarrhea and
constipation (8–11). A recent study (12) proved that the expression of AQPs in
colon tissues increased significantly in SAP, but in a diverse
manner. The aim of the present study was to investigate the
potential beneficial effect of fluid resuscitation via the rectum
in the early stages of SAP and the underlying mechanisms, including
the role of AQPs.
Materials and methods
Chemicals and reagents
Sodium taurocholate (purity, ≥97%) was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). PrimeScript RT
Master Mix and SYBR Premix Ex Taq were purchased from Takara Bio
Inc. (Otsu, Japan). Rabbit anti-rat
Na+-K+-ATPase (cat. no. SC-28800) was
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
Animals
Male Sprague Dawley (SD) rats (age, 8–10 weeks;
weight, 300±10 g) were purchased from the Shanghai Laboratory
Animal Center (Chinese Academy of Science, Shanghai, China). The
rats were provided rodent chow and tap water ad libitum for
at least 1 week and were allowed to acclimatize to the laboratory
environment. The rats were anesthetized via an intraperitoneal
injection of 3% pentobarbital sodium (50 mg/kg body weight), and an
additional injection of 1/5 of the initial dose was given every
hour throughout the entire experiment. Clinical signs, including
the mean arterial pressure (MAP), were continuously monitored with
a multi-channel physiological recorder (Powerlab 15T;
ADInstruments, Bella Vista, NSW, Australia). The rats were
sacrificed by CO2 inhalation in accordance with the
institutional criteria for euthanasia.
SAP model establishment and fluid
resuscitation
The SAP model was established as previously
described (5). The rats were fasted
overnight with free access to water prior to the experiment, and
were randomly divided into four groups as follows: The SHAM, no
fluid resuscitation (NFR), intravenous fluid resuscitation (IVFR)
and fluid resuscitation via the rectum (FRVR) groups. SAP was
induced by injection of 5% sterile sodium taurocholate (final dose,
0.1 ml/100 g) into the biliopancreatic duct of the SD rats, and the
MAP was continuously monitored via femoral artery catheterization
throughout the experiment. At 30 min after the induction of SAP,
the rats in the IVFR and FRVR groups were resuscitated with normal
saline at a rate of 4 ml/kg/h through the venous or the rectal
route (5), while the rats in the NFR
group were not administered any fluid therapy. In the rats of the
SHAM group, catheterization and laparotomy were performed, and the
duodenum and pancreas were flipped instead of inducing SAP. At 12 h
after the beginning of fluid resuscitation, the rats were
sacrificed by CO2 inhalation, except for model rats that
were sacrificed at 4, 8, 12 and 24 h for the determination of
Na+-K+-ATPase.
Detection of AQP-3, AQP-4 and AQP-8
mRNA levels by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis
Total RNA was extracted from colon tissues using
TRIzol reagent (Thermo Fisher Scientific, Inc.). Complementary DNA
was synthesized by RT according to the protocol of PrimeScript RT
Master Mix and then subjected to qPCR using β-actin as an internal
control. The primer sequences were as follows (5′-3′): AQP-3
forward, CCCCTTGTGATGCCTCTC and reverse, CCCTAGCTGGCAGAGTTC; AQP-4
forward, CAGAACCAAGGCGTAGACCG and reverse, TCCCTGGAAATGACTGAGAAA;
AQP-8 forward, GCCGATGTGTAGTATGGACCTA and reverse,
ACCCAATGAAGATGAAGAGAGC; β-actin forward, GCGCTCGTCGTCGACAACGG and
reverse, GTGTGGTGCCAAATCTTCTCC. The PCR was performed according to
the manufacturer's protocol using an Applied Biosystems 7500
Real-Time PCR System (Thermo Fisher Scientific, Inc.). The
thermocycling protocol was as follows: Denaturation for 30 sec at
95°C and 40 cycles of 5 sec at 95°C and 34 sec at 60°C. The
relative changes in gene expression were calculated as
2−ΔΔCq (13).
ELISA detection of
Na+-K+-ATPase
The concentration of
Na+-K+-ATPase was determined using ELISA kits
according to the manufacturer's protocol (cat. no. SC-28800; Santa
Cruz Biotechnology Inc.).
Sample staining
Histopathological examination was performed using
the hematoxylin and eosin (H&E) staining method. Fresh tissue
specimens were immersed in 4% paraformaldehyde for 12 h at room
temperature. Samples were dehydrated in room temperature through a
serial alcohol gradient (50%, 3×1 h; 70%, 1 h; 80%, 1 h; 90%, 1 h;
100%, 2×1 h), followed by immersing in xylene (2×20 min) at room
temperature and embedded in paraffin wax blocks (52–56°C; 3×2 h).
Paraffin-embedded tissue sections (4 µm) were prepared and dewaxed
in xylene (3× 10 min at room temperature), rehydrated through
decreasing concentrations of ethanol (100%, 2×10 min; 90%, 5 min;
80%, 5 min; 70%, 5 min), and washed with PBS. Samples were stained
using the H&E staining kit according to the manufacturer's
protocol (Sangon Biotech Co., Ltd., Shanghai, China) at room
temperature for 10 min and 30 sec, respectively. Samples were
observed under a light microscope (magnification, ×100).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean and compared using Student's t-tests or one-way analysis
of variance with Student-Newman-Keuls post hoc test. Pearson
correlation analysis was performed to assess the correlation
between the MAP and the mRNA levels of AQPs. P<0.05 was
considered to indicate a statistically significant difference.
Analysis was performed using SPSS version 19 (IBM Corp., Armonk,
NY, USA) and Prism (version 5.0; GraphPad Software, Inc., La Jolla,
CA, USA).
Results
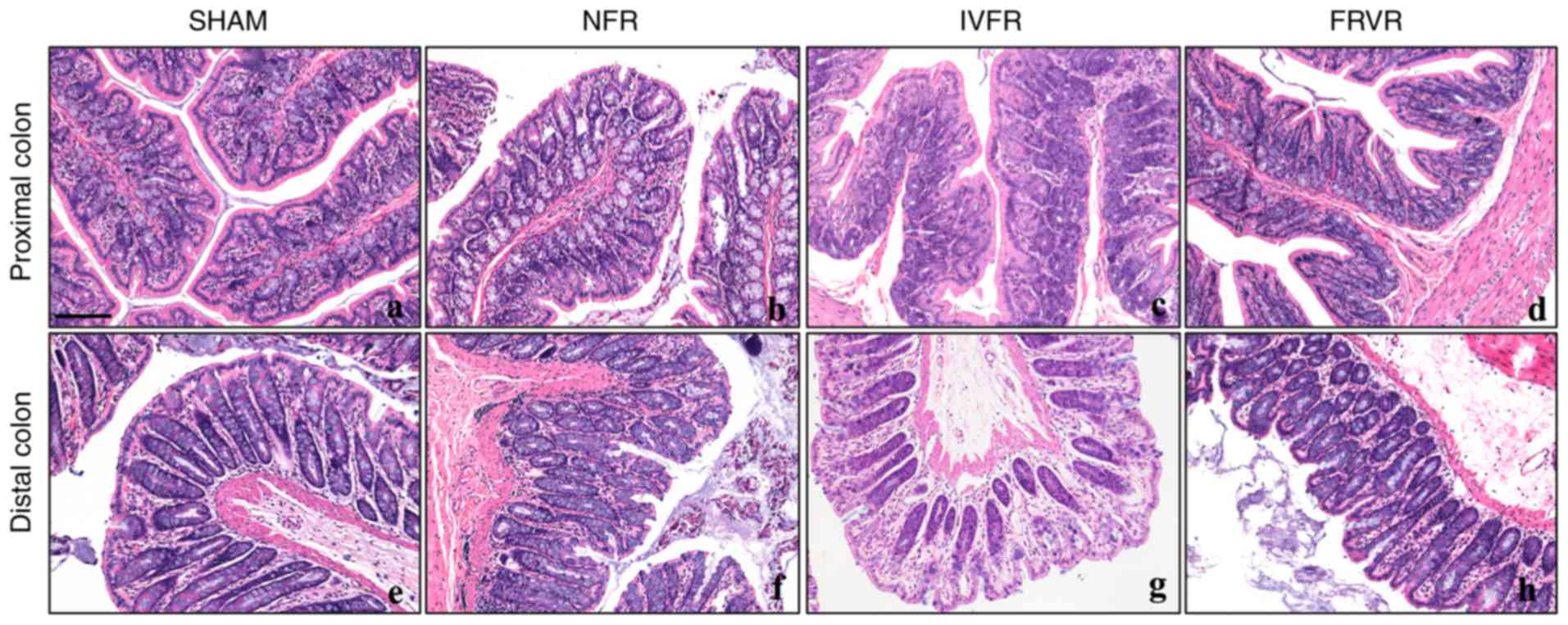
Pathological changes of the colon
After the establishment of SAP, the integrity of the
proximal colonic mucosa was continuously damaged and inflammatory
cell infiltration was observed in the submucosa. Compared with the
SHAM group, the structure of the epithelial cells was disturbed and
the colonic villi were destroyed in the NFR group (Fig. 1A and B). Following IVFR, the
condition of the lesions improved significantly (Fig. 1C), with only mild edema observed in
the proximal colon, whereas the inflammatory cell infiltration had
subsided. The pathological changes in the distal colon were almost
identical to those in the proximal colon (Fig. 1E, F). Similarly, between distal and
proximal colon, fluid resuscitation via the rectum also effectively
reduced the inflammatory response (Fig.
1D and H).
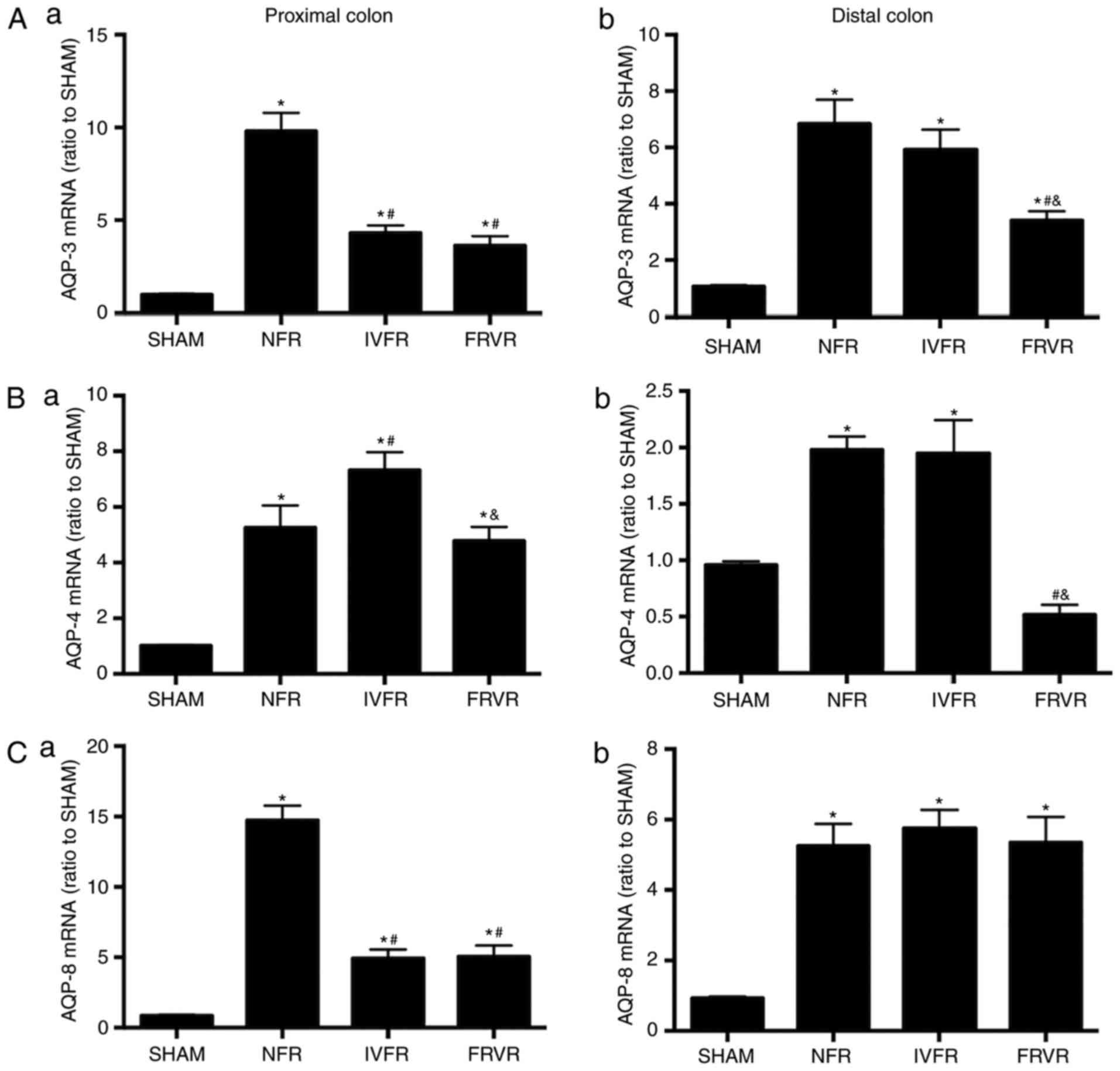
Changes in the mRNA levels of colonic
AQPs with fluid resuscitation in SAP
The expression of AQP-3, AQP-4 and AQP-8 mRNA in the
proximal and distal colon was significantly higher compared with
that in the SHAM group (Fig. 2). In
the proximal colon, the mRNA levels of AQP-3 and AQP-8 in the IVFR
and FRVR groups were significantly lower compared with those in the
NFR group, while they were still higher compared with those in the
SHAM group. Following fluid resuscitation, a different response was
observed regarding the changes in the levels of AQP-4. The
expression of AQP-4 mRNA in the IVFR group was higher compared with
that in the NFR group, but was decreased in the FRVR group.
The results obtained in the distal colon were
different from those in the proximal colon. Following fluid
resuscitation, although the expression of AQP-8 mRNA was higher
compared with that in the SHAM group, there was no significant
difference between the NFR group and the resuscitation groups.
Furthermore, FRVR effectively reduced the levels of AQP-3 and
AQP-4, while IVFR did not. Of note, the expression of AQP-4 mRNA
was lower compared with that in the SHAM group.
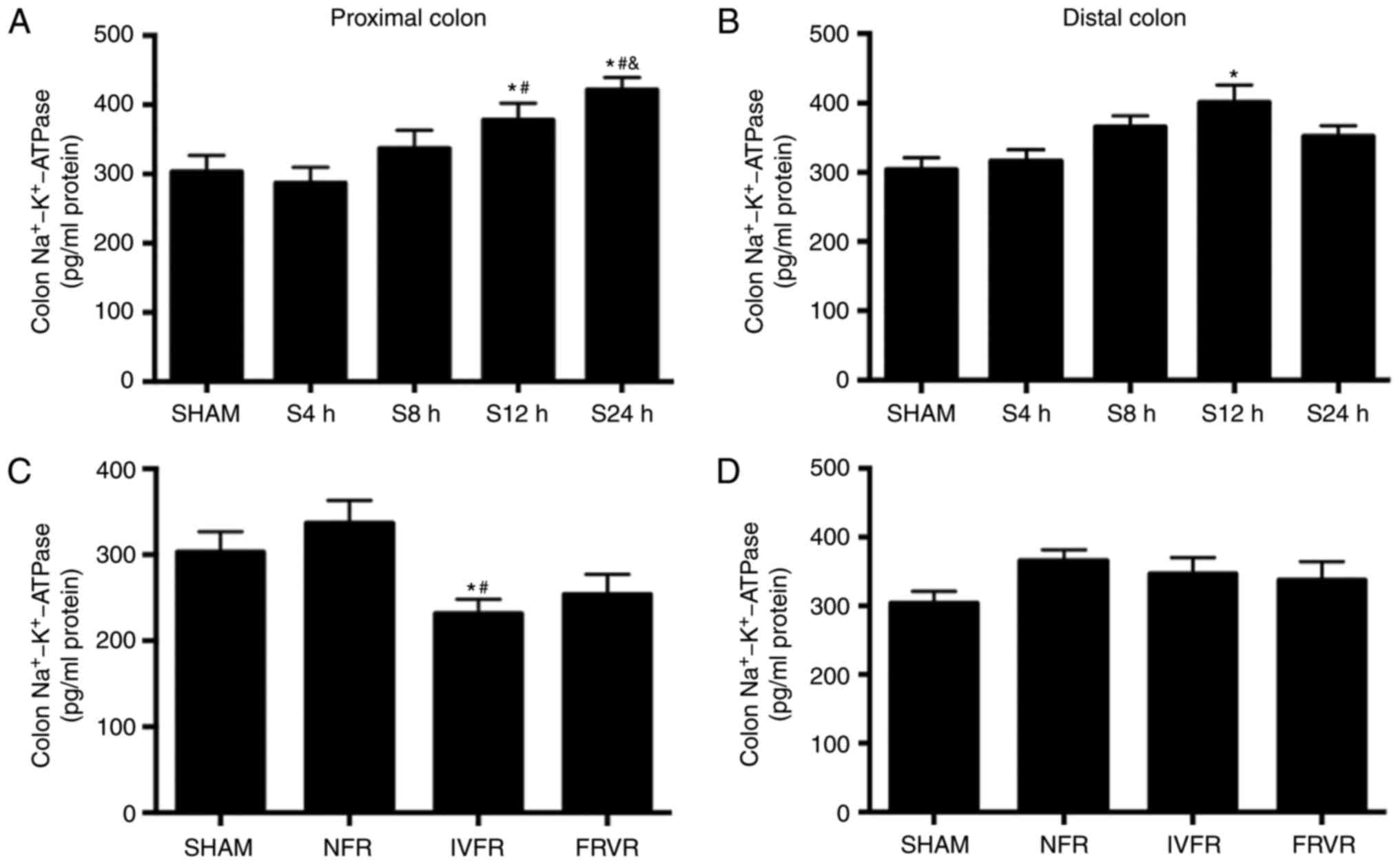
Changes in colon
Na+-K+-ATPase at the early stages of SAP
Na+-K+-ATPase is an important
ion channel on the cell membrane. It is used to transport sodium
and potassium ions through an inverse concentration gradient to
help the transmembrane diffusion of water. Thus, the expression of
Na+-K+-ATPase was investigated in the colon
tissues of SAP rats (Fig. 3). In the
SHAM group, the expression levels of
Na+-K+-ATPase in the proximal and distal
colon were similar. Furthermore, compared with the SHAM group, the
expression of Na+-K+-ATPase in the SAP model
maintained for 12 h (S12h group) was significantly higher
(P<0.05). In the S24h group, the results were different between
the proximal and distal colon: The proximal colon exhibited a
significant increase of Na+-K+-ATPase
compared with that in the S12h group, but a significant decrease in
the distal colon. Following fluid resuscitation, the
Na+-K+-ATPase expression in the proximal
colon of the IVFR group was significantly reduced compared with
that in the SHAM and NFR groups (P<0.05; Fig. 3C) while there is no difference
between the FRVR and NFR groups. However, no significant
differences were observed in the
Na+-K+-ATPase levels in the distal colon
among the four groups (P>0.05; Fig.
3D).
Correlation of MAP with colon AQP
expression at the early stages (12 h following onset) of SAP
(3/group)
A negative correlation was observed between AQP
expression and the MAP (Fig. 4). In
addition, AQP expression in the proximal colon was more
significantly correlated with the MAP compared with that in the
distal colon of SAP rats. In proximal colon tissues, the
correlation analysis between the mRNA levels of AQP-3, −4 and −8
and the MAP provided r values of −0.9124, −0.9335 and −0.9345,
respectively (Fig. 4A-C). In distal
colon tissues, the r values were −0.8228, −0.8847 and −0.8831,
respectively (Fig. 4D-F).
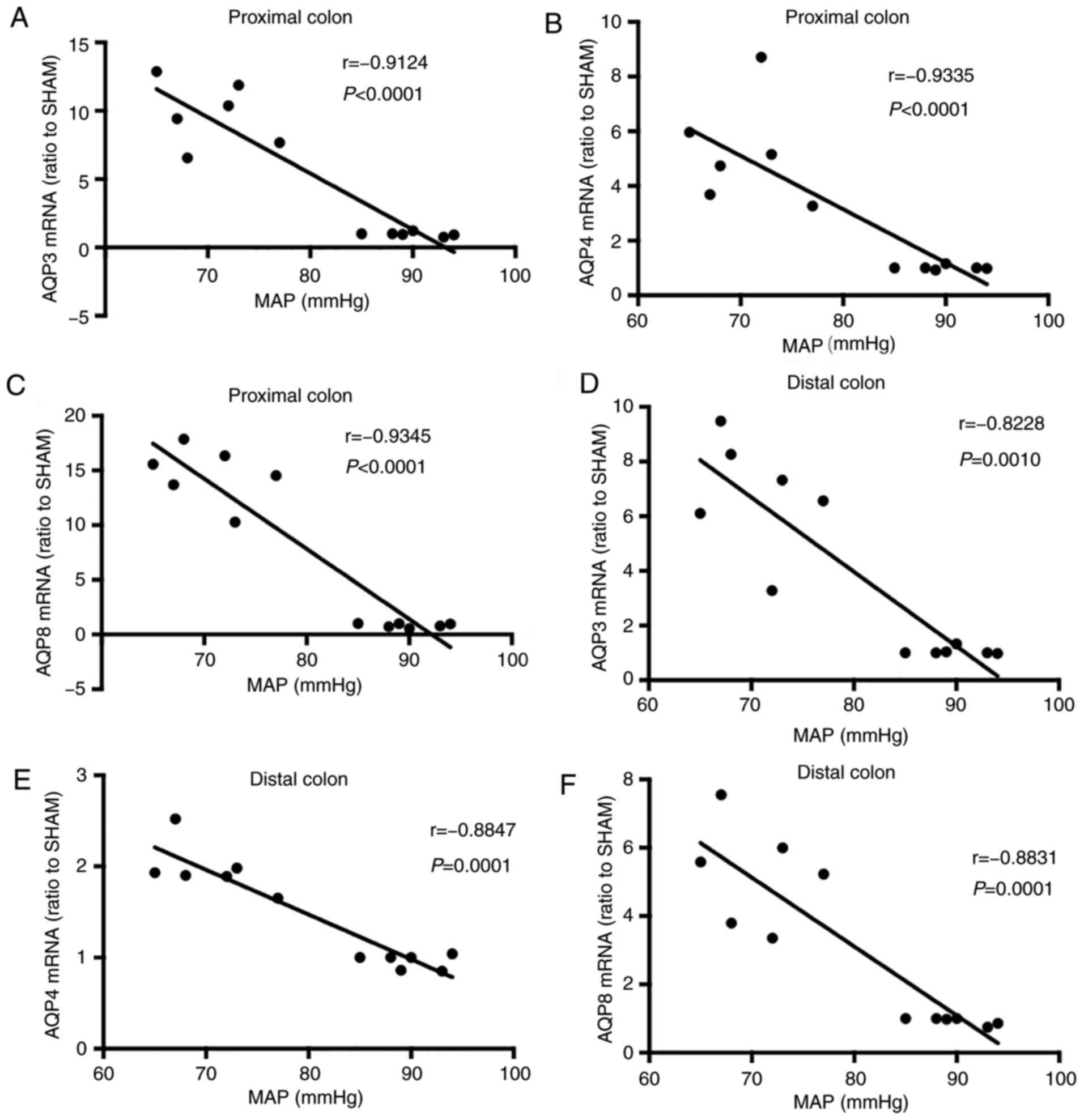 | Figure 4.Correlation of MAP with colonic AQP
expression at an early stage (12 h following onset) of severe acute
pancreatitis. MAP was continuously monitored via femoral artery
catheterization. In the proximal colon tissues, the r of (A) AQP3,
(B) AQP4 and (C) AQP8 was −0.9124, −0.9335 and −0.9345,
respectively (P<0.0001). In the distal colon tissues, the r of
(D) AQP3, (E) AQP4 and (F) AQP8 was −0.8228 (P=0.0010), −0.8847
(P=0.0001) and −0.8831 (P=0.0001), respectively. MAP, mean arterial
pressure; AQP, aquaporin. |
Discussion
SAP is an inflammation of the pancreas that is
associated with a significant amount of morbidity and mortality.
According to clinical experience, there are two mortality rate
peaks over the course of SAP, with most deaths occurring in the
early stages of the acute response, which is considered to be the
primary factor for the rapidly aggravated early systemic disease
leading to death. Hence, effective correction of systemic
circulation and microcirculation disorders shortens the time of
tissue hypoxia and control of sustained systemic inflammatory
response syndrome is key to the treatment of the acute response
(14,15). The most effective treatment is fluid
resuscitation, which is recognized as the primary measure in the
current guidelines (2,16). Chen et al (5), demonstrated that FRVR is a potential
supplementary method for fluid management at the early stages of
SAP. Following treatment with FRVR, the MAP and organ function
improved, suggesting that the colon may readily absorb a large
volume of water under conditions of shock. Hence, the aim of the
present study was to investigate the mechanisms underlying water
absorption through the colon during SAP.
AQPs are widely distributed in the cell membranes of
human tissues and visceral organs, and participate in almost all
pathophysiological processes of a variety of diseases (6,17). A
number of studies revealed that AQP-3, −4 and −8 are implicated in
water uptake and transport in the colon. AQPs may participate in
the adjustment of intestinal mucosal permeability and the rate of
water absorption. Abnormalities of AQPs are known to be associated
with Crohn's disease, ulcerative colitis, slow transit constipation
and cholera (18–24). A previous study (12) and the results of the present study
demonstrated that the expression of AQPs in colon tissues during
SAP increased significantly, but in a diverse manner, and a
negative correlation between AQP expression and the MAP was
determined. Thus, it may be hypothesized that a negative feedback
mechanism exists, which improves the intestinal water intake
ability in order to restore the blood volume. This may be the
mechanism through which FRVR exerts a therapeutic effect in
SAP.
Compared with the jejunum and ileum, AQP-3 is
abundantly expressed in the colon. Silberstein et al
(25), initially discovered that
AQP-3 was specifically expressed in epithelial microvilli of the
colon, indicating that it may be involved in water absorption. In
addition, suppression of AQP-3 function may lead to diarrhea by
increasing the fecal water content. Furthermore, the purgative
action of magnesium sulfate changes the osmotic pressure and
downregulates AQP-3 expression in mucosal epithelial cells of the
colon, resulting from activation of adenylate cyclase by increasing
the intracellular magnesium ion concentration (20,26). In
the present study, normal rat colon tissues expressed low levels of
AQP-3. With fluid resuscitation therapy, the level of colonic AQP-3
decreased in the proximal colon and distal colon.
A previous study indicated that AQP-4 is mainly
expressed in the ascending colon in humans (24). In rats, AQP-4 was reported to be
predominantly expressed in the epithelial cells of the proximal
colon (21,27). Wang et al (22), studied AQP-4 gene knockout mice and
identified that the expression of AQP-4 was positively correlated
with the water permeability of the colon. In addition, high
expression of AQP-4 accelerated water absorption through the colon,
which may be a key event in slow transit constipation. AQP-4 was
identified to be downregulated in a mouse model of allergic
diarrhea, which was explained by a dysfunction of colonic
absorption (21). In the present
study, following treatment with invasive fluid resuscitation, the
mRNA expression of AQP-4 was elevated; however, the expression was
significantly decreased in the FRVR group, particularly in the
distal colon.
AQP-8 is expressed in the colon and pancreas in
humans. In rats, this protein was identified to be abundantly
expressed in body parts including the duodenum, jejunum, rectum and
liver (28–31). In the present study, the AQP-8 mRNA
expression was altered not only in the IVFR group, but also in the
FRVR group: Similar to AQP-3 and AQP-4, the expression of AQP-8 was
increased in the NFR group. In the proximal colon, the expression
decreased significantly in IVFR group and FRVR group, as expected.
However, there was no significant change in distal colon following
the fluid resuscitation.
In the early stages of SAP, the expression of AQPs
was negatively correlated with the MAP, suggesting that, due to the
significant loss of body fluids, hemodynamic abnormalities may
increase the expression of colon AQPs. The upregulation of these
proteins may constitute a negative feedback mechanism, enhancing
the ability of the colon to absorb water in order to restore the
effective circulatory volume. In the present study, the expression
of AQP-3 and AQP-4 in the distal colon was only downregulated
following rectal fluid resuscitation, whereas intravenous
rehydration exerted a less prominent or no effect, suggesting that
rectal administration of fluids to improve the colon hypoperfusion
and restore the blood volume reduces the expression of AQPs in the
distal colon. This result indicates that rectal fluid resuscitation
may achieve active regulation of water absorption, while during
invasive intravenous fluid resuscitation, the body only passively
accepts the water. No significant change in distal colon AQP-8 was
detected after fluid resuscitation, suggesting that AQP-8 exerted
no effect on the regulation of distal colonic water absorption.
Various biological mechanisms participate in water
absorption and transport. The present study also examined the
association between Na+-K+-ATPase and SAP
(pathological and recovery process).
Na+-K+-ATPase is a vital ion channel, which
facilitates the movement of water molecules across the cell
membrane. Na+-K+-ATPase uses ATP for the
intracellular and extracellular transport of sodium and potassium
ions against a concentration gradient. The expression of
Na+-K+-ATPase was analyzed in the present
study. In the proximal and distal colon of the SHAM group,
Na+-K+-ATPase was expressed at similar
levels. During the pathological progression of SAP, the
Na+-K+-ATPase increased with time, but fluid
resuscitation had little or no effect on its expression,
particularly in the distal colon except, for the IVFR group and the
proximal colon.
SAP is a systemic inflammatory disease originating
in the pancreas but involving multiple organs, and features a high
mortality rate and multiple complications. Conventional treatment
includes intravenous fluid therapy to improve the effective
circulatory blood volume. However, a recent study (5) by our group indicated that fluid
resuscitation via the rectum may decrease the occurrence of
complications and, therefore, significantly improve the prognosis
of affected patients. In the present study, it was demonstrated
that fluid resuscitation via the rectum effectively reduced the
inflammatory response in a rat model of SAP. Furthermore, the
underlying mechanism was identified to be associated with AQPs
rather than with Na+-K+-ATPase. This provides
convincing evidence for the efficacy of fluid resuscitation via the
rectum in the treatment of SAP and furthermore, AQPs may be a
potential target in SAP research.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81670581, 81671901
and 81600501).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RX, JW, YC and JF designed the research. JW, YC, YY,
MQ, SH and ZZ performed the research. RX, YC, ZY, HS, EM and EC
analyzed the data. RX and YC prepared the manuscript. All of the
authors listed have approved the final version of the manuscript
submitted for publication.
Ethical approval and consent to
participate
The Institutional Animal Care and Use Committee
(IACUC) of Ruijin Hospital affiliated to Shanghai Jiao Tong
University School of Medicine (Shanghai, China) approved the study
protocol and the animal surgical procedures. All experimental
procedures were performed according to the Guide for the Care and
Use of Laboratory Animals developed by the Ruijin Hospital
affiliated to Shanghai Jiao Tong University School of Medicine
(Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tenner S, Baillie J, DeWitt J and Vege SS:
American College of Gastroenterology: American college of
gastroenterology guideline: Management of acute pancreatitis. Am J
Gastroenterol. 108:1400–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carnovale A, Rabitti PG, Manes G, Esposito
P, Pacelli L and Uomo G: Mortality in acute pancreatitis: Is it an
early or a late event? JOP. 6:438–444. 2005.PubMed/NCBI
|
|
4
|
Jacob AO, Stewart P and Jacob O: Early
surgical intervention in severe acute pancreatitis: Central
Australian experience. ANZ J Surg. 86:805–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y, Ma L, Song X, Fei J, Chen E and
Mao E: Beneficial effects of fluid resuscitation via the rectum on
hemodynamic disorders and multiple organ injuries in an
experimental severe acute pancreatitis model. Pancreatology.
15:626–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agre P: The aquaporin water channels. Proc
Am Thorac Soc. 3:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King LS and Agre P: Pathophysiology of the
aquaporin water channels. Annu Rev Physiol. 58:619–648. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koyama N, Ishibashi K, Kuwahara M, Inase
N, Ichioka M, Sasaki S and Marumo F: Cloning and functional
expression of human aquaporin8 cDNA and analysis of its gene.
Genomics. 54:169–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hasegawa H, Lian SC, Finkbeiner WE and
Verkman AS: Extrarenal tissue distribution of CHIP28 water channels
by in situ hybridization and antibody staining. Am J Physiol.
266:C893–C903. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zahn A, Moehle C, Langmann T, Ehehalt R,
Autschbach F, Stremmel W and Schmitz G: Aquaporin-8 expression is
reduced in ileum and induced in colon of patients with ulcerative
colitis. World J Gastroenterol. 13:1687–1695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujikawa T, Itoh A, Fukunaga T, Satoh J,
Yasuoka T and Fujiyama Y: Alteration of aquaporin mRNA expression
after small bowel resection in the rat residual ileum and colon. J
Gastroenterol Hepatol. 18:803–808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Xie R and Wang J: Expression and
role of aquaporin in the colon of acute necrotizing pancreatitis
rats. Chin J Pancreatol. 17:162–167. 2017.(In Chinese).
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Q, Li A, Xia Q, Liu X, Tian B, Mai G,
Huang Z, Chen G, Tang W, Jin X, et al: The role of organ failure
and infection in necrotizing pancreatitis: A prospective study. Ann
Surg. 259:1201–1207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trikudanathan G, Navaneethan U and Vege
SS: Current controversies in fluid resuscitation in acute
pancreatitis: A systematic review. Pancreas. 41:827–834. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou ZG, Chen YD, Sun W and Chen Z:
Pancreatic microcirculatory impairment in experimental acute
pancreatitis in rats. World J Gastroenterol. 8:933–936. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agre P and Kozono D: Aquaporin water
channels: Molecular mechanisms for human diseases. FEBS Lett.
555:72–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng YF, Liu CF, Lai WF, Xiang Q, Li ZF,
Wang H and Lin N: The laxative effect of emodin is attributable to
increased aquaporin 3 expression in the colon of mice and HT-29
cells. Fitoterapia. 96:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kon R, Ikarashi N, Nagoya C, Takayama T,
Kusunoki Y, Ishii M, Ueda H, Ochiai W, Machida Y, Sugita K and
Sugiyama K: Rheinanthrone, a metabolite of sennoside A, triggers
macrophage activation to decrease aquaporin-3 expression in the
colon, causing the laxative effect of rhubarb extract. J
Ethnopharmacol. 152:190–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikarashi N: The elucidation of the
function and the expression control mechanism of aquaporin-3 in the
colon. Yakugaku Zasshi. 133:955–961. 2013.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamamoto T, Kuramoto H and Kadowaki M:
Downregulation in aquaporin 4 and aquaporin 8 expression of the
colon associated with the induction of allergic diarrhea in a mouse
model of food allergy. Life Sci. 81:115–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang KS, Ma T, Filiz F, Verkman AS and
Bastidas JA: Colon water transport in transgenic mice lacking
aquaporin-4 water channels. Am J Physiol Gastrointest Liver
Physiol. 279:G463–G470. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhi H and Yuan WT: Expression of aquaporin
3, 4, and 8 in colonic mucosa of rat models with slow transit
constipation. Zhonghua Wei Chang Wai Ke Za Zhi. 14:459–461.
2011.(In Chinese). PubMed/NCBI
|
|
24
|
Wang XJ, Yuan WT, Song JM and Zhang ZY:
Expression and significance of aquaporin 4 in the colonic mucosa of
patients with slow transit constipation. Zhonghua Wei Chang Wai Ke
Za Zhi. 13:445–447. 2010.(In Chinese). PubMed/NCBI
|
|
25
|
Silberstein C, Kierbel A, Amodeo G, Zotta
E, Bigi F, Berkowski D and Ibarra C: Functional characterization
and localization of AQP3 in the human colon. Braz J Med Biol Res.
32:1303–1313. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikarashi N, Kon R, Iizasa T, Suzuki N,
Hiruma R, Suenaga K, Toda T, Ishii M, Hoshino M, Ochiai W and
Sugiyama K: Inhibition of aquaporin-3 water channel in the colon
induces diarrhea. Biol Pharm Bull. 35:957–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang WS, Li F and Bao JQ: Regulatory
effect of anthraquinone derivatives from rhubarb on aquaporin 4
expression in colon of rats and in LoVo cell line. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 28:818–823. 2008.(In Chinese). PubMed/NCBI
|
|
28
|
Matsuzaki T, Tajika Y, Ablimit A, Aoki T,
Hagiwara H and Takata K: Aquaporins in the digestive system. Med
Electron Microsc. 37:71–80. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koyama Y, Yamamoto T, Tani T, Nihei K,
Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K and
Kihara I: Expression and localization of aquaporins in rat
gastrointestinal tract. Am J Physiol. 276:C621–C627. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoque AT, Yamano S, Liu X, Swaim WD,
Goldsmith CM, Delporte C and Baum BJ: Expression of the aquaporin 8
water channel in a rat salivary epithelial cell. J Cell Physiol.
191:336–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JQ, Zhang L, Tao XG, Wei L, Liu B,
Huang LL and Chen YG: Tetramethylpyrazine upregulates the aquaporin
8 expression of hepatocellular mitochondria in septic rats. J Surg
Res. 185:286–293. 2013. View Article : Google Scholar : PubMed/NCBI
|