Introduction
Vitiligo is a dermatogic disease (1), whose pathogenesis is currently only
slightly understood. Vitiligo is a melanocyte-specific damage
disease, and many scholars have speculated that the occurrence of
vitiligo is very likely to be caused by the autoimmune dysfunction
of patients (2,3). There is a variety of cytokines in the
body which can form a very complex immune regulatory network
through the mutual feedback and regulatory effect of synthesis and
secretion, and the mutual influence, regulatory effect and
biological effect of the expression of cytokine receptors (4,5).
Abdellatif et al (6) have
proposed that the proliferation activity of T cells in the
peripheral blood of patients with vitiligo is abnormal, and the T
cell-mediated normal melanocyte antigen response plays an important
role in the occurrence of vitiligo. In the present study, in order
to further investigate the pathogenesis of vitiligo, the levels of
soluble intercellular adhesion molecule-1 (sICAM-1) and
granulocyte-macrophage colony stimulating factor (GM-CSF) in the
skin tissue fluid and the levels of interleukin (IL)-6, IL-17 and
tumor necrosis factor-α (TNF-α) in the blood of patients with
vitiligo were detected, and their correlations with the course of
disease and skin lesion area of patients were explored.
Materials and methods
General data
A total of 120 patients diagnosed with vitiligo and
treated in Daqing Long Nan Hospital (Daqing, China) from March 2014
to March 2016 were selected, according to the diagnostic and
staging criteria of vitiligo (7,8),
including 66 male patients and 54 female patients, 15–72 years of
age, with the course of disease being 3 months to 15 years. Among
them, 60 patients of 14–70 years of age, with the course of disease
being 2 months to 14 years, were in the progressive stage; and 60
patients of 16–72 years of age, with the course of disease being 4
months to 16 years, were in the stable stage. The skin lesion areas
of all patients enrolled were recorded and presented as percentage.
Another 80 healthy volunteers receiving physical examination in
Daqing Long Nan Hospital during the same period were selected as
control group, including 44 males and 36 females, 18–70 years of
age. The age and sex of all subjects in vitiligo and control group
were comparable (P>0.05).
Exclusion criteria: patients with severe
cardiovascular or cerebrovascular diseases, liver or kidney
disease, infection, tumor or other autoimmune diseases, or patients
receiving any immunotherapy within 1 month before detection.
The study was approved by the Ethics Committee of
Daqing Long Nan Hospital and informed consents were signed by the
patients or the guardians.
Methods
Collection of skin tissue fluid
The blebs at white and non-white spots of patients
with vitiligo were sucked via negative pressure suction using a
negative pressure suction instrument (Jiangsu Keling Medical
Appliances Co., Ltd., Jiangsu, China). More visible blebs could be
seen after 2 h. The bleb fluid at white and non-white spots was
collected using a sterile disposable syringe, and it was stored in
a refrigerator at −20°C to be detected.
Collection of serum
A total of 5 ml fasting blood was collected into
anticoagulant tubes from vitiligo patients and healthy volunteers
receiving physical examination in the morning. The samples were
placed in the refrigerator at −4°C for 2 h. The serum of the two
groups was collected from the blood via centrifugation at 1,300 × g
for 5 min at 4°C, labeled and placed in the refrigerator at −20°C
in order to be detected.
Detection of sICAM-1 levels in the
skin tissue fluid via radioimmunoassay (RIA) kit
The RIA kit was purchased from Shanghai Kemin
Biotechnology Co., Ltd. (Shanghai, China). DFM-96 RIA γ counter was
used as the measuring instrument and the detection was performed
strictly according to the manufacturer's instructions of the RIA
kit.
Detection of serum IL-6, IL-17, TNF-α
and GM-CSF levels via enzyme-linked immunosorbent assay (ELISA)
kit
IL-6, IL-17 and TNF-α ELISA kits were purchased from
Shanghai Jingkang Biological Engineering Co., Ltd. (Shanghai,
China), and the GM-CSF ELISA kit was purchased from Shanghai Beinuo
Biotech Co., Ltd. (Shanghai, China). Bio-Rad 550 full-automatic
enzyme-labeling counter (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used as the measuring instrument, and the detection was
performed strictly according to the manufacturer's instructions of
the ELISA kit.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (IBM Corp., Armonk, NY, USA) was used for the
statistical analysis of the data. Measurement data are presented as
mean ± standard deviation. t-test was used for the intergroup
comparison. ANOVA was used for comparison between multiple groups
and the post hoc test was LSD test. The Pearson's correlation test
was utilized to analyze correlation. α=0.05 was used as the
inspection level.
Results
Expression of IL-6, IL-17 and TNF-α in
the blood of patients of both groups
The expression levels of IL-6, IL-17 and TNF-α in
the blood of patients in the healthy control group were
significantly lower than those in the vitiligo group, and the
differences were statistically significant (P<0.05) (Table I).
 | Table I.Expression of IL-6, IL-17 and TNF-α in
the blood of patients of both groups (mean ± standard
deviation). |
Table I.
Expression of IL-6, IL-17 and TNF-α in
the blood of patients of both groups (mean ± standard
deviation).
| Group | n | IL-6 (ng/l) | IL-17 (g/l) | TNF-α (µg/l) |
|---|
| Healthy control | 80 | 104.29±37.61 | 9.74±3.48 | 0.88±0.25 |
| Vitiligo | 120 |
188.47±58.42a |
14.29±4.31a |
2.04±0.73a |
IL-6, IL-17 and TNF-α in the blood of
patients with vitiligo in different stages
There were no significant differences in the
expression levels of IL-6, IL-17 and TNF-α in the blood of patients
of the healthy control group and the patients at the stable stage;
but the levels were significantly lower than those in the blood of
patients in progressive stage, and the differences were
statistically significant (P<0.05) (Table II).
 | Table II.Expression of IL-6, IL-17 and TNF-α in
the blood of patients with vitiligo at different stages (mean ±
standard deviation). |
Table II.
Expression of IL-6, IL-17 and TNF-α in
the blood of patients with vitiligo at different stages (mean ±
standard deviation).
| Group | n | IL-6 (ng/l) | IL-17 (g/l) | TNF-α (µg/l) |
|---|
| Healthy controls | 80 |
104.29±37.61a |
9.74±3.48a |
0.88±0.25a |
| Patients in
progressive stage | 60 | 238.42±55.29 | 17.46±4.99 | 2.52±0.96 |
| Patients in stable
stage | 60 |
112.49±40.05a |
10.61±4.16a |
1.16±0.44a |
Levels of sICAM-1 and GM-CSF in the
skin tissue fluid of patients with vitiligo
The levels of sICAM-1 and GM-CSF in the skin tissue
fluid at white spots of patients with vitiligo vulgaris were
significantly higher than those in the skin tissue fluid at
non-white spots, and the differences were statistically significant
(P<0.05). There were no significant changes in the levels of
sICAM-1 and GM-CSF in the skin tissue fluid at white and non-white
spots of patients with segmental vitiligo, and the differences were
not statistically significant (P>0.05) (Table III).
 | Table III.Levels of sICAM-1 and GM-CSF in the
skin tissue fluid of patients with vitiligo (mean ± standard
deviation). |
Table III.
Levels of sICAM-1 and GM-CSF in the
skin tissue fluid of patients with vitiligo (mean ± standard
deviation).
| Group | n | sICAM-1 (ng/ml) | GM-CSF (ng/ml) |
|---|
| Vitiligo
vulgaris | 88 |
|
|
| White
spots | 44 | 285.42±39.71 | 0.57±0.08 |
| Non-white
spots | 44 |
164.27±31.38a |
0.24±0.05a |
| Segmental
vitiligo | 32 |
|
|
| White
spots | 16 | 151.49±41.36 | 0.51±0.07 |
| Non-white
spots | 16 |
147.52±46.03b |
0.48±0.06b |
Correlation of sICAM-1 levels in the
skin tissue fluid with the levels of IL-6, IL-17 and TNF-α in the
blood
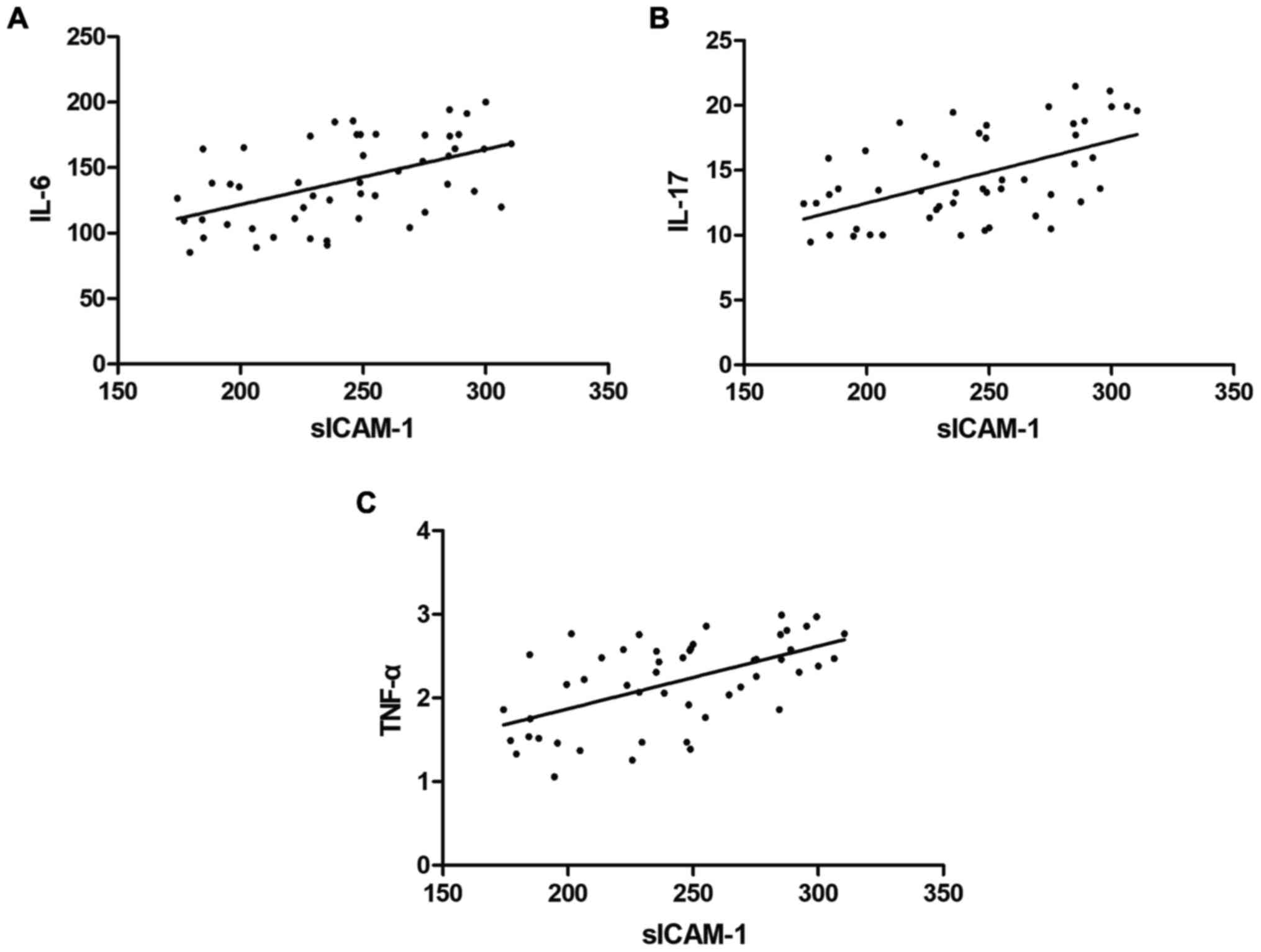
Pearson's correlation test showed that the sICAM-1
levels in the skin tissue fluid were significantly positive
correlated with the levels of IL-6, IL-17 and TNF-α in the blood
(correlation coefficient r=0.5141, 0.5423 and 0.5657, P<0.05)
(Fig. 1).
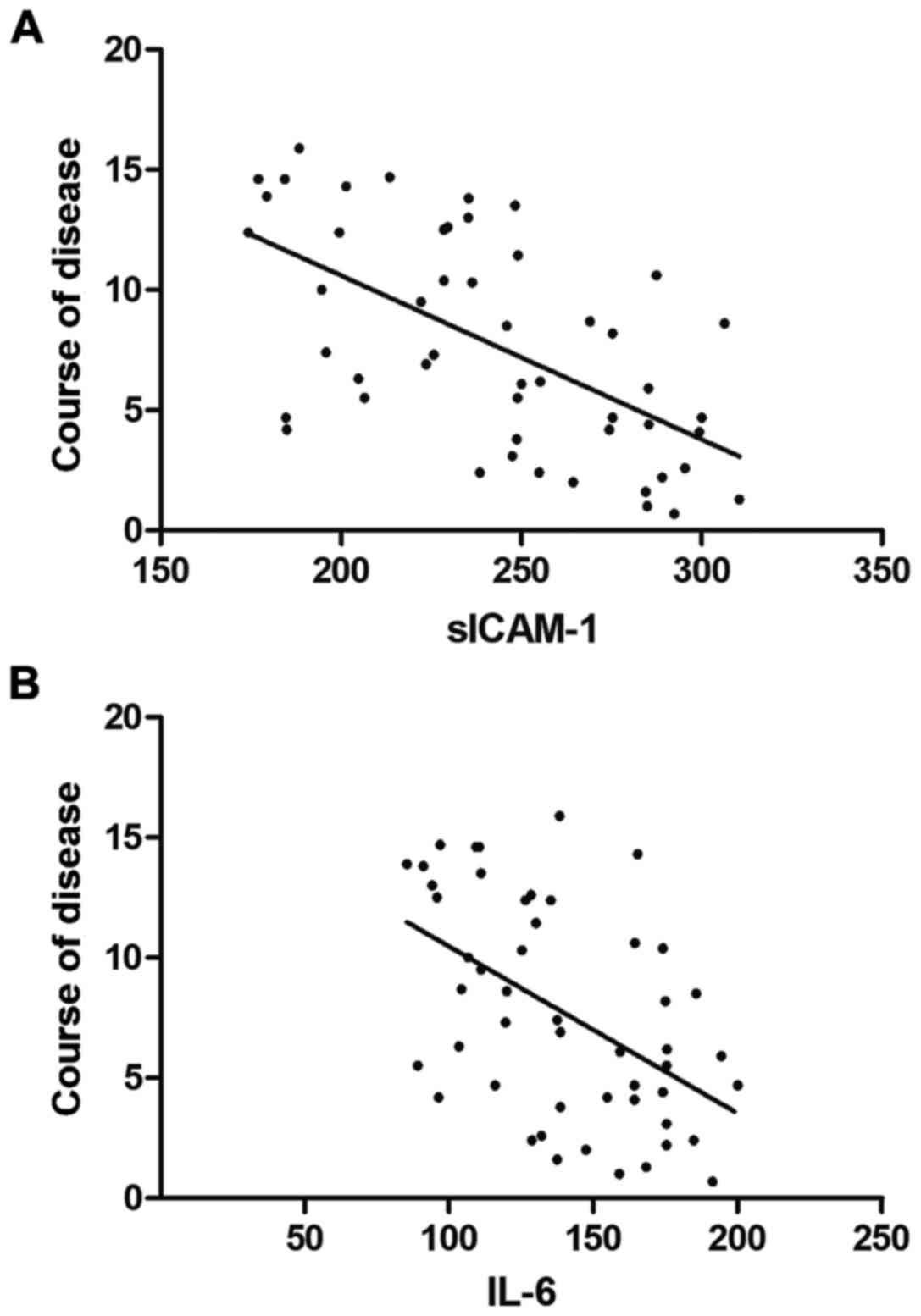
Correlation of sICAM-1 and IL-6 levels
with the course of disease of patients
Pearson's correlation test showed that the levels of
sICAM-1 in the skin tissue fluid and IL-6 in the blood of patients
with vitiligo were negatively correlated with the course of disease
(r=−0.5999 and −0.5013, P<0.05 and P=0.0002) (Fig. 2).
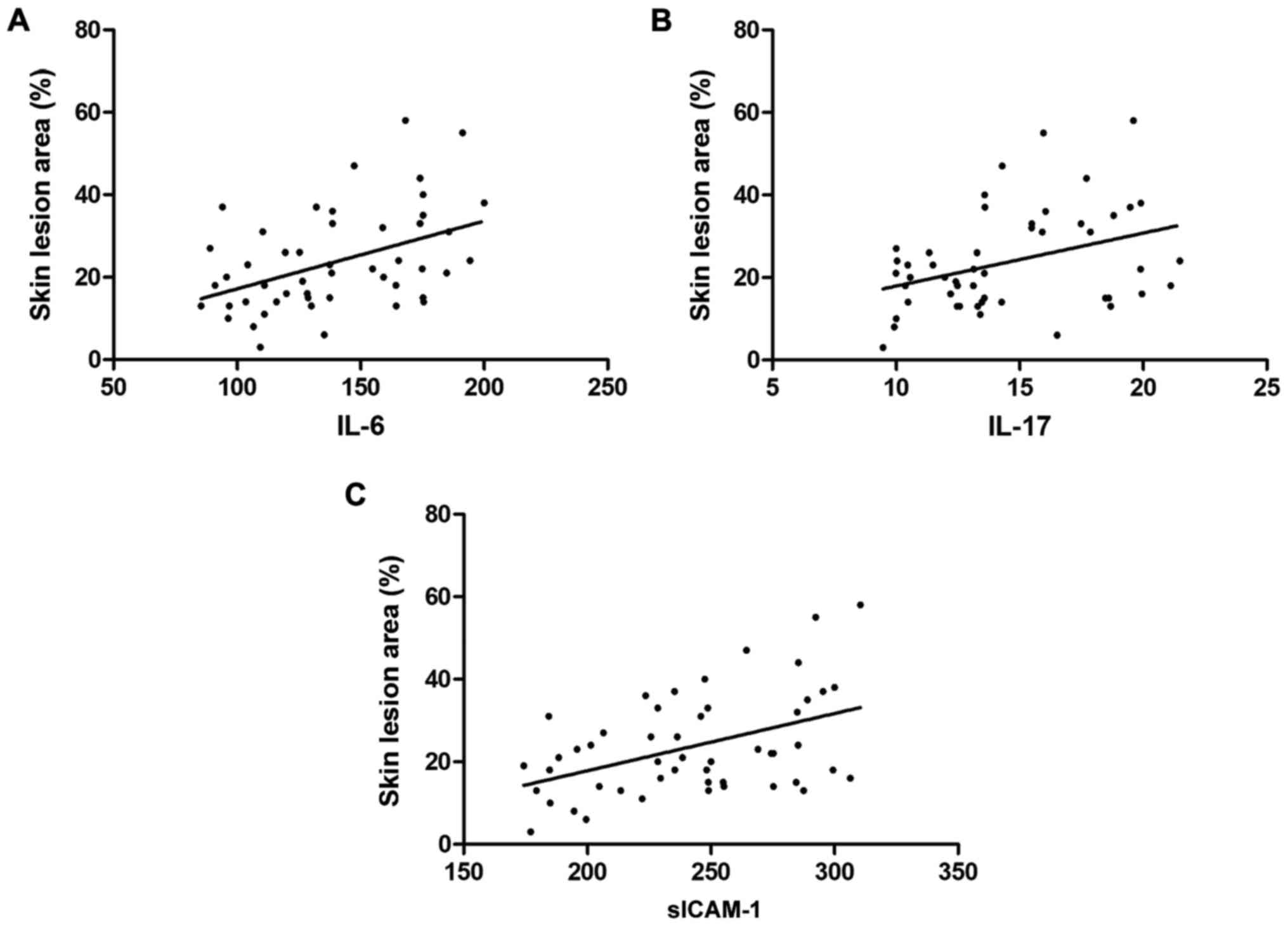
Correlation of the factor levels with
the skin lesion area of patients
Pearson's correlation test showed that the levels of
sICAM-1 in the skin tissue fluid and IL-6 and IL-17 in the blood of
patients with vitiligo were positively correlated with the skin
lesion area of patients (r=0.3772, 0.4353 and 0.3668, P=0.0069,
0.0016 and 0.0088) (Fig. 3).
Discussion
sICAM-1 is a shedding cycle mode of ICAM-1 which
mainly mediates the inflammatory response among cells, as a kind of
adhesion molecule (9). Aydıngöz
et al (10) have found that
there is a significant positive correlation between ICAM-1 and
sICAM-1. Camara-Lemarroy and Salas-Alanis (11) have shown that the expression level of
sICAM-1 in the blood of patients with progressive vitiligo is
higher, and it is speculated that sICAM-1 can be used as one of the
indexes of vitiligo activity. Farhan et al (12) have found that lymphocyte relocation
can be seen in the skin lesion, and this relocation process is
synchronized with the destruction of melanocytes. Further studies
have shown that ICAM-1 can promote the adhesion process in
vivo, leading to the destruction of melanocytes. IL-17 in
vivo can effectively promote the synthesis of interferon and
TNF, further inducing the ICAM-1 expression. In this study, the
levels of sICAM-1 in the skin tissue fluid at white spots of
patients with vitiligo vulgaris were found to be significantly
higher than that in the skin tissue fluid at non-white spots, but
there were no significant changes in the levels of sICAM-1 in the
skin tissue fluid at white and non-white spots of patients with
segmental vitiligo, suggesting that depigmentation of skin in
patients with vitiligo vulgaris is correlated with sICAM-1. The
levels of sICAM-1 showed significant positive correlations with the
levels of IL-6, IL-17 and TNF-α, so it is speculated that with the
increase of sICAM-1 level in the tissue fluid, the secretion of
IL-6, IL-17 and TNF-α is promoted via cell adhesion and receptor
binding, eventually leading to increased expression levels in the
blood.
GM-CSF, as the mitogen of melanocytes, can provide
endogenous promoters for the growth of melanocytes (13,14).
Zhou et al (15) have shown
via animal experiments that GM-CSF can induce autoimmune gastritis
in rats. However, there have been no reports on its correlation
with human vitiligo so far. In this study, the levels of GM-CSF in
the skin tissue fluid at white spots of patients with vitiligo
vulgaris were found to be significantly higher than that in the
skin tissue fluid at non-white spots, but there were no significant
changes in the levels of GM-CSF in the skin tissue fluid at white
and non-white spots of patients with segmental vitiligo, and the
levels of GM-CSF had no significant correlations with other factors
selected in this study.
IL-6, as a kind of stimulating factor, shows a
variety of biological functions in vivo (16). Miniati et al (17) have found that the expression of serum
IL-6 in patients with vitiligo is significantly higher than that in
normal subjects. It is speculated that the increase of IL-6 levels
may be due to the destruction of cellular network in patients with
vitiligo. When cells secrete IL-6 continuously, IL-6 can bind to
the receptor and further stimulate the proliferation of cells,
thereby leading to local skin damage. It was also found in this
study that the expression of serum IL-6 in patients with vitiligo
is increased, the expression level of sICAM-1 is significantly
related to IL-6, and the larger the skin lesion area of patients
is, the higher the expression of IL-6 will be. Moreover, the longer
the course of disease is, the lower the level of IL-6 will be,
presumably because the disease gradually becomes stable, and the
IL-6 level is decreased with the extension of the course of
disease.
IL-17 is mainly produced by T cells (18). In this study, it was found that the
expression of serum IL-17 in patients with vitiligo is increased,
and it is positively correlated with the skin lesion area. Some
scholars speculate that IL-17 in the body may change the local
microenvironment of patients, leading to melanocyte damage and
vitiligo. TNF-α is a kind of cytokine with dual effects, which can
be involved in the immune damage of the body (19,20). It
was found in this study that the level of serum TNF-α in patients
with vitiligo is increased, presumably because TNF-α causes immune
suppression in the body, so lymphocytes cannot exert normal immune
response.
In conclusion, the levels of sICAM-1 and GM-CSF in
the skin tissue fluid, and the expression of IL-6, IL-17 and TNF-α
in the blood of patients with vitiligo are abnormal, but there are
no significant correlations of GM-CSF with the expression of other
factors, and the expression of sICAM-1 and IL-6 are correlated with
the course of disease and skin lesion area of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY wrote the manuscript and collected the skin
tissue fluid. LY collected the serum. DH and LQ detected the
sICAM-1 levels. LL and YT performed ELISA. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Daqing Long Nan Hospital (Daqing, China) and informed consents were
signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baldini E, Odorisio T, Sorrenti S, Catania
A, Tartaglia F, Carbotta G, Pironi D, Rendina R, D'Armiento E,
Persechino S, et al: Vitiligo and autoimmune thyroid disorders.
Front Endocrinol (Lausanne). 8:2902017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ezzedine K and Eleftheriadou V: Vitiligo
and quality of life: The dark face of whiteness. Br J Dermatol.
178:28–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dwivedi M, Laddha NC, Shah K, Shah BJ and
Begum R: Involvement of interferon-gamma genetic variants and
intercellular adhesion molecule-1 in onset and progression of
generalized vitiligo. J Interferon Cytokine Res. 33:646–659. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XG, Hong WS and Xu A: GM-CSF: a
possible prognostic serum biomarker of vitiligo patients'
considered for transplantation treatment with cultured autologous
melanocytes: A pilot study. J Eur Acad Dermatol Venereol.
30:1409–1411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra S, De Sarkar S, Pradhan A, Pati AK,
Pradhan R, Mondal D, Sen S, Ghosh A, Chatterjee S and Chatterjee M:
Levels of oxidative damage and proinflammatory cytokines are
enhanced in patients with active vitiligo. Free Radic Res.
51:986–994. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdellatif AA, Zaki AM, Abdo HM, Aly DG,
Emara TA, El-Toukhy S, Emam HM and Abdelwahab MS: Assessment of
serum levels of granulocyte-macrophage colony-stimulating factor
(GM-CSF) among non-segmental vitiligo patients: A pilot study. Acta
Dermatovenerol Alp Pannonica Adriat. 24:43–45. 2015.PubMed/NCBI
|
|
7
|
Laddha NC, Dwivedi M, Gani AR, Mansuri MS
and Begum R: Tumor necrosis factor B (TNFB) genetic variants and
its increased expression are associated with vitiligo
susceptibility. PLoS One. 8:e817362013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kemp EH: Tumour necrosis factor-α
antagonists as therapies for vitiligo. Br J Dermatol. 173:6352015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Webb KC, Tung R, Winterfield LS, Gottlieb
AB, Eby JM, Henning SW and Le Poole IC: Tumour necrosis factor-α
inhibition can stabilize disease in progressive vitiligo. Br J
Dermatol. 173:641–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aydıngöz IE, Kanmaz-Özer M, Gedikbaşi A,
Vural P, Doğru-Abbasoğlu S and Uysal M: The combination of tumour
necrosis factor-α −308A and interleukin-10 −1082G gene
polymorphisms and increased serum levels of related cytokines:
Susceptibility to vitiligo. Clin Exp Dermatol. 40:71–77. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camara-Lemarroy CR and Salas-Alanis JC:
The role of tumor necrosis factor-α in the pathogenesis of
vitiligo. Am J Clin Dermatol. 14:343–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farhan J, Al-Shobaili HA, Zafar U, Al
Salloom A, Meki AR and Rasheed Z: Interleukin-6: A possible
inflammatory link between vitiligo and type 1 diabetes. Br J Biomed
Sci. 71:151–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manga P, Elbuluk N and Orlow SJ: Recent
advances in understanding vitiligo. F1000Res. 5:22342016.
View Article : Google Scholar
|
|
14
|
Bhardwaj S, Rani S, Srivastava N, Kumar R
and Parsad D: Increased systemic and epidermal levels of IL-17A and
IL-1β promotes progression of non-segmental vitiligo. Cytokine.
91:153–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou L, Shi YL, Li K, Hamzavi I, Gao TW,
Huggins RH, Lim HW and Mi QS: Increased circulating Th17 cells and
elevated serum levels of TGF-beta and IL-21 are correlated with
human non-segmental vitiligo development. Pigment Cell Melanoma
Res. 28:324–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Speeckaert R, Lambert J, Grine L, Van Gele
M, De Schepper S and van Geel N: The many faces of interleukin-17
in inflammatory skin diseases. Br J Dermatol. 175:892–901. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miniati A, Weng Z, Zhang B, Therianou A,
Vasiadi M, Nicolaidou E, Stratigos AJ, Antoniou C and Theoharides
TC: Stimulated human melanocytes express and release interleukin-8,
which is inhibited by luteolin: Relevance to early vitiligo. Clin
Exp Dermatol. 39:54–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elela MA, Hegazy RA, Fawzy MM, Rashed LA
and Rasheed H: Interleukin 17, interleukin 22 and FoxP3 expression
in tissue and serum of non-segmental vitiligo: A case- controlled
study on eighty-four patients. Eur J Dermatol. 23:350–355.
2013.PubMed/NCBI
|
|
19
|
Toussirot É and Aubin F: Paradoxical
reactions under TNF-α blocking agents and other biological agents
given for chronic immune-mediated diseases: An analytical and
comprehensive overview. RMD Open. 2:e0002392016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meurer M and Ceric-Dehdari P: Systemic
treatment of vitiligo: Balance and current developments. Hautarzt.
68:876–884. 2017.(In German). View Article : Google Scholar : PubMed/NCBI
|