Introduction
Kaposi's sarcoma (KS) is a vascular malignant tumor
type that is characterized by abnormal angiogenesis and
inflammation. KS usually occurs in the skin of the face and may
affect viscera, mucosa, the lungs and oral cavity (1). Xinjiang Autonomous Region in China is a
region with high morbidity of KS and acquired immune deficiency
syndrome (AIDS)-associated Kaposi's sarcoma (AIDS-KS) and the
disease is most commonly seen in the Uygur and Kazakh ethnic groups
(2). However, KS is rarely found in
other regions or ethnic groups in China. Toll-like receptor 4
(TLR4) is a recognition receptor of lipopolysaccharide that
participates in the recognition process of protein components in
viruses. It is mainly expressed in immune cells, such as
macrophages, T cells, B lymphocytes and endothelial cells.
Activation of TLR4 induces a series of inflammatory mediators,
including cytokines and chemokines, to produce a strong
inflammatory reaction. TLR4 has an important role in
anti-bacterial, inflammatory, anti-viral and stress processes. TLR4
recognizes pathogen-associated molecular patterns. It participates
in innate immune responses via the activation of cell signaling
pathways that lead to the transcription of pro-inflammatory
cytokine genes, such as interleukin (IL)-12, IL-6, tumor necrosis
factor-α and type I interferon (IFN). As a result, TLR4 is involved
in the pathogenesis of various viral infections. TLR4 also has
important roles in innate and adaptive immunity, such as
KS-associated herpesvirus (KSHV) infection (3). However, the role and regulation of TLRs
in innate immunity against AIDS-KS have remained elusive. The
present study investigated the expression of TLR4 and its signaling
proteins in classic KS and AIDS-KS.
Materials and methods
Patients
A total of 35 patients with KS were included in the
present study between May 2011 and July 2013, including 26 cases of
AIDS-KS and 9 cases of classic KS. All patients with KS were of
Uygur ethnicity. Another 10 Uygur healthy subjects were included in
the normal control group. The age and gender composition were not
significantly different (P>0.05; Table I). Inclusion ecriteria for AIDS-KS
were as follows: i) Uygur patients with HIV infection; ii)
purple-brown plaque above the surface of the skin occurring on the
face and verifiable by immunohistochemistry as KS; and iii) HIV
infection confirmed by western blot analysis by Xinjiang Uygur
Autonomous Region Center for Disease Control and Prevention.
Exclusion criteria were as follows: i) Patients who suffer from
sever skin diseases; ii) no definite diagnosis of KS; iii) patients
with a history of mental illness or other circumstances due to
which they did not cooperate with the experiment or sign the
informed consent form. All procedures were approved by the Ethics
Committee of Xinjiang Medical University. Written informed consent
was obtained from all patients or their families.
 | Table I.General data of included subjects. |
Table I.
General data of included subjects.
| Group | Cases (n) | Males n (%) | Age (years) | CD4+
T-lymphocyte count (n/µl) |
|---|
| AIDS-KS | 26 | 21 (80.8) | 43.3±11.6 | 197.5±80.9 |
| Classical KS | 9 | 7 (77.8) | 60.7±16.5 | 663.0±137.9 |
| Normal control | 10 | 6 (60.0) | 45.9±15.1 | 745.4±145.4 |
Tissues
KS tissues were obtained from the patients during
surgery, while skin tissues (0.6×0.3 cm) were obtained from healthy
subjects. After fixation with formaldehyde, the tissues were
paraffin-embedded, followed by preparation of 5-µm serial sections.
The tissues underwent hematoxylin and eosin staining and
immunohistochemical staining.
Hematoxylin and eosin staining
KS tissue samples were fixed, paraffin-embedded and
cut into 5-µm serial slices. The specimens were baked at 60°C over
night and then de-paraffinized with xylene and re-hydrated,
followed by washing under flowing water. Specimens were then placed
in Harris-acidified hematoxylin for 2 min, followed by a brief
rinse in water. Slides were placed in eosin solution for 30 sec,
followed by serial rinsing with ethanol and xylene, before
coverslips were applied. The stained specimens were examined under
a microscope (Olympus BX51, Tokyo, Japan).
Immunohistochemistry
Paraffin-embedded slices were de-waxed and
de-hydrated according to standard procedures. The slices were
treated with 3% H2O2-methanol at room
temperature to digest endogenous peroxidase. Bovine serum (10%;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used to block
non-specific antibody binding. After removing the serum, TLR4 mouse
anti-human monoclonal antibody (1:150 dilution; cat. no. sc-13593;
Santa Cruz Biotechnology, Inc.) was added, followed by incubation
at 4°C overnight. The samples were then incubated with horseradish
peroxidase-conjugated rabbit anti-mouse monoclonal second antibody
(1:1,000; cat. no. sc-516102; Santa Cruz Biotechnology, Inc.) at
37°C for 40 min. The sections were developed using diaminobenzidine
(Jinshan Reagent Company, Beijing, China) and counterstained using
hematoxylin, followed by dehydration and mounting.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from ground tissues using
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
mRNA was reversely transcribed to complementary (c)DNA using a
superscript III kit (Thermo Fisher Scientific, Inc.). First, RNA
(10 µl), Oligo-dT (1 µl) and random primer (1 µl) were mixed
together prior to incubation at 65°C for 5 min, followed by cooling
on ice for 2 min. Subsequently, 10 µM deoxynucleotide triphosphate
(1 µl), 0.1 M dithiothreitol (2 µl), 5× RT buffer (4 µl) and
reverse transcriptase (1 µl) were added (all from Takara
Biotechnology Co., Ltd., Dalian, China), followed by incubation at
50°C for 50 min in water bath. The synthesized cDNA was stored at
−20°C.
The qPCR assays were performed on an ABI 7500 FAST
Real-Time System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR reaction system contained cDNA (1 µl), 10X buffer (2
µl), MgCl2 (1 µl), SYBR PrimeScript RT-PCR kit assay mix
(1 µl), double distilled H2O (14.8 µl) and Taq
polymerase (0.2 µl) (SYBR PrimeScript RT-PCR kit; Takara
Biotechnology Co., Ltd.). PCR conditions were as follows: 95°C for
2 min, followed by 40 cycles of 94°C for 20 sec, 60°C for 20 sec
and 72°C for 30 sec, and a final elongation at 72°C for 10 min.
Primers were as follows: TLR4 forward, 5′-AAGCCGAAAGGTGATTGTTG-3′
and reverse, 5′-CTGAGCAGGGTCTTCTCCAC-3′; β-actin (reference gene)
forward, 5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse,
5′-GGGCACGAAGGCTCATCATT-3′. Quantitative evaluation was performed
using the ΔΔCq method (4).
Western blot analysis
Tissues were lysed for 30 min prior to
centrifugation at 1,204 × g and 4°C for 5 min. The protein
concentration in supernatant was determined using the bicinchoninic
acid method using a BCA kit (Biotek China, Beijing, China). Total
protein (10 µg per lane) was subjected to 10% SDS-PAGE at a
constant 80 V for 30 min followed by 120 V until the markers
(Takara Biotechnology Co., Ltd.) reached the edge of the gel. The
samples were electrotransferred onto a polyvinylidene difluoride
membrane (Takara Biotechnology Co., Ltd.) at constant 80 V at 4°C
for 2 h. The membrane was blocked with skimmed milk (1%) for 1 h at
room temperature. Mouse anti-human RAF, AKT, ERK, IκB-α, p105/P50,
RAS, P-AKT, FAK, MEK, p65 (1:1,000 dilution) and β-actin primary
antibodies (1:5,000 dilution; Santa Cruz Biotechnology, Dallas, TX,
USA) were added, followed by incubation with shaking overnight at
4°C. After rinsing with PBS containing Tween-20 (3×10 min), the
rabbit anti-mouse monoclonal secondary antibody (1:3,000; Santa
Cruz Biotechnology, Dallas, TX, USA) labeled with horseradish
peroxidase was added prior to incubation at room temperature for 2
h, followed by rinsing with PBS containing Tween 20 (3×10 min). The
immunoreactive bands were visualized by enhanced chemiluminescence
(ECL enhanced chemiluminescence kit; Takara Biotechnology Co.,
Ltd.).
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Values are expressed as the mean ± standard
deviation. Analysis of variance was used for comparisons among
multiple groups. Comparisons between samples were performed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
AIDS-KS is mainly distributed in the
face and limbs, while classic KS is mainly distributed in the
limbs
To assess the distribution of KS tissues on the
bodies of patients, the locations of KS tissues were determined.
AIDS-KS tissues were mainly distributed in the face (9 cases,
34.6%), upper limb (6 cases, 23.1%), lower limbs (7 cases, 26.9%),
back (2 cases, 7.7%) and oral mucosa (2 cases, 7.7%). Classic KS
tissues were mainly distributed in the limbs (7 cases, 77.8%) and
face (2 cases, 22.2%). The results indicated that AIDS-KS is mainly
distributed in the face and limbs, while classic KS is mainly
distributed in the limbs.
Histopathological characteristics of
AIDS-KS and classic KS tissues are different from those of normal
tissues
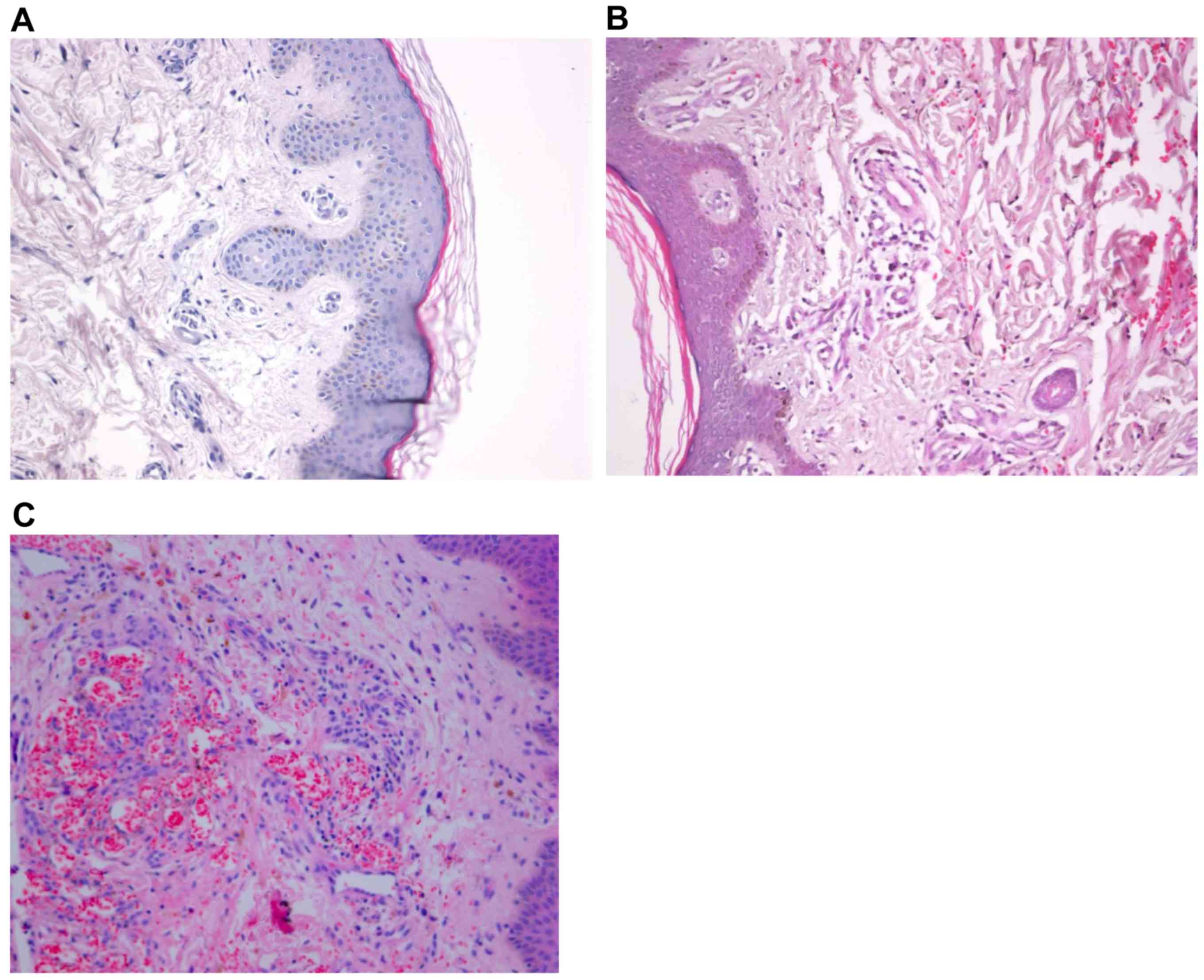
To investigate the histopathology of KS tissues,
hematoxylin and eosin staining was performed. Microscopy showed
extravasation of red cells in the dermis, endothelial hyperplasia,
tube obliteration, vascular labyrinth and vascular gap formation in
AIDS-KS and classic KS tissues (Fig. 1A
and B). In addition, large shuttle-type endothelial cells were
observed in the vascular wall and undifferentiated abnormal cells
with giant nuclei were observed around the blood vessels in AIDS-KS
and classic KS tissues (Fig. 1C and
D). These results suggested that the histopathological
characteristics of AIDS-KS and classic KS tissues are different
from those of normal tissues.
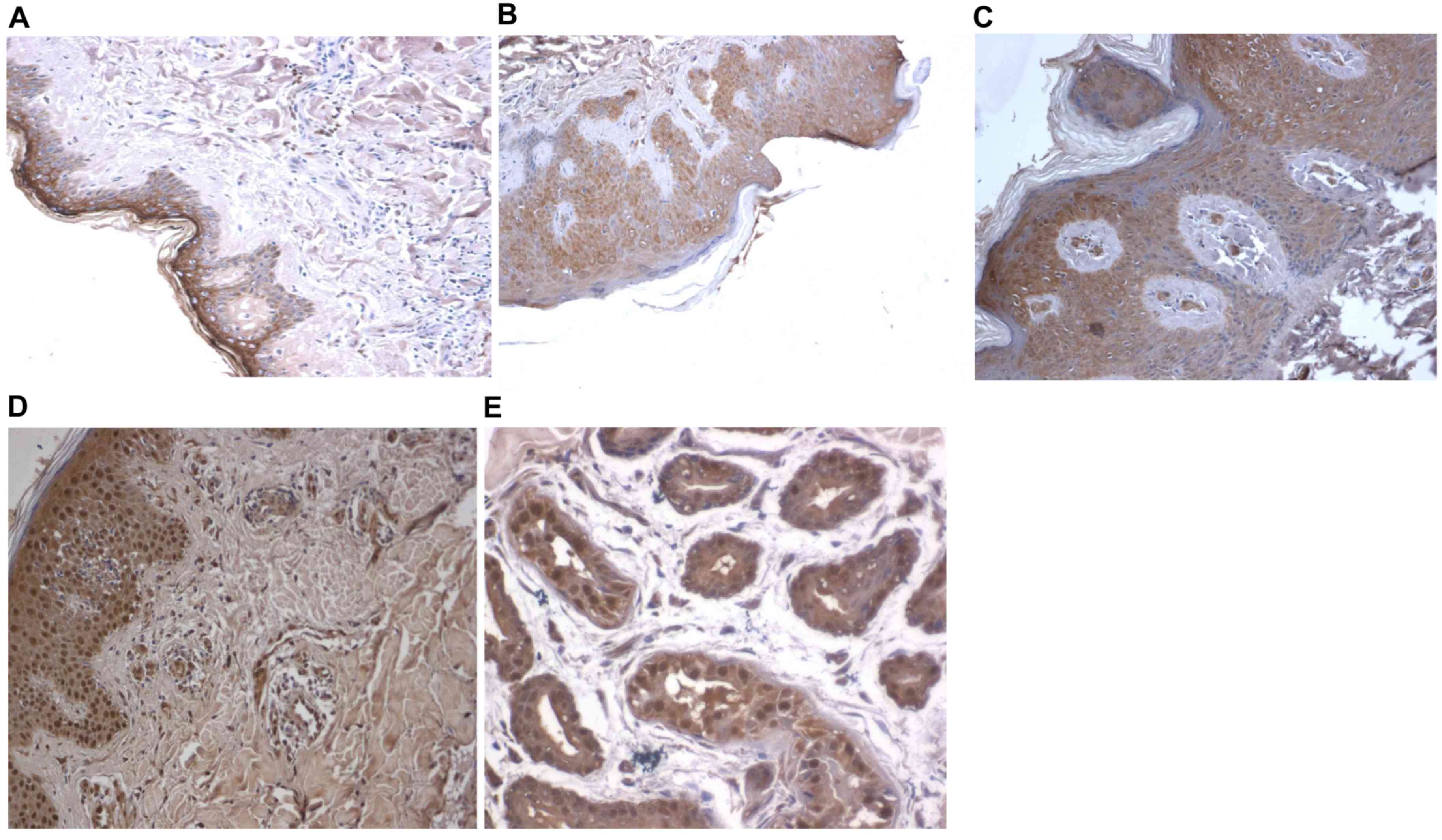
TLR4 is mainly distributed in the
dermis of KS tissues
To visualize the distribution of TLR4 in KS tissues,
immunohistochemical staining was used. TLR4-positive cells were
observed in the dermis of KS tissues. TLR4 expression was mainly
present in the cell membrane, cytoplasm and nuclei of endothelial
cells and abnormal tumor cells, with the levels of expression being
higher in the epidermis. The expression of TLR4 in gland ducts was
also high. However, expression of TLR4 in skin tissues of normal
subjects and AIDS patients was relatively low (Fig. 2). These results indicated that TLR4
is mainly distributed in the dermis of KS tissues.
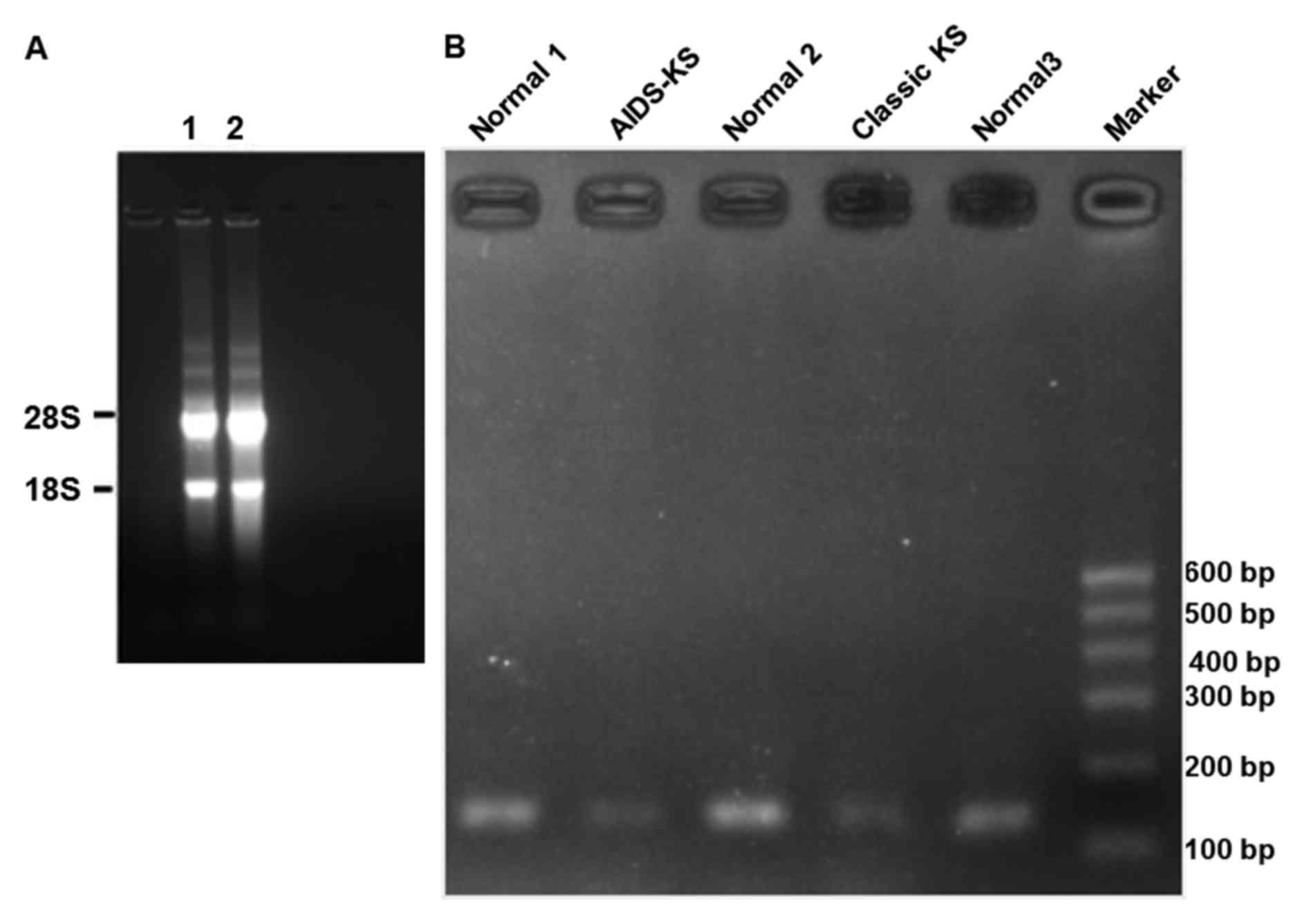
TLR4 mRNA is downregulated in classic
KS and AIDS-KS
To measure the expression of TLR4 mRNA in classic
KS, AIDS-KS and normal tissues, agarose gel electrophoresis and
RT-qPCR were performed. In the agarose gel, bands of 28S and 18S
were clearly visualized, while no obvious 5S band was observed
(Fig. 3A). In addition,
electrophoresis of PCR products of the TLR4 gene showed that target
bands (112 bp) of AIDS-KS and classic KS were thinner than those of
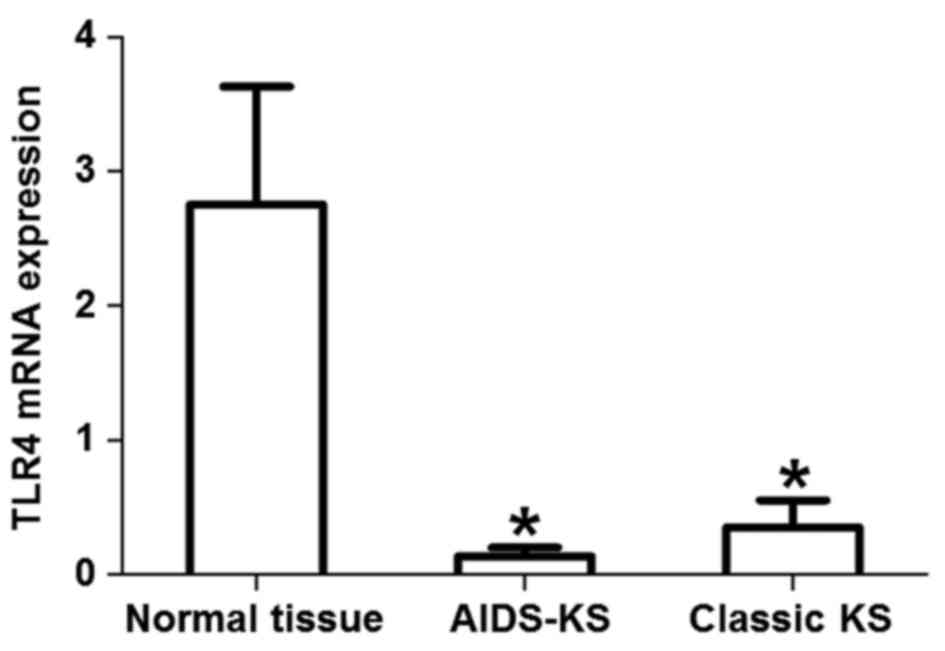
normal tissues (Fig. 3B). RT-qPCR
revealed that the expression of TLR4 mRNA in AIDS-KS tissues was
significantly lower than that in normal tissues (t=3.368,
P=0.0017). Similarly, the level of TLR4 mRNA in classic KS tissues
was significantly lower than that in normal tissues (t=2.076,
P=0.0497). However, the expression of TLR4 mRNA in classic KS was
not significantly different from that in AIDS-KS (t=1.230, P=0.46;
Fig. 4). These results suggested
that TLR4 mRNA expression levels were reduced in classic KS and
AIDS-KS.
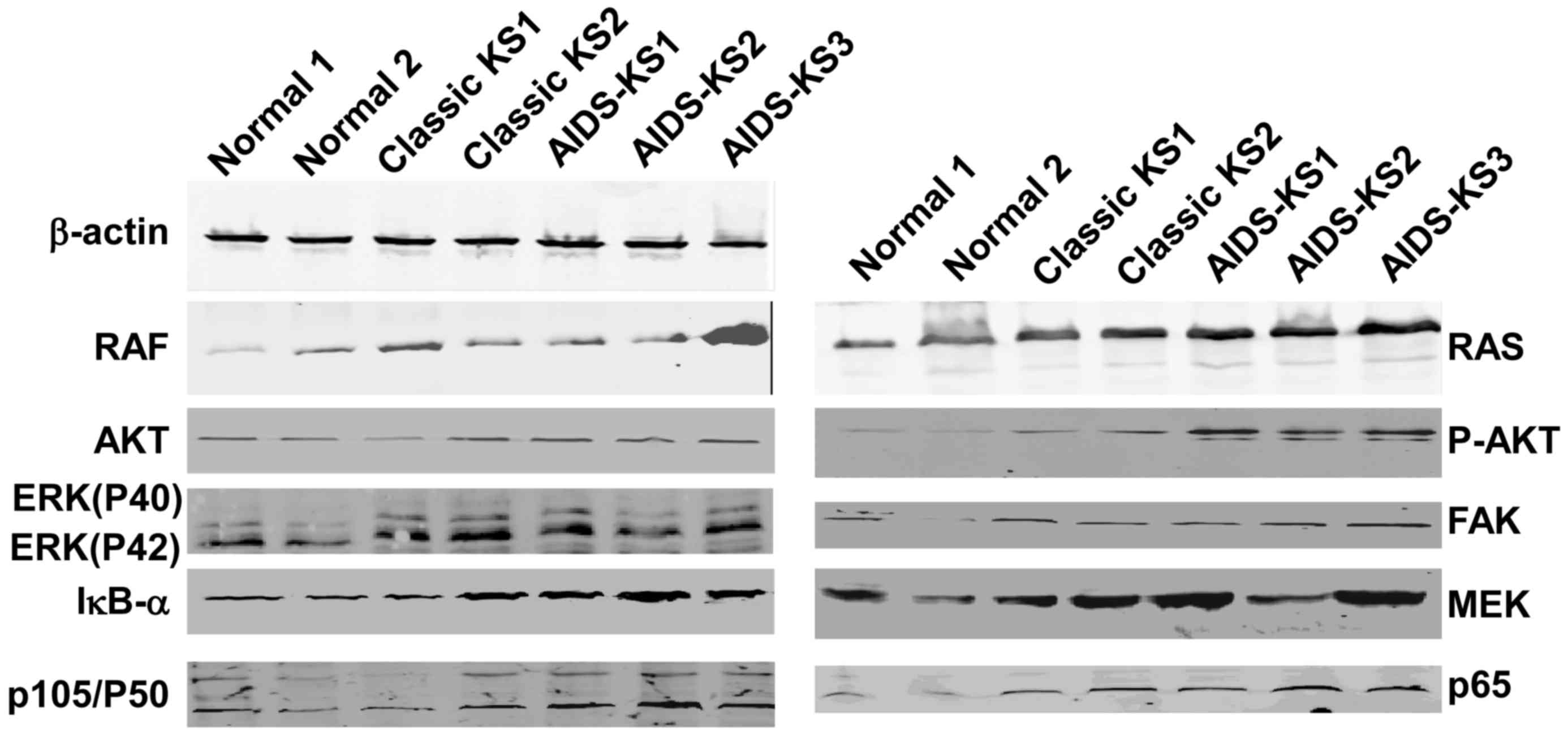
RAS, RAF, ERK, p-AKT, IκB-α, P50 and
P65 as proteins of the TLR4 signaling pathway in AIDS-KS and KS
tissues are higher than those in normal tissues
To determine the expression of proteins involved in
the TLR4 signaling pathway, western blot analysis was used. The
results demonstrated that the protein expression levels of RAS,
RAF, ERK (P40 and P42), inhibitor of nuclear factor (NF)-κB
(IκB)-α, P50 and P65 in classic KS tissues were significantly
higher than those in normal tissues (P<0.05; Fig. 5 and Table
II). Protein levels of RAS in AIDS-KS tissue were significantly
higher than that of normal tissues (P<0.05). Furthermore, ERK
protein expression (P40 and P42), P50 and P65 in classic KS tissues
were significantly higher than those in AIDS-KS tissues
(P<0.05). These results indicated that the expressions of RAS,
RAF, ERK (P40 and P42), IκB-α, P50 and P65 proteins of TLR4
signaling pathways in AIDS-KS and KS tissues was upregulated
compared to normal tissues. Additionally, results revealed
significant differences in ERK (P40 and P42), P50 and P65 protein
levels between AIDS-KS and classic KS tissues.
 | Table II.Expression of proteins involved in
Toll-like receptor 4 signaling pathway. |
Table II.
Expression of proteins involved in
Toll-like receptor 4 signaling pathway.
| Proteins | Normal tissues | Classic Kaposi's
sarcoma tissues | AIDS Kaposi's sarcoma
tissues |
|---|
| RAF | 0.54±0.08 | 1.69±
0.96a | 0.95±0.57 |
| RAS | 0.69±0.13 |
1.32±0.12a |
1.30±0.41a |
| AKT | 1.12±0.10 | 1.27±0.24 | 1.17±0.20 |
| P-AKT | 1.54±0.27 | 2.33±1.03 | 1.73±0.55 |
| P40 | 0.65±0.06 | 1.48±0.49 |
0.94±0.42b |
| P42 | 0.79±0.10 | 1.13±0.50 |
0.87±0.30b |
| FAK | 1.06±0.08 | 1.35±0.46 | 1.17±0.32 |
| IκB-α | 1.28±0.14 |
2.07±0.66a | 1.61±0.50 |
| MEK | 1.71±0.18 | 1.81±0.41 | 2.09±0.54 |
| P105 | 1.32±0.12 | 1.75±0.67 | 1.27±0.42 |
| P50 | 1.21±0.06 | 2.03±0.58 |
1.53±0.43b |
| P65 | 1.31±0.18 | 1.82±0.50 |
1.62±0.47b |
Discussion
KS may be clinically divided into four types: i)
Classic KS, ii) African KS, iii) AIDS-KS and iv)
immunosuppression-associated KS (5).
KS is an opportunistic tumor type in patients with advanced AIDS
(6). Human herpesvirus-8 (HHV-8)
represents the etiological basis of KS to cause KSHV (7). HHV-8 is detected in nearly all KS
tissues (8). If HHV-8 is detected in
AIDS patients, it is very likely that the patient will develop KS
within 4 years (9). The present
study found that AIDS-KS was distributed primarily in the face.
However, classic KS was mainly distributed in the limbs. In
addition, Uygur males accounted for the majority of patients with
KS. A large amount of TLR4-positive cells were observed in the
dermis of AIDS-KS patients, indicating that TLR4 expression is
associated with the occurrence and development of AIDS-KS. However,
in the present study, TLR4 expression was not measured at different
time-points of tumor growth in AIDS-KS patients. Therefore, the
association between TLR4 expression and AIDS-KS growth remains
elusive. At the early stage of AIDS-KS, TLR4 may possibly aid in
the resistance to HHV-8 infection and propagation, but may promote
the growth of KS as the disease develops (10–13).
Consistently, low TLR4 expression was also observed in the dermis
of classic KS tissues, suggesting that TLR4 participates in the
occurrence of AIDS-KS as well as classic KS. A previous study
showed that the infection rate of HHV-8 is high in classic KS of
Xinjiang Autonomous Region (14).
HHV-8 is the common cause of AIDS-KS and classic KS, explaining the
similarity of TLR4 expression in the two types of KS.
Consistent with the study by Lagos et al
(15), the present study showed that
TLR4 mRNA expression was significantly downregulated in classic KS
and AIDS-KS, suggesting that HHV-8 inhibits inflammatory pathways
after infection. Zhou et al (16) reported that TLR4 expression in
peripheral blood is upregulated in AIDS patients and that TLR4 mRNA
expression in peripheral blood mononuclear cells in untreated AIDS
patients is significantly increased. After using highly active
anti-retroviral therapy to inhibit the virus, the expression of
TLRs was reduced to a normal level, suggesting that human
immunodeficiency virus (HIV) directly affects the expression of
TLRs and the function of immune cells (17). Bosinger et al (18) reported that the expression of TLR4 in
the peripheral blood of Rhesus monkeys with acute 89.6P (HIV)
infection is reduced. In the acute phase of HIV infection, TLR4 is
downregulated. However, TLR4 expression is upregulated during the
development of AIDS, in which opportunistic infections induce local
or systemic inflammatory responses. KS occurs in the AIDS phase of
HIV infection, mainly on the back, prothorax, limbs, oral cavity
and nose (19,20). The pathology of AIDS-KS is similar to
that of classic KS, being characterized by abnormal angiogenesis
and spindle cell proliferation (21).
In the present study, expression of RAS, RAF, ERK
(P40/P42), IκB-α, P50 and P65 protein in classic KS was
significantly higher than that in normal tissues. Furthermore, RAS
levels were significantly higher in AIDS-KS tissues when compared
with normal tissues. The levels of ERK (P40/P42), P50 and P65
protein were also significantly different between AIDS-KS and
classic KS. The expression of other proteins involved in TLR4
signaling pathways in KS tissues was not significantly different
from that in normal tissues. RAS, RAF, ERK, focal adhesion kinase
(FAK) and mitogen-associated protein kinase/ERK kinase (MEK)
proteins exist in the cytoplasm (22). Ras protein is the upstream protein of
the RAF/MEK/ERK pathway, which may be activated by the secretion of
numerous stimulating factors (23).
After the activation of RAS protein, it binds to the RAF-1 domain
to activate RAF, which subsequently activates MEK. Activation of
ERKs by MEK stimulates phosphorylation of downstream substrates
(24). ERKs are downstream proteins
of multiple growth factors, regulating the cell proliferation,
differentiation and survival, as well as gene expression.
Overexpression of ERK is found in numerous human cancer types
(25). In the present study,
expression of RAS, RAF and ERK in KS tissues was higher than that
in normal tissues, possibly due to HHV-8 infection. AKT has
important roles in cell survival and apoptosis (26). The present study determined that the
expression of ERK (P40/P42), P50 and P65 in AIDS-KS was lower than
that in classic KS. FAK is important in tumor progression.
Inhibition of FAK expression or activity suppresses cell
proliferation and promotes apoptosis (27). In the present study, FAK expression
in KS tissues was not significantly different from that in normal
tissues. This may be due to the limited number of patients. NF-κB
is a dimer of p50 and p65 and the activation of NF-κB promotes
tumor growth (28). The expression
of P50 and P65 in KS was higher than that in normal tissues. HHV-8
infection activates ERK and its downstream signaling pathway,
enhances NF-κB expression and regulates the expression of tumor
genes (29). IκB-α binds to the P50
and P65 dimer and prevents the binding of the dimer with nuclear
DNA. When the classic pathway is activated, the dimer is released
and activated, and NF-κB then enters the nucleus (30).
The expression of TLR4 mRNA in KS tissues of AIDS-KS
patients was lower compared with that in normal tissues, while the
expression of RAS, RAF, ERK, IκB-α, P50 and P65 in AIDS-KS tissues
was higher than that in normal tissues. This may be due to the
following mechanism. HHV-8 encodes G protein-coupled receptors
(vGPCR) and IFN regulatory factor-1 (vIRF1), and partially inhibits
the expression of TLR4 (31). HHV-8
structural protein and vGPCR activates ERK, and phosphorylated ERK
acts on the conserved ETS binding site on the TLR4 promoter,
reducing TLR4 mRNA levels and TLR4 expression (32). vIRF1 blocks IFN mediated by TLR. Each
vIRF1 has its unique function and mechanism that inhibits
anti-viral responses of IFN (33).
Increase of HHV-8-TIR-domain-containing adapter-inducing IFN-β
(TRIF) inhibits the TLR-TRIF signaling pathway and downregulates
TLR4 (34). Prior to the occurrence
of tumors, the sustained activation of the TLR4 signaling pathway
indicates the existence of chronic inflammation. Studies have
indicated that sustained chronic inflammation is the key driver of
malignant tumor development (11,35).
Another study showed that TLR4 expression is high in lung,
laryngeal and colon cancer, and activation of the TLR4 signaling
pathway is important in specific anti-tumor immunity (36). Combined with previous studies, the
results demonstrate that the expression of TLR4 and its associated
signaling pathway proteins do not differ between AIDS-KS and
classic KS.
In conclusion, the present study demonstrated the
close association between TLR4 and AIDS-KS. In AIDS-KS, TLR4 mRNA
levels were downregulated. Interactions between HHV-8 and ERK may
have induced the high expression of proteins of the TLR4 signaling
pathway in AIDS-KS tissues. As TLR4 is downregulated in AIDS-KS and
classic KS, its restoration may inhibit the occurrence or growth of
KS as a novel therapeutic strategy. However, the effect of TLR4
overexpression was not assessed in the present study. Future
studies should thus be performed to examine this.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81060131 and
81260246).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XLu collected data from subjects, performed H&E
staining, immunohistochemistry, RT-qPCR, western blotting and
statistical analysis, and wrote the manuscript; XW and XLi
participated in H&E staining and immunohistochemistry; KP and
YZ also performed RT-qPCR and western blotting; and WM conceived
and designed the present study and revised the manuscript. All
authors have read and approved this manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of Xinjiang Medical University (Xinjiang, China) and written
informed consent was obtained from all patients or their
families.
Patient consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest to
declare.
References
|
1
|
Cancian L, Hansen A and Boshoff C:
Cellular origin of Kaposi's sarcoma and Kaposi's sarcoma-associated
herpesvirus-induced cell reprogramming. Trends Cell Biol.
23:421–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Zhang Z, Liu T, Li X, Zhang Q,
Wang L, Lu X, Lin R and Wen H: Analysis of infection rate of human
herpes virus 8 in blood donors in Xinjiang Autonomous Region. Chin
J Infect Dis. 27:502–504. 2009.(In Chinese).
|
|
3
|
Lester SN and Li K: Toll-like receptors in
antiviral innate immunity. J Mol Biol. 426:1246–1264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laresche C, Fournier E, Dupond AS,
Woronoff AS, Drobacheff-Thiebaut C, Humbert P and Aubin F: Kaposi's
sarcoma: A population-based cancer registry descriptive study of 57
consecutive cases diagnosed between 1977 and 2009. Int J Dermatol.
53:e549–e554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alcendor DJ: KSHV Down-regulates
Tropoelastin in Both an in-vitro and in-vivo Kaposi's Sarcoma
Model. J Oncobiomarkers. 2:1–7. 2015.PubMed/NCBI
|
|
7
|
Machado PR, Farias KJ, Pereira MG, Freitas
PP and Fonseca BA: Human herpesvirus 8 (HHV-8) detected by nested
polymerase chain reaction (PCR) in HIV patients with or without
Kaposi's sarcoma. An analytic cross-sectional study. Sao Paulo Med
J. 134:187–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandes F, Eloy C, Carimo A, Pinto P,
Graves S, Simões J, Carrilho C and Lopes JM: Simultaneous
presentation of Kaposi sarcoma and HHV8-associated large B-cell
lymphoma in the same lymph node: A rare diagnosis in an
HIV-negative patient. Am J Case Rep. 14:263–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boivin G, Côté S, Cloutier N, Abed Y,
Maguigad M and Routy JP: Quantification of human herpesvirus 8 by
real-time PCR in blood fractions of AIDS patients with Kaposi's
sarcoma and multicentric Castleman's disease. J Med Virol.
68:399–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HW, Trotter MW, Lagos D, Bourboulia
D, Henderson S, Mäkinen T, Elliman S, Flanagan AM, Alitalo K and
Boshoff C: Kaposi sarcoma herpesvirus-induced cellular
reprogramming contributes to the lymphatic endothelial gene
expression in Kaposi sarcoma. Nat Genet. 36:687–693. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer AK, Dixon D, DeGraff LM, Cho HY,
Walker CR, Malkinson AM and Kleeberger SR: Toll-like receptor 4 in
butylated hydroxytoluene-induced mouse pulmonary inflammation and
tumorigenesis. J Natl Cancer Inst. 97:1778–1781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang K, Zhou B, Wang Y, Rao L and Zhang
L: The TLR4 gene polymorphisms and susceptibility to cancer: A
systematic review and meta-analysis. Eur J Cancer. 49:946–954.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Yang L, Tan XH, Li F, Qin J, Guo S,
Pu X and Xie J: Detection of HHV-8 DNA in Serum of 29 Xinjiang
Classic Kaposi Sarcoma by Nested PCR. Chin J Dermatovenereol.
19:329–220. 2005.(In Chinese).
|
|
15
|
Lagos D, Vart RJ, Gratrix F, Westrop SJ,
Emuss V, Wong PP, Robey R, Imami N, Bower M, Gotch F and Boshoff C:
Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma
herpesvirus. Cell Host Microbe. 4:470–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou L, Shang H, Wang Y, Li G, Ding H,
Zhang X, Dai D and Shi W: The expression of TLR4 on peripheral
blood monocyte and TNF-α concentration of plasma in Chinese
HIV/AIDS patients. Chin J Microbiol Immunol. 27:1016–1019. 2007.(In
Chinese).
|
|
17
|
Lester RT, Yao XD, Ball TB, McKinnon LR,
Kaul R, Wachihi C, Jaoko W, Plummer FA and Rosenthal KL: Toll-like
receptor expression and responsiveness are increased in viraemic
HIV-1 infection. AIDS. 22:685–694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bosinger SE, Hosiawa KA, Cameron MJ,
Persad D, Ran L, Xu L, Boulassel MR, Parenteau M, Fournier J, Rud
EW and Kelvin DJ: Gene expression profiling of host response in
models of acute HIV infection. J Immunol. 173:6858–6863. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Speicher DJ, Sehu MM, Johnson NW and Shaw
DR: Successful treatment of an HIV-positive patient with unmasking
Kaposi's sarcoma immune reconstitution inflammatory syndrome. J
Clin Virol. 57:282–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamburro KM, Yang D, Poisson J, Fedoriw Y,
Roy D, Lucas A, Sin SH, Malouf N, Moylan V, Damania B, et al:
Vironome of Kaposi sarcoma associated herpesvirus-inflammatory
cytokine syndrome in an AIDS patient reveals co-infection of human
herpesvirus 8 and human herpesvirus 6A. Virology. 433:220–225.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maimaitiaili W, Pan K, Lu X, Sun X,
Yibaguli A, Yang K, Zuohela T, Adili K and Zhang Y: Clinical,
pathological and prognosis analyses of AIDS patients with Kaposi
sarcoma. Chin J Infect Dis. 30:368–370. 2012.(In Chinese).
|
|
22
|
Rea K, Sensi M, Anichini A, Canevari S and
Tomassetti A: EGFR/MEK/ERK/CDK5-dependent integrin-independent FAK
phosphorylated on serine 732 contributes to microtubule
depolymerization and mitosis in tumor cells. Cell Death Dis.
4:e8152013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buscà R, Christen R, Lovern M, Clifford
AM, Yue JX, Goss GG, Pouysségur J and Lenormand P: ERK1 and ERK2
present functional redundancy in tetrapods despite higher evolution
rate of ERK1. BMC Evol Biol. 15:1792015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh A, Ruan Y, Tippett T and Narendran
A: Targeted inhibition of MEK1 by cobimetinib leads to
differentiation and apoptosis in neuroblastoma cells. J Exp Clin
Cancer Res. 34:1042015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamminen JA, Yin M, Rönty M, Sutinen E,
Pasternack A, Ritvos O, Myllärniemi M and Koli K: Overexpression of
activin-A and -B in malignant mesothelioma-attenuated Smad3
signaling responses and ERK activation promote cell migration and
invasive growth. Exp Cell Res. 332:102–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HS, You GE, Yang KH, Kim JY, An S,
Song JY, Lee SJ, Lim YK and Nam SY: Role of AKT and ERK pathways in
controlling sensitivity to ionizing radiation and adaptive response
induced by low-dose radiation in human immune cells. Eur J Cell
Biol. 9:653–660. 2015. View Article : Google Scholar
|
|
27
|
Nguyen MP, Lee D, Lee SH, Lee HE, Lee HY
and Lee YM: Deguelin inhibits vasculogenic function of endothelial
progenitor cells in tumor progression and metastasis via
suppression of focal adhesion. Oncotarget. 6:16588–16600. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shostak K and Chariot A: EGFR and NF-κB:
Partners in cancer. Trends Mol Med. 21:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cannon M, Philpott NJ and Cesarman E: The
Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor
has broad signaling effects in primary effusion lymphoma cells. J
Virol. 77:57–67. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pati S, Cavrois M, Guo HG, Foulke JS Jr,
Kim J, Feldman RA and Reitz M: Activation of NF-kappaB by the human
herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine
model of Kaposi's sarcoma pathogenesis. J Virol. 75:8660–8673.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gregory SM, West JA, Dillon PJ, Hilscher
C, Dittmer DP and Damania B: Toll-like receptor signaling controls
reactivation of KSHV from latency. Proc Natl Acad Sci USA.
106:11725–11730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hävemeier A, Gramolelli S, Pietrek M,
Jochmann R, Stürzl M and Schulz TF: Activation of NF-κB by the
Kaposi's sarcoma-associated herpesvirus K15 protein involves
recruitment of the NF-κB -inducing kinase, IκB kinases, and
phosphorylation of p65. J Virol. 88:13161–13172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobs SR, Gregory SM, West JA, Wollish
AC, Bennett CL, Blackbourn DJ, Heise MT and Damania B: The viral
interferon regulatory factors of kaposi's sarcoma-associated
herpesvirus differ in their inhibition of interferon activation
mediated by toll-like receptor 3. J Virol. 87:798–806. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meyer F, Ehlers E, Steadman A, Waterbury
T, Cao M and Zhang L: TLR-TRIF pathway enhances the expression of
KSHV replication and transcription activator. J Biol Chem.
288:20435–20442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmad H, Gubbels R, Ehlers E, Meyer F,
Waterbury T, Lin R and Zhang L: Kaposi sarcoma-associated
herpesvirus degrades cellular Toll-interleukin-1 receptor
domain-containing adaptor-inducing beta-interferon (TRIF). J Biol
Chem. 286:7865–7872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Y, Li Y, Wei X, Feng Z and Yang S:
Expression of TLR2 and TLR4 in primary liver cancer. Chin J
Pathophysiol. 24:1912–1915. 2008.(In Chinese).
|