Introduction
Myasthenia gravis (MG) is an autoimmune disease,
which is caused by anti-nicotinic acetylcholine receptor antibody,
resulting in a defect in neuromuscular transmission (1). Clinically, MG is manifested by weakness
of voluntary muscles on prolonged exercise, which is restored
rapidly after rest (1). Although the
trigger of autoimmunity in MG is unknown, it is well documented
that the thymus has an important role in the pathogenesis of MG
(2,3). An estimated 80–90% of MG patients have
thymic abnormalities, of which lymphoid follicular hyperplasia
(LFH) accounts for 65–75% (4).
Thymectomy has almost always been routinely performed for patients
with MG and is effective in most cases of LFH (5–8). From
the position of the thoracic surgeon and under consideration of the
cost, pre-operative computed tomography (CT) is a more valuable
method than magnetic resonance imaging and 201Tl-single
photon emission CT in diagnosing the abnormalities of thymus,
including LFH and thymoma. However, CT has its own limitations in
differentiating LFH from normal and involuted thymus considering
the radiology standards for thymus LFH (9–13).
Whether a patient with non-thymomatous MG requires a thymectomy is
currently difficult to decide for the surgeon. As described
previously, the overall volume of the thymus remains stable after
birth and the volume of the thymus epithelial space diminishes
during aging, while thymopoiesis is encountered in thymus tissues
of normal individuals aged >60 years (14,15).
However, most patients with MG have an LFH thymus, which is
enlarged and contains B-cell germinal centers (4,16,17). The
present study assessed the chest CT of MG patients who received
thymectomy after one week following admission. The removed thymuses
were then confirmed as either normal/involuted or LFH by
pathological analysis. On the basis of the pathological results, a
comparison of the CT appearance between the LFH thymus group and
the normal/involuted thymus group was performed in order to
determine the best indicators of thymus abnormalities of LFH for
the thoracic surgeon.
Materials and methods
Patients and thymuses
Of the 80 MG patients included, 54 were diagnosed as
having LFH thymuses (21 males and 33 females; age range, 11–73
years; mean age, 40.2±17.28 years). The other 26 patients were
diagnosed by the pathologist as having normal or involuted thymuses
(20 males and 6 females; age range, 42–72 years; mean age,
58.57±8.21 years). All of the patients received transsternal
thymectomy between January 2001 and January 2011 at the Affiliated
General Hospital of Tianjin Medical University (Tianjin, China).
Written informed consent was obtained from all of the patients who
participated and the Ethics Committee of the Affiliated General
Hospital of Tianjin Medical University (Tianjin, China) approved
the study protocols.
The diagnosis of MG was made based on the patients'
history, the results of neurological examinations,
electrophysiological studies (repetitive nerve stimulation, single
fiber electromyography) and a prostigmin test. The patients did not
receive any immunosuppressants, including corticosteroids, prior to
the operation. Patients with other diagnoses, including Grave's
disease, malignant tumors receiving chemotherapy, polyneuropathy,
myopathy, multiple sclerosis or neurasthenic syndrome, were
excluded.
The pathological diagnoses were made by a senior
pathologist without any information from imaging and clinical
studies. There are two histopathological diagnoses for the thymus
in MG: LFH and normal/involuted thymus. Hyperplasia is
characterized by the appearance of lymphoid follicles, which are
usually round-shaped accumulations of B lymphocytes with or without
germinal centers on microscopic examination. Normal thymus was
considered when no lymphoid follicles were present in the
perivascular space with or without thymic atrophy or adipose tissue
alone (18).
Spiral CT
Spiral CT images were acquired on a single scanner
with contiguous 1.3–10.0 mm thick slices using a whole-body CT (GE
Lightspeed 64; GE Healthcare, Little Chalfont, UK) in the
mediastinal field condition. Standard parameters for spiral CT of
the chest were 120 kVp and 200–340 mAsec. The images were
reconstructed with a standard soft-tissue-kernel algorithm.
Contrast-enhanced CT scans were obtained after intravenous
injection of 120–150 ml contrast material (iohexol; 140 mg/ml; GE
Healthcare) by using a power injector at a rate of 2.0–4.0 ml/sec.
The injection volume and rate of contrast medium delivery varied
depending on the patient's weight and vascular access. Radiological
findings were determined by a senior radiologist prior surgery.
Analysis of CT images
CT images were retrieved from the institutional
picture archiving and communication system and analyzed on a
clinical workstation (GE Healthcare). The upper border of the
mediastinal tissue was fixed at the level of the aortic arch and
the lower border was fixed at the junction of the pulmonary artery
and the heart (19).
According to St Amour et al (20) and Francis et al (21), the shape (quadrilateral or
triangular) and density of the thymus were determined in all
patients. The use of the terms triangular and quadrilateral was
chosen for simplicity and conformity with prior studies (22). Thymic measurements, including
anteroposterior (AP) and transverse dimensions, width (the longest
axis of the lobe on a transverse scan) and thickness (the largest
dimension perpendicular to the long axis of the lobe), were also
obtained for each of the patients (20). The craniocaudal dimension of the
thymus was not determined, since precise measurement was usually
not possible due to variations in the degree of inspiration on
contiguous CT scan (20).
The pixels of the thymus region, muscle and adipose
tissue in the chest wall in the same CT slice were measured using
the workstation to compare the pixel attenuation of different
tissues. The mean CT attenuation of each region in each group of
patients (LFH and normal thymus) was compared, by studying
differences in the tissues within each group and between the groups
of patients for the same tissue.
Statistical analysis
Values are expressed as the mean ± standard
deviation unless otherwise specified. The Kolmogorov-Smirnov test
was used to test if the quantitative data had a normal
distribution. The independent-samples t-test was used to examine
the differences in thymic thickness, AP dimension and perpendicular
dimension between the two MG groups. Analysis of variance followed
by the Student-Neuman-Keuls post-hoc test was used to examine
differences in mean CT attenuation among thymus tissue, fat tissue
and muscle in the two groups. Pearson's rank correlation was used
to test the association between the patients' age and the CT
attenuation. Analyses were performed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
CT dimensions of the thymus
region
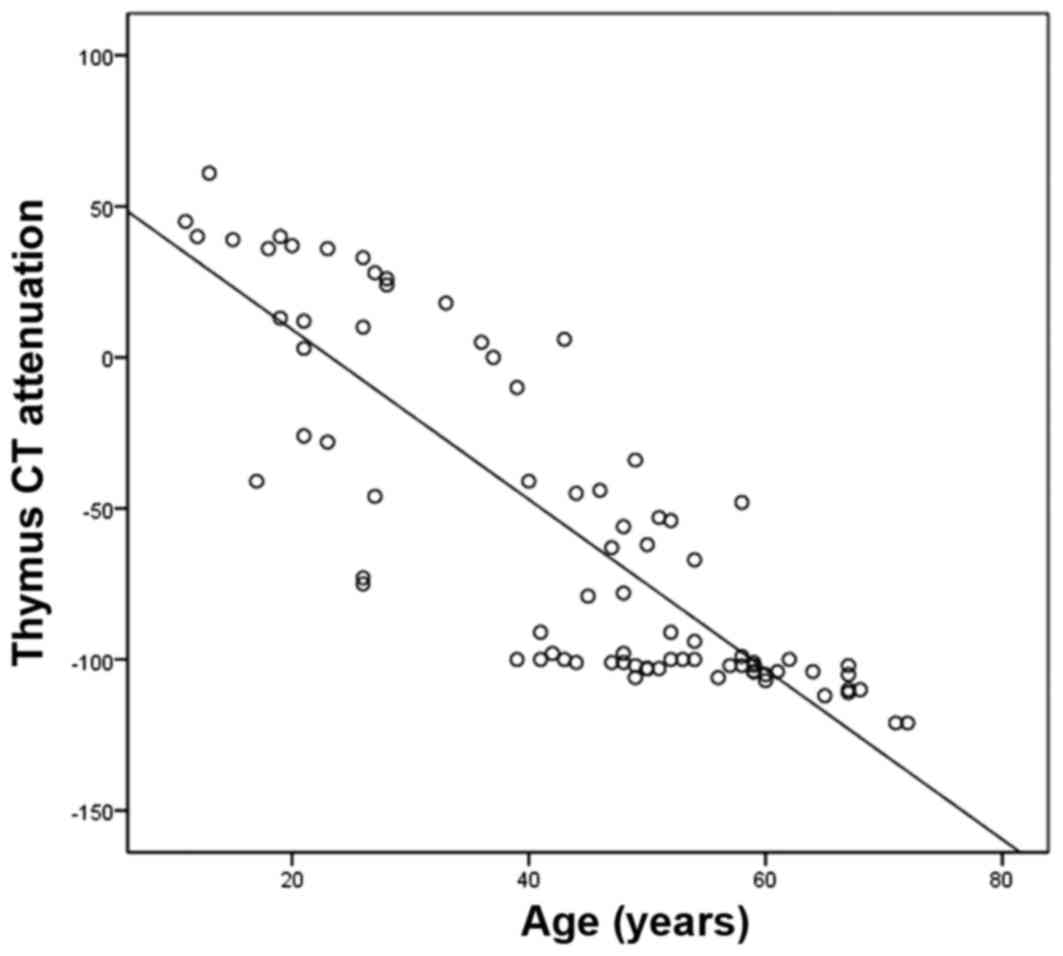
First, when all of the patients were analyzed as a
whole and not grouped according to their histopathological results,
a negative correlation between the patients' age and the CT
attenuation in Hounsfield units (HU) of the thymus region was
identified (r=−0.779; P<0.05; Pearson's rank correlation test;
Fig. 1), which means the that the CT
attenuation in HU of the thymus region of MG patients decreases
with age. The mean age at disease onset in the LFH thymus group was
lower than that in the normal thymus group (40.2±17.3 vs. 59.2±9.3
years; P<0.001; Table I). In the
LFH group, the thymus or its remnant was detected in all of the MG
patients. All of the thymuses remained in their usual triangular or
quadrilateral cross-sectional shape and the two types of shape were
observed in patients in each decade of life. Even in patients in
their fifties, it was possible to distinguish the thymus region
from adipose tissue. However, in the cases where the thymus had
been replaced by adipose tissue, certain nodules were present in
the thymus region, which did not occur in normal thymus of MG
patients. Typically, the gland was located anterior to the
ascending aorta, pulmonary outflow tract and distal superior vena
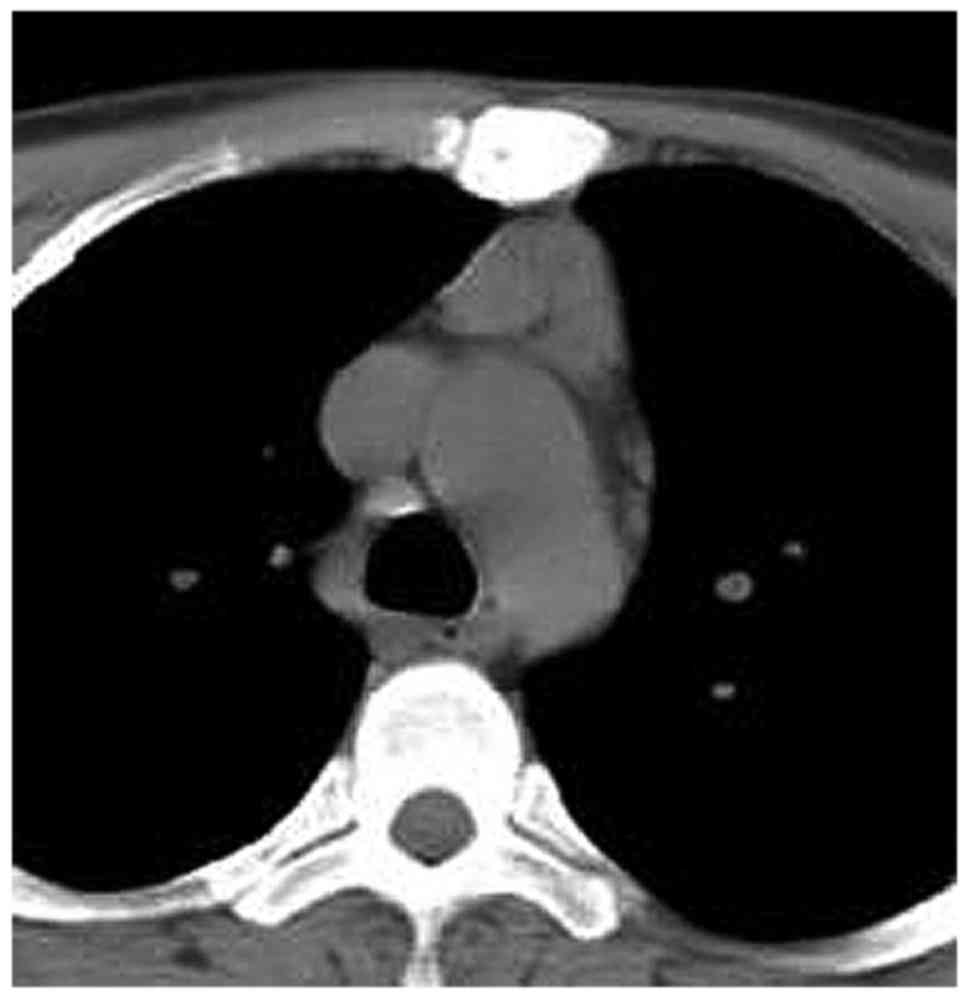
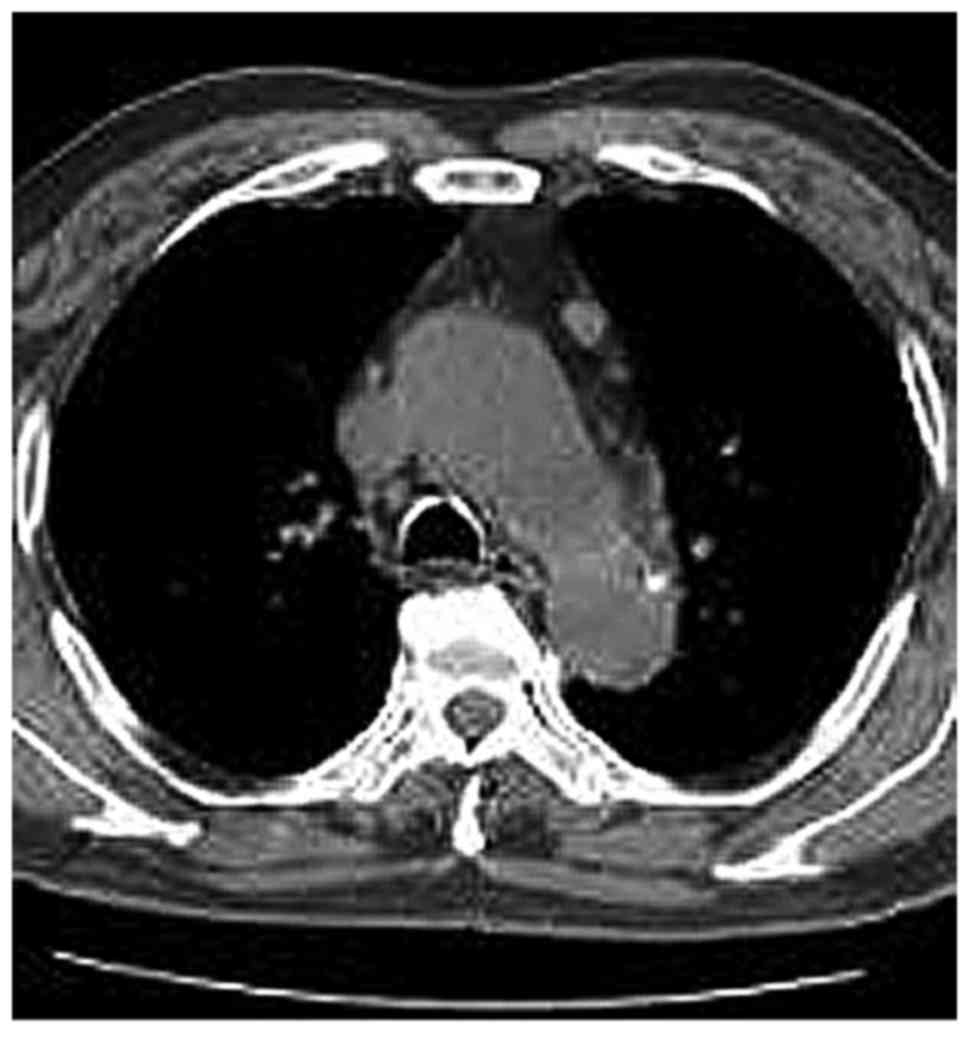
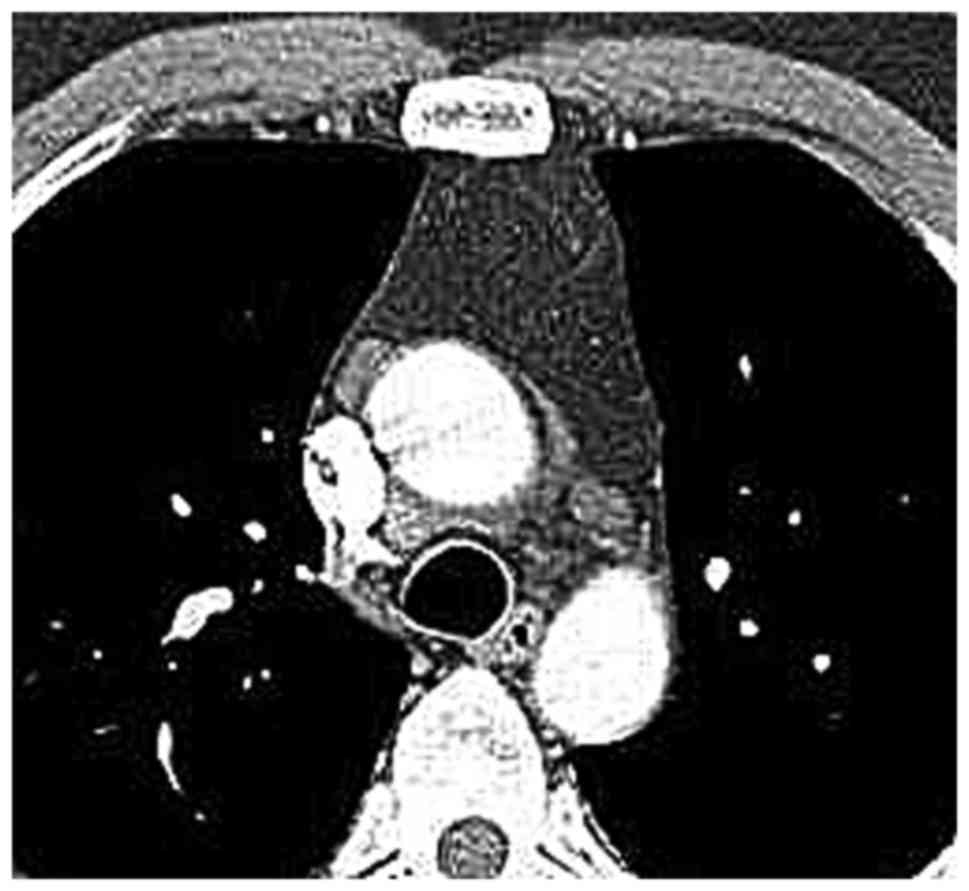
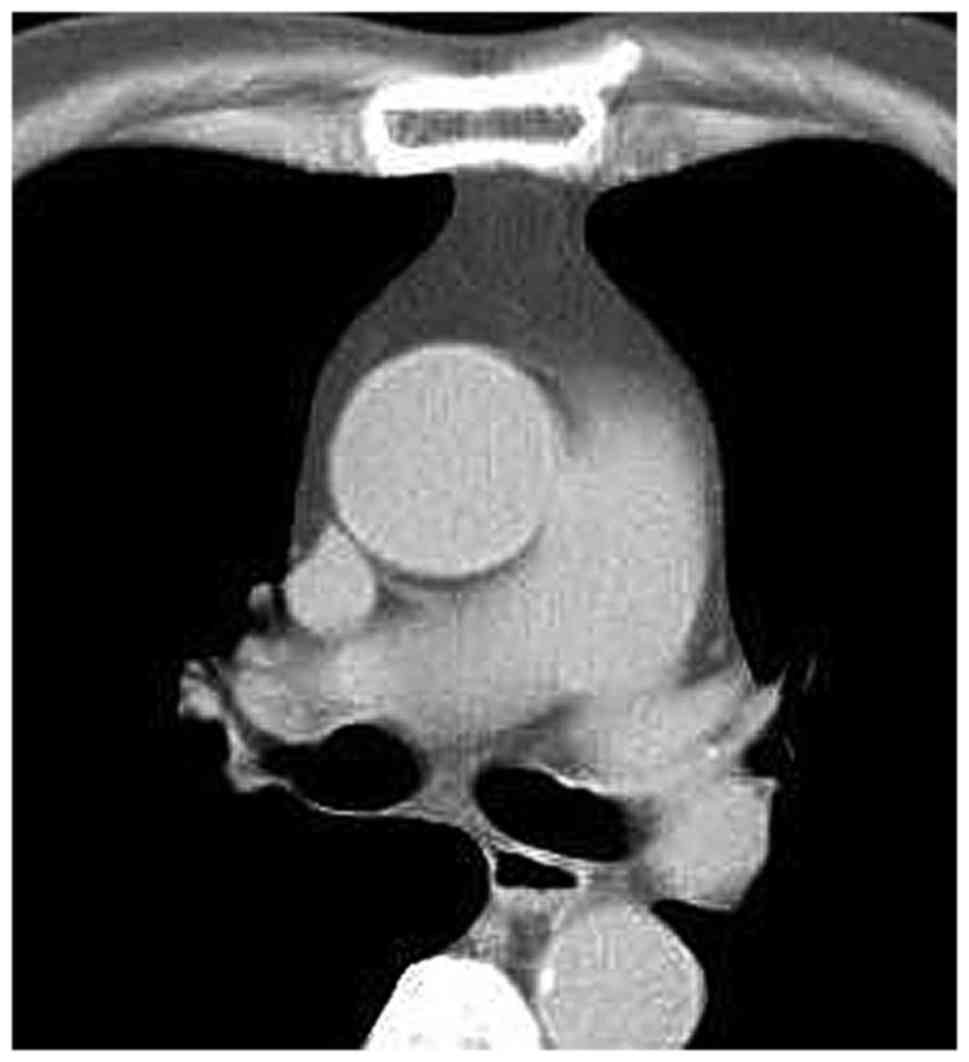
cava within the anterior mediastinal fat. Among the MG patients
with LFH (Figs. 2–9), no case of aberrantly positioned thymus
was encountered. On the other hand, in the MG patients of the
normal thymus group, it was not possible to detect the thymus or
its remnant, as the thymus tissue had been completely infiltrated
by adipose tissue. In all MG patients with normal/involute thymus,
the shape of the thymus region on CT was quadrilateral (Figs. 10–12), which may be due to the patients' age
or a different pathogenesis from that in the LFH MG group.
 | Table I.Clinicopathological features of the
patients. |
Table I.
Clinicopathological features of the
patients.
| Parameter | LFH thymus group
(n=54) | Normal/involuted
thymus group (n=26) | P-value |
|---|
| Gender |
|
|
0.001 |
| Male | 21 (26.3%) | 20 (25.0%) |
|
|
Female | 33 (41.3%) | 6 (7.5%) |
|
| Age of disease onset
(years) |
|
|
|
|
Range | 11–73 | 40–72 |
|
| Mean ±
standard deviation | 40.2±17.3 | 59.2±9.3 | <0.001 |
Regarding the mean measures of thymic thickness,
width, AP dimension and transverse dimension, there were no
statistical differences between the LFH group and normal/involuted
thymus group (Table II).
 | Table II.Thymic measurements of myasthenia
gravis patients with LFH or normal/involuted thymus (cm). |
Table II.
Thymic measurements of myasthenia
gravis patients with LFH or normal/involuted thymus (cm).
| Type of
measurement | LFH thymus group
(n=54) | Normal/involuted
thymus group (n=26) | P-value |
|---|
| Thickness | 2.03±0.71 | 2.57±0.65 | 0.41 |
| Width | 3.70±1.37 | 4.48±2.29 | 0.22 |
| AP dimension | 2.48±0.99 | 2.42±0.75 | 0.86 |
| Transverse
dimension | 4.76±1.54 | 5.76±1.26 | 0.16 |
CT attenuation of thymus region on
unenhanced CT
A total of 43 and 18 patients were subjected to
unenhanced CT in the LFH group and the normal/involuted thymus
group, respectively. The mean values of CT attenuation of different
regions are listed in Table III.
In the LFH group, when comparing the CT attenuation of different
regions (thymus region vs. adipose tissue, thymus region vs. muscle
tissue, adipose tissue vs. muscle tissue), the differences were all
significant (P<0.05). In the normal/involuted thymus group,
there was no significant difference in the mean CT attenuation
between thymus region and adipose tissue, while the differences
were significant between other tissues (thymus region vs. muscle
tissue, adipose tissue vs. muscle tissue, P<0.05). Most
importantly, there was a significant difference in the CT
attenuation of the thymic region between the two groups (Table III).
 | Table III.Unenhanced computed tomography
attenuation of different types of soft tissue in non-thymomatous
myasthenia gravis patients. |
Table III.
Unenhanced computed tomography
attenuation of different types of soft tissue in non-thymomatous
myasthenia gravis patients.
| CT attenuation
(HU) | LFH thymus group
(n=43) | Normal/involuted
thymus group (n=18) | P-value |
|---|
| Thymus region | −41.21±54.42 | −108.13±8.7 | 0.02 |
| Adipose tissue |
−107.61±14.16a | −110.24±4.17 | 0.72 |
| Muscle tissue |
53.62±19.83a,b |
48.5±17.94a,b | 0.62 |
CT attenuation of thymus region on
contrast-enhanced CT
In the LFH group and the normal/involuted thymus
group, 11 and 8 patients received contrast-enhanced CT,
respectively. The mean values of CT attenuation of different
regions are presented in Table IV.
Similar to the trends of the unenhanced CT, a significant
difference in the CT attenuation of the thymic region was
identified between the two groups (Table IV). Within each group, when
comparing the CT attenuation of different regions (thymus region
vs. adipose tissue, thymus region vs. muscle tissue, adipose tissue
vs. muscle tissue), the differences were all significant
(P<0.05).
 | Table IV.Contrast-enhanced CT attenuation of
different types of soft tissue in non-thymomatous myasthenia gravis
patients. |
Table IV.
Contrast-enhanced CT attenuation of
different types of soft tissue in non-thymomatous myasthenia gravis
patients.
| CT attenuation
(HU) | LFH thymus group
(n=11) | Normal/involuted
thymus group (n=8) | P-value |
|---|
| Thymus region | −25.57±58.65 | −117.40±6.20 | 0.01 |
| Adipose tissue |
−105.66±16.13a |
−123.00±6.45a | 0.17 |
| Muscle tissue |
60.63±7.20a,b |
57.64±3.60a,b | 0.59 |
Discussion
In general, the thymus in a healthy human subject is
a central lymphoid organ that is gradually replaced by adipose
tissue, which results in thymic involution. The processes/timing of
the weight and volume of the thymus reaching its apex and the
beginning of involution are inconsistently described among previous
studies (19–21). However, it is known that when the
involution starts, the epithelial component of the thymus
atrophies, resulting in scattered small lymphocytes in abundant
adipose tissue (18,19,22).
LFH, also known as autoimmune thymitis, is characterized by a
normal size and weight of the thymus with chronic inflammation and
proliferation of lymphoid follicles, active germinal centers and
increased numbers of lymphocytes and epithelial cells (23,24). The
symptoms of MG, including the impairment of ocular (extra-ocular
muscles, eyelids), bulbar (ingestion function, voice/speech
function, respiratory function, facial muscles) and limb-axial
muscles (arms, legs and neck), always improve after thymectomy in
patients with thymoma or LFH thymus (5–8).
Furthermore, in MG patients with LFH, the thymus also frequently
appears as atrophic on CT scan and gross examination, particularly
in elderly patients. Differentiation of LFH from the normal thymus
may be difficult for the radiologist on the basis of morphologic
features alone. These all present obstacles for the thoracic
surgeon when determining whether an MG patient requires a
thymectomy.
In the present study, the data of CT scans of
non-thymomatous MG patients with LFH thymus or normal/involuted
thymus, which had been pathologically confirmed, were analyzed. The
results indicated that the mean age of disease onset in the LFH
thymus group was lower than that in the normal/involuted thymus
group (40.2±17.28 vs. 58.57±8.21 years). In the LFH group, the age
was distributed more widely (from 11 to 73 years) than in the
normal/involuted thymus group (42–72 years). This result may
possibly indicate a different mechanism between younger and older
MG patients (25). In fact, the
observation that there were no significant differences between the
two groups in six views of the thymus exactly coincides with the
fact that the autoimmune LFH thymus is characterized by a normal
size and weight, including those of lymphoid follicles (24).
In the present study, the CT attenuation of the
thymus region of MG patients gradually decreased with age, although
for the spiral CT mentioned above, the parameters diversified. This
observation coincides with the histological results that MG
patients always have a thymus with atrophy and varying degrees of
fat infiltration, even in LFH MG patients with B-cell lymphoid
follicles and germinal centers in the thymus (18,25).
Regardless of whether unenhanced or contrast-enhanced CT was used,
a significant difference in the mean CT attenuation was present
between the LFH group and the normal/involuted thymus group
(−41.21±54.42 vs. −108.13±8.71 on unenhanced CT; −25.57±58.65 vs.
−117.40±6.20 on contrast-enhanced CT). Furthermore, in the LFH
group, the difference in the mean unenhanced and contrast-enhanced
CT attenuation, between the thymus region and the adipose tissue
was significant, while the normal/involuted thymus group did not
exhibit such a difference. This result should be interpreted with
the differentiation of LFH from normal thymus in MG patients prior
to thymectomy in mind. A larger study is necessary to assess more
MG patients with a normal/involuted thymus to identify differences
from LFH patients. Only in this way, thymectomy may be used for the
patients with immune system abnormalities to cure associated
diseases, including MG.
In conclusion, either unenhanced or
contrast-enhanced CT may be used to distinguish LFH MG thymus from
normal/involuted MG thymus, even though there were no significant
differences in the dimensional measurements between the two
groups.
Acknowledgements
The authors would like to thank Mr. Wang Qing, Dr
Zhang Le and Mr. GuoHong at the Radiological Department of the
Affiliated General Hospital of Tianjin Medical University (Tianjin,
China) for their excellent technical assistance and collection of
CT data. The authors also thank Mr. Wang Yuanguo, Dr Liu Yimei, Mr.
Song Shihui, Mr. Zheng Kai, Mr. Wang Zhongyi and Mr. Shang
Zhongliang at the Cardiac and Thoracic Surgery Department of the
Affiliated General Hospital of Tianjin Medical University (Tianjin,
China) for their advice and guidance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ performed the structural determination. TLY
contributed to the conception and design. HZ collected, analyzed
and interpreted the data and participated in the preparation of the
manuscript. All authors read and approved the final version of the
manuscript.
Ethical approval and informed consent
Written informed consent was obtained from all of
the patients who participated and the Ethics Committee of the
Affiliated General Hospital of Tianjin Medical University (Tianjin,
China) approved the study protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Gihus NE and Verschuuren JJ: Myasthenia
gravis: Subgroup classification and therapeutic strategies. Lancet
Neurol. 14:1023–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song Y, Zhou L, Miao F, Chen G, Zhu Y, Gao
X, Wang Y, Pang L, Zhao C, Sun X and Chen Z: Increased frequency of
thymic T follicular helper cells in myasthenia gravis patients with
thymoma. J Thorac Dis. 8:314–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Truffault F, de Montreville V, Eymard B,
Sharshar T, Le Panse R and Berrih-Aknin S: Thymic germinal centers
and corticosteroids in myasthenia gravis: An immunopathological
study in 1035 cases and a critical review. Clin Rev Allergy
Immunol. 52:108–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Domeier PP, Schell SL and Rahman ZS:
Spontaneous germinal centers and autoimmunity. Autoimmunity.
50:4–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rückert JC, Ismail M, Badakhshi H, Meisel
A and Swierzy M: Thymectomy in myasthenia and/or thymoma. Zentralbl
Chir. 139:121–134. 2014.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie Y, Li HF, Sun L, Kusner LL, Wang S,
Meng Y, Zhang X, Hong Y, Gao X, Li Y and Kaminski HJ: The role of
osteopontin and its gene on glucocorticoid response in myasthenia
gravis. Front Neurol. 8:2302017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moser B, Janik S, Schiefer AI, Müllauer L,
Bekos C, Scharrer A, Mildner M, Rényi-Vámos F, Klepetko W and
Ankersmit HJ: Expression of RAGE and HMGB1 in thymic epithelial
tumors, thymic hyperplasia and regular thymic morphology. PLoS One.
9:e941182014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yablonsky P, Pischik V, Tobina MG and
Atiukov M: The results of video-assisted thoracoscopic thymectomies
in Saint Petersburg, Russia: 20-year of experience. J Vis Surg.
3:1132017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ottlakan A, Borda B, Morvay Z, Maraz A and
Furak J: The effect of diagnostic imaging on surgical treatment
planning in diseases of the thymus. Contrast Media Mol Imaging.
2017:93072922017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Roxas RC, Bagnas MA, Baldonado JJ,
Rivera JP and Roxas AA: Clinical profile and outcome of
postthymectomy versus non-thymectomy myasthenia gravis patients in
the Philippine general hospital: A 6-year retrospective study.
Front Neurol. 7:962016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li HF, Hong Y, Xie Y, Hao HJ and Sun RC:
Precision medicine in myasthenia graves: Begin from the data
precision. Ann Transi Med. 4:1062016. View Article : Google Scholar
|
|
12
|
Mao ZF, Mo XA, Qin C, Lai YR and Hackett
ML: Incidence of thymoma in myasthenia gravis: A systematic review.
J Clin Neurol. 8:161–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Araki T, Nishino M, Gao W, Dupuis J,
Washko GR, Hunninghake GM, Murakami T, O'Connor GT and Hatabu H:
Anterior mediastinal masses in the framingham heart study:
Prevalence and CT image characteristics. Eur J Radiol Open.
2:26–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tajima A, Pradhan I, Trucco M and Fan Y:
Restoration of thymus function with bioengineered thymus organoids.
Curr Stem Cell Rep. 2:128–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagakubo D, Krauth B and Boehm T: Genetic
and non-genetic determinants of thymic epithelial cell number and
function. Sci Rep. 7:103142017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kranich J and Krautler NJ: How follicular
dendritic cells shape the B-cell antigenome. Front Immunol.
7:2252016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mlika M, Gattoufi W, Zribi H, Braham E,
Marghli A and El Mezni F: A unilocular thymic cyst associated with
true thymic hyperplasia: A challenging diagnosis especially in a
child. Int Med Case Rep J. 8:215–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pereira BI and Akbar AN: Convergence of
innate and adaptive immunity during human aging. Front Immunol.
7:4452016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sidler C, Kovalchuk O and Kovalchuk I:
Epigenetic regulation of cellular senescence and aging. Front
Genet. 8:1382017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
St Amour TE, Siegel MJ, Glazer HS and
Nadel SN: CT appearances of the normal and abnormal thymus in
childhood. J Comput Assist Tomogr. 11:645–650. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Francis IR, Glazer GM, Bookstein FL and
Gross BH: The thymus: Reexamination of age-related changes in size
and shape. AJR Am J Roentqenol. 145:249–254. 1985. View Article : Google Scholar
|
|
22
|
Nishino M, Ashiku SK, Kocher ON, Thurer
RL, Boiselle PM and Hatabu H: The thymus: A comprehensive review.
Radiographics. 26:335–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manchanda S, Bhalla AS, Jana M and Gupta
AK: Imaging of the pediatric thymus: Clinicoradiologic approach.
World J Clin Pediatr. 6:10–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimamoto A, Ashizawa K, Kido Y, Hayashi
H, Nagayasu T, Kawakami A, Mukae H, Hayashi T, Ohtsubo M,
Shigematsu K, et al: CT and MRI findings of thymic carcinoid. Br J
Radiol. 90:201503412017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa N, Nagai M, Tsujii T, Kyaw WT,
Tanabe N, Iwaki H, Yabe H, Ando R and Nomoto M: Treatment of
myasthenia gravis in patients with elderly onset at advanced age.
Jpn Clin Med. 6:9–13. 2015. View Article : Google Scholar : PubMed/NCBI
|