Introduction
Tooth orthodontics is to exert the external
mechanical force to the deformed tooth in clinical treatment, so as
to remodel the periodontal tissues around the tooth, thus moving
and correcting the tooth. Periodontal tissues have rich structures,
including alveolar bone, periodontal membrane and gingiva. During
the tooth orthodontic treatment, periodontal membrane remodeling is
the most important, which is a dynamic equilibrium state between
bone formation and bone resorption (1). The periodontal membrane, as the
mediator of orthodontic treatment, is a kind of variant periosteum.
Bone morphogenetic protein-3 (BMP-3) is one of the important
members of the transforming growth factor-β (TGF-β) family, and
mainly involved in the bone activity (2). Moreover, BMP-3, as an important
cytokine, plays an important regulatory role in bone remodeling
(3). At first, scholars believed
that BMP-3 is a kind of bone response factor, but they recognized
that BMP-3 is a negative regulatory factor of bone formation with
the deepening of research on BMP-3 (4,5).
However, the role and expression characteristics of BMP-3 in the
process of orthodontic tooth movement remain unclear. Therefore,
this study investigated the role and expression characteristics of
BMP-3 in the process of orthodontic tooth movement of rats.
Materials and methods
Experimental animals and grouping
A total of 48 Sprague-Dawley rats (half male and
half female) weighing 220±20 g were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China) [license no.: SCXK
(Shanghai) 2014-0003]. The rats were kept in cages with controlled
temperature and light cycles (24°C and 12/12 light/dark cycle). The
humidity was 60±10% and had free access to food and water. The
above 48 rats were randomly divided into the the 3-day group
(n=12), the 7-day group (n=12), the 14-day group (n=12) and the
21-day group (n=12) using the random number table method. The
maxillary left molar of each rat was used to establish the
orthodontic tooth movement model, while the contralateral tooth
received no treatment as the control group. The study was approved
by the Ethics Committee of Qingdao Women and Children's Hospital
(Qingdao, China).
Experimental reagents
Anti-BMP-3 antibody (Abcam, Cambridge, MA, USA),
ethylene diamine tetraacetic acid (EDTA) (Sinopharm Group),
hematoxylin and eosin (H&E) staining kit (Solarbio, Beijing,
China), immunohistochemistry kit (Maxim, Fuzhou, China), AceQ
quantitative polymerase chain reaction (qPCR) SYBR Green Master Mix
kit (Vazyme, Nanjing, China), HiScript II Q RT SuperMix for qPCR
(+gDNA wiper) kit (Vazyme), optical microscope (Leica DMI
4000B/DFC425C; Leica Microsystems GmbH, Wetzlar, Germany),
fluorescence qPCR instrument (ABI 7500, USA), Image-lab image
analysis system and Image-Pro image analysis system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), orthodontic
clinically-special nickel-titanium push spring (Dalian Tongdali
Co., Ltd., Dalian, China) and orthodontic clinically-special micro
implant (Dentaurum, Ispringen, Germany).
Establishment of orthodontic tooth
movement model
After successful anesthesia via intraperitoneal
injection of 7% chloral hydrate into rats (5 ml/kg), the maxilla of
rats was fully exposed, and the micro-implant was implanted as the
support into the left upper incisor at a speed of 1,020 × g at 20°C
for 5 min. A 0.02 mm orthodontic ligature wire was passed between
the maxillary left first molar and the second molar, one end of
which was connected to the nickel-titanium push spring, and the
other end was connected to the implant. An orthodontic dynamometer
was connected to the other end of the nickel-titanium push spring
to measure its length at 40 g, and the length of both ends of the
nickel-titanium push spring should be the same.
Treatment in each group
The maxillary left molar of rats in each group was
used as the experimental tooth and the incisor as the anchorage
tooth to prepare the orthodontic tooth movement model. The
corresponding maxillary right molar received no treatment as the
control group. The tooth movement distance of each rat was measured
using a vernier caliper at day 1, 3, 7, 14 and 21 after modeling.
The rats were sacrificed at day 3 after modeling in the 3-day
group, at day 7 after modeling in the 7-day group, at day 14 after
modeling in the 14-day group and at day 21 after modeling in the
21-day group.
Material acquisition
After successful anesthesia, the maxillary left and
right molars of each rat were taken directly. The molars of 6 rats
in each group were fixed in 4% paraformaldehyde at 4°C for 48 h,
decalcified in EDTA solution for 2 months, and prepared into
paraffin-embedded tissue sections for H&E staining and
immunohistochemical detection. The molars of the remaining 6 rats
were placed into Eppendorf (EP) tubes for western blotting and
qPCR.
H&E staining
After 5 µm-thick paraffin-embedded tissue sections
were routinely deparaffinized with xylene, they were dehydrated
with gradient alcohol, stained with hematoxylin for 5 min, rinsed
with tap water, differentiated with hydrochloric acid alcohol for
30 sec, soaked in tap water for 15 min, stained with eosin for 2
min, routinely dehydrated again with gradient alcohol, made
transparent with xylene and sealed with neutral gum.
Immunohistochemistry
Tissues were fixed with 10% formal-dehyde at 20°C
for 16 h. Paraffin-embedded tissue sections (5 µm in thickness)
were routinely deparaffinized, placed into water, added with the
citrate buffer and heated in a microwave oven for antigen
retrieval. After sections were rinsed with phosphate-buffered
saline (PBS), the endogenous peroxidase blocker was added for
incubation for 10 min, followed by rinsing with PBS. Then, sections
were sealed with goat serum for 20 min, the serum sealing solution
was removed, and rabbit anti-rat BMP-3 primary polyclonal antibody
(1:200; cat no. ab134724; Abcam) was added for incubation at 4°C
overnight. After being rinsed with PBS, sections were incubated
with goat anti-rabbit secondary antibody for 10 min, rinsed again
with PBS and added with streptavidin-peroxidase solution for 10 min
of incubation, followed by color development via diaminobenzidine
(DAB), counterstaining via hematoxylin, sealing with neutral gum
and observation and photography under the microscope (Olympus,
Tokyo, Japan).
Western blotting
Tissues stored at −20°C to be used were added with
lysis solution, followed by ice bath for 60 min and centrifugation
at 14,000 × g for 10 min at 4°C. The protein was extracted with
ProteoPrep® Total Extraction Sample Kit (Sigma-Aldrich,
Darmstadt, Germany) and quantified using the bicinchoninic acid
(BCA) method. The standard curve and absorbance were obtained in a
microplate reader (Bio-Rad Laboratories, Inc.), based on which the
protein concentration was calculated. After protein denaturation,
12 µl sample was added and separated via sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) in the corresponding
concentration. When the Marker protein ran to the bottom of the
glass plate, and the sample protein sank to the bottom basically in
a straight line, the gel running was terminated. The protein was
transferred onto a polyvinylidene fluoride (PVDF) membrane, sealed
with 5% milk at 20°C for 1.5 h and washed with TPBS 3 times. Then,
anti-BMP-3 primary antibody (1:1,000) and secondary antibody
(1:1,000) were added successively, and the membrane was rinsed with
Tris-buffered saline with Tween®−20 (TBST) once between
every two steps. The color was developed after the secondary
antibody was removed using TBST. The membrane was placed into the
chemiluminesence reagent for reaction for 1 min, and the color was
developed in the dark, followed by densitometry using the gel
scanning imaging system Quantity One software (Bio-Rad,
Laboratories, Inc., Hercules, CA, USA).
qPCR
The total ribonucleic acid (RNA) was extracted from
the tissues stored at −20°C using the RNA extraction kit and it was
reverse transcribed into complementary deoxyribonucleic acid (cDNA)
using the reverse transcription kit. The reaction system was 20 µl,
and reaction conditions were as follows: reaction at 51°C for 2
min, predenaturation at 96°C for 10 min, denaturation at 96°C for
10 sec and annealing at 60°C for 30 sec, a total of 40 cycles. The
relative expression level of BMP-3 messenger RNA (mRNA) was
analyzed using the 2−ΔΔCq method (6). Primer sequences are shown in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Name | Primer sequences |
|---|
| BMP-3 | F:
5′-ACATCGCTAACCAAGTCTGA-3′ |
|
| R:
5′-GAGCAATAATAGGCATCAAAG-3′ |
| GAPDH | F:
5′-ACGGCAAGTTCAACGGCACAG-3′ |
|
| R:
5′-GAAGACGCCAGTAGACTCCACGAC-3′ |
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 software (IBM Corp., Armonk, NY, USA) was used for statistical
analysis. Enumeration data are presented as mean ± standard
deviation. The t-test was used for data in line with the normal
distribution and homogeneity of variance, corrected t-test was used
for data in line with the normal distribution and heterogeneity of
variance, and non-parametric test was used for data not in line
with the normal distribution and homogeneity of variance.
Chi-square test was used for enumeration data. ANOVA was used for
comparison between multiple groups and the post hoc test was LSD
test.
Results
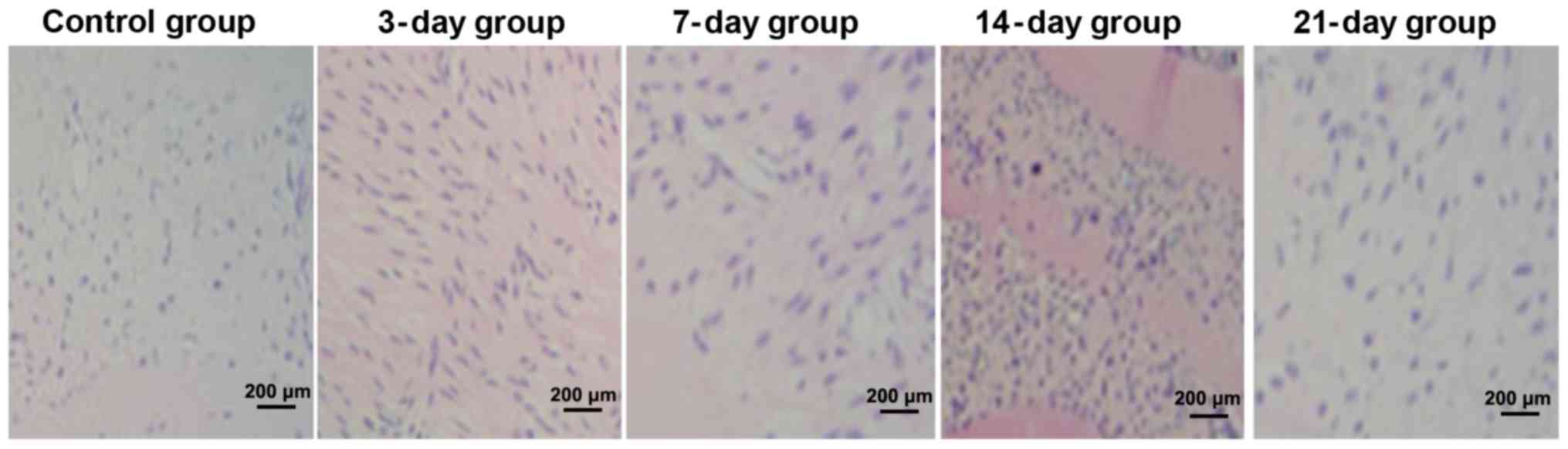
H&E staining
In the control group, the periodontal space was
uniform, the periodontal membrane was tidy and distributed in an
orderly manner without compression and traction, and there were no
obvious multinucleated osteoclasts. In the 3- and 7-day groups, the
periodontal space was not uniform, the periodontal membrane was not
tidy with slight compression and traction, and multinucleated
osteoclasts were visible. In the 14- and 21-day groups, the
periodontal space was uneven, the periodontal membrane was not
uniform and distributed in a disorderly manner with obvious
compression and traction, and there were many multinucleated
osteoclasts (Fig. 1).
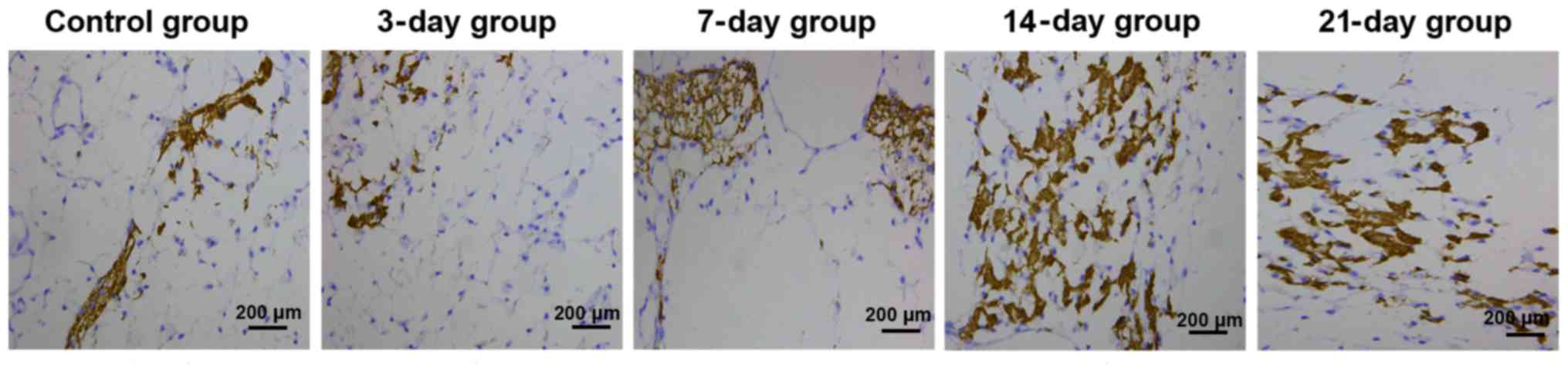
BMP-3 expression level detected
through immunohistochemistry
In the control group, there was no significant
positive expression of BMP-3, and the color of periodontal membrane
was uniform. In the 3-day group, slightly positive expression of
BMP-3 could be seen on the tension side. In the 7-day group, the
positive expression of BMP-3 was enhanced on the tension side,
showing bead string-like distribution. In the 14-day group, there
was significantly positive expression of BMP-3 on the tension side,
with deep color and numerous quantity, showing plate-like
distribution. In the 21-day group, the positive expression of BMP-3
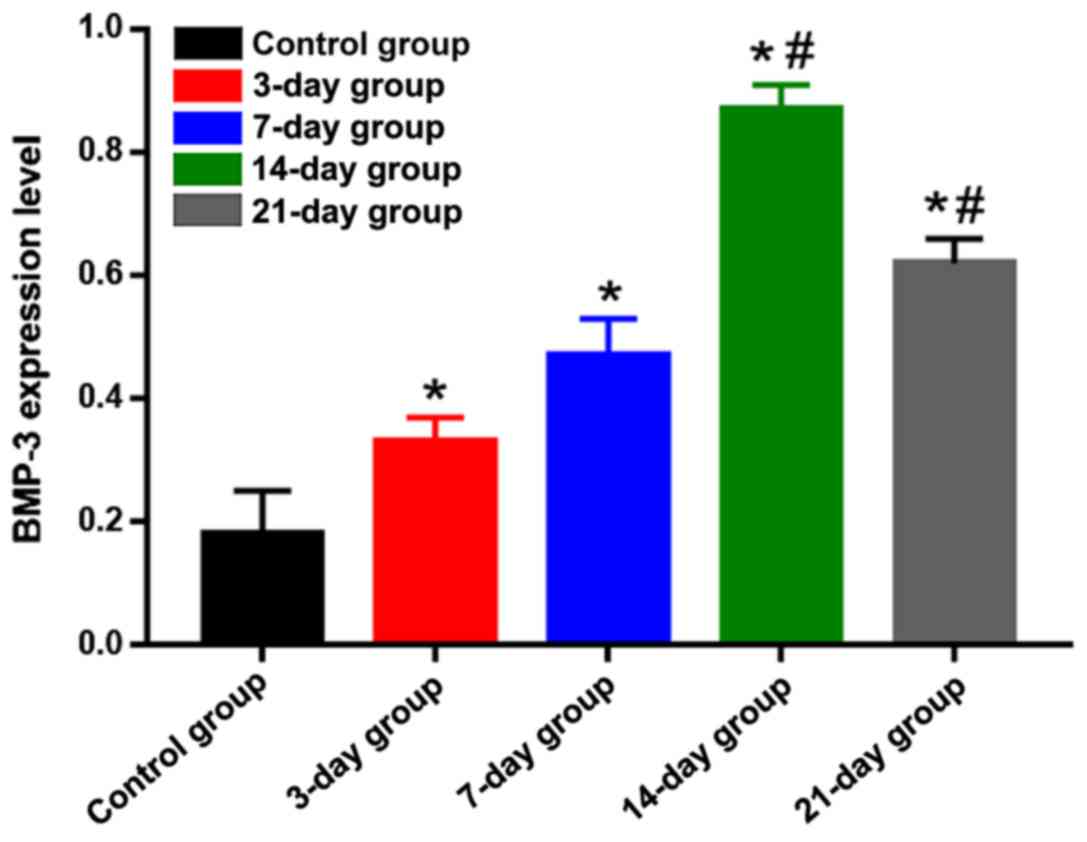
on the tension side was weakened and light brown (Fig. 2). Statistical analysis results of the
positive expression are shown in Fig.
3. Compared with that in the control group, the positive
expression of BMP-3 on the tension side in the other groups was
significantly increased, and differences were statistically
significant (P<0.05). The positive expression of BMP-3 on the
tension side in the 14-day group was the highest, which was
significantly higher than those in the 3-, 7- and 21-day groups,
suggesting that the expression level of BMP-3 on the tension side
is gradually increased in the process of orthodontic tooth
movement, and reaches the peak at day 14 and begins to decline
gradually after that.
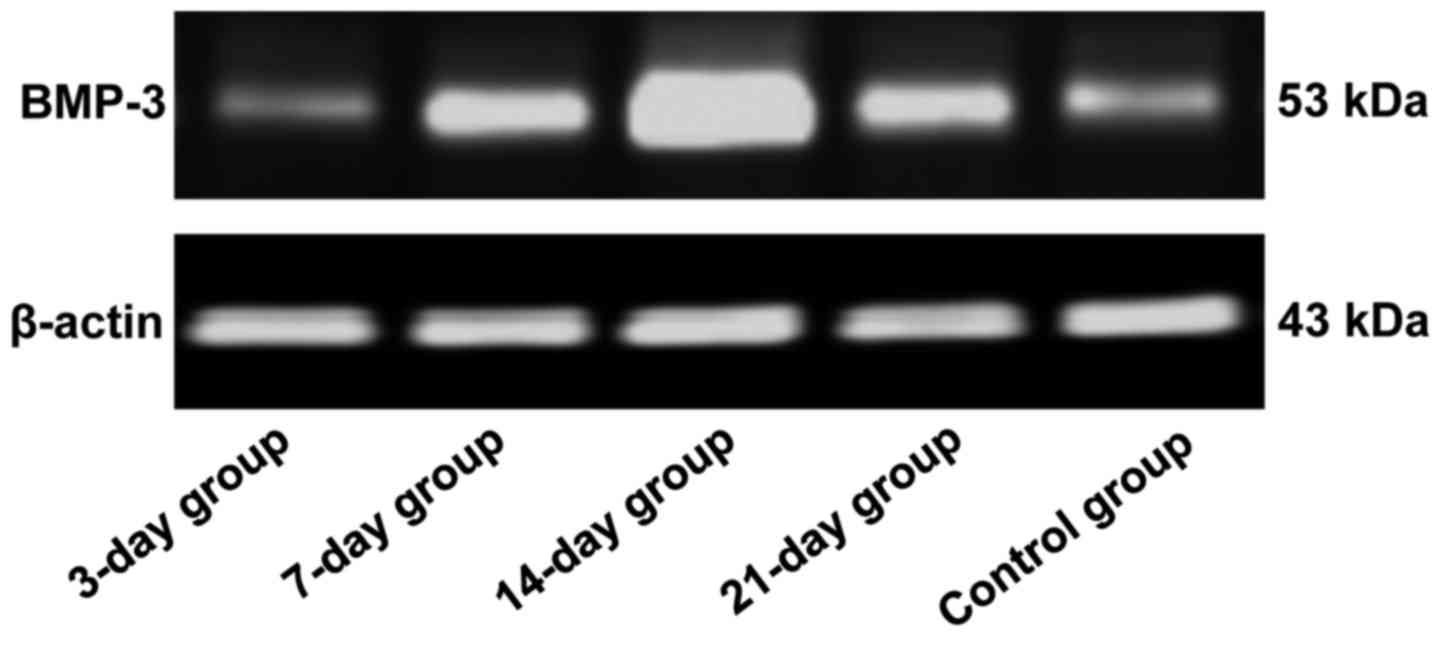
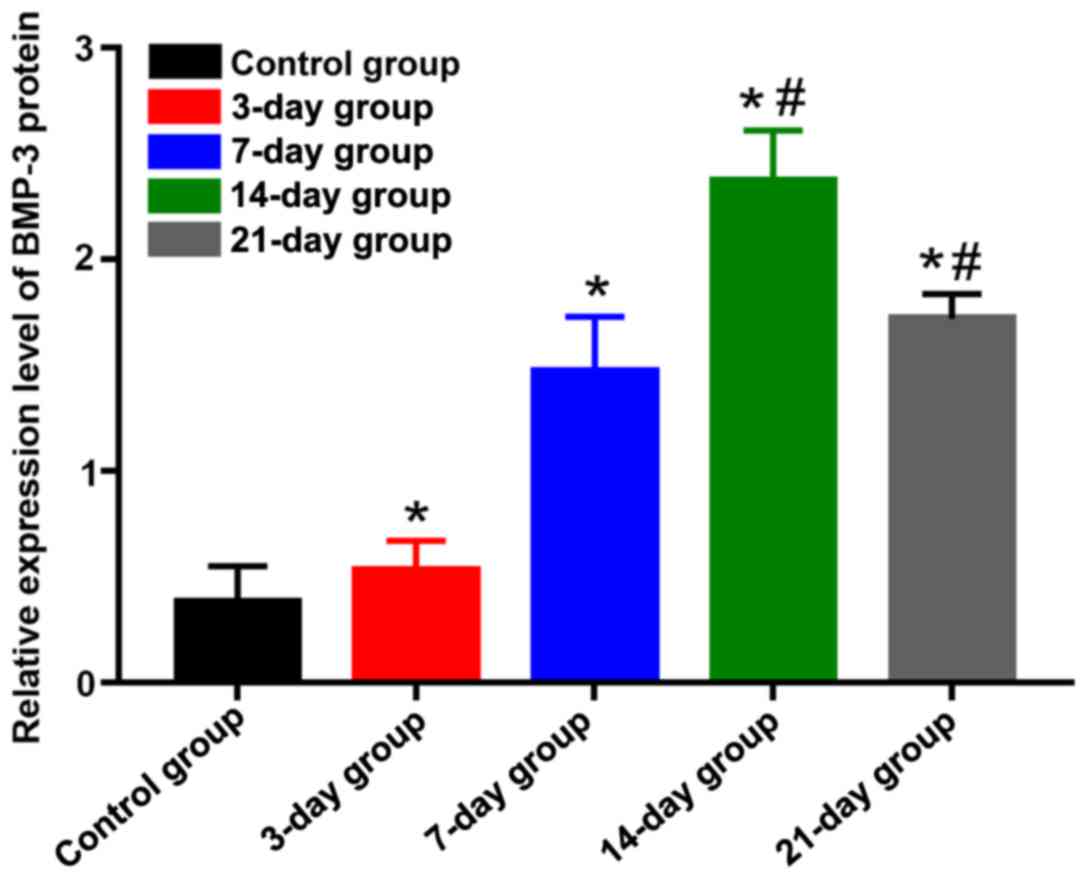
BMP-3 protein expression level
detected via western blotting
The protein expression level of BMP-3 was lower in
the control group, but significantly increased in the other groups,
displaying statistically significant differences (P<0.05). The
protein expression level of BMP-3 in the 14-day group was
significantly higher than those in the 3-, 7- and 21-day groups,
and differences were of statistical significance (P<0.05)
(Figs. 4 and 5), indicating that the protein expression
level of BMP-3 is gradually increased, and the translation ability
is enhanced in the process of orthodontic tooth movement. The
protein expression level reached the peak at day 14 and began to
decline gradually after that.
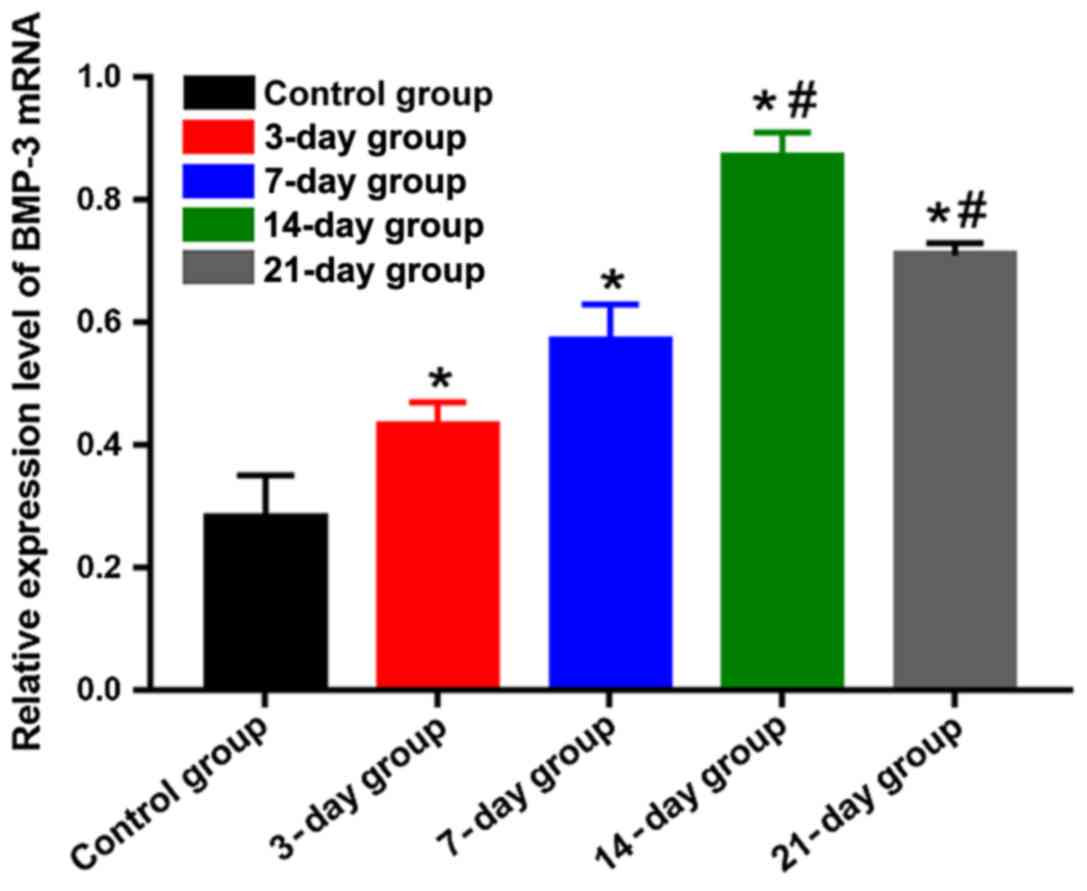
BMP-3 mRNA expression level detected
using qPCR
The mRNA expression level of BMP-3 was lower in the
control group, but it had obvious increases in the other groups,
showing statistically significant differences (P<0.05). The mRNA
expression level of BMP-3 in the 14-day group was the highest,
which was obviously higher than those in the 3-, 7- and 21-day
groups, and differences were statistically significant (P<0.05)
(Fig. 6), indicating that the mRNA
expression level of BMP-3 is gradually elevated, and the
transcription ability is enhanced in the process of orthodontic
tooth movement. The mRNA expression level reached the peak at day
14 and began to decline gradually after that.
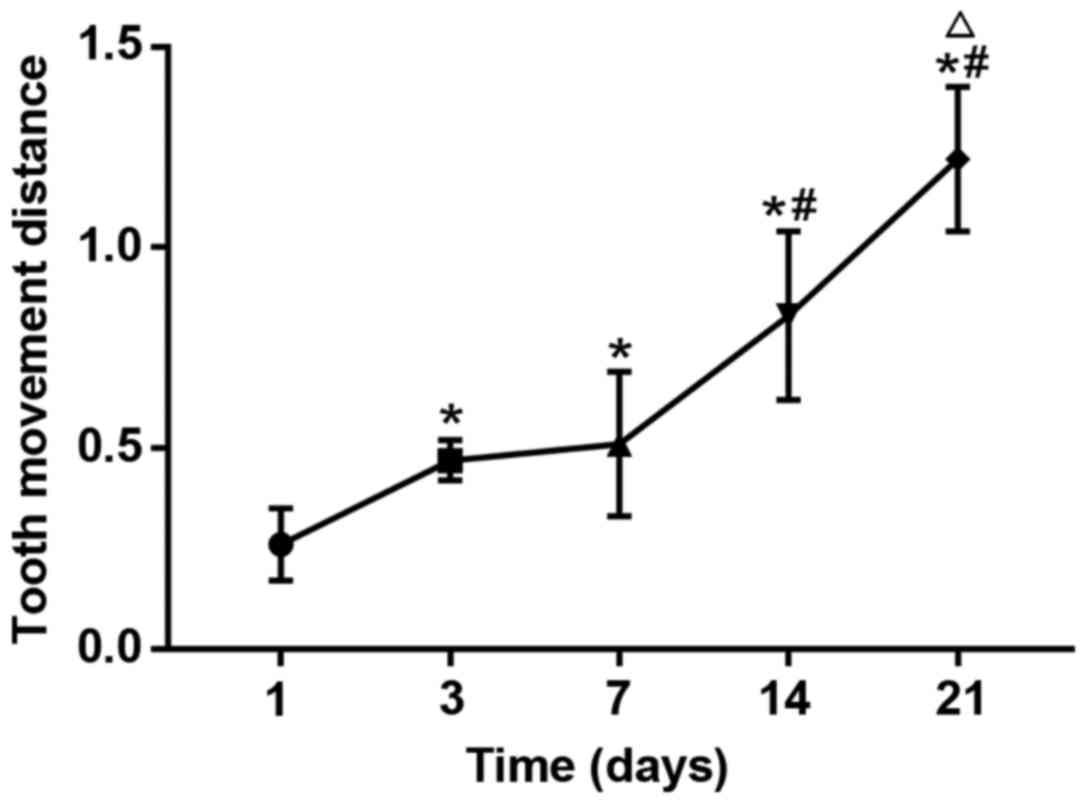
Average tooth movement distance
The tooth movement distance at day 3 after modeling
was remarkably longer than that at day 1 after modeling, and there
was a statistically significant difference (P<0.05). The tooth
movement distances at day 14 and 21 after modeling were increased
significantly, which were obviously longer than those at day 3 and
7 after modeling, and differences were statistically significant
(P<0.05). The tooth movement distance at day 21 after modeling
was remarkably longer than that at day 14 after modeling, and there
was a statistically significant difference (P<0.05) (Fig. 7), suggesting that the tooth movement
distance is shorter in the early stage of orthodontic treatment,
and it is gradually increased with the extension of time. The
longer the time is, the greater the movement distance will be.
Discussion
Tooth orthodontics is a commonly-used oral
orthodontic method in clinic, which is widely used with a good
curative effect (7). When
orthodontic external forces are exerted on the tooth to be
corrected, osteoblasts will accumulate in the alveolar bone on the
tension side, while osteoclasts will accumulate in the alveolar
bone on the pressure side, thereby mediating bone formation and
bone resorption (7–9). In this study, the movement distance of
the rat's molars was measured using the vernier caliper. It was
found that the rat molars moved rapidly in a near-medium distance
in the early ortho-dontic stage, namely within 1–3 days, moved
slowly within 3–7 days and moved significantly quickly from 7 to 14
days. The reason is that the orthodontic external forces lead to
periodontal compression on the pressure side of the rat's molars in
the early orthodontic stage, and the rapid tooth movement is
closely related to the concession of periodontal membrane (10). The hyalinization of periodontal
membrane at 3–7 days after orthodontic treatment is a possible
cause of the slow movement of the rat's molars (11). The external force is positively
correlated with the scope of hyalinization, so the tooth
displacement is difficult, with underlying absorption (12). The rapid tooth movement at 7–14 days
after orthodontic treatment is related to the maximum physiological
remodeling function of periodontal membrane and alveolar bone
(13).
BMP is one of the important subfamilies of TGF-β
superfamily. BMP-3, as a member of the family, has only
approximately 40% similarity to other BMP members (14). BMP-3 has an effect of chemotaxis of
dorsal development (15), which is
similar to the antagonists of other BMP members (16). Therefore, BMP-3 has a certain
inhibitory effect on BMP-2 and BMP-4, two members with the bone
induction effect in the BMP family, thereby inhibiting the BMP-2-
and BMP-4-mediated downstream signaling pathways and suppressing
osteoblasts (17,18). BMP-3, through regulating
TGF-β/activin signaling pathways, can act on downstream Smad-2,
Smad-3 and BMP-2- and BMP-4-activated Smad-1, Smad-5 and Smad-8 to
compete for Smad-4, thus inhibiting BMP-2 and BMP-4 (17,19). In
addition, ActRII-β, as a co-receptor of BMP-2, BMP-3 and BMP-4,
binds to BMP-3 via the chemical bond and is invulnerable to
hydrolysis, and there is a high affinity between them (20). Therefore, BMP-3 competitively
combines with the ActRII-β receptor of BMP-2 and BMP-4 to inhibit
BMP-2 and BMP-4. In this study, the implant anchorage was used to
pull the rat's molars to study the process of tooth movement under
the orthodontic external forces. BMP-3 antagonized the orthodontic
tooth movement process and was expressed more on the tension side
(21). Results of this study
indicated that the staining of osteoclasts in the rat alveolar bone
was deepened with time, and there were increasingly more
osteoclasts, indicating that the orthodontic tooth becomes loose
under the external force, thus facilitating the tooth movement. At
the same time, the expression level of BMP-3 on the tension side of
the alveolar bone was increased with time, and BMP-3 accumulated on
the tension side, inhibiting osteoblasts, so that the
microenvironment of the alveolar bone is conducive to tooth
loosening, facilitating the tooth movement (22). Moreover, the increase of BMP-3
expression was displayed not only at the protein translation level,
but also at the mRNA transcription level, suggesting that BMP-3
plays a positive role of inhibiting the osteogenic components in
the alveolar bone and facilitating the tooth movement in the
process of tooth orthodontic treatment. As a result, in the process
of orthodontic tooth movement, the expression level of BMP-3 is
gradually increased with time and reaches the peak at day 14,
promoting the increase of osteoclasts and benefiting the tooth
movement.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG drafted the manuscript. YG and MZ were mainly
devoted to the establishment of orthodontic tooth movement model.
XT and MW helped with immunohistochemistry and H&E staining. YG
and FZ were responsible for qPCR and western blotting. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingdao Women and Children's Hospital (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krishnan V and Davidovitch Z: Cellular,
molecular, and tissue-level reactions to orthodontic force. Am J
Orthod Dentofacial Orthop. 129(469): e1–32. 2006.
|
|
2
|
Ducy P and Karsenty G: The family of bone
morphogenetic proteins. Kidney Int. 57:2207–2214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen S, Gluhak-Heinrich J, Martinez M, Li
T, Wu Y, Chuang HH, Chen L, Dong J, Gay I and MacDougall M: Bone
morphogenetic protein 2 mediates dentin sialophosphoprotein
expression and odontoblast differentiation via NF-Y signaling. J
Biol Chem. 283:19359–19370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daluiski A, Engstrand T, Bahamonde ME,
Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V and Lyons KM: Bone
morphogenetic protein-3 is a negative regulator of bone density.
Nat Genet. 27:84–88. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bahamonde ME and Lyons KM: BMP3: To be or
not to be a BMP. J Bone Joint Surg Am. 83-A Suppl 1:S56–S62.
2001.
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2 (Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren Y, Maltha JC and Kuijpers-Jagtman AM:
Optimum force magnitude for orthodontic tooth movement: A
systematic literature review. Angle Orthod. 73:86–92.
2003.PubMed/NCBI
|
|
8
|
Dibart S, Yee C, Surmenian J, Sebaoun JD,
Baloul S, Goguet-Surmenian E and Kantarci A: Tissue response during
Piezocision-assisted tooth movement: A histological study in rats.
Eur J Orthod. 36:457–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wise GE and King GJ: Mechanisms of tooth
eruption and orthodontic tooth movement. J Dent Res. 87:414–434.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagaie M, Nishiura A, Honda Y, Fujiwara S
and Matsumoto N: A comprehensive mixture of tobacco smoke
components retards orthodontic tooth movement via the inhibition of
osteoclastogenesis in a rat model. Int J Mol Sci. 15:18610–18622.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garlet TP, Coelho U, Silva JS and Garlet
GP: Cytokine expression pattern in compression and tension sides of
the periodontal ligament during orthodontic tooth movement in
humans. Eur J Oral Sci. 115:355–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dilsiz A, Kiliç N, Aydin T, Ates FN, Zihni
M and Bulut C: Leptin levels in gingival crevicular fluid during
orthodontic tooth movement. Angle Orthod. 80:504–508. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weltman B, Vig KW, Fields HW, Shanker S
and Kaizar EE: Root resorption associated with orthodontic tooth
movement: A systematic review. Am J Orthod Dentofacial Orthop.
137:462–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allendorph GP, Vale WW and Choe S:
Structure of the ternary signaling complex of a TGF-beta
superfamily member. Proc Natl Acad Sci USA. 103:7643–7648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bami M, Mavrogenis AF, Angelini A,
Milonaki M, Mitsiokapa E, Stamoulis D and Soucacos PN: Bone
morphogenetic protein signaling in musculoskeletal cancer. J Cancer
Res Clin Oncol. 142:2061–2072. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hino J, Nishimatsu S, Nagai T, Matsuo H,
Kangawa K and Nohno T: Coordination of BMP-3b and cerberus is
required for head formation of Xenopus embryos. Dev Biol.
260:138–157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsubara T, Kida K, Yamaguchi A, Hata K,
Ichida F, Meguro H, Aburatani H, Nishimura R and Yoneda T: BMP2
regulates Osterix through Msx2 and Runx2 during osteoblast
differentiation. J Biol Chem. 283:29119–29125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Sayed Fawzy KM, Dörfer C, Ungefroren H,
Kassem N, Wiltfang J and Paris S: Effect of Emdogain enamel matrix
derivative and BMP-2 on the gene expression and mineralized nodule
formation of alveolar bone proper-derived stem/progenitor cells. J
Craniomaxillofac Surg. 42:568–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muraoka R, Nakano K, Kurihara S, Yamada K
and Kawakami T: Immunohistochemical expression of heat shock
proteins in the mouse periodontal tissues due to orthodontic
mechanical stress. Eur J Med Res. 15:475–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koch FP, Weinbach C, Hustert E, Al-Nawas B
and Wagner W: GDF-5 and BMP-2 regulate bone cell differentiation by
gene expression of MSX1, MSX2, Dlx5, and Runx2 and influence OCN
gene expression in vitro. Int J Periodontics Restorative Dent.
32:285–293. 2012.PubMed/NCBI
|
|
21
|
Wang Y, Gao S, Jiang H, Lin P, Bao X,
Zhang Z and Hu M: Lithium chloride attenuates root resorption
during orthodontic tooth movement in rats. Exp Ther Med. 7:468–472.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bao X, Hu M, Zhang Y, Machibya F, Zhang Y,
Jiang H and Yu D: Effect of fangchinoline on root resorption during
rat orthodontic tooth movement. Korean J Orthod. 42:138–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|