Introduction
The pathogenesis of gout is an inflammatory joint
disease caused by sodium urate crystal deposited in the joint in
vitro. The disease is mainly triggered by hyperuricemia
(1), which occurs more frequently in
men aged >40 years and post-menopausal women (2). Previous studies have suggested that
~10% patients with raised uric acid have inflammatory responses
resulting from deposition of serum uric acid in the form of sodium
salt at the joints, finally having the onset of gout (3).
Currently, the treatment of gout mainly focuses on
lowering the uric acid level, and it is generally advised to
control the uric acid level at 6.0 mg/ml or below, so as to
alleviate the patients' clinical symptoms (4). Allopurinol is the most commonly applied
medicine in clinic, which can effectively suppress the production
of uric acid in the patients. However, the clinical application of
the drug is restricted among the yellow race, especially the
Chinese Han population, because of the existence of positive rate
of human leukocyte antigen-B (HLA-B)*5801 allele that may result in
hypersensitivity reactions and even death of the patients after the
use of allopurinol (5). Studies have
confirmed that febuxostat, as a new type of xanthine oxidase
inhibitor, can effectively decrease the uric acid level in the body
of patients, and it becomes increasingly recognized in clinical
practice (6). In order to better
investigate the clinical effects of febuxostat on treating gout,
the major purpose of this study is to analyze the influence of
febuxostat on the primary inflammation-associated cytokines and
cyclooxygenase-2 (COX-2) in the serum of gout patients.
Patients and methods
General data
A total of 80 patients with gout admitted and
treated in the Affiliated Zhongshan Hospital of Dalian University
(Dalian, China) from January 2015 to September 2017 were selected.
The diagnosis of all patients was confirmed by virtue of clinical
manifestations and laboratory examinations. The patients themselves
or their authorized persons signed the consent before enrollment,
and this study was approved by the Ethics Committee of the
Affiliated Zhongshan Hospital of Dalian University. Patients with
the following conditions were enrolled: full capacity for civil
conduct, normal mental status, normal audition, language and other
expression abilities as well as educational level at or higher than
primary education. Patients complicated with other endocrine system
diseases, systemic immune system diseases, diabetes mellitus, limb
fracture or allergy to the drugs applied, or pregnant and
breast-feeding women were excluded. All the patients were divided
into two groups by means of a random number table, with 40 patients
in each group. In the observation group, there were 30 males and 10
females aged 18–60 years, with an average age of 42.3±2.1 years.
The course of disease was 3 months to 15 years, with an average
course of 4.1±0.3 years. A total of 25 patients had apparent
crystals of gout, and 15 patients only had pain of limb joints. The
control group included 31 males and 9 females aged 18–60 years,
with an average age of 42.4±2.0 years. The course of disease ranged
from 3 months to 15 years, with an average course of 4.0±0.3 years.
There were 24 cases of apparent crystals of gout and 16 cases of
mere pain of limb joints. The differences in sex, age, course of
disease and major clinical manifestations between the two groups
were not statistically significant (p>0.05).
Methods
All the enrolled patients received strict gout diet
adjustment and took colchicine [national medicine permission number
(NMPN) H53021798; Yunnan Haopy Pharmaceutical Co., Ltd., Yunnan,
China] (0.5 mg/time, 3 times a day) at the same time. Symptomatic
and supporting therapy with non-steroidal anti-inflammatory drugs
was conducted when patients had obvious pain. Patients in the
control group were additionally treated with allopurinol (NMPN
H31020334; Shanghai Sine Pharmaceutical Co., Ltd., Shanghai, China)
(100 mg/time, 3 times a day). Furthermore, the consent of
medication was signed by the patients before laparotomy and drug
administration, and they were informed of the possibility of
hypersensitivity reactions in detail. In the observation group,
patients were administered with febuxostat (NMPN H20130081; Jiangsu
Hengrui Medicine Co., Ltd., Jiangsu, China) (40 mg/time, once a
day). Treatment conducted for 8 consecutive weeks was regarded as a
course of treatment.
Observation indexes
All the enrolled patients were followed up and
observed through out- and in-patient follow-up for 3 consecutive
months. The serum uric acid levels before treatment and at 1 week,
1 and 3 months after treatment were compared between the two
groups. Moreover, the levels of interleukin (IL)-1, IL-4, IL-6 and
IL-8 at 3 months after treatment as well as changing trends of IL-1
and tumor necrosis factor-α (TNF-α) during the observation period
in both groups were compared. The gout attacks that needed medical
intervention during the follow-up period were recorded, and the
variations in COX-2 positive value integral before and after
treatment were clarified.
Evaluation criteria
Serum uric acid (phosphotungstic acid deoxidizing
method: 149–416 µmol/l). Testing methods and normal values of
related inflammatory factors: IL-1 [enzyme-linked immunosorbent
assay (ELISA): 0.13–0.25 µg/l], IL-4 (ELISA: ≤31.2 µg/l), IL-6
(ELISA: 67.37–142.33 ηg/l), IL-8 (ELISA: 0.317–0.329 µg/l) and
TNF-α (ELISA: 1.10–1.18 g/l). The COX-2 level was detected using
reverse transcription-polymerase chain reaction (RT-PCR), whose
expression was evaluated through positive scores, that is, the
staining distribution under every high-power field was scored: 0
point (no staining), 1 point (light yellow staining), 2 points
(yellowish brown staining), 3 points (tawny staining) and 4 points
(brown staining). Higher scores indicated stronger COX-2
expression.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
21.0 software (IBM Corp., Armonk, NY, USA) was applied. Measurement
data in the enrolled information, such as inflammatory cytokine
(IL-1, IL-4, IL-6, IL-8 and TNF-α) levels and COX-2, were presented
as mean ± standard deviation (means ± SD). t-test was adopted for
intergroup comparisons, repeated measures analysis of variance was
conducted for intragroup comparison of means and the post hoc test
was Dunnetts test. χ2 test was performed for comparison
of adverse reaction rate. P<0.05 suggested that the difference
was statistically significant.
Results
Comparison of serum uric acid during
follow-up between the two groups
There was no statistically significant difference in
the serum uric acid among all the enrolled patients before
treatment (p>0.05). At 1 week, 1 and 3 months after treatment,
the serum uric acid level was decreased markedly in all the
enrolled patients (p<0.05), and the level in the observation
group was obviously lower than that in the control group
(p<0.05) (Table I).
 | Table I.Comparison of serum uric acid during
follow-up between the two groups (means ± SD). |
Table I.
Comparison of serum uric acid during
follow-up between the two groups (means ± SD).
| Groups | Before treatment | 1 week after
treatment | 1 month after
treatment | 3 months after
treatment | F | P-value |
|---|
| Observation | 635.6±15.9 | 415.6±12.1 | 321.1±10.0 | 256.3±5.6 | 13.589 | <0.001 |
| Control | 636.9±16.0 | 568.9±13.3 | 451.5±12.3 | 329.8±7.9 |
8.693 |
0.013 |
| t | 0.364 | 53.923 | 52.026 | 48.005 | – | – |
| P-value | 0.716 | <0.001 | <0.001 | <0.001 | – | – |
Comparison of IL-1, IL-4, IL-6 and
IL-8 levels at 3 months after treatment between the two groups
Compared with those in the control group, the levels
of IL-1, IL-4, IL-6 and IL-8 were notably lower at 3 months after
treatment in the observation group (p<0.05) (Table II).
 | Table II.Comparison of IL-1, IL-4, IL-6 and
IL-8 levels at 3 months after treatment between the two groups
(means ± SD). |
Table II.
Comparison of IL-1, IL-4, IL-6 and
IL-8 levels at 3 months after treatment between the two groups
(means ± SD).
| Groups | IL-1 (µg/l) | IL-4 (µg/l) | IL-6 (ng/l) | IL-8 (µg/l) |
|---|
| Observation | 0.16±0.01 | 28.5±1.5 | 124.1±2.7 | 0.312±0.001 |
| Control | 0.86±0.11 | 59.8±2.6 | 205.3±5.9 | 0.419±0.012 |
| t | 40.082 | 65.950 | 79.149 | 56.199 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
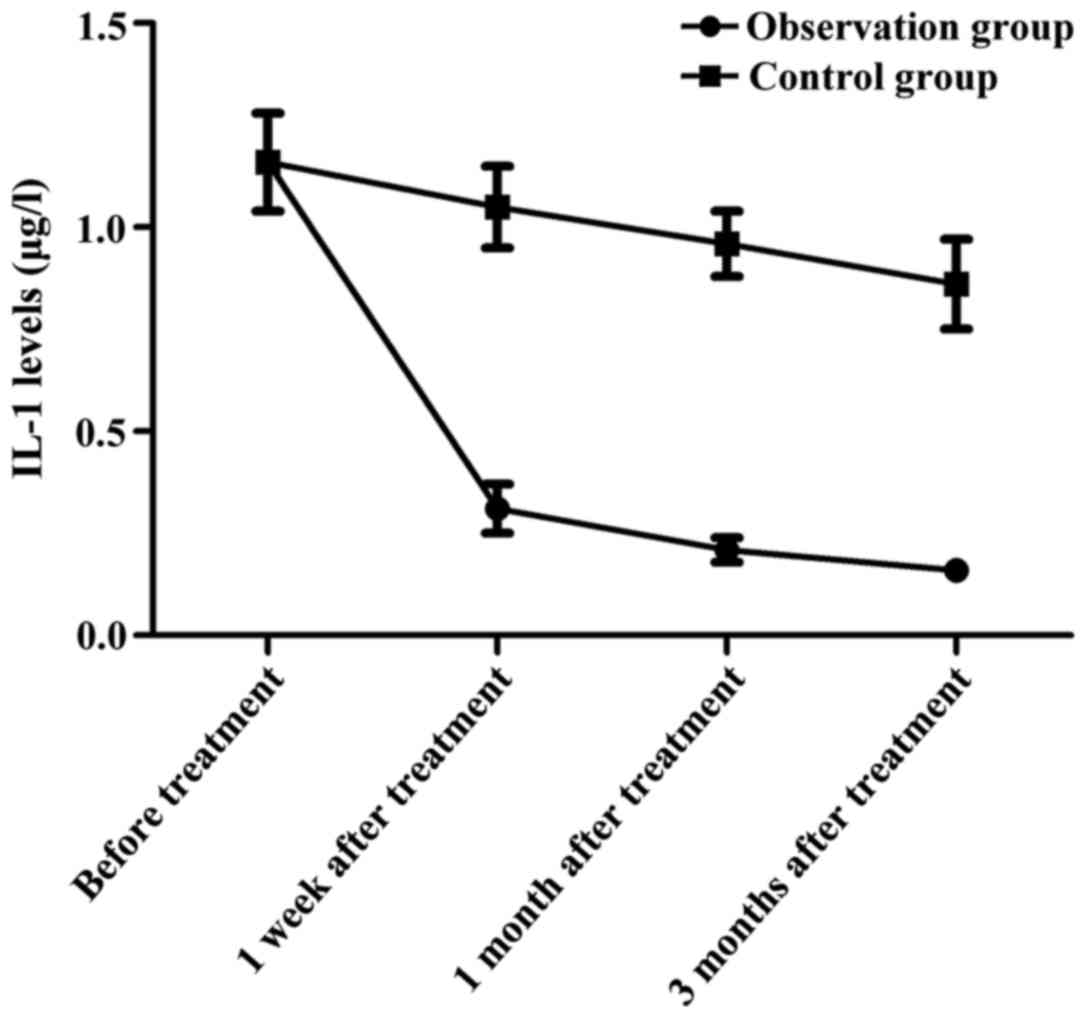
Change in trends of IL-1 at different
time-points of observation in both groups
In the observation group, the IL-1 level was
1.16±0.12 µg/l before treatment, 0.31±0.06 µg/l at 1 week after
treatment, 0.21±0.03 µg/l at 1 month after treatment and 0.16±0.01
µg/l at 3 months after treatment. In the control group, the IL-1
levels before treatment and at 1 week, 1 and 3 months after
treatment were 1.16±0.12, 1.05±0.10, 0.96±0.08 and 0.86±0.11 µg/l,
respectively. At 1 week, 1 and 3 months after treatment, the
observation group had significantly lower IL-1 levels than the
control group in the same time period (p<0.05) (Fig. 1).
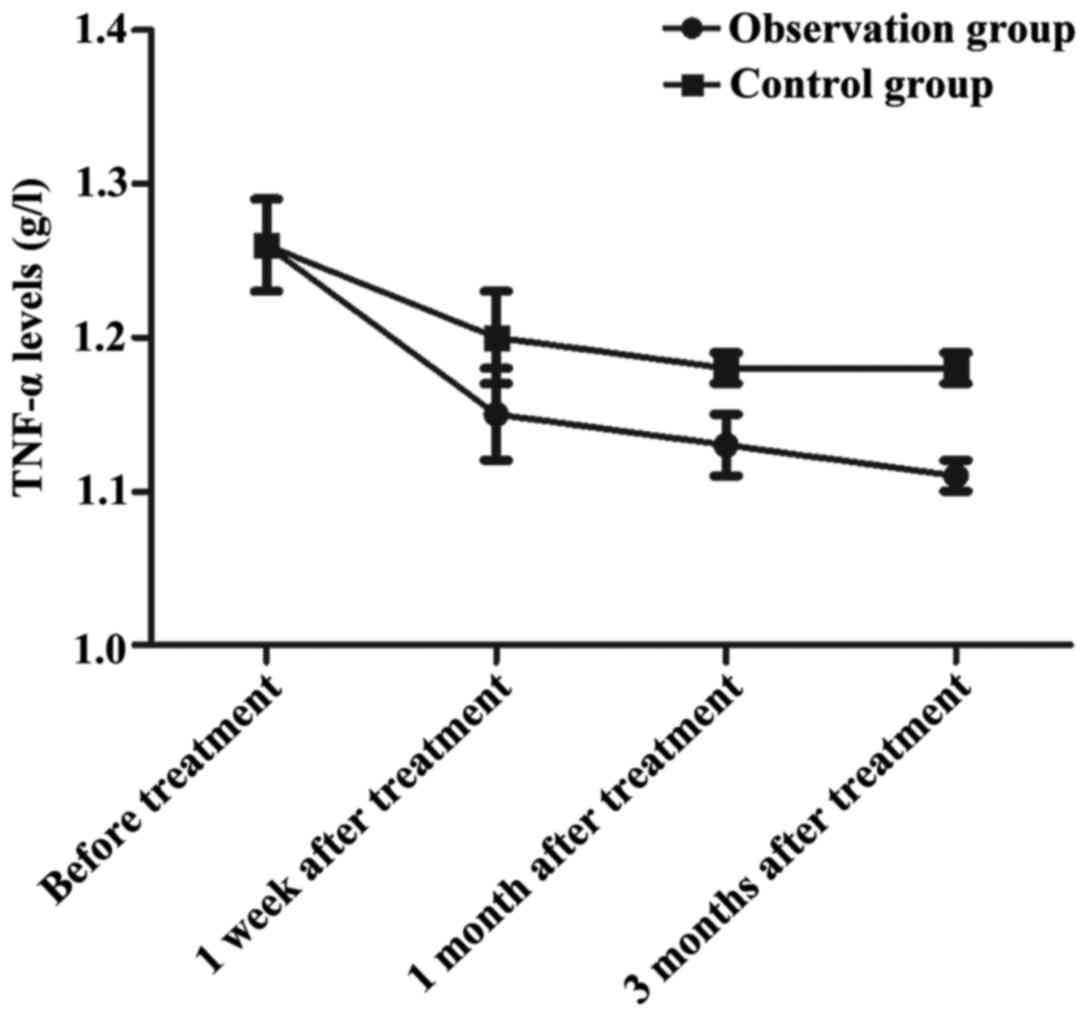
Change in trends of TNF-α at different
time-points of observation in the groups
In the observation group, the TNF-α level was
1.26±0.03 g/l before treatment, 1.15±0.03 g/l at 1 week after
treatment, 1.13±0.02 g/l at 1 month after treatment and 1.11±0.01
g/l at 3 months after treatment. In the control group, the TNF-α
levels before treatment and at 1 week, 1 and 3 months after
treatment were 1.26±0.03, 1.20±0.03, 1.18±0.01 and 1.18±0.01 g/l,
respectively. The TNF-α levels in the observation group at 1 week,
1 and 3 months after treatment were decreased remarkably compared
with those in the control group in the same time period (p<0.05)
(Fig. 2).
Comparison of COX-2 positive value
integrals before and after treatment between the two groups
The difference in the COX-2 positive value integral
was not statistically significant between the two groups before
treatment (p>0.05). After treatment, however, the COX-2 positive
value integral in the observation group was better than that before
treatment and that in the control group after treatment (p<0.05)
(Table III).
 | Table III.Comparison of COX-2 positive value
integrals before and after treatment between the two groups (point,
means ± SD). |
Table III.
Comparison of COX-2 positive value
integrals before and after treatment between the two groups (point,
means ± SD).
| Groups | Before treatment | After treatment | F | P-value |
|---|
| Observation | 3.1±0.2 | 0.6±0.1 | 70.711 | <0.001 |
| Control | 3.1±0.2 | 2.1±0.3 | 17.541 | <0.001 |
| t | 0.000 | 30.000 | – | – |
| P-value | >0.001 | <0.001 | – | – |
Comparison of gout attacks that needed
medical intervention during follow-up
There was no statistically significant difference in
the frequency of gout attacks that needed medical intervention
among the enrolled patients before treatment (p>0.05). At 1
week, 1 and 3 months after treatment, the number of gout attacks
that needed medical intervention was decreased markedly in all the
enrolled patients compared with that before treatment (p<0.05),
and the number in the observation group was apparently smaller than
that in the control group (p<0.05) (Table IV).
 | Table IV.Comparison of gout attacks that needed
medical intervention during follow-up (time/month, (means ±
SD). |
Table IV.
Comparison of gout attacks that needed
medical intervention during follow-up (time/month, (means ±
SD).
| Groups | Before treatment | 1 week after
treatment | 1 month after
treatment | 3 months after
treatment | F | P-value |
|---|
| Observation | 3.1±0.2 | 1.3±0.2 | 1.1±0.1 | 0.9±0.1 | 21.362 | <0.001 |
| Control | 3.1±0.3 | 2.3±0.1 | 1.8±0.1 | 1.5±0.2 | 12.305 | <0.001 |
| t | 0.000 | 28.284 | 31.305 | 16.971 | – | – |
| P-value | >0.001 | <0.001 | <0.001 | <0.001 | – | – |
Comparison of adverse reactions that
occurred during treatment between the two groups
Comparisons of adverse reactions or complications
detected during treatment between the two groups showed no
statistically significant differences, with no comparability
(p>0.05) (Table V).
 | Table V.Comparison of adverse reactions that
occurred during treatment between the two groups [n (%)]. |
Table V.
Comparison of adverse reactions that
occurred during treatment between the two groups [n (%)].
| Groups | Hyperlipidemia | Gastrointestinal
discomfort | Liver and kidney
injury | Hypersensitivity
reaction | Total incidence |
|---|
| Observation
group | 1 | 2 | 1 | 2 | 6 (15.0%) |
| Control group | 1 | 1 | 1 | 1 | 4 (10.0%) |
| t |
| – |
| 0.114 |
| χ2 |
| – |
| 0.735 |
Discussion
In recent years, with the increase in China's
national economy and changes in people's living standard and diet
style, the incidence rate of hyperuricemia is obviously increasing
(7). Studies have demonstrated that
(8) hyperuricemia is associated with
inheritance, medicine, past history of renal diseases, and intake
of high purine and protein diet. When purine metabolism disorder
occurs in the body, it causes overproduction and excretion
reduction of uric acid in vitro at the same time (9). At this time, ~1/10 patients with
hyperuricemia have uric acid deposited in the joints, soft tissues
and kidneys in the form of sodium salt, which triggers inflammatory
responses at the above-mentioned positions with uric acid
deposition, further manifesting as gout attacks (10). As a kind of metabolism-related joint
disease induced by sodium urate deposition, gout is mainly a local
inflammatory response that results from disorders of the purine
metabolism (11). In severe cases,
it can lead to the occurrence of renal lesions and damage to joint
functions, thus affecting the quality of life and even threatening
the life safety of the patients (12). As a result, inflammatory response
factors have close correlations with the occurrence and development
of gout. In previous treatments, the two most classic and important
drugs (allopurinol and colchicine) are utilized, of which
allopurinol is especially widely applied in clinic. However, it is
used with vigilance in clinical practice due to its inevitable
hypersensitivity reactions (13).
Therefore, it is urgent in clinic to find an alternative drug for
patients unsuitable for allopurinol.
In this study, all the enrolled patients were
definitely diagnosed with gout, and colchicine as well as
symptomatic and supporting therapy with non-steroidal
anti-inflammatory drug was utilized on the basis of diet for gout.
Patients in the control group were treated with allopurinol, while
those in the observation group were given febuxostat. The study on
the serum uric acid level after intervention discovered that in
spite of the urate-lowering effects of the drugs, the serum uric
acid levels in the observation group were decreased significantly
compared with those in the control group at 1 week, 1 and 3 months
after treatment. It demonstrated that patients treated with
febuxostat have better effectiveness in lowering the uric acid. The
changes in the inflammation-associated cytokines at 3 months after
treatment in both groups were investigated, and it was revealed
that the levels of IL-1, IL-4, IL-6 and IL-8 at 3 months after
treatment in the observation group were remarkably lower than those
in the control group, implying that febuxostat plays a positive
role in reducing inflammatory responses in the body. Moreover, the
study findings of the change in trends of IL-1 and TNF-α at
different time-points of observation in the two groups indicated
that at 1 week, 1 and 3 months after treatment, the observation
group had significantly decreased IL-1 and TNF-α levels than the
control group in the same time period. Furthermore, it indicated
that as for gout patients, the febuxostat therapy is more effective
in suppressing inflammatory responses in the body, and the effects
start at 1 week after medication. In addition, the COX-2 positive
value integrals before and after treatment were compared between
the two groups, and the results revealed that the COX-2 positive
value integral in the observation group after treatment was
superior to that before treatment and that in the control group
after treatment. It implied that on treating patients with gout,
febuxostat can selectively inhibit the activity of
prostaglandin-endoperoxide synthase 2 in the body, thereby reducing
local inflammatory responses and relieving the clinical symptoms of
the patients. Finally, the comparison of gout attack frequency and
adverse reactions that occurred during the treatment indicated that
during follow-up, the number of gout attacks in the observation
group was obviously smaller than that in the control group, and the
incidence of adverse reactions of medication were <15% in both
groups. Besides, the differences were not statistically
significant.
For gout patients, febuxostat was applied in the
observation group based on the conventional treatment in this
research (14), and it had more
prominent clinical effects in patients than allopurinol. As a novel
xanthine oxidase inhibitor (15),
febuxostat can repress the activity of xanthine oxidase in an
efficient manner and further avoid the allopurinol-induced adverse
reactions in a selective way (16).
The possible mechanism of action is that it inhibits the
transformation of hypoxanthine into xanthine by means of oxidation
(17) and reduces the formation of
uric acid as much as possible. Compared with allopurinol (18), febuxostat averts the inhibitory
effects on nucleotidase and deaminase in the processes of purine or
pyrimidine metabolism through the selective inhibitory effect
(19), enhances the efficacy of
medical treatment and reduces the occurrence of hypersensitivity
reactions of allopurinol (20).
In conclusion, compared with allopurinol therapy,
febuxostat therapy can remarkably inhibit inflammatory responses in
the body, relieve clinical symptoms and reduce relapse of the
patients with gout.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GH and WD were responsible for treating patients and
collecting data of patients. JS detected the serum uric acid
levels. JL contributed to PCR. GH, WD and BP contributed to ELISA.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Zhongshan Hospital of Dalian University (Dalian,
China) and informed consents were signed by the patients or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richette P, Latourte A and Bardin T:
Cardiac and renal protective effects of urate-lowering therapy.
Rheumatology (Oxford). 57 suppl_1:i47–i50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foody J, Turpin RS, Tidwell BA, Lawrence D
and Schulman KL: Major cardiovascular events in patients with gout
and associated cardiovascular disease or heart failure and chronic
kidney disease initiating a xanthine oxidase inhibitor. Am Health
Drug Benefits. 10:393–401. 2017.PubMed/NCBI
|
|
3
|
Kim Y, Oh HC, Park JW, Kim IS, Kim JY, Kim
KC, Chae DS, Jo WL and Song JH: Diagnosis and treatment of
inflammatory joint disease. Hip Pelvis. 29:211–222. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anandh U and Jayanna K: Nontubercular
mycobacterial infection in a renal allograft recipient. Indian J
Nephrol. 27:478–481. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beslon V, Moreau P, Maruani A, Maisonneuve
H, Giraudeau B and Fournier JP: Effects of discontinuation of
urate-lowering therapy: A systematic review. J Gen Intern Med.
33:358–366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruiz Perez F, Sanchez-Piedra CA,
Sanchez-Costa JT, Andres M, Diaz-Torne C, Jimenez-Palop M, De
Miguel E, Moragues C and Sivera F: Improvement in diagnosis and
treat-to-target management of hyperuricemia in gout: Results from
the GEMA-2 transversal study on practice. Rheumatol Ther.
5:243–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones G, Panova E and Day R: Guideline
development for the management of gout: Role of combination therapy
with a focus on lesinurad. Drug Des Devel Ther. 11:3077–3081. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamanaka H, Tamaki S, Ide Y, Kim H, Inoue
K, Sugimoto M, Hidaka Y, Taniguchi A, Fujimori S and Yamamoto T:
Stepwise dose increase of febuxostat is comparable with colchicine
prophylaxis for the prevention of gout flares during the initial
phase of urate-lowering therapy: Results from FORTUNE-1, a
prospective, multicentre randomised study. Ann Rheum Dis.
77:270–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou C, Xue C, Yang B, Wang W, Xu Y, Huang
F and Wang Y: Amputation of the first metatarsophalangeal joint due
to a giant gouty tophi: A case report. Medicine (Baltimore).
96:e84412017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sheer R, Null KD, Szymanski KA, Sudharshan
L, Banovic J and Pasquale MK: Predictors of reaching a serum uric
acid goal in patients with gout and treated with febuxostat.
Clinicoecon Outcomes Res. 9:629–639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collison J: Crystal arthritis: Febuxostat
reduces synovitis in early gout. Nat Rev Rheumatol. 13:6942017.
View Article : Google Scholar
|
|
12
|
Cutolo M, Cimmino MA and Perez-Ruiz F:
Potency on lowering serum uric acid in gout patients: A pooled
analysis of registrative studies comparing febuxostat vs.
allopurinol. Eur Rev Med Pharmacol Sci. 21:4186–4195.
2017.PubMed/NCBI
|
|
13
|
Huneycutt E, Board C and Clements JN:
Lesinurad, a selective URAT-1 inhibitor with a novel mechanism in
combination with a xanthine oxidase inhibitor, for hyperuricemia
associated with gout. J Pharm Pract. Jan 1–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sanchez-Niño MD, Zheng-Lin B, Valiño-Rivas
L, Sanz AB, Ramos AM, Luño J, Goicoechea M and Ortiz A: Lesinurad:
What the nephrologist should know. Clin Kidney J. 10:679–687. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalbeth N, Saag KG, Palmer WE, Choi HK,
Hunt B, MacDonald PA, Thienel U and Gunawardhana L: Effects of
febuxostat in early gout: A randomized, double-blind,
placebo-controlled study. Arthritis Rheumatol. 69:2386–2395. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies K and Bukhari MAS: Recent
pharmacological advances in the management of gout. Rheumatology
(Oxford). 2017:1093–1096. 2017.
|
|
17
|
Plumpton CO, Alfirevic A, Pirmohamed M and
Hughes DA: Cost effectiveness analysis of HLA-B*58:01 genotyping
prior to initiation of allopurinol for gout. Rheumatology (Oxford).
56:1729–1739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Britnell SR, Chillari KA and Brown JN: The
role of xanthine oxidase inhibitors in patients with history of
stroke: A systematic review. Curr Vasc Pharmacol. 2017:128–135.
2017.
|
|
19
|
Kim S, Kim HJ, Ahn HS, Oh SW, Han KH, Um
TH, Cho CR and Han SY: Renoprotective effects of febuxostat
compared with allopurinol in patients with hyperuricemia: A
systematic review and meta-analysis. Kidney Res Clin Pract.
36:274–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hill-McManus D, Soto E, Marshall S, Lane S
and Hughes D: Impact of non-adherence on the safety and efficacy of
uric acid-lowering therapies in the treatment of gout. Br J Clin
Pharmacol. 84:142–152. 2018. View Article : Google Scholar : PubMed/NCBI
|