Introduction
Keratinized gingiva extends from the gingival margin
to the muco-gingival junction and is a specialized mucosa that
maintains the underlying osseous stability and a healthy
dentogingival junction (1). Recently
in clinical practice, soft tissue augmentation procedures have
received increasing attention due to their benefits. Thus, gingival
augmentation improves patient comfort, prevents recession of the
gingiva, and facilitates plaque control of teeth where plaque
accumulation leads to inflammation of the surrounding tissues and
mucosal recession (2,3). On the other hand, the use of collagen
matrices instead of connective tissue grafts for root coverage
results in good healing and significantly reduced recession
(4). Various surgical methods and
materials have been described in the literature, but the most
frequent is the repositioned flap and application of an autogenous
free gingival graft or subepithelial connective tissue graft
harvested from the palatal mucosa (5). Acellular dermal matrix grafts and
human-derived dermal substitutes were initially proposed as viable
alternatives (6,7). A two-layer recombinant xenogeneic
collagen matrix and a porcine-derived dermal matrix have been used
more recently, with good results for augmenting keratinized tissue
and recession coverage procedures in teeth and dental implants
(8,9).
Wound healing following periodontal surgery and
placement of the collagen matrix is a complex process, where a
multitude of factors, including the immune system, interact
(10). Many factors can modulate the
regeneration outcome of the oral mucosa once the immune system
contacts the collagen matrix. Among these, T cells are one of the
most important immune cellular effectors, and their role in wound
healing has been described in detail in the oral cavity as well as
other organs and tissues (11–13). T
cells accumulate in the skin and oral mucosa early after a wound
occurs or is created, and specific ablation of these cells results
in delayed wound re-epithelialization and kinetics of wound closure
(14).
However, the molecular interactions between these
matrices, the surrounding tissues, and their cellular components,
such as keratinocytes and immune cells, have not been fully
explored. In a previous study, we showed that resorbable collagen
matrices stimulate the healing process post-surgery, allowing the
formation of new oral epithelia, while being incompletely
integrated into the newly formed soft tissue (15). To the best of our knowledge there are
no studies that explored the interactions between a resorbable
three-dimensional (3D) collagen matrix and T cells. Previous
experimental models showed that activation of the immune system,
particularly T cells, is required for optimal healing following
wounds (16) or surgery in the oral
cavity. Therefore, studies to explore the interactions between the
immune system and the collagen matrix are mandated.
The aim of the present study was to investigate the
effect of a resorbable 3D collagen matrix on activation of
autologous primary T cells and activation-induced cell death in
these cells.
Materials and methods
Patient selection
The present study was designed as a pilot study of
five patients selected from subjects under treatment at the
Department of Periodontology of the Victor Babes University of
Medicine and Pharmacy, Timisoara, Romania. All patients met several
inclusion criteria: Young adults with no local or systemic diseases
and not taking any medication over the past 6 months prior to the
study. The exclusion criteria included smokers, pregnancy, chronic
or acute inflammatory disease or infection, past alcohol abuse,
active periodontal disease, and allergies. The protocol and patient
informed consent process were approved by the Commission of
Research Ethics of the ‘Victor Babes’ University of Medicine and
Pharmacy of Timisoara, Romania (Timisoara, Romania), and written
informed consent was obtained from all patients.
Isolation of mononuclear cells from
peripheral venous blood
Samples of peripheral venous blood were collected
from subjects in anticoagulant (heparin 15,000 IU/5 ml; Biochemie
GmbH, Kundl, Austria). The peripheral blood mononuclear cells
(PBMCs) were separated from peripheral blood by centrifugation on a
Ficoll-Paque™ Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden)
gradient. The blood samples were transferred to 16 ml Falcon
(Becton-Dickinson, Brea, CA, USA) sterile tubes and diluted at 1:1
with PBS. Each sample was transferred to a 50 ml Falcon sterile
tube with Ficoll-Paque, so that the ratio of the diluted sample:
Ficoll was 1:1. The transfer of diluted biological samples over the
Ficoll layer was gentle, without mixing the two components,
allowing the formation of a density gradient. The samples were
centrifuged at 400 × g for 30 min. After centrifugation, the ring
of mononuclear cells at the interface of the components was
harvested and moved to a sterile tube. This step was followed by
two successive washes with PBS and consecutive centrifugations at
100 × g for 10 min. After the second centrifugation, the
supernatant was removed, and the cell pellet was used for further
analysis, or frozen at −80°C (or −196°C) and kept for further
investigation.
3D collagen matrix
PBMCs were seeded on a fully resorbable 3D collagen
matrix specially designed for soft tissue regeneration (Geistlich
Mucograft®; Geistlich Pharma AG, Wolhusen, Switzerland).
This collagen matrix is routinely used for various periodontal
surgical procedures in the Department of Periodontology of the
Victor Babes University of Medicine and Pharmacy.
In vitro growth of PBMCs
The PBMCs were cultured for 48 h in T cell expansion
Stemline media (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany),
supplemented, as specified by the producer, with 10 ml of 200 mM
L-glutamine/500 ml medium to increase the T cell population. After
48 h of in vitro culture, the cells (1×105 T
cells/mm3) were placed in contact with the 3D collagen
matrix and were transferred to 24-well plates. A comparative
analysis was performed between the cells in contact with the 3D
collagen matrix and untreated cells.
Viability tests - Annexin V/propidium
iodide (PI)
The Annexin V/PI method was used in order to detect
late apoptosis. This assay measures changes in the plasma membrane
using Annexin V labeled with a fluorochrome, which binds
specifically to phosphatidylserine normally present on the internal
face of the plasma membrane. In accordance with the manufacturer's
recommendations, 1×106 cells were counted using a
hemocytometer, washed in 1X Annexin V Binding Buffer (BD
Pharmingen, San Diego, CA, USA), centrifuged at 300 × g for 10 min,
resuspended in the same solution, and incubated in 10 ml of Annexin
V-FITC for 15 min in the dark. After washing the cells with 1 ml of
specific buffer followed by centrifugation, the cell pellet was
resuspended in 500 µl of binding buffer, and a 1 µg/ml PI solution
was added before the flow cytometric analysis. Data acquisition was
performed with FACSCalibur flow cytometer and the data analysis was
performed using Flow Software 2.5 (Becton-Dickinson).
Immunophenotyping analysis of T cells
by flow cytometry
A lymphocyte immunophenotyping analysis was carried
out for the lymphocytes cultured in the main culture medium which
represented the control cells, as well as for lymphocytes that were
in contact with the collagen matrix for 5 days which represented
our target cells. Surface markers were evaluated by flow cytometry
after preparing the cells for this purpose. The cells
(105 cells/ml) were washed in PBS, resuspended in PBS,
and incubated for 30 min in the dark with fluorochrome-conjugated
monoclonal antibodies using the manufacturer's recommended
dilutions. After washing with a specific solution (Cell Wash
Solution; BD Biosciences, San Diego, CA, USA), the cells were
resuspended in 500 µl Cell Wash and analyzed with a FACSCalibur
flow cytometer. Data were acquired using CellQuest Pro software
(Becton-Dickinson), and the data analysis was performed using Flow
Software 2.5. The primary antibodies conjugated with fluorochromes
(BD Pharmingen) used for immunophenotyping characterization of the
PBMCs were: CD3 (FITC)/CD16 + 56 (PE)/CD45 (PerCP)/CD19 (APC); CD3
(FITC)/CD8 (PE)/CD45 (PerCP)/CD4 (APC); CD25 (FITC)/CD38 (PE); and
CD69 (FITC)/CD166 (PE).
Statistical analysis
All data are representative of three separate
experiments. The statistical analysis was performed using the PASW
Statistics 18.0 software (SPSS Inc., Chicago, IL, USA). The paired
t-test was used to analyze the differences between the two groups.
P-values <0.05 were considered to indicate statistically
significant differences.
Results and Discussion
Comparative immunophenotyping analysis
between unstimulated T cells (control) and collagen
matrix-activated T cells
A comparative analysis was performed between cells
in contact with the 3D collagen matrix and a control group of
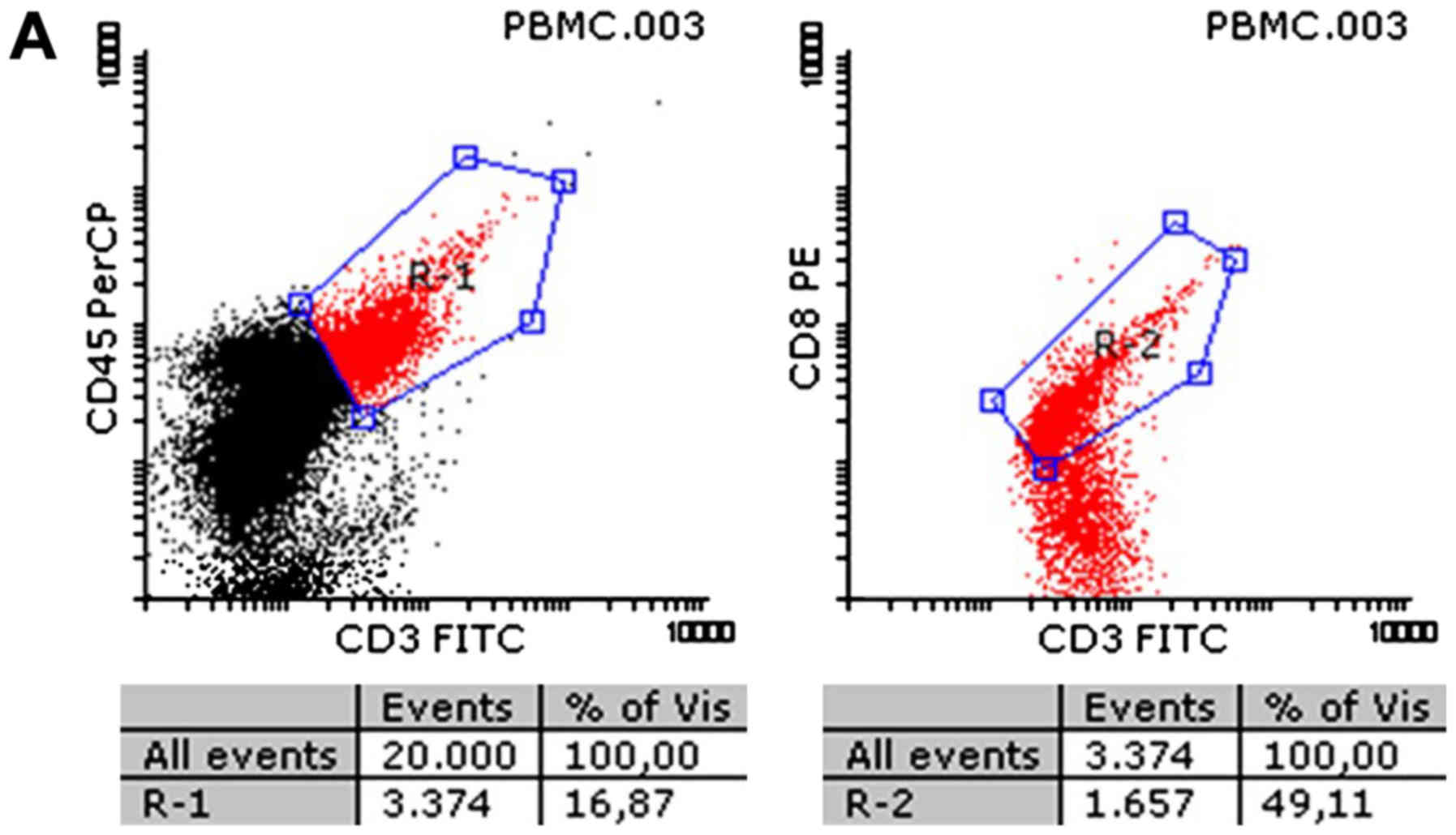
cells. As shown in Fig. 1 for
CD3+ and in Fig. 3 for
CD8+, both cytotoxic T cell groups were increased
significantly due to contact with the collagen matrix, compared to
lymphocytes cultured in growth medium. Growth was seen in all
samples, regardless of the initial immune status of the subject
from which the blood sample was taken. Thus, the flow cytometry
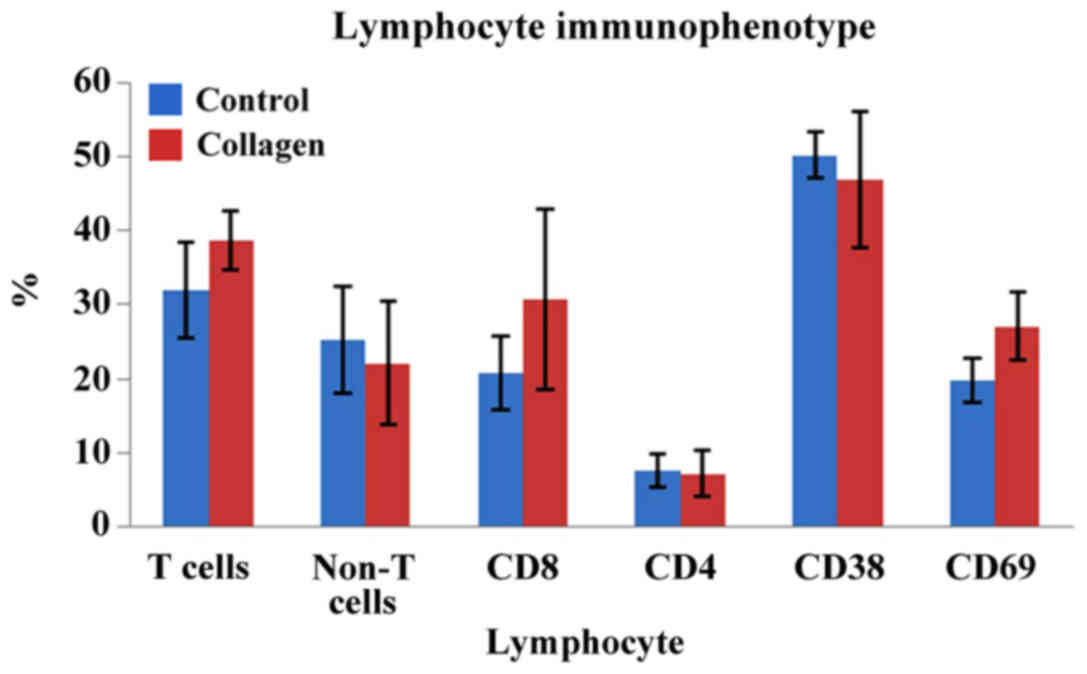
analysis showed values of 31.9±6.5 vs. 38.7±3.8% for T cells in the
control vs. collagen samples, respectively.
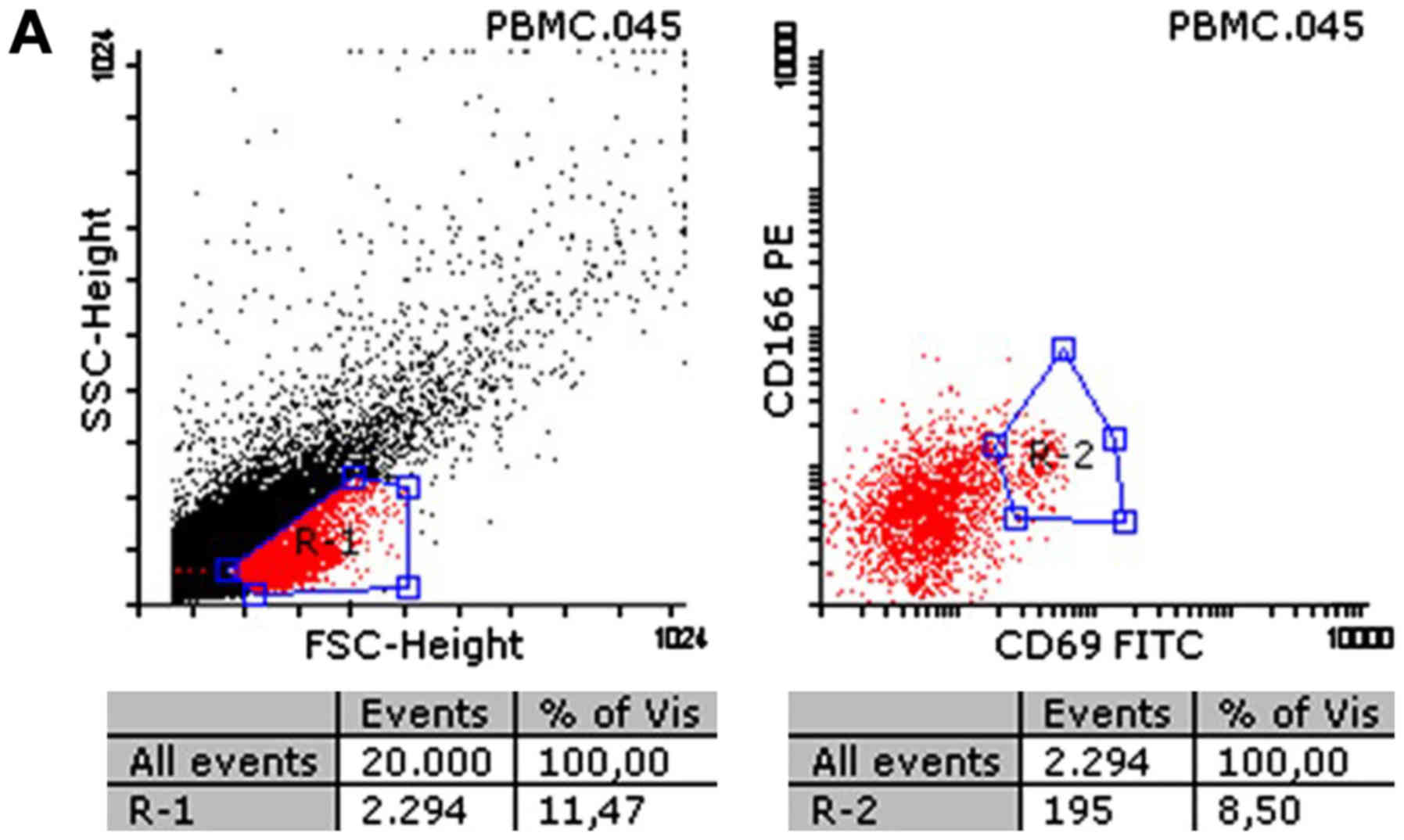
As illustrated in Figs.
2 and 3, the CD69 surface marker
increased significantly on the surface of T cells in contact with
the collagen matrix (19.7±3.0 vs. 27.1±4.5% for control and
collagen sample group, respectively).
The present results showed inter-individual
variations in expression of CD38 surface marker, but >50% of the
samples demonstrated decreased expression during contact of
lymphocytes with the collagen matrix (50.3±3.0 vs. 46.8±9.1% for
controls and collagen samples, respectively; P>0.05; no
statistical significance) (Fig.
3).
At the same time, CD8 increased significantly
(20.7±5.5 vs. 30.7±12.1% in controls vs. collagen samples;
P<0.05), but no difference was found in the CD4 lymphocytes
(7.4±2.2 vs. 7.1±3.3%; P>0.05). Both CD4 and CD8 cells are
usually found in the wound bed at all stages of the wound healing
process and are particularly involved in late stages of
inflammation and early stages of tissue remodeling and
proliferation.
Apoptosis and viability levels
following T cell contact with the 3D collagen matrix
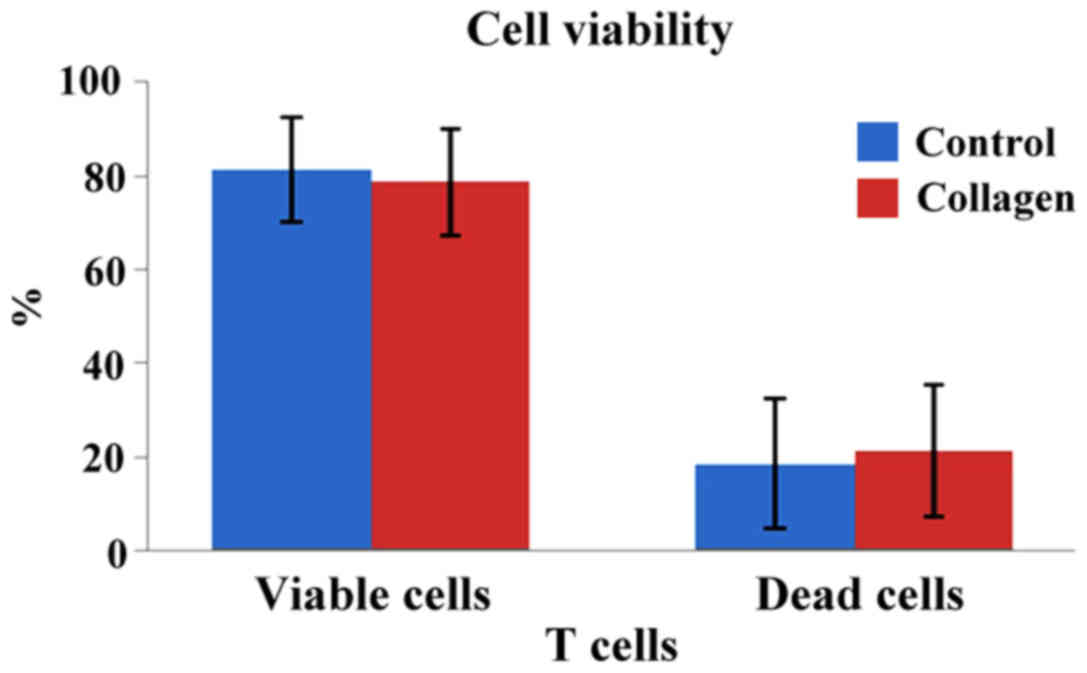
As shown in Fig. 4,
the 3D matrix induced no significant apoptotic cell death in T
cells, compared to their respective controls. Moreover, the
percentages of viable and dead cells were similar in both samples.
A comparative analysis was performed between the cells in contact
with the 3D collagen matrix and the control group cells (viable
cells: 81.2±11.1 vs. 78.5±13.9% in the control vs. collagen groups;
P<0.05), whereas necrotic/late apoptotic cells were 18.5±11.1
vs. 21.2±13.9% in the control vs. collagen groups, respectively
(P<0.05).
Over the past decades, complete regeneration of the
oral mucosa following surgery has been the focus of intense
exploratory research. Allograft materials, such as acellular dermal
matrix grafts, have been used as alternatives to avoid morbidity at
the donor site. However, the complex interactions between the
immune system and the artificial biomaterials used for oral soft
tissue regeneration have not been thoroughly studied. The specific
aim of the present study was to analyze for the first time the
interactions between a resorbable 3D collagen matrix used for oral
soft tissue regeneration and T cells, one of the most important
immune players in the inflammatory microenvironment following oral
soft tissue regenerative surgical procedures.
Apoptosis and migration of keratinocytes are vital
for wound repair, and the T cells were demonstrated to ameliorate
the wound-healing condition by increasing keratinocyte migration
and proliferation and decreasing apoptosis of keratinocytes. More
specifically, several subsets of T cells promote wound healing,
which is a critical and complex process that occurs in the skin and
other barrier sites. Activation of T cells at the injury site
initiates massive infiltration of the wound with αβ T cells that
likely facilitate the transition from the inflammatory to the
proliferative phase of healing, and defects in dermal γδ T cells
may be an important mechanism underlying delayed wound healing
(17). A crucial role has been
demonstrated for CD100-plexin B2 interactions in the wound healing
response mediated by murine γδ T cells and in the T cell
morphological changes associated with this process (18). T cell receptor ligands are not
constitutively expressed in healthy tissues but are rapidly
upregulated following wounding of keratinocytes bordering wound
edges. Ligand expression is tightly regulated, with down-modulation
following T cell activation. Early inhibition of T cell
receptor-ligand interactions delays wound repair in vivo,
highlighting T cells as rapid responders to injury (19).
The results of this study show that the T cell
population increased significantly when exposed to 5-day incubation
with the selected collagen matrix compared to cultured lymphocytes.
Stimulating the overall T cell population suggests that the immune
system's effector cell subsets are activated when in contact with
the collagen scaffold. This increase was detected in all analyzed
samples, with no connection with the initial immune status of the
patient from which the sample was collected. We infer that the
efficiency of tissue repair may be increased.
CD69 is a cell activation antigen present on active
immune system cells in the very early stage of the lymphocyte
activation process (20,21). Lymphocytes usually have very low
levels of CD69, but nearly all bone-marrow derived cells express
the marker within the first 1–3 h following activation. Once
upregulated, CD69 affects T cell differentiation and modulates the
inflammatory response. Our data show that the CD69 surface marker
increased significantly on cells in contact with the collagen
matrix in all five samples, suggesting that the matrix initiates
the appropriate immune response needed for tissue repair.
CD38 is another cell surface marker present on T
cells and is responsible for cellular adhesion, migration, and
local angiogenesis (22,23). The marker is usually expressed during
inflammatory processes and appears later in the wound healing
process. In this study, CD38 levels decreased slightly but not
significantly in the T cell group exposed to the collagen matrix
compared to the controls. The marker displayed variations between
patients, but the finding that expression was similar in both
groups suggests that longer incubation times are needed for this
marker to be significantly expressed. Future studies are necessary
to assess the expression of CD38 on the T cell surface following
exposure to collagen.
Our study also analyzed CD4 and CD8 as two key
surface markers of the inflammatory process of wound healing. These
markers are present in the wound at all four stages of wound
healing and have their highest levels during the early phase of
proliferation and the late phase of wound inflammation (24,25). A
decreased concentration of CD8 T cells and CD4 T cells can thus be
associated with impaired or delayed wound healing. Our results show
that CD8 increased significantly in the collagen sample cells,
whereas CD4 levels were similar with the controls.
Annexin V is a 36 kDa protein with a high affinity
for phosphatidylserine, which is located on the cytoplasmic surface
of the cell membrane. During execution of the apoptotic program,
phosphatidylserine is externalized, and the cells become rapidly
positive for Annexin V (26,27). Translocation of phosphatidylserine
occurs on the external surface membrane during apoptosis and
phosphatidylserine is exposed to the outside as the cell membrane
is destroyed during necrosis and becomes a trigger for activation
of macrophages. This phenomenon is also present in activated
platelets, during mast cell degranulation, and in several
erythrocyte abnormalities. In the presence of calcium ions, Annexin
V binds with high affinity to phosphatidylserine exposed by
apoptotic cells. The labeling technique using Annexin V conjugated
with fluorochrome was developed to detect and identify apoptotic
cell and dead T cells (28). A
viability marker (PI) was used distinguish apoptotic cells from
cells with a permeabilized plasma membrane during late
non-apoptotic death, so that only apoptotic cells appeared positive
for Annexin V, and double positive cells (Annexin
V+/PI+) were eliminated from the statistics.
Apoptotic cells are detected by staining with Annexin V, dead cells
are identified by Annexin V and PI, while the viable cells cannot
be detected by any of these markers (29). Our present results show that the
selected 3D collagen matrix does not include T cells in early or
late apoptosis. The percentage of viable cells was the same in the
samples and controls. These findings demonstrate that the collagen
matrix is nontoxic to immune system cells and does not hinder the
healing process following periodontal surgery for soft tissue
regeneration. A further study should address the compatibility of
the collagen matrix with another important component of the healing
process, the cells belonging to the epithelial compartment, namely
epithelial cells. It is known that prolonged healing times or
chronic inflammation can initiate or potentiate tumor processes
both in the oral mucosa and in skin in general (30–33).
This study had several limitations that need to be
addressed. One limitation is the limited number of clinical cases
available for investigation. In addition, it can be difficult to
associate the present in vitro results with the in
vivo mechanisms responsible for the complex wound healing
processes initiated after surgical placement of the xenogeneic
collagen matrix. Another drawback is that the present results are
based on a short 5-day incubation period. More studies involving
larger groups of patients and longer incubation times of the
collagen matrix are needed to better expand and explain the present
findings.
In conclusion, the interaction between T cells and
the 3D collagen matrix did not impair the healing process, it may
accelerate oral wound healing following surgery, and increase the
activity of immune system cells. However, these results need
further research to fully explore the implications of the
interactions between the resorbable 3D collagen matrix used for
oral soft tissue regeneration and the T cells.
Acknowledgements
Not applicable.
Funding
This study was partially supported by internal
grants from the ‘Victor Babes’ University of Medicine and Pharmacy,
Timisoara, Romania: DUROTOM-P-IV-CI-PDCC 2015/2016, contract
7199/01.07.2015 and DENTALOCT PIII-C2-PCFI 2015/2016. The
manuscript was reviewed by native English-speaking experts from
BioMed Proofreading, LLC (Cleveland, OH, USA).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RD, SIS, RS and SP participated in study design and
sample collection, as well as in acquisition of data. BF and PV
performed cell analysis of T-lymphocytes and aquisition of data. MB
drafted the manuscript and revised it critically for intellectual
content. CB, CC and DA carried out data analysis and statistical
analysis. MS and CH contributed to data acquisition and drafting
the manuscript. AR and NPG contributed to the design and critical
revision of the study. RD, CH and MS contributed equally to this
study and can be considered first authors. All authors read and
approved the final version of manuscript.
Ethics approval and consent to
participate
The protocol and patient informed consent process
were approved by the Commission of Research Ethics of the ‘Victor
Babes’ University of Medicine and Pharmacy (06/27.06.2013;
Timisoara, Romania). Written informed consent was obtained from all
the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nevins M, Nevins ML, Kim SW, Schupbach P
and Kim DM: The use of mucograft collagen matrix to augment the
zone of keratinized tissue around teeth: A pilot study. Int J
Periodontics Restorative Dent. 31:367–373. 2011.PubMed/NCBI
|
|
2
|
Lin GH, Chan HL and Wang HL: The
significance of keratinized mucosa on implant health: A systematic
review. J Periodontol. 84:1755–1767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall WB and Lundergan WP: Free gingival
grafts. Current indications and techniques. Dent Clin North Am.
37:227–242. 1993.PubMed/NCBI
|
|
4
|
Tonetti MS and Jepsen S: Working Group 2
of the European Workshop on Periodontology: Clinical efficacy of
periodontal plastic surgery procedures: Consensus report of Group 2
of the 10th European Workshop on Periodontology. J Clin
Periodontol. 41 Suppl 15:S36–S43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuhr O, Bäumer D and Hürzeler M: The
addition of soft tissue replacement grafts in plastic periodontal
and implant surgery: Critical elements in design and execution. J
Clin Periodontol. 41 Suppl 15:S123–S142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scarano A, Barros RR, Iezzi G, Piattelli A
and Novaes AB Jr: Acellular dermal matrix graft for gingival
augmentation: A preliminary clinical, histologic, and
ultrastructural evaluation. J Periodontol. 80:253–259. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGuire MK, Scheyer ET, Nunn ME and Lavin
PT: A pilot study to evaluate a tissue-engineered bilayered cell
therapy as an alternative to tissue from the palate. J Periodontol.
79:1847–1856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanz M, Lorenzo R, Aranda JJ, Martin C and
Orsini M: Clinical evaluation of a new collagen matrix (Mucograft
prototype) to enhance the width of keratinized tissue in patients
with fixed prosthetic restorations: A randomized prospective
clinical trial. J Clin Periodontol. 36:868–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McGuire MK and Scheyer ET: Randomized,
controlled clinical trial to evaluate a xenogeneic collagen matrix
as an alternative to free gingival grafting for oral soft tissue
augmentation. J Periodontol. 85:1333–1341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teller P and White TK: The physiology of
wound healing: Injury through maturation. Surg Clin North Am.
89:599–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Portou MJ, Baker D, Abraham D and Tsui J:
The innate immune system, Toll-like receptors and dermal wound
healing: A review. Vascul Pharmacol. 71:31–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dasu MR and Isseroff RR: Toll-like
receptors in wound healing: Location, accessibility, and timing. J
Invest Dermatol. 132:1955–1958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sorg H, Tilkorn DJ, Hager S, Hauser J and
Mirastschijski U: Skin wound healing: An update on the current
knowledge and concepts. Eur Surg Res. 58:81–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun BK, Siprashvili Z and Khavari PA:
Advances in skin grafting and treatment of cutaneous wounds.
Science. 346:941–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rusu D, Stratul SI, Festila D, Surlin P,
Kasaj A, Baderca F, Boariu M, Jentsch H, Locovei C and Calenic B:
Histology and surface ultrastructure during early healing after
gingival augmentation with a three-dimensional collagen matrix: A
report of six cases. Quintessence Int. 48:57–67. 2017.PubMed/NCBI
|
|
16
|
Martin P and Nunan R: Cellular and
molecular mechanisms of repair in acute and chronic wound healing.
Br J Dermatol. 173:370–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kabashima K: Basic and clinical sciences
in skin immune responses. Immunology of the Skin. 1st. Springer;
Japan: pp. 95–111. 2016
|
|
18
|
Zhang C, Xiao C, Dang E, Cao J, Zhu Z, Fu
M, Yao X, Liu Y, Jin B, Wang G, et al: CD100-Plexin-B2 promotes the
inflammation in psoriasis by activating NF-κB and inflammasome in
keratinocytes. J Invest Dermatol. 138:375–383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sena LA, Li S, Jairaman A, Prakriya M,
Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman
H, et al: Mitochondria are required for antigen-specific T cell
activation through reactive oxygen species signaling. Immunity.
38:225–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mackay LK, Braun A, Macleod BL, Collins N,
Tebartz C, Bedoui S, Carbone FR and Gebhardt T: Cutting edge: CD69
interference with sphingosine-1-phosphate receptor function
regulates peripheral T cell retention. J Immunol. 194:2059–2063.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Etich J, Bergmeier V, Frie C, Kreft S,
Bengestrate L, Eming S, Mauch C, Eckes B, Ulus H, Lund FE, et al:
PECAM1(+)/Sca1(+)/CD38(+) vascular cells transform into
myofibroblast-like cells in skin wound repair. PLoS One.
8:e532622013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Obaldia ME and Bhandoola A:
Transcriptional regulation of innate and adaptive lymphocyte
lineages. Annu Rev Immunol. 33:607–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Mehta ND, Zhao Y and DiPietro LA:
Absence of CD4 or CD8 lymphocytes changes infiltration of
inflammatory cells and profiles of cytokine expression in skin
wounds, but does not impair healing. Exp Dermatol. 23:189–194.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nosbaum A, Prevel N, Truong HA, Mehta P,
Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK and
Rosenblum MD: Cutting edge: Regulatory T cells facilitate cutaneous
wound healing. J Immunol. 196:2010–2014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugimoto MA, Vago JP, Teixeira MM and
Sousa LP: Annexin A1 and the resolution of inflammation: Modulation
of neutrophil recruitment, apoptosis, and clearance. J Immunol Res.
2016.82392582016.doi: 10.1155/2016/8239258. PubMed/NCBI
|
|
27
|
Yeh SH, Kong FL and Lin MH: Visualization
of apoptosis: Annexin V imaging. Personalized Pathway-Activated
Systems Imaging in Oncology. Inoue T, Yang D and Huang G: Springer;
Singapore: pp. 233–243. 2017, View Article : Google Scholar
|
|
28
|
Pietkiewicz S, Schmidt JH and Lavrik IN:
Quantification of apoptosis and necroptosis at the single cell
level by a combination of imaging flow cytometry with classical
Annexin V/propidium iodide staining. J Immunol Methods. 423:99–103.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weyd H, Abeler-Dörner L, Linke B, Mahr A,
Jahndel V, Pfrang S, Schnölzer M, Falk CS and Krammer PH: Annexin
A1 on the surface of early apoptotic cells suppresses
CD8+ T cell immunity. PLoS One. 8:e624492013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Boda D: Cellomics as integrative omics for
cancer. Curr Proteomics. 10:237–245. 2013. View Article : Google Scholar
|
|
31
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Copenh). 10:545–558.
2014.
|
|
32
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LM, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ion A, Popa IM, Papagheorghe LM, Lisievici
C, Lupu M, Voiculescu V, Caruntu C and Boda D: Proteomic approaches
to biomarker discovery in cutaneous T-cell lymphoma. Dis Markers.
2016.96024722016.doi: 10.1155/2016/9602472. PubMed/NCBI
|