Introduction
Keloid formation is a fibroproliferative disorder
caused by abnormal cutaneous wound healing that is characterized by
the aggressive growth of fibroblasts and excessive extracellular
matrix (ECM) deposition (1).
Although keloid formation is a nonmalignant disease, keloid-derived
fibroblasts (KFs) can undergo malignant manifestations such as
hyperproliferation, changes in collagen morphology, and excessive
collagen deposition (1,2). Keloid formation in the dermis is
primarily driven by upregulated transforming growth factor-β
(TGF-β) signaling pathways and the excessive deposition of collagen
type I α 1 chain (COL1A1) and collagen type 3 α1 chain (COL3A1),
which are the main structural components of the ECM (1–4). These
cross-linked long-chain fibers form a strong network in the ECM,
thereby maintaining the integrity of the keloid scar. However, this
is accompanied with lower flexibility and malformation of the ECM.
The etiology and underlying mechanism of keloid formation are still
poorly understood (2).
microRNAs (miRNAs or miRs) serve critical roles in
fibrosis and in malignant biological processes, such as excessive
cell proliferation and ECM deposition (5). The synthesis and degradation of the ECM
in fibrotic tissues are controlled by numerous factors, such as
cell signaling pathways, certain collagenases and miRNAs (6,7). Certain
malignant miRNAs, such as miR-21, miR-181c and miR-196a, have
previously been reported to participate in fibrosis and ECM
metabolism (8–10). Among them, miR-21 and miR-181c have
been demonstrated to mediate collagen deposition in hypertrophic
scars (8,9). miR-96 has also been demonstrated to
contribute to the malignant features in prostate and colorectal
cancer and was found to be associated with TGF-mediated signaling
pathways (11,12). In addition, overactivation of TGF-β
signaling has been reported to be required for the initiation and
progression of keloids (12). The
TGF-β pathway modulates tumorigenesis and progression through
mothers against decapentaplegic homolog (Smad)2/3
phosphorylation-mediated fibroblast proliferation and collagen
deposition in keloids (13).
However, the role of miR-96 in keloid pathogenesis and its effect
on the TGF-β signaling pathway remains poorly understood.
Smad7, an important inhibitory cytokine in the TGF-β
signaling pathway, was predicted to be a target of miR-96,
according to the bioinformatics algorithm TargetScan Human7.2. As
such, the aim of the present study was to determine the correlation
between miR-96 and Smad7, and compare their expression in KFs with
that in normal skin-derived fibroblasts (NFs). In addition, an
antagomir-treated keloid organ culture (OC) model was used to
determine the therapeutic effects of miR-96 downregulation.
Patients and methods
Patient recruitment and keloid OC
establishment
Patients with typical keloid characteristics who had
no prior treatment were recruited for the present study, as
previously described (14). The
diagnosis of keloid pathogenesis in the patient specimens was
confirmed through routine pathological examination by an
experienced dermatopathologist. The present study was conducted
with the consent of each recruited patient and with the approval of
the Ethics Committee of the Lanzhou General Hospital of Chinese
People's Liberation Army (Lanzhou, China). A total of 10 patients
with KFs, aged 17–42 years, were recruited from February 2016 to
June 2017 at Burns and Plastic Surgery Center of PLA in Lanzhou
General Hospital of Chinese People's Liberation Army, and the
control group consisted of corresponding tissue sections obtained
from the NFs adjacent to the lesion in these 10 keloid cases
(Table I). As described previously
(15), the excised 5-mm3
tissue biopsies (keloid explants or keloid OC) were embedded in a
collagen gel matrix and were then preserved in serum-free William's
medium E (WE medium; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
(15).
 | Table I.Profile of each sample for primary
culture. |
Table I.
Profile of each sample for primary
culture.
| Patient | Sex | Age (years) | Biopsy site | Duration of the
lesion (months) | Etiology |
|---|
| 1 | Male | 21 | Shoulder | 6 | Burn |
| 2 | Female | 17 | Chest | 8 | Scald |
| 3 | Female | 27 | Arm | 13 | Scald |
| 4 | Female | 42 | Shoulder | 11 | Burn |
| 5 | Male | 38 | Chest | 17 | Scald |
| 6 | Female | 24 | Shoulder | 9 | Burn |
| 7 | Male | 36 | Buttock | 7 | Burn |
| 8 | Female | 22 | Cheek | 10 | Burn |
| 9 | Female | 21 | Shoulder | 5 | Scald |
| 10 | Male | 18 | Chest | 14 | Scald |
Cell culture and transfection of miRNA
mimics and miRNA inhibitors
The primary fibroblasts were cultured as described
previously (9). Fibroblasts that had
undergone 0–3 passages were used in the present study. All
synthetic miRNAs and miRNA inhibitors (including the scrambled RNA
as a negative control) were purchased from Shanghai GeneChem Co.,
Ltd. (Shanghai, China). The scrambled RNA was used as a control for
mimic or inhibitor transfection experiments. All sequences are
presented in Table II. Cells were
transduced with miR-96 inhibitor (70 nM) or control molecules (70
nM) with Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), 48 h prior to subsequent experiments. miR-96
mimics or control molecules (50 nM) were transfected using
Lipofectamine® 2000 (16).
 | Table II.Sequences of miR-96 mimic, miR-96
inhibitor, scrambled RNA, si-Smad7 and Si-NC. |
Table II.
Sequences of miR-96 mimic, miR-96
inhibitor, scrambled RNA, si-Smad7 and Si-NC.
|
| Sequence
(5′→3′) |
|---|
| miR-96 mimic |
UUUGGCACUAGCACAUUUUUGCU |
| miR-96
inhibitor |
AGCAAAAAUGUGCUAGUGCCAAA |
| scrambled RNA |
CAGUACUUUUGUGUAGUACAA |
| si-Smad7
forward |
AAGATAATTCGTTCCCCCTGTCCTGTCTC |
| si-Smad7
reverse |
AAACAGGGGGAACGAATTATCCCTGTCTC |
| si-NC forward |
GCAAACAUCCCAGAGGUAU′ |
| si-NC reverse |
AUACCUCUGGGAUGUUUGC |
Masson's staining
The degree of fibrosis was examined using Masson's
trichrome staining, according to standard protocols (1,17), and
the stained tissue sections were then examined with a light
microscope at magnification, ×400 (Olympus Corporation, Tokyo,
Japan).
Luciferase reporter assay
TargetScan Human 7.2 (www.targetscan.org/vert_72) was used to predict Smad7
as a target of miR-96. The wild-type (wt) or mutant (mut) Smad7
3′-untranslated region (UTR) was cloned into the pGL3 vector
(Promega Corporation, Madison, WI, USA). Subsequently, the
pGL3-Smad7-3′UTR-wt or pGL3-Smad7-3′UTR-mut vector, along with the
miR-96 mimic or the mimic-control (as described above), were
transfected with Lipofectamine® 2000 into 293T cells
(ATCC, Rockville, MD, USA). Following 48 h, the luciferase activity
was measured with a Luciferase Reporter Gene Assay kit (Promega
Corporation), using a GloMax-Multi Jr Single Tube Multimode Reader
(Promega Corporation). Renilla luciferase activity was used
for normalization of the firefly luciferase activity (18).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the mRNA of cells and
tissues, according to the manufacturer's protocol. Universal primer
and the miScript reverse transcription kit (both Qiagen GmbH,
Hilden, Germany) were used for reverse transcription of miRNA.
RETROscript™ reverse transcription kit (cat. no. AM1710; Ambion;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used for mRNA
reverse transcription. Triplicate RT-qPCR reactions and analyses
were performed using a Bio-Rad C1000 Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The qPCR reactions were
performed with miScript SYBR Green PCR kit (cat. no. 218200; Qiagen
GmbH) using the following thermocycling conditions: Initial
denaturation for 15 sec at 95°C; 45 cycles of denaturation at 94°C
for 15 sec, annealing at 55°C for 30 sec and extension at 70°C for
30 sec. The internal loading controls used for the mRNAs and miRNAs
were GAPDH and RNU6B, respectively. The PCR primers used for mRNA
and miR quantification are listed in Table III. Expression levels were
determined using Applied Biosystems 7500 software version 2.0.1
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and analyzed
using the 2−ΔΔCq method (19).
 | Table III.Primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table III.
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence
(5′→3′) |
|---|
| COL1A1 forward |
GTGGAAACCCGAGCCCTGCC |
| COL1A1 reverse |
TCCCTTGGGTCCCTCGACGC |
| COL3A1 forward |
TCCCACTATTATTTTGGCACAACA |
| COL3A1 reverse |
TCATCGCAGAGAACGACGGATCC |
| Smad7 forward |
ATGDTGTGCCTTCCTCCGCT |
| Smad7 reverse |
CGTCCACGGCTGCTGCATAA |
| GAPDH forward |
GTCGCCAGCCGAGCCACATC |
| GAPDH reverse |
CCAGGCGCCCAATACGACCA |
| miR-96 forward |
TCGTTTTTACACGATCACGGTTT |
| RNU6B RNA |
ACGCAAATTCGTGAAGCGTT |
Western blotting
The proteins were extracted from KF and NF tissues
using a mammalian protein extraction reagent (M-PER™; Thermo Fisher
Scientific, Inc.) and concentrations were determined using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). The
lysates were mixed with laemmli buffer (2X; Bio-Rad Laboratories,
Inc.) supplemented with 5% β-mercaptoethanol (1:1 ratio, Thermo
Fisher Scientific, Inc.). Samples were boiled for 5 min at 95°C.
Proteins (15 µg/lane) were separated using SDS-PAGE on 10% gel and
subsequently the proteins were transferred to polyvinylidene
fluoride membranes by cold transfer (25V at 4°C overnight). The
membranes were blocked by a solution of 5% non-fat dried milk in
Tris buffered saline with Tween (TBST; 25 mM Tris-HCl, pH 7.5, 150
mM NaCl, 0.05% Tween-20) for 1 h at room temperature (20). The membrane was then incubated at 4°C
overnight with primary antibodies for COL1A1 (cat. no. sc-293184,
1:1,000), COL3A1 (cat. no. sc-271249, 1:500), Smad7 (cat. no.
sc-365846, 1:300; all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), and β-actin (cat. no. sc-70319, 1:5,000; Sigma-Aldrich; Merck
KGaA). All primary antibodies were detected using anti-mouse- or
anti-rabbit-horseradish peroxidase conjugated secondary antibodies
(1:3,000; cat. nos. sc-390944 and sc-5162, respectively; Santa Cruz
Biotechnology, Inc.) at 37°C for 2 h. Membranes were washed three
times with TBST and the chemiluminescent signals were detected
using Clarity ECL Western Blotting substrate (cat. no. 1705061;
Bio-Rad Laboratories, Inc.) and detected using the Odyssey Infrared
Imaging System (LI-COR Biosciences, Lincoln, NE, USA). The relative
protein expression of COL1A1 and COL3A1 was normalized to that of
β-actin with Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA).
ELISA
The level of Col1 supernatant protein was assessed
using COL1A1 and COL3A1 ELISA kits (cat. nos. ab210966 and ab7778,
respectively; Abcam, Cambridge, MA, USA) according to the
manufacturer's protocol. Briefly, the conditioned medium from cell
culture was added to an ELISA kit plate precoated with an
anti-COL1A1 and COL3A1 antibody. Subsequently a biotinylated
secondary antibody was added and the plate was incubated at room
temperature for 2 h. The optical density was measured at a
wavelength of 450 nm (21).
Treatment of keloid OC with the miR-96
inhibitor, antagomir
The chemically synthesized miR-96 inhibitor,
antagomir, functions to decrease the miR-96 expression level in the
keloid OC (22–24). miR-96 antagomir (100 µg; Guangzhou
RiboBio Co., Ltd., Guangzhou, China) was added to 1 ml keloid OC in
serum-free WE medium every 72 h for a period of five weeks at 37°C,
while the control group received an equal amount of scrambled RNA
in the same antagomir medium, and the medium was changed every
three days. Keloid shrinkage in vitro was assessed through
measuring the dry weight of the 5-µm Masson's trichrome-stained
sections on an analytical balance.
siRNA transfection
si-Smad7 sequences were chemically synthesized and
purified by high-performance liquid chromatography (Shanghai
GenePharma Co., Ltd., Shanghai, China). All the oligonucleotides
were 2′-OMe modified. Briefly, cells were transfected with
siRNA-Smad7 at a final concentration of 50 nM using
Lipofectamine® 2000. siRNA-NC was transfected as a
negative control. At 24 h post-transfection, the culture medium was
changed according to the protocols of manufacturer. After 48 h,
cells were harvested for analysis. All transfections were performed
in triplicate (25).
Statistical analysis
Statistical analysis was performed using a
two-tailed unpaired Student's t-test, paired Student's t-test,
Pearson's linear correlation test and one-way analysis of variance
with Tukey's post hoc test. All data were obtained from triplicate
experiments and were presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Altered expression of miRNAs and Smad7
in KFs
The expression levels of Smad7 and miR-96 were
compared between the KFs and NFs obtained from 10 keloid patients.
It was demonstrated that the mRNA expression of miR-96 was
increased whereas that of Smad7 was decreased in the KFs, as
compared with the NFs (Fig. 1A-C).
Furthermore, their expression levels were inversely correlated
(Fig. 1D). Given that type I
collagen is the major component of the ECM that participates in
keloid formation, the correlation between miR-96 and COL1A1 was
also investigated. Endogenous miR-96 was demonstrated to be
positively correlated with the COL1A1 expression levels (Fig. 1E). Masson's staining of KF tissues
revealed relatively high COL1A1 and miR-96 expression levels in
case #5, yet a low Smad7 expression level (Fig. 1F); whereas case #9 exhibited
relatively low expression levels of miR-96 and COL1A1, yet a high
Smad7 expression level (Fig. 1G).
Examination of the structural characteristics of the keloid scars
of cases #5 and #9 revealed that case #5, which had a higher
expression level of miR-96 and a lower level of Smad7, exhibited a
thicker collagen fiber deposition and a relatively non-uniform
collagen density distribution (Fig.
1F). Conversely, case #9, which had a lower level of miR-96 and
a higher level of Smad7, exhibited a thinner collagen fiber
deposition and a relatively uniform density distribution (Fig. 1G).
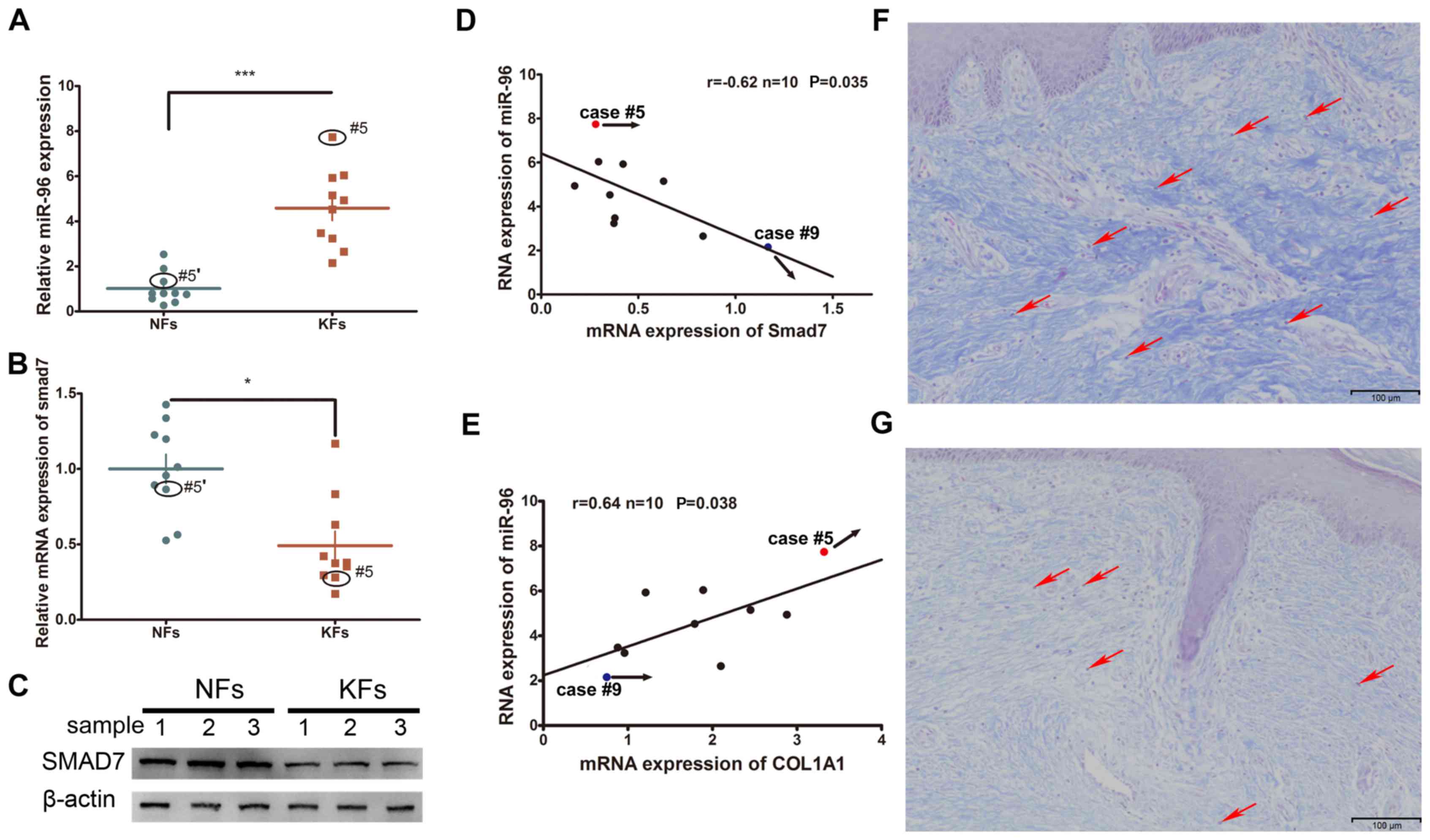 | Figure 1.The expression of miR-96, Smad7 and
COL1A1 in KFs and NFs. (A) The miR-96 expression level was
upregulated in KFs compared with that in NFs. The expression of
Smad7 was decreased in KFs compared with that in NFs at both the
(B) mRNA and (C) protein levels. Black circles indicate the Smad7
expression level in the keloid of case #5 and in the autologous
normal skin control (#5′) of case #5. (D) An inverse correlation of
Smad7 and the miR-96 mRNA level was observed in the keloid samples.
(E) A positive correlation between Smad7 and miR-96 was revealed in
the keloid samples. Masson's staining of two keloid tissue samples:
(F) Case #5 and (G) case #9. Case #5 exhibited a relatively thicker
dermis and stronger staining of the collagen fibers, a higher
miR-96 expression, and a relatively lower Smad7 expression,
compared with that of case #9. Red arrows indicate the KFs in the
tissue samples of cases #5 and #9. *P<0.05 and ***P<0.001, as
indicated. miR, microRNA; Smad, mothers against decapentaplegic
homolog; COL1A1, collagen subunit 1α1; KF, keloid-derived
fibroblast; NF, normal skin-derived fibroblast. |
Identification of Smad7 genes as
direct targets of miR-96
The putative binding sites of miR-96 in the region
of the 3′UTR of Smad7 were detected through bioinformatics
analysis. Therefore, the predicted region, containing the wt or mut
seed sequence of miR-96 in the 3′UTR of Smad7, was cloned into the
luciferase reporter plasmid (Fig. 2A and
B). The 293T cells transfected with the miR-96 mimic and the
Smad7-3′-UTR had a lower luciferase intensity, but the mutant
reporter-transfected group did not (Fig.
2C), thus indicating that Smad7 may be a direct target of
miR-96. Western blotting and RT-qPCR were used to confirm the role
of miR-96 in regulating Smad7 expression in the KFs and NFs. The
miR-96 expression level was higher in the KFs than in the NFs
(Fig. 1A), thus, the miR-96 mimic
was transfected into NFs, whereas the miR-96 inhibitor was
transfected into the KFs (Fig. 2D).
The results demonstrated that Smad7 was decreased in the miR-96
mimic-transfected NFs at the mRNA and protein levels; however, the
Smad7 expression level was increased in the KFs transfected with
the miR-96 inhibitor, compared with the corresponding control
groups (Fig. 2E and F). These
results suggest that miR-96 may regulate Smad7 expression in KFs
and NFs at both the mRNA and protein levels.
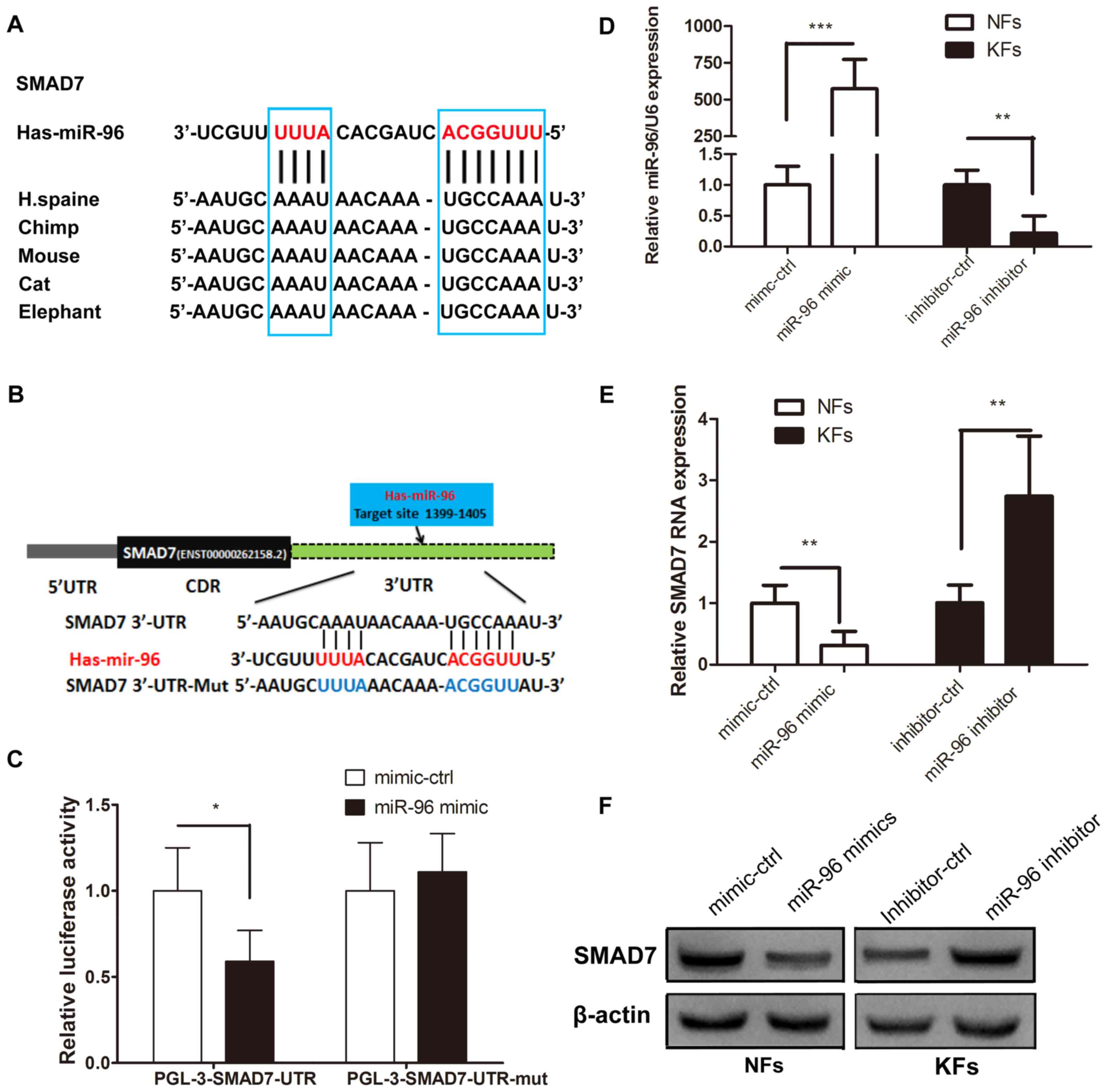 | Figure 2.miR-96 targets Smad7. (A) The
predicted Smad7 binding site on miR-96, which is highly conserved
in different species. (B) Using pGL3 vectors, the wild-type
luciferase reporter plasmid contains the Smad7 3′UTR binding site,
whereas the mutant plasmid does not. (C) Reduced luciferase
activity in the wild-type group compared with that of the mutant
group at 48 h following transfection into 293T cells. (D) The
miR-96 mimic and its control were transfected into NFs, whereas the
miR-96 inhibitor and its control were transfected into KFs. (E)
Reverse transcription-quantitative polymerase chain reaction and
(F) western blotting analyses of the mRNA and protein levels of
Smad7 in the NFs comprising the miR-96 mimic and the KFs comprising
the miR-96 inhibitor at 48 h following transfection. All values
were normalized to their mimic control or inhibitor control,
respectively. *P<0.05, **P<0.01 and ***P<0.001, as
indicated. miR, microRNA; Smad, mothers against decapentaplegic
homolog; UTR, untranslated region; NF, normal skin-derived
fibroblast; KF, keloid-derived fibroblast; ctrl, control. |
miR-96 inhibition leads to decreased
type I and Ш collagen production in KFs, which may be reversed by
Smad7 reintroduction
Type I and Ш collagen are the most common types of
collagen that are deposited in the abnormal ECM of KFs (1–3,10,26).
Therefore, these two collagen types were chosen to evaluate the
regulatory effect of miR-96 on ECM deposition. As Smad7 is a
well-known inhibitory cytokine in the TGF-β pathway and is under
the regulation of miR-96, it was assumed that miR-96 inhibition may
reduce the COL1A1 and COL3A1 expression levels in KFs. ELISA was
used to confirm that transfection of the miR-96 inhibitor into KFs
led to a reduction in COL1A1 and COL3A1 expression (Fig. 3A and B). However, the introduction of
siRNA-Smad7 was demonstrated to significantly reverse the
miR-96-inhibitor-induced Smad7 upregulation (Fig. 3C and D), thereby leading to increased
COL1A1 and COL3A1 production (Fig. 3E
and F).
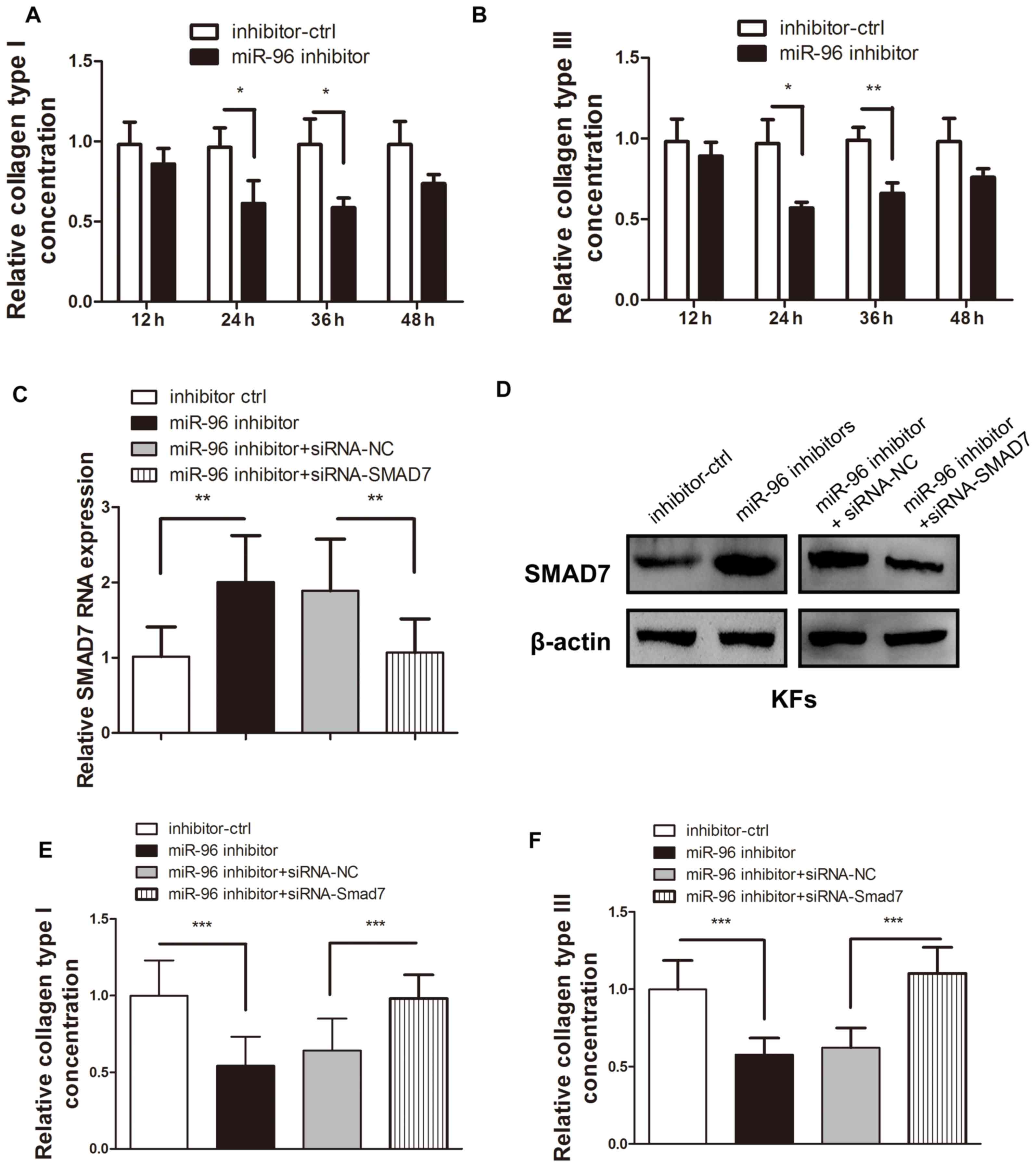 | Figure 3.Smad7 inhibition reversed the
upregulation of COL1A1 and COL3A1 expression induced by the miR-96
inhibitor in the KFs. Following transfection of the miR-96
inhibitor, both the (A) type I and (B) type Ш collagen in the
supernatant of the cultured KFs were detected by ELISA. The
addition of siRNA-Smad7 reversed the effect of the upregulated (C)
mRNA and (D) protein expression of Smad7, which was initially
upregulated by the miR-96 inhibitor. The miR-96 inhibitor-mediated
reduction of (E) COL1A1 and (F) COL3A1 expression levels in the
supernatant of the cultured KFs was reversed by transfection of
siRNA-Smad7. *P<0.05, **P<0.01 and ***P<0.001, as
indicated. Smad, mothers against decapentaplegic homolog; COL1A1,
collagen subunit 1α1; COL3A1, collagen subunit 3α1; miR, microRNA;
KF, keloid-derived fibroblast; siRNA, small interfering RNA; ctrl,
control. |
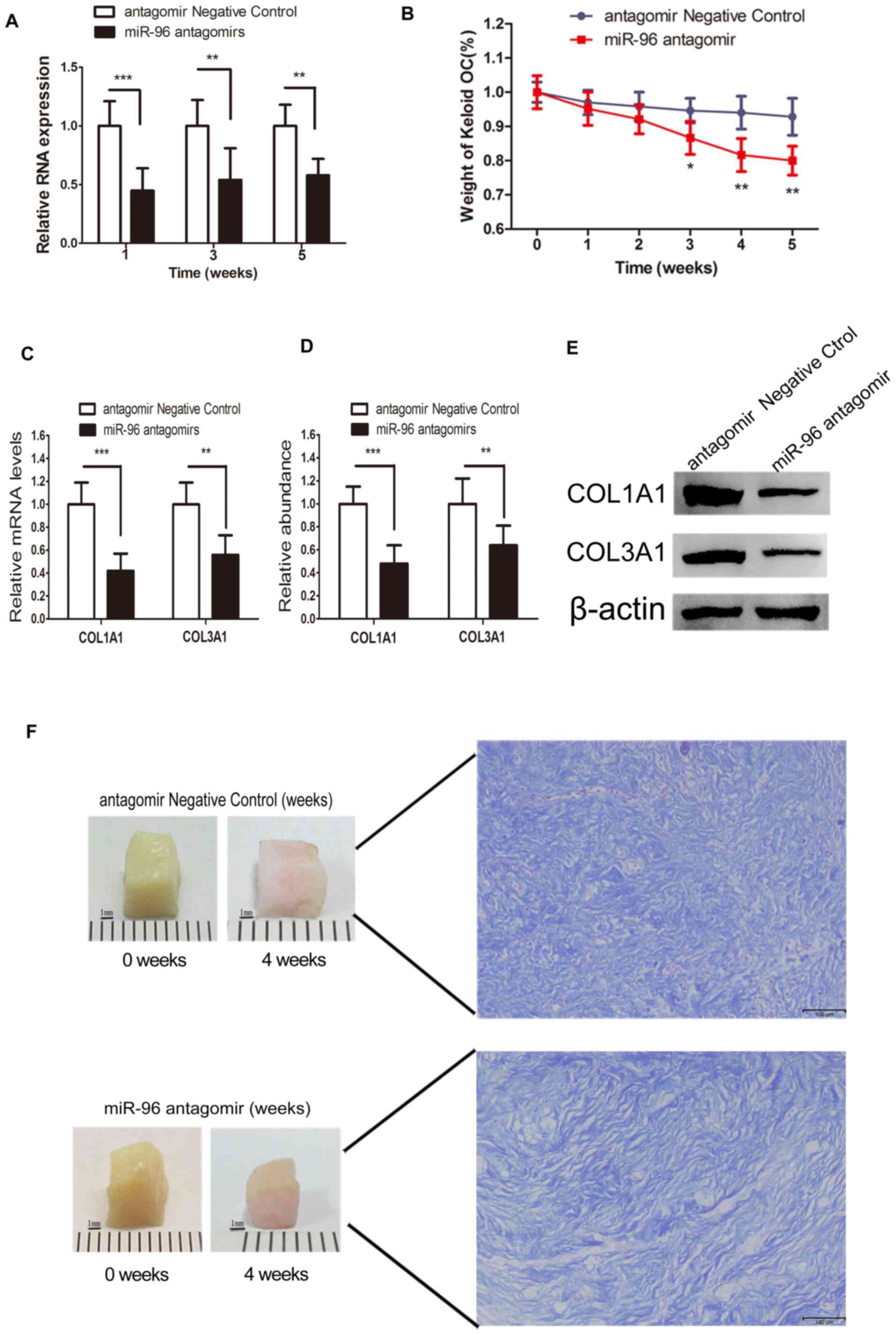
Direct delivery of miR-96 antagomir
into the keloid OC decreased the expression level of miR-96
Local gene therapies targeted at reducing fibrosis
through the application of liposome-complex small interfering RNA
or an miRNA mimic, or through the direct administration of plasmid,
have been demonstrated to be effective and successful (22). Given the validation of the
degradation ability of antagomirs (23), miRNA antagomir was added to the
keloid OC media, as described previously (22–24). The
RT-qPCR data demonstrated that the miR-96 expression level was
barely changed at each time point in the control group. However,
the miR-96 antagomir-treated keloid OC tissues exhibited decreased
miR-96 expression levels at each time point, compared with the
control groups (Fig. 4A). These
findings demonstrated that the direct delivery of miR-96 antagomir
into the keloid OC may lead to a reduction in miR-96
expression.
miR-96 antagomir promotes type I and Ш
collagen degradation and keloid shrinkage in the keloid OC
model
Compared with the scrambled RNA-treated control, the
dry weight of the keloid OC was significantly reduced by 8% at week
3, and by 14% at week 5 (Fig. 4B).
The keloid OC models were treated with miR-96 antagomir to
investigate its effect on collagen degradation. In the miR-96
antagomir-treated group, both the COL1A1 and COL3A1 mRNA expression
levels in the keloid tissues at the end of the 4th week of OC were
significantly reduced (Fig. 4C).
Similarly, the protein expression levels of COL1A1 and COL3A1
(Fig. 4D and E) were also
significantly inhibited in the miR-96 antagomir-treated group.
These findings indicated that the addition of miR-96 antagomir to
the keloid OC decreased the quantity of type I and Ш collagen. The
weight of the keloid OC was measured weekly for a period of 5 weeks
post-treatment to assess the antifibrotic potential of miR-96
antagomir. Visible keloid shrinkage was observed at the end of week
4 (Fig. 4F).
Improved dermal architecture in the
miR-96 antagomir-treated keloid OC
At the end of 4 weeks of OC, the harvested keloid
tissues were subjected to Masson's staining. Thinner and more
loosely arranged collagen fibers were observed in the miR-96
antagomir-treated keloid tissues, compared with the control
(Fig. 4F).
Discussion
Keloid pathogenesis is characterized by the
aggressive proliferation and excessive deposition of type I and Ш
collagen in the ECM (27).
Furthermore, ECM proteins, including collagen, are synthesized by
dermal fibroblasts; therefore, KFs were used for the present study
(28). During wound healing, the
expression of type I and Ш collagen increases significantly,
thereby accelerating wound repair (17). However, the persistent overexpression
of collagen can lead to skin fibrosis, which can eventually result
in keloid pathogenesis.
It has been indicated that miRNAs serve an important
role in fibrotic diseases (29) by
promoting aggressive fibroblast proliferation and collagen
deposition. Our previous study revealed that miR-21 overexpression
within the hypertrophic scar, which is one of the two types of
pathological scars, may promote fibroblast proliferation and
inhibit apoptosis (8). Conversely, a
number of previous studies have reported that certain miRNAs have
an antifibrotic ability, thereby preventing organ fibrosis. For
example, a lower miR-29 expression level is observed in the
fibrotic tissues of the lungs, myocardium, and skin; hence,
fibrotic diseases may be ameliorated by miR-29 overexpression
(29–31). miR-10a attenuated collagen type I
generation in hypertrophic scars by targeting PAI-1 (9). miR-196a overexpression decreases the
expression of type I and Ш collagens in keloid fibroblasts
(10).
miR-96 has been the focus of previous studies on
diseases associated with aggressive proliferation, particularly
cancers. One previous study has demonstrated the correlation
between miR-96 upregulation and chemoresistance in non-small cell
lung cancer cells through the downregulation of Smad9 (32). The miR-200c-mediated regulatory
mechanism of keloid formation, which is a well-known antitumor
miRNA correlated with the TGF-β pathway, has been elucidated in our
previous study (13). However, the
biological relevance and mechanism of action of miR-96 in keloid
pathogenesis have not yet been investigated, to the best of our
knowledge. Therefore, the present study focused on the
investigation of miR-96 and the TGF-β pathway in keloid
formation.
In the present study, miR-96 upregulation was
observed in KFs and compared with that of NFs. Furthermore, the
positive correlation between miR-96 and COL1A1 as well as the
negative correlation between miR-96 and Smad7 were revealed for the
first time, to the best of our knowledge. Furthermore, a luciferase
reporter assay demonstrated the direct targeting of Smad7 by
miR-96. The overexpression or inhibition of miR-96 in the primary
fibroblast cells and the keloid OC models validated the results of
the reporter assay. These findings supported the hypothesis that
Smad7 is a direct target of miR-96 and that it is regulated by
miR-96 in the keloid. Also, it was demonstrated that miR-96
inhibited the production of COL1A1 and COL3A1 proteins, which could
be reversed with the transfection of siRNA-Smad7, indicating that
miR-96 in KFs can regulate collagen deposition through the
targeting of Smad7 expression. However, it was noted that the
expression level of Smad7 was slightly decreased following
transfecting the miR-96 mimic into the KFs, but the difference was
not statistically significant (data not shown).
The lack of an in vivo animal model that can
mimic human keloid scars has limited the study of potential keloid
therapeutic agents (33,34). Therefore, the ex vivo OC of
skin tissue and keloids according were evaluated, according to
reference protocols (15,24,35).
Treatment with the miRNA inhibitor, miR-96 antagomir, revealed
shrinkage of the keloid OC. In addition, a reduction in COL1A1 and
COL3A1 expression was observed following treatment.
In summary, the present study revealed the
upregulation of miR-96 expression in the keloid, and that Smad7 is
a direct target of miR-96. Furthermore, the addition of miR-96
antagomir into the keloid OC model revealed an effective reduction
of type I and Ш collagen expression, which further led to keloid
shrinkage. These findings suggest that miR-96 upregulation is
correlated with keloid formation and that miR-96 may be a
therapeutic target in keloid pathogenesis.
Acknowledgements
Not applicable.
Funding
The present study was conducted with the support of
the National Natural Science Foundation of China (grant nos.
81501684 and 1506RJZA303).
Availability of data and materials
The datasets used and/or analyzed in this study can
be obtained from the corresponding author upon request. Genbank
(www.ncbi.nlm.nih.gov/genbank) is
recommended for performing the DNA and RNA sequence searches.
Authors' contributions
LC made substantial contributions to the conception
and design of experiments and was a major contributor in the
writing of the manuscript. H-YZ was involved in the writing of the
manuscript and revisions to ensure important intellectual content.
W-DB performed data acquisition and prepared the figures. BX
performed histological examination of the keloid and the skin. MS
performed the cell culture and transfection experiments. D-HH
conceived the study, analyzed data, and wrote the manuscript. YL
analyzed and interpreted the data. All the authors read and
approved the final manuscript. Each author participated actively in
the study and takes responsibility for the respective work
conducted.
Ethics approval and consent to
participate
The present study was conducted with the approval of
the Medical Ethics Committee of Lanzhou General Hospital of Chinese
People's Liberation Army (approval no. LB1706472). All patients
provided written informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
COL1A1
|
collagen subunit 1α1
|
|
COL3A1
|
collagen subunit 3α1
|
|
TGF-β
|
transforming growth factor β
|
|
Smad7
|
mothers against decapentaplegic
homolog 7
|
References
|
1
|
Verhaegen PD, van Zuijlen PP, Pennings NM,
van Marle J, Niessen FB, van der Horst CM and Middelkoop E:
Differences in collagen architecture between keloid, normotrophic
scar, and normal skin: An objective histopathological analysis.
Wound Repair Regen. 17:649–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: A review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman DW, Boyd CD, Mackenzie JW, Norton
P, Olson RM and Deak SB: Regulation of collagen gene expression in
keloids and hypertrophic scars. J Surg Res. 55:214–222. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng J, Wang Y, Wang D and Wu Y:
Identification of collagen 1 as a post-transcriptional target of
miR-29b in skin fibroblasts: Therapeutic implication for scar
reduction. Am J Med Sci. 346:98–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang X, Tsitsiou E, Herrick SE and
Lindsay MA: MicroRNAs and the regulation of fibrosis. FEBS J.
277:2015–2021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chau BN and Brenner DA: What goes up must
come down: The emerging role of microRNA in fibrosis. Hepatology.
53:4–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu HY, Li C, Bai WD, Su LL, Liu JQ, Li Y,
Shi JH, Cai WX, Bai XZ, Jia YH, et al: MicroRNA-21 regulates hTERT
via PTEN in keloid fibroblasts. PLoS One. 9:e971142014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Zhu HY, Bai WD, Su LL, Liu JQ, Cai
WX, Zhao B, Gao JX, Han SC, Li J and Hu DH: MiR-10a and miR-181c
regulate collagen type I generation in hypertrophic scars by
targeting PAI-1 and uPA. FEBS Lett. 589:380–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kashiyama K, Mitsutake N, Matsuse M, Ogi
T, Saenko VA, Ujifuku K, Utani A, Hirano A and Yamashita S:
miR-196a downregulation increases the expression of type I and Ш
collagens in keloid fibroblasts. J Invest Dermatol. 132:1597–1604.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F,
Chen WY and Liu YN: Transforming growth factor-β promotes prostate
bone metastasis through induction of microRNA-96 and activation of
the mTOR pathway. Oncogene. 34:4767–4776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Mao Y, Zhu J, Meng F, Chen Q, Tao
L, Li R, Fu F, Liu C, Hu Y, et al: TGF-β1 promotes colorectal
cancer immune escape by elevating B7-H3 and B7-H4 via the
miR-155/miR-143 axis. Oncotarget. 7:67196–67211. 2016.PubMed/NCBI
|
|
13
|
Zhu HY, Bai WD, Li C, Zheng Z, Guan H, Liu
JQ, Yang XK, Han SC, Gao JX, Wang HT and Hu DH: Knockdown of
lncRNA-ATB suppresses autocrine secretion of TGF-β2 by targeting
ZNF217 via miR-200c in keloid fibroblasts. Sci Rep. 6:247282016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ZF, Zhang YG, Hu DH, Shi JH, Liu JQ,
Zhao ZT, Wang HT, Bai XZ, Cai WX, Zhu HY and Tang CW: Smad
interacting protein 1 as a regulator of skin fibrosis in
pathological scars. Burns. 37:665–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bagabir R, Syed F, Paus R and Bayat A:
Long-term organ culture of keloid disease tissue. Exp Dermatol.
21:376–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen Y, Xu H, Pan X, Wu W, Wang H, Yan L,
Zhang M, Liu X, Xia S and Shao Q: miR-34a and miR-125b are
upregulated in peripheral blood mononuclear cells from patients
with type 2 diabetes mellitus. Exp Ther Med. 14:5589–5596.
2017.PubMed/NCBI
|
|
17
|
O'Reilly S: MicroRNAs in fibrosis:
Opportunities and challenges. Arthritis Res Ther. 18:112016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou W, He L, Dai Y, Zhang Y, Wang J and
Liu B: MicroRNA-124 inhibits cell proliferation, invasion and
migration by targeting CAV1 in bladder cancer. Exp Ther Med.
16:2811–2820. 2018.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing T, Du L, Zhuang X, Zhang L, Hao J and
Wang J: Upregulation of microRNA-206 induces apoptosis of vascular
smooth muscle cells and decreases risk of atherosclerosis through
modulating FOXP1. Exp Ther Med. 14:4097–4103. 2017.PubMed/NCBI
|
|
21
|
Nie JM and Li HF: Therapeutic effects of
Salvia miltiorrhiza injection combined with telmisartan in patients
with diabetic nephropathy by influencing collagen IV and
fibronectin: A case-control study. Exp Ther Med. 16:3405–3412.
2018.PubMed/NCBI
|
|
22
|
Yang LL, Liu JQ, Bai XZ, Fan L, Han F, Jia
WB, Su LL, Shi JH, Tang CW and Hu DH: Acute downregulation of
miR-155 at wound sites leads to a reduced fibrosis through
attenuating inflammatory response. Biochem Biophys Res Commun.
453:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krützfeldt J, Rajewsky N, Braich R, Rajeev
KG, Tuschl T, Manoharan M and Stoffel M: Silencing of microRNAs in
vivo with ‘antagomirs’. Nature. 438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Syed F, Bagabir RA, Paus R and Bayat A: Ex
vivo evaluation of antifibrotic compounds in skin scarring: EGCG
and silencing of PAI-1 independently inhibit growth and induce
keloid shrinkage. Lab Invest. 93:946–960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou D, Wang J, He LN, Li BH, Ding YN,
Chen YW and Fan JG: Prolyl oligopeptidase attenuates hepatic
stellate cell activation through induction of Smad7 and PPAR-γ. Exp
Ther Med. 13:780–786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sidgwick GP and Bayat A: Extracellular
matrix molecules implicated in hypertrophic and keloid scarring. J
Eur Acad Dermatol Venereol. 26:141–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikeda M, Naitoh M, Kubota H, Ishiko T,
Yoshikawa K, Yamawaki S, Kurokawa M, Utani A, Nakamura T, Nagata K
and Suzuki S: Elastic fiber assembly is disrupted by excessive
accumulation of chondroitin sulfate in the human dermal fibrotic
disease, keloid. Biochem Biophys Res Commun. 390:1221–1228. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi
S, Hyakusoku H and Shimada T: siRNA knockdown of tissue inhibitor
of metalloproteinase-1 in keloid fibroblasts leads to degradation
of collagen type I. J Invest Dermatol. 134:818–826. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pandit KV, Milosevic J and Kaminski N:
MicroRNAs in idiopathic pulmonary fibrosis. Transl Res.
157:191–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Rooij E, Sutherland LB, Thatcher JE,
DiMaio JM, Naseem RH, Marshall WS, Hill JA and Olson EN:
Dysregulation of microRNAs after myocardial infarction reveals a
role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA.
105:13027–13032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maurer B, Stanczyk J, Jüngel A,
Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE,
Michel BA, Distler JH, et al: MicroRNA-29, a key regulator of
collagen expression in systemic sclerosis. Arthritis Rheum.
62:1733–1743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Pu X, Wang Q, Cao J, Xu F, Xu LI and
Li K: miR-96 induces cisplatin chemoresistance in non-small cell
lung cancer cells by downregulating SAMD9. Oncol Lett. 11:945–952.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Williams FN, Herndon DN and Branski LK:
Where we stand with human hypertrophic and keloid scar models. Exp
Dermatol. 23:811–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van den Broek LJ, Limandjaja GC, Niessen
FB and Gibbs S: Human hypertrophic and keloid scar models:
Principles, limitations and future challenges from a tissue
engineering perspective. Exp Dermatol. 23:382–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu Z, Hasse S, Bodo E, Rose C, Funk W and
Paus R: Towards the development of a simplified long-term organ
culture method for human scalp skin and its appendages under
serum-free conditions. Exp Dermatol. 16:37–44. 2007. View Article : Google Scholar : PubMed/NCBI
|