Introduction
Idiopathic generalized epilepsy (IGE) is a type of
epilepsy in which patients experience generalized tonic-clonic,
myoclonic and absence seizures (1).
Patients undergoing generalized tonic-clonic seizures (GTCS) are
unresponsive as they sustain convulsions. Scalp
electroencephalography (EEG) reveal that such patients exhibit
generalized spike-and-wave discharges (GSWDs) of 2.5–5.0 Hz, which
are thought be propagated, at least in part, via the
corticothalamic circuit (2).
Regional homogeneity (ReHo) is a voxel-based measure of brain
activity, which evaluates the synchronization between different
brain regions by calculating the similarity between the time series
of a voxel and its proximal voxels. Requiring no prior knowledge
about hemodynamic response, as a data-driven method, the major
advantage is its ability to detect the changes of deoxyhemoglobin
in venous blood. Abnormal regional homogeneity is relevant to the
changes of temporal aspects of neural activity in the regional
brain (3).
ReHo may be used clinically to evaluate functional
modulations when the brain is in its resting state; many valuable
findings have been obtained from ReHo conducted in patients with
neuropsychological diseases, including epilepsy (4–6),
schizophrenia (7) and amnestic mild
cognitive impairment (8). The
results of previous studies support that there is increased
synchronization in the epileptogenic zones during seizures and the
when patients are in the interictal state, which may be involved in
the generation of interictal activity (5). It is also notable that ReHo analyses in
patients with generalized epilepsy experiencing absence seizures
(6) or patients with juvenile
myoclonic epilepsy (4) have
identified abnormalities in the striato-thalamo-cortical network
and the thalamo-motor cortical network, respectively. In addition,
convulsions experienced by patients with IGE may be caused by the
hyperexcitability of motor neuron circuits. Thus, the thalamus,
basal ganglia and motor cortex may all be involved in the
development of IGE-GTCS. To evaluate changes in the spontaneous
brain activity of patients with IGE-GTCS, the present study
investigated the ReHo features of such patients compared with
healthy controls. The effects of changes in ReHo on the clinical
factors of IGE-GTCS were also assessed.
Although numerous studies have focused on the gray
matter of patients with IGE-GTCS, few studies have assessed the
white matter of these patients. Diffusion tensor imaging (DTI)
studies have been used to determine structural abnormalities in
patients with IGE-GTCS (9). However,
it has been demonstrated that diffusional kurtosis imaging (DKI) is
more sensitive than conventional DTI at evaluating tissue
microstructure (10), even in the
presence of crossing fibers. DKI is a novel magnetic resonance
imaging (MRI)-based, noninvasive technique able to measure
fractional anisotropy (FA), mean diffusivity (MD) and mean kurtosis
(MK), and therefore may identify microstructural changes in the
cerebral white matter (WM) (11).
Tract-based spatial statistics (TBSS) represents a novel approach
and provides a fully automated and independent analysis of multiple
subjects, allowing for clear observation of localized changes in
diffusion (12). Therefore,
combining DKI with TBSS (DKI-TBSS) in patients with IGE-GTCS may
allow the detection of alterations that were previously
undetectable in the microstructure of the cerebral WM. Indeed,
previous studies have identified changes in kurtosis-based
diffusion metrics in several neuropathologies, including acute
cerebral infarction (13),
idiopathic normal pressure hydrocephalus (14) and epilepsy (15).
Most research into epilepsy has been conducted in
patients with IGE or has used a single method to study a specific
subtype of IGE. GTCS is the most common phenotype of IGE (16); therefore, the aim of the present
study was to use DKI-TBSS to determine non-Gaussian diffusion
patterns in the whole-brain WM of patients with IGE-GTCS. The
characterization of such anatomical and functional connectivity
abnormalities may improve the diagnosis and treatment of patients
with early stage IGE-GTCS.
Materials and methods
Participants
A total of 28 right-handed patients (mean age,
22.85±5.61 years; 11 females and 17 males) with IGE-GTCS from the
Epilepsy Clinic at the First Affiliated Hospital of China Medical
University (Shenyang, China) were recruited between March 2016 and
December 2016. IGE-GTCS was diagnosed based on the electroclinical
criteria of the International League Against Epilepsy (17). The inclusion criteria for patients in
the current study were as follows: i) The presence of typical
clinical symptoms of IGE-GTCS, apart from partial seizures
secondary to GTCS; ii) ≥1 EEG examination identifying typical GSWDs
on a normal background; iii) no abnormal or unusual clinical MRI
findings; and iv) no typical GTCS seizures for 7 days. A total of
28 right-handed healthy participants (mean age, 24.52±4.34 years;
12 females and 16 males) with no previous neurological or
psychiatric problems were recruited as controls. There were no
differences in the sex, age, education level and handedness between
patients and controls (Table I). The
present study was approved by the Ethics Committee of the First
Hospital of China Medical University and written informed consent
was obtained from all participants.
 | Table I.Demographic and clinical
characteristics of patients with IGE-GTCS and HCs. |
Table I.
Demographic and clinical
characteristics of patients with IGE-GTCS and HCs.
| Clinical
characteristics | Patients with
IGE-GTCS (n=28) | Patients with HC
(n=28) | P-values |
|---|
| Age (years) | 22.85±5.61 | 24.52±4.34 |
0.253 |
| Sex
(female:male) | 11:17 | 12:16 |
0.766 |
| Duration of
epilepsy (years) |
3.47±1.68 | n/a | n/a |
| Duration of
education (years) | 11.06±1.96 | 11.89±2.12 |
0.570 |
| Handedness
(right/left) | 28/0 | 28/0 | >0.99 |
Image acquisition
Data were obtained on a SIEMENS Verio 3.0 Tesla MRI
scanner (Siemens Healthineers, Erlangen, Germany). T2-weighted
axial/oblique coronal images and fluid-attenuated inversion
recovery oblique coronal images were acquired as part of the
examination. All images underwent visual analysis by experienced
radiologists and were found to be normal. Resting-state functional
data were obtained using an echo-planar imaging sequence
[repetition time (TR), 2,000 msec; echo time (TE), 30 msec]. The
following imaging parameters were used: Field of view, 240×240
mm2; slice thickness, 4 mm with a 0.8 mm gap; and
matrix, 64×64. DKI was applied to a single shot echo-planar imaging
sequence (TR, 9,500 msec; TE, 104 msec) with the following
diffusion weightings applied along 30 non-collinear directions:
b-value, 0, 1,000 and 2,000 sec/mm2. A total of 45 axial
slices were collected from each participant. The field of view was
222×222 mm2 and slice thickness was 2 mm with no gap.
Three-dimensional T1-weighted images using a magnetization-prepared
rapid gradient echo sequence (TR, 2,250 msec; TE, 4.18 msec) were
obtained for each participant. Imaging parameters were as follows:
256×256 mm2 field of view, 1-mm slice thickness and
256×256 acquisition matrix.
ReHo analysis
All resting-state functional MRI (fMRI) data were
pre-processed using the Data Processing Assistant for Resting-State
fMRI (DPARSF_V2.2; restfmri.net/forum/dparsf_v2_2), which was based on
Statistical Parametric Mapping (SPM; http://www.fil.ion.ucl.ac.uk/spm). To ensure magnetic
field stabilization, the first 10 points were discarded. The
remaining 230 points were corrected for slice timing and head
motion, and then spatially normalized. T1-weighted images were
spatially normalized based on the Montreal Neurological Institute
(MNI) standard brain space (18),
followed by detrending and bandpass filtering (0.01–0.08 Hz). Using
Kendall's coefficient of concordance, ReHo was computed using REST
software (REST plus version 1.2; www.restfmri.net/forum/REST). ReHo was used to measure
local synchronization of blood-oxygen-level-dependent fluctuations
within 27 voxels in a voxel-wise manner. All ReHo maps were
smoothed with a Gaussian kernel of 6×6×6 mm3 (full-width
at half-maximum). To evaluate local spontaneous brain activity in
each group of participants, a one-sample t test was used to compare
ReHo values. Two-sample t-tests based on ReHo maps were used to
evaluate differences between patients with IGE-GTCS and healthy
controls. P<0.05 was considered to indicate a significant
difference. False discovery rate correction was used for multiple
comparisons as well as cluster correction for a cluster size ≥21
voxels. The REST Toolbox was used to obtain cluster sizes,
locations and their respective t-values. The t-value represented
the statistical value of the peak voxel that exhibited differences
in ReHo and compared these values between patients with IGE-GTCS
and healthy controls. Finally, the correlation between ReHo values
and epilepsy duration were determined using Pearson's correlation
for patients with IGE-GTCS.
DKI image processing with TBSS
All diffusion images without lesions, artifacts or
severe atrophy for brain distortion were corrected using the
‘eddy-current’ toolbox in the Functional MRI of the Brain (FMRIB)
Software Library (FSL) tools (version 5.0; www.fmrib.ox.ac.uk/fsl) for Linux. Subsequently, the
Diffusion Kurtosis Estimator (DKE) (www.nitrc.org/projects/dke) was used to calculate DKI
parametric maps, including MK, FA and MD. DKI data were normalized
to the Montreal Neurological Institute (MNI) standard brain space
(18) using the FMRIB Software
Library (FSL) tools. FA maps of all participants were aligned to
the MNI152 space. Subsequently, the mean FA image was generated and
thinned to create the mean FA skeleton, which represented the
centers of all tracts common to each group. An FA threshold of 0.2
was applied to exclude further prevent partial-volume effects, and
peripheral tracts and gray matter were excluded (15,19). A
threshold was applied to the mean FA skeleton and a skeletonized FA
map was made by projecting each participant's FA map onto the mean
FA skeleton with local maximum FA values. This projection was also
applied to other non-FA diffusion metrics to create skeletonized MD
and MK maps. Permutation-based testing was used with 5,000
permutations and statistical inferences by threshold-free cluster
enhancement, with a threshold of P<0.05, corrected for multiple
comparisons (family wise error) using random field theory.
Significant differences in the anatomic locations of white matter
tracts were revealed by TBSS. The JHU ICBM-DTI-81 White-Matter
Labels Atlas (20) was used to
evaluate results. The values of MK, FA, and MD were calculated for
each cluster using in-house Matlab scripts (Matlab 2012b, Math
Works, Inc., Natick, MA, USA). Student's t test was used to compare
the values of MK, FA and MD in the area of the WM between IGE-GTCS
patients and healthy controls. The t-value represented the
statistical value of peak voxel identifying differences in MK, FA
and MD differences between patients with IGE-GTCS and healthy
controls. FA, MD and MK values from each participant were extracted
from regions exhibiting significant differences between patients
and controls in TBSS. Partial Spearman's correlation analysis was
performed to evaluate the association between extracted values and
epilepsy duration following controlling for age and sex.
Results
Within-group and between-group ReHo
analyses
Differences in ReHo values within each group were
analyzed using a one-sample t-test. Compared with healthy controls,
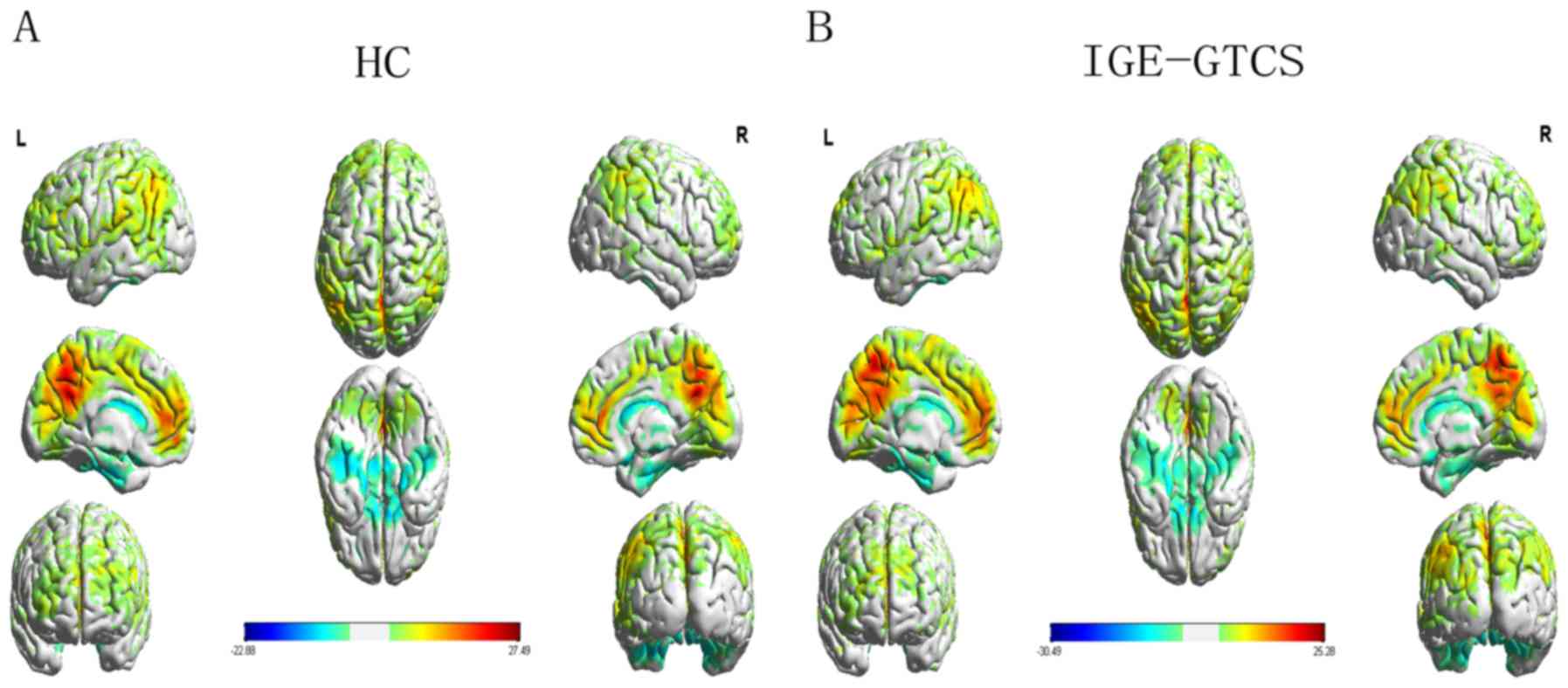
ReHo values were markedly increased in patients with IGE-GTCS in
bilateral regions of the basal-thalamus, including the putamen,
thalamus, right pallidum, right supplementary motor area and
bilateral paracentral lobules (Fig.
1 and Table II). However, ReHo
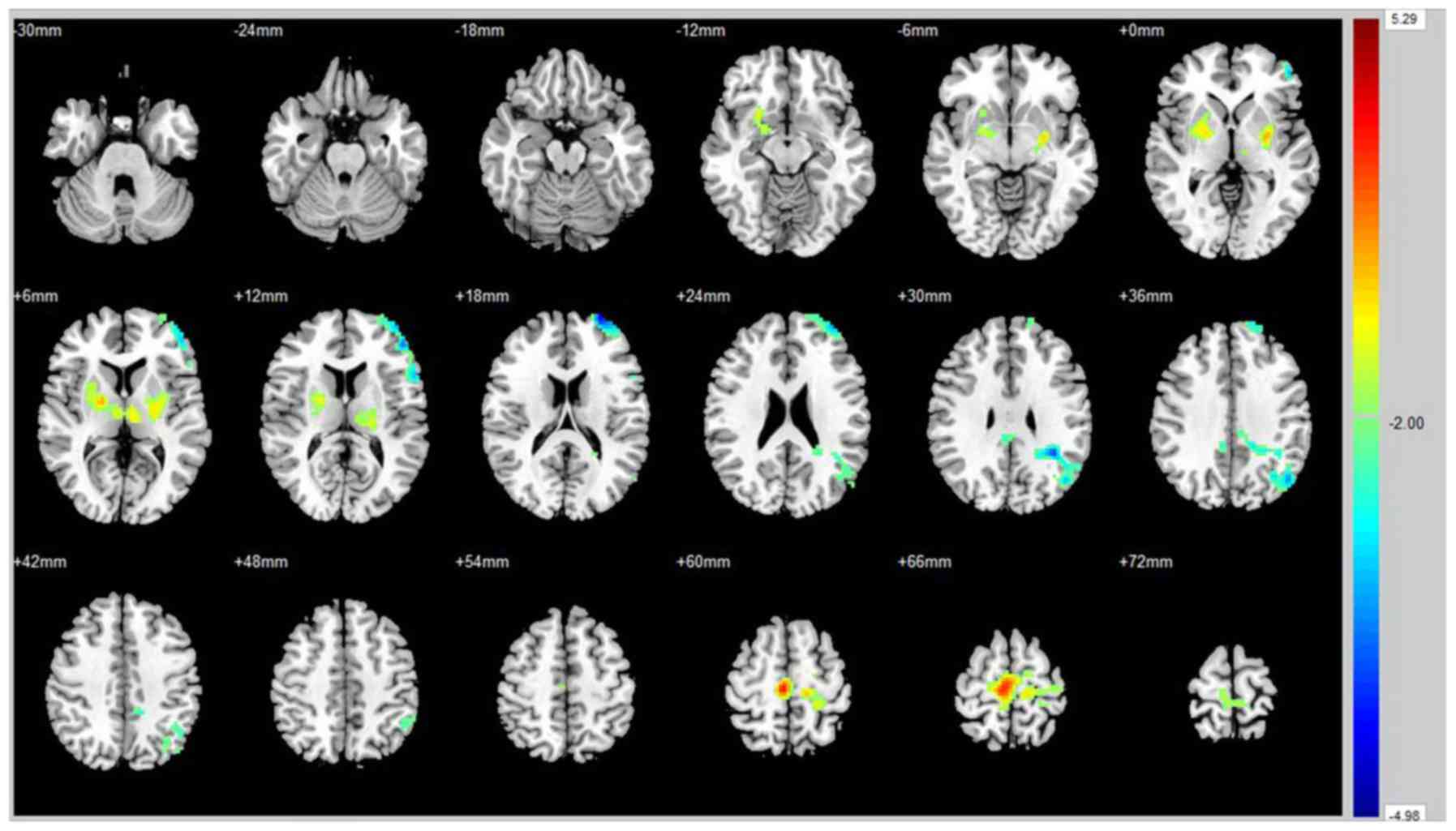
were values were markedly decreased in the posterior cingulate
cortex, left angular gyrus, left middle frontal gyrus and left
superior fontal gyrus (Fig. 2 and
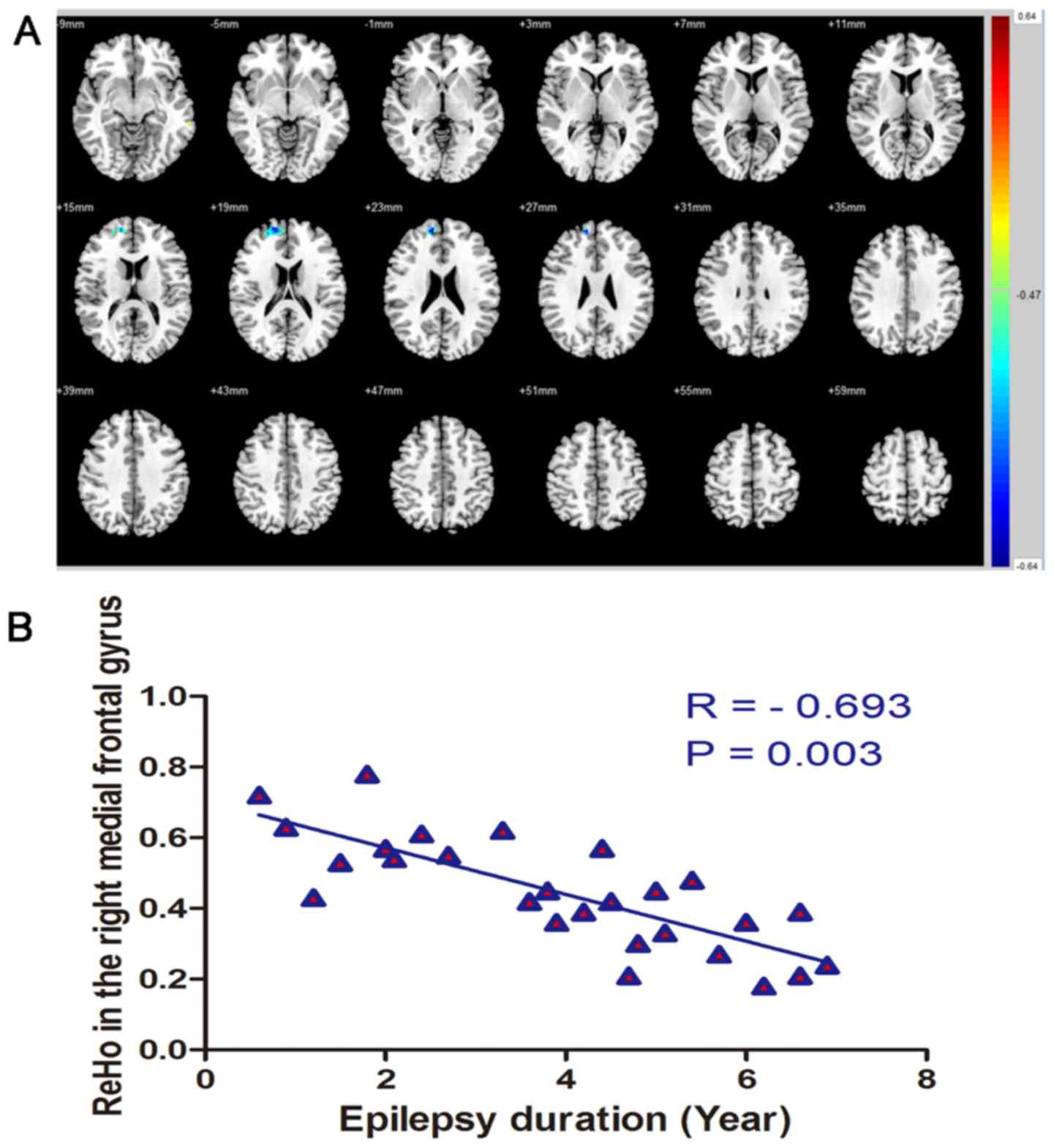
Table II). ReHo and epilepsy
duration were negatively correlated in regions of the right
superior frontal gyrus (r=−0.693, P=0.003; Fig. 3).
 | Table II.Brain regions of increased/decreased
ReHo in patients with IGE-GTCS. |
Table II.
Brain regions of increased/decreased
ReHo in patients with IGE-GTCS.
|
|
|
|
|
| MNI
coordinates |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Brain region | Voxels | AAL | BA | Side | X | Y | Z | t-value |
|---|
| Putamen | 95 | 73 |
| L | −29 | −4 | −3 | 3.029 |
| Putamen | 71 | 74 |
| R | 24 | −1 | 8 | 2.522 |
| Pallidum | 42 | 76 |
| R | 21 | −5 | 8 | 2.003 |
| Thalamus | 69 | 77 |
| L | −7 | −17 | 8 | 2.753 |
| Thalamus | 32 | 78 |
| R | 6 | −12 | 8 | 2.221 |
| Supplementary motor
area | 55 | 20 | 6 | R | 3 | −21 | 63 | 4.431 |
| Paracentral
lobule | 92 | 69 |
| L | −5 | −32 | 70 | 2.327 |
| Paracentral
lobule | 73 | 70 |
| R | 5 | −32 | 69 | 2.510 |
| Posterior cingulate
cortex | 33 | 35 | 23 | L | −1 | −36 | 30 | −2.132 |
| Posterior cingulate
cortex | 31 | 36 | 23 | R | 5 | −36 | 31 | −2.450 |
| Angular gyrus | 151 | 65 | 39 | L | −45 | −68 | 35 | −3.7735 |
| Middle frontal
gyrus | 116 | 7 | 46 | L | −41 | 54 | 9 | −2.946 |
| Superior frontal
gyrus | 43 | 3 | 10 | L | −27 | 63 | 17 | −4.311 |
DKI-TBSS
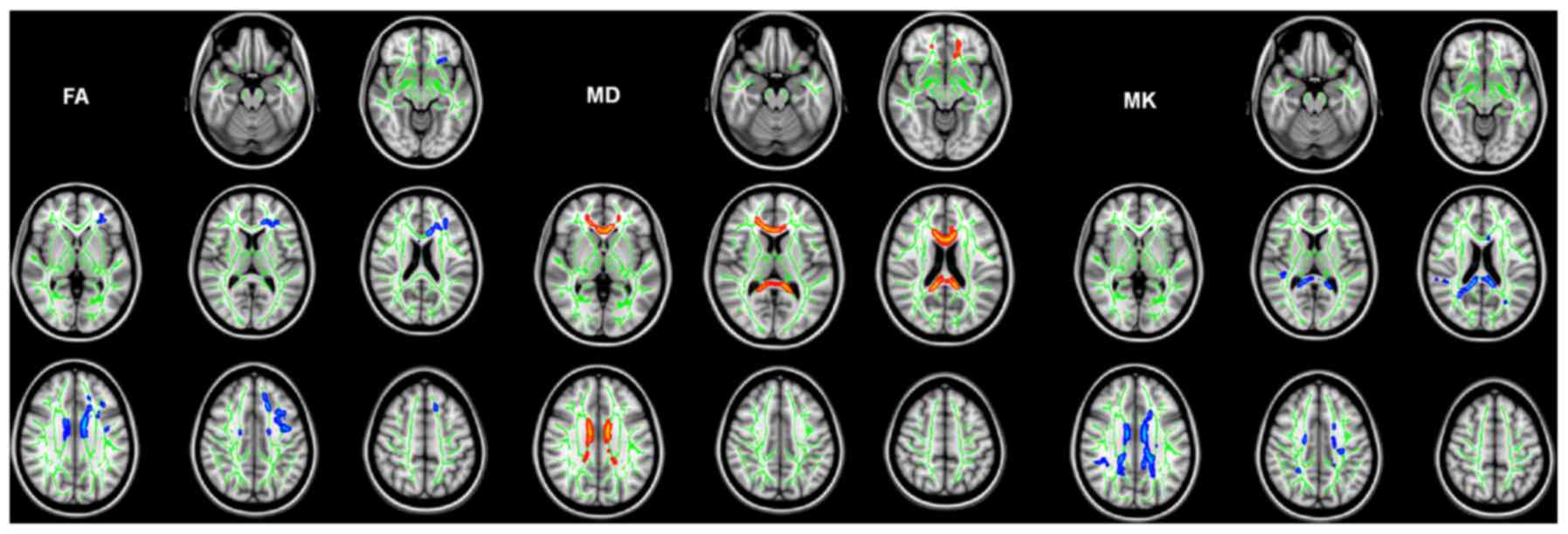
Compared with healthy controls, patients with
IGE-GTCS exhibited microstructural abnormalities in their WM. A
large cluster of significantly reduced FA was detected in patients
with IGE-GTCS compared with controls (Table III). Affected WM tracts included
the left anterior corona radiata, left superior corona radiata,
left superior longitudinal fasciculus and genu/body of the corpus
callosum (MNI coordinates of local maxima, −8/7/26). Two large
clusters of significantly increased MD were observed in the WM
tracts of patients with IGE-GTCS compared with healthy controls
(Table IV). Affected WM tracts
included the bilateral anterior corona radiata, left superior
corona radiata, left cingulum, genu/body of the corpus callosum
(MNI coordinates of local maxima, 5/27/0); and left splenium/body
of the corpus callosum (MNI coordinates of local maxima,
−12/-41/11). Four large clusters of significantly decreased MK
values were observed in the WM tracts of patients with IGE-GTCS
compared with controls (Table V).
Affected WM tracts included the right superior longitudinal
fasciculus (MNI coordinates of local maxima, 41/-37/32); left
posterior thalamic radiation (including optic radiation; MNI
coordinates of local maxima=−28/-65/17); bilateral superior corona
radiata, bilateral posterior corona radiata, left anterior corona
radiata, left cingulum and splenium/genu/body of the corpus
callosum (MNI coordinates of local maxima, −12/-41/21); and left
superior corona radiata (MNI coordinates of local maxima,
−28/-14/23). Notably, the abnormalities that manifested as
decreased MK values were more extensive. However, the abnormalities
revealed by kurtosis metrics did not fully explain the
abnormalities that manifested as reduced FA and increased MD
(Fig. 4). There was no correlation
observed between DKI metrics and epilepsy duration (Table VI).
 | Table III.MNI coordinates of the brain with
reduced FA identified by TBSS. |
Table III.
MNI coordinates of the brain with
reduced FA identified by TBSS.
|
|
|
|
|
| MNI
coordinates |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Cluster number | Voxels | Atlas | Side | P-values | X | Y | Z | t-value |
|---|
| 1 | 4,027 | Body of corpus
callosum |
| 0.01 | −8 | 7 | 26 | 2.06 |
|
| 1,170 | Anterior corona
radiata | L | 0.01 |
|
|
|
|
|
| 623 | Genu of corpus
callosum |
| 0.01 |
|
|
|
|
|
| 310 | Superior corona
radiata | L | 0.01 |
|
|
|
|
|
| 107 | Superior
longitudinal fasciculus | L | 0.01 |
|
|
|
|
 | Table IV.MNI coordinates of the brain with
increased MD identified by TBSS. |
Table IV.
MNI coordinates of the brain with
increased MD identified by TBSS.
|
|
|
|
|
| MNI
coordinates |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Cluster number | Voxels | Atlas | Side | P-values | X | Y | Z | t-value |
|---|
| 1 | 2,986 | Genu of corpus
callosum |
| 0.01 | 5 | 27 | 0 | 2.50 |
|
| 4,476 | Body of corpus
callosum |
| 0.01 |
|
|
|
|
|
| 541 | Anterior corona
radiata | R | 0.01 |
|
|
|
|
|
| 327 | Anterior corona
radiata | L | 0.01 |
|
|
|
|
|
| 176 | Superior corona
radiata | L | 0.01 |
|
|
|
|
|
| 110 | Cingulum | L | 0.01 |
|
|
|
|
| 2 | 3,585 | Splenium of corpus
callosum | L | 0.01 | −12 | −41 | 11 | 3.06 |
|
| 131 | Body of corpus
callosum | L | 0.01 |
|
|
|
|
 | Table V.MNI coordinates of the brain with
reduced MK identified by TBSS. |
Table V.
MNI coordinates of the brain with
reduced MK identified by TBSS.
|
|
|
|
|
| MNI
coordinates |
|
|---|
| Cluster number | Voxels | Atlas | Side | P-values | X | Y | Z | t-value |
|---|
| 1 | 4,861 | Splenium of corpus
callosum |
| 0.01 | −12 | −41 | 21 | 2.97 |
|
| 3,927 | Body of corpus
callosum |
| 0.01 |
|
|
|
|
|
| 572 | Superior corona
radiata | L | 0.01 |
|
|
|
|
|
| 328 | Posterior corona
radiata | L | 0.01 |
|
|
|
|
|
| 319 | Cingulum | L | 0.01 |
|
|
|
|
|
| 152 | Superior corona
radiata | R | 0.01 |
|
|
|
|
|
| 107 | Genu of corpus
callosum |
| 0.01 |
|
|
|
|
|
| 104 | Anterior corona
radiata | L | 0.01 |
|
|
|
|
|
| 100 | Posterior corona
radiata | R | 0.01 |
|
|
|
|
| 2 | 390 | Superior
longitudinal fasciculus | R | 0.01 | 41 | −37 | 32 | 2.60 |
| 3 | 248 | Superior corona
radiata | L | 0.01 | −28 | −14 | 23 | 2.73 |
| 4 | 225 | Posterior thalamic
radiation | L | 0.01 | −28 | −65 | 17 | 4.73 |
 | Table VI.Association between epilepsy duration
and DKI in patients with IGE-GTCS. |
Table VI.
Association between epilepsy duration
and DKI in patients with IGE-GTCS.
|
|
| MNI
coordinates |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Cluster number | DKI metrics
(µm2/ms) | X | Y | Z | IGE-GTCS
(n=28) | Duration of
epilepsy (years) | P-values |
|---|
|
| FA |
|
|
|
|
|
|
| 1 |
| −8 | 7 | 26 | 0.45±0.03 | 3.47±1.68 | 0.87 |
|
| MD |
|
|
|
|
|
|
| 1 |
| 5 | 27 | 0 | 0.94±0.03 | 3.47±1.68 | 0.38 |
| 2 |
| −12 | −41 | 11 | 0.90±0.04 | 3.47±1.68 | 0.19 |
|
| MK |
|
|
|
|
|
|
| 1 |
| −12 | −41 | 21 | 0.94±0.04 | 3.47±1.68 | 0.56 |
| 2 |
| 41 | −37 | 32 | 0.88±0.05 | 3.47±1.68 | 0.63 |
| 3 |
| −28 | −14 | 23 | 0.95±0.04 | 3.47±1.68 | 0.50 |
| 4 |
| −28 | −65 | 17 | 0.98±0.04 | 3.47±1.68 | 0.71 |
Discussion
Amplitude of low-frequency fluctuation (21), functional connectivity (22) and ReHo are resting-state fMRI methods
that may be used to assess patients with IGE-GTCS. ReHo reflects
the synchronization of neural activity in local brain regions
(3). In the present study, analysis
of within- and between-group ReHo measurements identified
widespread cortical and subcortical region involvement. Compared
with healthy controls, patients with IGE-GTCS presented with
significantly increased ReHo values in bilateral basal
ganglia-thalamus regions and regions of the cortex associated with
motor function, including the supplementary motor area and
bilateral paracentral lobules. The thalamus, which regulates
susceptibility to and propagation of seizures, is the major relay
station of the cortical and subcortical projection systems. The
thalamus may serve as a stronger driver of cortical activity, from
initiation to propagation of GSWDs (23) and may be critical for the generation
of epileptic tonic-clonic motor activity and impairment of
consciousness (24). Paz et
al (25) used optogenetics in a
rat model to reveal the crucial role of the thalamus within the
cortico-thalamo-cortical network in sustaining seizures.
Furthermore, structural investigations centered on voxel-based
morphometry demonstrated that the volume of gray matter is reduced
in the thalami of patients with IGE-GTCS (26).
Basal ganglia are widely considered to mediate
epileptic discharge regulation (27)
and their dysfunction is associated with locomotor disturbances,
which may be linked to motor responses as uncontrolled jerking
movements in tonic-clonic seizures. It has been reported that
patients with idiopathic generalized epilepsy syndromes exhibit
reduced putamen volumes (28).
Furthermore, reduced dopamine transporter binding has been detected
in the putamen of patients with IGE-GTCS, who primarily exhibited
impairments in motor control and speed, which suggests that
dopamine may be neuroprotective and may inhibit the onset of
seizures (29). In addition, the
substantia nigra may mediate the spread of epileptic activity
(30). The imaging threshold set by
SPM may result in brain areas appearing smaller than they actually
are and may account for the absence in the results of the current
study regarding small basal ganglia nuclei. Somatotopic
organization of movement-related neurons is maintained throughout
the basal ganglia-thalamo-cortical circuit (31). Additionally, functional connectivity
between the putamen and the motor and premotor cortices has been
previously reported (32).
Furthermore, the pallidum and putamen comprise a resting state
network in the basal ganglia, which also project into the
supplementary motor area, functioning as the motor control circuit
of the basal ganglia (31). Most
patients with IGE-GTCS present with sustained muscle rigidity and
rapid muscle contractions during seizures; therefore, the current
study hypothesized that there would be disruptions in the basal
ganglia-thalamo-cortical circuit. It is likely that the basal
ganglia themselves do not generate seizures; however, cortical
feedback loops mediated by basal ganglia circuits may impact on
cortical epileptic activity (33).
In the present study, ReHo values were decreased in
the posterior cingulate cortex (PCC) and left angular gyrus. These
areas overlap with the default mode network (DMN), which influences
self-awareness, episodic memory and interactive modulation
(34). In the current study,
decreased activity was observed in the dorsolateral prefrontal
cortex (DLPFC), which is the central brain region of the central
executive network (CEN) that mediates cognitive and emotional
circuits (35). Wei et al
(36) used Granger causality
analysis to identify alterations in direct causal relationships
across the key nodes PCC and DLPFC, of the DMN and CEN,
respectively, in patients with IGE-GTCS. Relative to the healthy
control group, patients with IGE-GTCS demonstrated a significantly
enhanced Granger causal influence from the DLPFC to the PCC, which
is coherent in both time and frequency domain analysis, and the
results were consistent with the results of the current study. The
altered efficacy in connectivity between the DLPFC and the dorsal
anterior cingulate cortex may be a key factor that affects
cognitive dysfunction in patients with IGE-GTCS (36). It has also been demonstrated that PCC
is primarily involved in determining consciousness. Laureys et
al (37) identified that the PCC
serves a pivotal role in regulating consciousness due to its
anatomic location, as it links to the anterior thalamic nucleus and
thalamic arousal system of the brainstem. In the present study, a
negative correlation was identified between ReHo in the right
superior frontal gyrus regions and epilepsy duration. This suggests
that patients with IGE-GTCS exhibit an increased vulnerability to
seizure in the right superior frontal gyri.
In the present study, the DKI metrics MK, FA, and MD
served as quantitative measures of microstructural changes in the
brains of patients with IGE-GTCS. FA quantifies preferred direction
and MD presents the average extent of water diffusion in WM. MK
values are quantitative measures of non-Gaussian water diffusion,
which results from barriers to diffusion (13). The pathophysiological mechanisms
involved in determining the changes in FA and MD in patients with
IGE-GTCS remain unknown. Decreased FA in WM is indicative of
disrupted microstructural integrity; FA is affected by cell
membrane and myelin integrity, as well as fiber density. Increased
MD is similarly indicative of disruption in microscopic barriers
and accumulation of extracellular fluid, and has been observed in
neuropathological diseases associated with tissue degeneration and
edema (38). It is noteworthy that
changes in FA and MD were primarily confined to the bilateral
anterior and left superior corona radiate, the corpus callosum,
cingulum and superior longitudinal fasciculus in the current study.
As projection fibers, the anterior and superior corona radiata
reciprocally connect the cerebral cortex to the thalamus.
Similarly, the genu and body of the corpus callosum comprise
interhemispheric commissural fibers that interconnect the
prefrontal, premotor and supplementary motor areas (39). A previous EEG study revealed that the
corpus callosum facilitates the epileptogenic susceptibility of the
hemispheres via bisynchronous and bisymmetrical epileptiform
discharges (40). The results of
clinical studies support this hypothesis: Corpus callosotomy
reduces the frequency and severity of generalized seizures in
patients with medically intractable IGE (41).
In the current study, brain regions with changes in
MK values were distinct from regions with changes in MD and FA.
Reduced MK values were not limited to the WM tracts of the corpus
callosum or corona radiata, but also included the WM of the
posterior thalamic radiation (including optic radiation) and the
right superior longitudinal fasciculus (SLF). Lower MK values
indicate reduced diffusion heterogeneity, and there is a weak
correlation between MK and MD in the brains of humans (11). In particular, reduced MK values in
the brains of patients with IGE-GTCS may be associated with reduced
cell compartmentalization and increased membrane permeability
(42). It may also be useful to
assess MK to evaluate crossing fibers, while FA and MD in WM may be
affected by the presence of crossing fibers. MK is able to identify
areas of crossing fibers and this may explain the sensitivity of
DKI to changes in cerebral WM (43).
Reduced MK values in the posterior thalamic radiation (including
optic radiation) may explain the visual aura experienced by some
patients with IGE (44). SLF is
involved in executive (inhibitory control) and language function
(45). IGE-GTCS is traditionally
considered to be a genetic form of epilepsy (17). However, no correlation was identified
between DKI metrics and epilepsy duration in patients with
IGE-GTCS. The results of the current study demonstrate that
microstructural abnormalities may represent subtle
neurodevelopmental alterations that precede the onset of epilepsy
in IGE-GTCS (15). In general, the
functional connectivity network is thought to be more flexible
whilst the structural connectivity network is relatively stable.
Therefore, the structural connectivity network may be affected less
in patients with IGE-GTCS (16).
An unexpected result of the current study was that
spatial distributions of FA were asymmetric in patients with
IGE-GTCS. It is generally considered that changes in the brain are
bilateral and symmetrical in IGE-GTCS; however, the results of the
current study indicated that the left hemisphere was more affected
than the right. These results are consistent with those of a
previous study (46) that indicated
that patients with IGE-GTCS exhibit brain asymmetry and
lateralization of features, the tendency for some neural functions
or cognitive processes to be specialized to one side of the brain
or the other. Forced ictal head version or asymmetric tonic limb
posturing are highly informative regarding seizure lateralization
(47). The cause of seizure
lateralization in IGE-GTCS is not well understood. Epileptic
activity affecting the two hemispheres unequally may lead to
lateralization of features that can be observed in IGE-GTCS
(47).
In conclusion, the current study investigated the
pattern of regional hemodynamic synchronization in the brains of
patients with IGE-GTCS by performing ReHo analysis of resting-state
fMRI scans. Patients with IGE-GTCS exhibited altered regional
synchronization in the bilateral thalami, the basal ganglia,
motor-related cortex, posterior DMN regions and the CEN regions.
Different DKI indices indicated different sensitivities for the
detection of changes in diffusion. Regions exhibiting MK
alterations were distinct from the regions with MD and FA
abnormalities, suggesting that the role of DKI-TBSS in the
characterization of microstructural characteristics of the brains
of patients with IGE-GTCS differs from that of conventional DTI.
These results, which support the potential of ReHo and DKI-TBSS as
techniques for detecting intrinsic epileptic activity, provide
important insights into the understanding of the pathophysiological
mechanisms involved in IGE-GTCS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Program of Shenzhen (grant no.
JCYJ20150731154850923).
Availability of data and materials
All datasets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
GF, GLi and GLy designed the study. GLi and NY
collected the data. GLi, GLy, BC, JY, YH, YL, JX and FL analyzed
the data. GF and GLi prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the First Affiliated Hospital of China Medical
University (Shenyang, China). All patients provided written
informed consent.
Patient consent for publication
All participants provided written informed consent
for publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferrie CD: Idiopathic generalized
epilepsies imitating focal epilepsies. Epilepsia. 46 Suppl
9:S91–S95. 2005. View Article : Google Scholar
|
|
2
|
Szaflarski JP, Kay B, Gotman J, Privitera
MD and Holland SK: The relationship between the localization of the
generalized spike and wave discharge generators and the response to
valproate. Epilepsia. 54:471–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zang Y, Jiang T, Lu Y, He Y and Tian L:
Regional homogeneity approach to fMRI data analysis. NeuroImage.
22:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang S, Luo C, Liu Z, Hou C, Wang P, Dong
L, Zhong C, Lai Y, Xia Y and Yao D: Altered local spontaneous brain
activity in juvenile myoclonic epilepsy: A preliminary
resting-state fMRI study. Neural Plast. 2016:35472032016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeng H, Pizarro R, Nair VA, La C and
Prabhakaran V: Alterations in regional homogeneity of resting-state
brain activity in mesial temporal lobe epilepsy. Epilepsia.
54:658–666. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang T, Fang Z, Ren J, Xiao F, Li Q, Liu
L, Lei D, Gong Q and Zhou D: Altered spontaneous activity in
treatment-naive childhood absence epilepsy revealed by regional
homogeneity. J Neurol Sci. 340:58–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwabuchi SJ and Palaniyappan L:
Abnormalities in the effective connectivity of visuothalamic
circuitry in schizophrenia. Psychol Med. 47:1300–1310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan X, Han Y, Wei Y, Xia M, Sheng C, Jia
J and He Y: Regional homogeneity changes in amnestic mild cognitive
impairment patients. Neurosci Lett. 629:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji GJ, Zhang Z, Xu Q, Zang YF, Liao W and
Lu G: Generalized tonic-clonic seizures: Aberrant interhemispheric
functional and anatomical connectivity. Radiology. 271:839–847.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Gao Y, Zhou M, Wu J, Zee C and
Wang D: A diffusional kurtosis imaging study of idiopathic
generalized epilepsy with unilateral interictal epileptiform
discharges in children. J Neuroradiol. 43:339–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jensen JH, Helpern JA, Ramani A, Lu H and
Kaczynski K: Diffusional kurtosis imaging: The quantification of
non-gaussian water diffusion by means of magnetic resonance
imaging. Magn Reson Med. 53:1432–1440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith SM, Johansen-Berg H, Jenkinson M,
Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC,
Bartsch AJ and Behrens TE: Acquisition and voxelwise analysis of
multi-subject diffusion data with tract-based spatial statistics.
Nat Protoc. 2:499–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo YL, Li SJ, Zhang ZP, Shen ZW, Zhang
GS, Yan G, Wang YT, Rao HB, Zheng WB and Wu RH: Parameters of
diffusional kurtosis imaging for the diagnosis of acute cerebral
infarction in different brain regions. Exp Ther Med. 12:933–938.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamiya K, Kamagata K, Miyajima M, Nakajima
M, Hori M, Tsuruta K, Mori H, Kunimatsu A, Arai H, Aoki S and
Ohtomo K: Diffusional kurtosis imaging in idiopathic normal
pressure hydrocephalus: Correlation with severity of cognitive
impairment. Magn Reson Med Sci. 15:316–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CY, Tabesh A, Spampinato MV, Helpern
JA, Jensen JH and Bonilha L: Diffusional kurtosis imaging reveals a
distinctive pattern of microstructural alternations in idiopathic
generalized epilepsy. Acta Neurol Scand. 130:148–155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Liao W, Chen H, Mantini D, Ding
JR, Xu Q, Wang Z, Yuan C, Chen G, Jiao Q and Lu G: Altered
functional-structural coupling of large-scale brain networks in
idiopathic generalized epilepsy. Brain. 134:2912–2928. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Engel J Jr; International League Against
Epilepsy (ILAE), : A proposed diagnostic scheme for people with
epileptic seizures and with epilepsy: Report of the ILAE task force
on classification and terminology. Epilepsia. 42:796–803. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chau W and McIntosh AR: The Talairach
coordinate of a point in the MNI space: How to interpret it.
NeuroImage. 25:408–416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li S, Tian J, Bauer A, Huang R, Wen H, Li
M, Wang T, Xia L and Jiang G: Reduced integrity of right
lateralized white matter in patients with primary insomnia: A
diffusion-tensor imaging study. Radiology. 280:520–528. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rohlfing T: Incorrect ICBM-DTI-81 atlas
orientation and white matter labels. Front Neuroscience. 7:42013.
View Article : Google Scholar
|
|
21
|
Liao W, Zhang Z, Mantini D, Xu Q, Wang Z,
Chen G, Jiao Q, Zang YF and Lu G: Relationship between large-scale
functional and structural covariance networks in idiopathic
generalized epilepsy. Brain Connect. 3:240–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu L, Li Y, Wang Y, Li R, Zhang Z, Lu G
and Chen H: Aberrant long-range functional connectivity density in
generalized tonic-clonic seizures. Medicine (Baltimore).
95:e38932016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang CH, Sha Z, Mundahl J, Liu S, Lu Y,
Henry TR and He B: Thalamocortical relationship in epileptic
patients with generalized spike and wave discharges-a multimodal
neuroimaging study. NeuroImage Clin. 9:117–127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong Y, Lu G, Zhang Z, Jiao Q, Li K and
Liu Y: Altered regional synchronization in epileptic patients with
generalized tonic-clonic seizures. Epilepsy Res. 97:83–91. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paz JT, Davidson TJ, Frechette ES, Delord
B, Parada I, Peng K, Deisseroth K and Huguenard JR: Closed-loop
optogenetic control of thalamus as a tool for interrupting seizures
after cortical injury. Nat Neurosci. 16:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang W, Lu G, Zhang Z, Zhong Y, Wang Z,
Yuan C, Jiao Q, Qian Z, Tan Q, Chen G, et al: Gray-matter volume
reduction in the thalamus and frontal lobe in epileptic patients
with generalized tonic-clonic seizures. J Neuroradiol. 38:298–303.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo C, Li Q, Xia Y, Lei X, Xue K, Yao Z,
Lai Y, Martínez-Montes E, Liao W, Zhou D, et al: Resting state
basal ganglia network in idiopathic generalized epilepsy. Hum Brain
Mapp. 33:1279–1294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciumas C and Savic I: Structural changes
in patients with primary generalized tonic and clonic seizures.
Neurology. 67:683–686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ciumas C, Wahlin TB, Espino C and Savic I:
The dopamine system in idiopathic generalized epilepsies:
Identification of syndrome-related changes. NeuroImage. 51:606–615.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akman O, Gulcebi MI, Carcak N, Ketenci
Ozatman S, Eryigit T, Moshé SL, Galanopoulou AS and Onat FY: The
role of the substantia nigra pars reticulata in kindling resistance
in rats with genetic absence epilepsy. Epilepsia. 56:1793–1802.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robinson S, Basso G, Soldati N, Sailer U,
Jovicich J, Bruzzone L, Kryspin-Exner I, Bauer H and Moser E: A
resting state network in the motor control circuit of the basal
ganglia. BMC Neurosci. 10:1372009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang D, Snyder AZ, Fox MD, Sansbury MW,
Shimony JS and Raichle ME: Intrinsic functional relations between
human cerebral cortex and thalamus. J Neurophysiol. 100:1740–1748.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rektor I, Kuba R, Brazdil M and Chrastina
J: Do the basal ganglia inhibit seizure activity in temporal lobe
epilepsy? Epilepsy Behav. 25:56–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mohan A, Roberto AJ, Lorenzo A, Jones K,
Carney MJ, Liogier-Weyback L, Hwang S and Lapidus KA: The
significance of the default mode network (DMN) in neurological and
neuropsychiatric disorders: A review. Yale J Biol Med. 89:49–57.
2016.PubMed/NCBI
|
|
35
|
Menon V: Large-scale brain networks and
psychopathology: A unifying triple network model. Trends Cogn Sci.
15:483–506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei H, An J, Shen H, Zeng LL, Qiu S and Hu
D: Altered effective connectivity among core neurocognitive
networks in idiopathic generalized epilepsy: An fMRI evidence.
Front Hum Neurosci. 10:4472016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Laureys S, Owen A and Schiff N: Coma
science: Clinical and ethical implications. Preface. Prog Brain
Res. 177:xiii–xiv. 2009.PubMed/NCBI
|
|
38
|
Yoo NJ, Kim HR, Kim YR, An CH and Lee SH:
Somatic mutations of the KEAP1 gene in common solid cancers.
Histopathology. 60:943–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anastasopoulou S, Kurth F, Luders E and
Savic I: Generalized epilepsy syndromes and callosal thickness:
Differential effects between patients with juvenile myoclonic
epilepsy and those with generalized tonic-clonic seizures alone.
Epilepsy Res. 129:74–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsuo A, Ono T, Baba H and Ono K:
Callosal role in generation of epileptiform discharges:
Quantitative analysis of EEGs recorded in patients undergoing
corpus callosotomy. Clin Neurophysiol. 114:2165–2171. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jenssen S, Sperling MR, Tracy JI, Nei M,
Joyce L, David G and O'Connor M: Corpus callosotomy in refractory
idiopathic generalized epilepsy. Seizure. 15:621–629. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee CY, Bennett KM and Debbins JP:
Sensitivities of statistical distribution model and diffusion
kurtosis model in varying microstructural environments: A monte
carlo study. J Magn Reson. 230:19–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamagata K, Tomiyama H, Hatano T, Motoi Y,
Abe O, Shimoji K, Kamiya K, Suzuki M, Hori M, Yoshida M, et al: A
preliminary diffusional kurtosis imaging study of parkinson
disease: Comparison with conventional diffusion tensor imaging.
Neuroradiology. 56:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gungor-Tuncer O, Baykan B, Altindag E,
Bebek N, Gurses C and Gokyigit A: Prevalence and characteristics of
visual aura in idiopathic generalized epilepsy. Epilepsy Behav.
25:573–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sasson E, Doniger GM, Pasternak O,
Tarrasch R and Assaf Y: White matter correlates of cognitive
domains in normal aging with diffusion tensor imaging. Front
Neurosci. 7:322013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Du H, Zhang Y, Xie B, Wu N, Wu G, Wang J,
Jiang T and Feng H: Regional atrophy of the basal ganglia and
thalamus in idiopathic generalized epilepsy. J Magn Reson Imaging.
33:817–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Walser G, Unterberger I, Dobesberger J,
Embacher N, Falkenstetter T, Larch J, Kuchukhidze G, Gotwald T,
Ortler M, Bauer G and Trinka E: Asymmetric seizure termination in
primary and secondary generalized tonic-clonic seizures. Epilepsia.
50:2035–2039. 2009. View Article : Google Scholar : PubMed/NCBI
|