Introduction
Laryngocarcinoma is one of the most common malignant
tumors in otolaryngology requiring head and neck surgery,
accounting for ~1–5% of all types of cancer and 7.9–35% of
otolaryngologic malignancies (1).
With increasing incidence, laryngocarcinoma ranks third in
otolaryngologic malignancies and is a squamous cell carcinoma in
90% of cases (2).
The mechanism underlying the occurrence of
laryngocarcinoma remains to be elucidated. Progress in gene
research indicated that certain genes serve important roles in the
occurrence and development of laryngocarcinoma. Among these,
epidermal growth factor receptor (EGFR) (3), cyclin D1 (4) and KRAS proto-oncogene, GTPase (KRAS)
(5) attracted attention in the field
of head and neck cancer research due to their strong association
with tumorigenesis, invasion, lymph node metastasis, recurrence and
prognosis. Furthermore, EGFR and cyclin D1 are recognized as
regulators of cancer cell proliferation, migration and patient
survival in laryngocarcinoma (6–8). In
addition, EGFR expression is markedly increased during the
progression from laryngeal dysplasia to carcinoma (9). In addition, the Ras signaling pathway
is involved in the formation of tumor microenvironment and is
closely associated with tumorigenesis and progression (7,10).
However, correlation between EGFR, cyclin D1 and KRAS is rarely
reported in laryngocarcinoma, and whether the three genes serve a
synergistic role in the occurrence and development of
laryngocarcinoma requires further investigation. To the best of our
knowledge, the expression and synergy of EGFR, cyclin D1 and KRAS
in patients with laryngocarcinoma have not been previously
reported. Studies on the association between the expression of
EGFR, cyclin D1 and KRAS in laryngocarcinoma and patient clinical
features, and the correlation between the expression levels of
these three genes, may elucidate the biological features of
laryngocarcinoma.
In the current study, the expression levels of EGFR,
cyclin D1 and KRAS were investigated to determine the association
between their expression in laryngocarcinoma tissues and clinical
features of the disease using immunohistochemical staining and
statistical analysis. In addition, the current study aimed to
elucidate whether there is a synergistic association among these
genes and whether their expression levels are associated with
prognosis. The results of the present may aid in evaluating the
severity of laryngocarcinoma and selecting the most appropriate
treatment option and provides a theoretical basis for gene targeted
drug discovery and therapy.
Materials and methods
General patient information
Paraffin-embedded tissue samples from 46 patients
with primary laryngeal squamous cell carcinoma (LSCC) and 20
patients diagnosed with vocal cord polyps (VCPs) were collected as
the study group and control group, respectively, at the Shantou
Central Hospital (Shantou, China) from 2005 to 2011. All cases had
>5 years of follow-up results. Among the 46 cases of LSCC
(Table I), 45 (97.83%) were male and
1 (2.17%) was female; the age of onset was 43–70 years old, with 9
cases (19.57%) at the age <50, 37 cases (80.43%) ≥50, and the
median age was 60 years old. Prior to treatment, the cancer stage
was determined using electronic laryngoscopy, B-mode ultrasound,
X-ray analysis, computed tomography, magnetic resonance imaging,
pathology and classified using the Union for International Cancer
Control Tumor Node Metastasis (TNM) Classification System for
laryngocarcinoma, the 8th Edition (2017) (11). A total of 19 cases were stage T1-T2
(41.30%), 22 cases (47.83%) stage T3 and 5 cases (10.87%) stage T4.
Prior to treatment, there were 43 cases (93.48%) free of cervical
lymph node metastasis (N0), and 3 cases with cervical lymph node
metastasis (6.52%), all of which were stage N1. Following
treatment, there were 28 cases (60.87%) with recurrent disease or
metastasis. The 46 patients with LSCC were treated with surgery,
including cordectomy, partial laryngectomy, total laryngectomy and
cervical lymph node dissection. Stage T4 patients and certain
patients with stage T3 were treated with Cobalt-60 radiotherapy
and/or 5-fluorouracil and cisplatin chemotherapy as adjuvant
therapy.
 | Table I.Baseline status of patients with
LSCC. |
Table I.
Baseline status of patients with
LSCC.
| Characteristic | Patients with LSCC
(n=46) |
|---|
| Age (mean ± SD,
years) | 58.0±9.5 |
|
<50 | 9
(19.57%) |
|
≥50 | 37 (80.43%) |
| Sex |
|
|
Male | 45 (97.83%) |
|
Female | 1 (2.17%) |
| BMI (mean ± SD,
kg/m2) | 23.34±2.33 |
| Smoking status |
|
|
Yes | 24 (52.17%) |
| No | 22 (47.83%) |
| Drinking
status |
|
|
Yes | 20 (43.48%) |
| No | 26 (56.42%) |
| Years since
diagnosis, mean | 0.62 |
| Clinical stage |
|
|
I–II | 19 (41.30%) |
|
III | 22 (47.83%) |
| IV | 5
(10.87%) |
| Metastasis status
before treatment |
|
|
Yes | 3 (6.52%) |
| No | 43 (93.48%) |
Immunohistochemical detection
A total of 46 samples of laryngocarcinoma tissues
and 20 control samples of vocal cord polyp tissues were collected.
Immunohistochemical (IHC) staining was performed using a Super
Vision IHC kit (cat. no. SV0002; Wuhan Boster Biological
Technology, Ltd., Wuhan, China). The samples were fixed with 4%
paraformaldehyde at 4°C for ~24 h, dehydrated through a graded
series of ethanol, washed with xylene and embedded in paraffin.
Serial 5 µm sections were then cut, conventionally dewaxed and
rehydratedin xylene and a descending alcohol series, and rinsed in
deionized water. Endogenous peroxidase was quenched by 3% hydrogen
peroxide in deionized water. Antigen retrieval was performed by
incubating the slides in pH 6.0 citrate buffer (0.01 M sodium
citrate-citric acid buffer; EDTA buffer for cyclin D1) at 98°C for
10 min, followed by incubation with 10% goat serum blocking
solution (cat. no. AR0009; Wuhan Boster Biological Technology,
Ltd.) for 20 min at room temperature and primary antibodies against
EGFR (1:50; cat. no. ZA-0505; OriGene Technologies, Inc.,
Rockville, MD, USA), CyclinD1 (1:50; cat. no. RMA-0541; Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) and K-ras (1:50; cat. no.
bs-1033R; Bioss Antibodies, Inc., Woburn, MA, USA) at 4°C
overnight. After two washes with PBS, polymeric horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G secondary
antibody (provided in the Super Vision IHC kit) was incubated with
the slides for 30 min in a 37°C. Following 3,3′-diaminobenzidine
staining, sections were counterstained with hematoxylin for 2 min
at room temperature, dehydrated and attached to coverslips.
Results assessment
Brown and tan color indicated positive EGFR, cyclin
D1 and KRAS staining. Yellow staining in the nucleus, cytoplasm or
cell membrane was considered a positive signal. For the
semiquantitative scoring analysis, each section was observed in
five randomly selected high-power fields (×200) with a light
microscope (Axioplan 2; Carl Zeiss AG, Oberkochen, Germany). The
total number of cells and the number of positive cells were counted
in each field of view, the percentage of positive cells was
calculated and averaged, and the percentages were divided into four
grades and recorded as 0, 1, 2, or 3 points. The following scoring
system was used: i) 0, <25% positive cells; ii) 1, 25–49%
positive cells; iii) 2, 50–74% positive cells; and iv) 3, ≥75%
positive cells. The following scoring system was used to for
staining intensity: i) 0, no color development; ii) 1, light
yellow; iii) 2, orange; iv) 3 tan. The final scores were obtained
by adding the two score types, which were subsequently divided by
2. Negative (−) result was defined as 0 points; 0.5 or 1.0
indicated a weakly positive (+) result; 1.5 or 2.0 indicated a
positive (++) result; and 2.5 or 3.0 indicated a strong positive
(+++) result. Negative and weakly positive expression was defined
as low expression; positive and strongly positive expression was
defined as high expression.
Statistical analyses
The experimental data were analyzed using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). A
χ2 test was used to analyze the association between gene
expression and sex, age, clinical stage of patients with
laryngocarcinoma, and metastasis status following treatment.
Spearman's correlation was used to analyze the association between
gene expression levels. The mean survival time and survival rate of
each group were calculated using the Kaplan-Meier method for
univariate analysis of prognostic factors for overall survival.
Log-rank test was used to compare the survival rate of each group,
and multivariate analysis of prognostic factors was performed using
the Cox proportional hazards model. P<0.05 was considered to
indicate a statistically significant difference.
Results
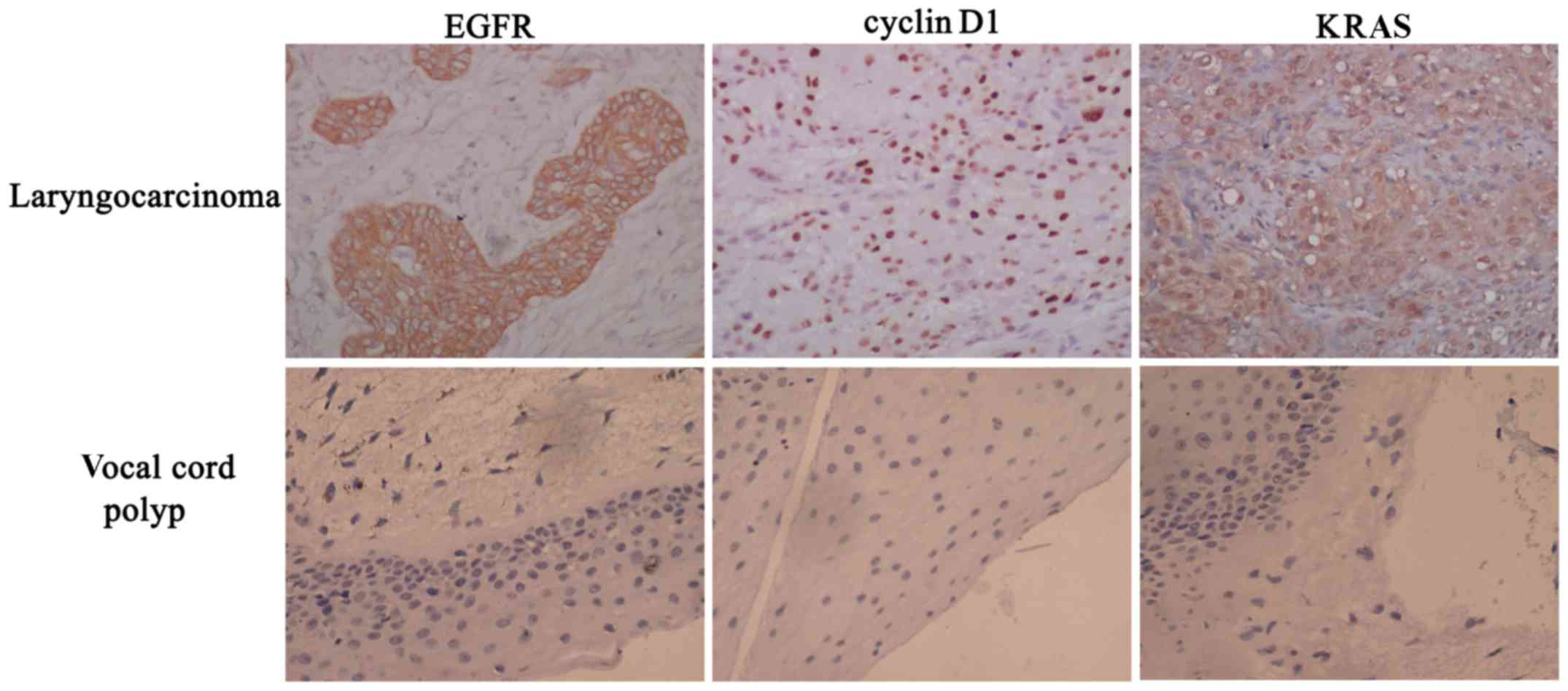
EGFR, cyclin D1 and KRAS staining
results and their association with clinical features
EGFR was mainly located in the membrane and
cytoplasm of laryngocarcinoma cells, as indicated by brown
staining. Cyclin D1 was primarily located in the nucleus, as
indicated by tan staining, and KRAS was mainly observed as tan or
brown staining located in the cytoplasm and nucleus. The expression
of EGFR, cyclin D1 and KRAS in vocal cord polyp tissues were very
low (Fig. 1).
The expression rates of EGFR, cyclin D1 and KRAS in
laryngocarcinoma tissues were 71.7, 52.2 and 39.1%, respectively,
while those in vocal cord polyps were only 10.0, 5.0 and 10.0%,
respectively. The differences in EGFR, cyclin D1 and KRAS
expression levels between the laryngocarcinoma tissues and the
vocal cord polyp tissues were statistically significant,
(P<0.001, P<0.001 and P=0.021, respectively; Tables II–IV). The expression levels of EGFR, cyclin
D1 and KRAS were significantly associated with the clinical stage
and treatment response (P<0.05). There was no association
between the expression of EGFR, cyclin D1 and KRAS and the age of
patients, and no significance in the different expression levels
between the laryngocarcinoma and vocal cord polyp groups
(P>0.05).
 | Table II.Association between EGFR expression
and clinical features. |
Table II.
Association between EGFR expression
and clinical features.
|
|
| EGFR |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical
parameter | Total | Low | High | High expression
rate, % | χ2 | P-value |
|---|
| Tissue source |
| Polyp
of vocal cord | 20 | 18 | 2 | 10 | 21.332 | <0.001 |
|
Laryngocarcinoma | 46 | 13 | 33 | 71.7 |
|
|
| Age group |
|
<50 | 9 | 1 | 8 | 88.9 |
1.623 | 0.203 |
|
≥50 | 37 | 12 | 25 | 67.6 |
|
|
| TNM
classification |
|
I–II | 19 | 8 | 11 | 57.9 | 63.773 | <0.001 |
|
III | 22 | 5 | 17 | 77.3 |
|
|
| IV | 5 | 0 | 5 | 100.0 |
|
|
| Recurrence or
metastasis after treatment |
| No | 28 | 11 | 17 | 60.7 |
4.290 | 0.049 |
|
Yes | 18 | 2 | 16 | 88.9 |
|
|
 | Table IV.Association between KRAS expression
and clinical features. |
Table IV.
Association between KRAS expression
and clinical features.
|
|
| KRAS |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical
parameter | Total | Low | High | High expression
rate, % | χ2 | P-value |
|---|
| Tissue source |
| Polyp
of vocal cord | 20 | 18 | 2 | 10.0 | 5.601 | 0.021 |
|
Laryngocarcinoma | 46 | 28 | 18 | 39.1 |
|
|
| Age group |
|
<50 | 9 | 6 | 3 | 22.2 | 0.158 | 0.999 |
|
≥50 | 37 | 22 | 15 | 40.5 |
|
|
| TNM
classification |
|
I–II | 19 | 16 | 3 | 15.8 | 8.943 | 0.011 |
|
III | 22 | 11 | 11 | 50.0 |
|
|
| IV | 5 | 1 | 4 | 80.0 |
|
|
| Recurrence or
metastasis after treatment |
| No | 28 | 20 | 8 | 28.6 | 4.041 | 0.044 |
|
Yes | 18 | 8 | 11 | 61.1 |
|
|
Correlation between EGFR, cyclin D1
and KRAS expression in laryngocarcinoma tissues
As presented in Tables
V–VII, positive co-expression
of EGFR and cyclin D1, EGFR and KRAS, and cyclin D1 and KRAS in
laryngocarcinoma tissues was identified in 33 (33/46), 34 (34/46)
and 33 (33/46) of the cases, respectively. Spearman's correlation
analysis indicated that the expression of EGFR was positively
correlated with the expression of cyclin D1 (r=0.356; P=0.015) and
KRAS (r=0.479; P=0.001) and the expression of cyclin D1 was
positively correlated with the expression of KRAS (r=0.604;
P<0.001) in laryngocarcinoma tissues.
 | Table V.Correlation between EGFR and cyclin
D1 in laryngocarcinoma tissues. |
Table V.
Correlation between EGFR and cyclin
D1 in laryngocarcinoma tissues.
|
| Cyclin D1 |
|
|---|
|
|
|
|
|---|
|
| − | + | ++ | +++ | Total |
|---|
| EGFR |
| − | 3 | 2 | 2 | 0 | 7 |
| + | 2 | 2 | 1 | 1 | 6 |
| ++ | 3 | 4 | 9 | 0 | 16 |
|
+++ | 1 | 5 | 7 | 4 | 17 |
| Total | 9 | 13 | 19 | 5 | 46 |
 | Table VII.Correlation between cyclin D1 and
KRAS in laryngocarcinoma tissues. |
Table VII.
Correlation between cyclin D1 and
KRAS in laryngocarcinoma tissues.
|
| KRAS |
|
|---|
|
|
|
|
|---|
|
| − | + | ++ | +++ | Total |
|---|
| Cyclin D1 |
| − | 5 | 3 | 1 | 0 | 9 |
| + | 3 | 9 | 0 | 1 | 13 |
| ++ | 1 | 6 | 11 | 1 | 19 |
|
+++ | 0 | 1 | 3 | 1 | 5 |
| Total | 9 | 19 | 15 | 3 | 46 |
Association between the expression
levels of EGFR, cyclin D1 and KRAS in laryngocarcinoma tissues and
patient prognosis
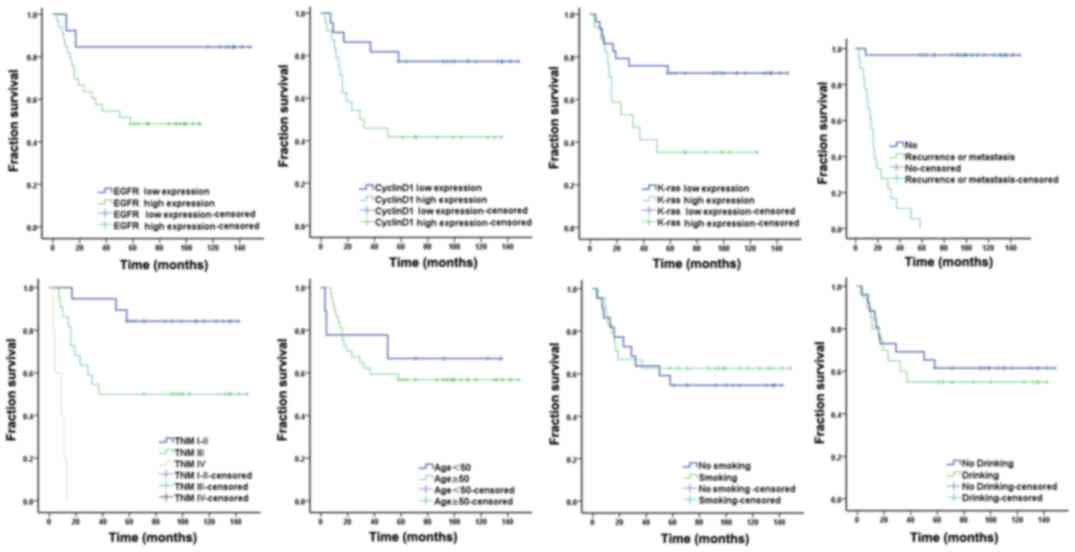
Survival analysis of patients with laryngocarcinoma
with low and high EGFR, cyclin D1 and KRAS expression indicated
that the prognosis of patients was associated with the expression
levels of these proteins (log rank value, 4.261, 6.219 and 5.916,
respectively; Tables VIII,
IX and Fig. 2; P=0.039, P=0.013 and P=0.015,
respectively; Tables VIII,
IX and Fig. 2).
 | Table VIII.Mean and median survival time. |
Table VIII.
Mean and median survival time.
| Variable | Median survival
time (month) | Mean survival time
(month) |
|---|
| EGFR |
| Low
expression | − | 127.31 |
| High
expression | 58.00 |
63.91 |
| Cyclin D1 |
| Low
expression | − | 120.18 |
| High
expression | 29.00 |
66.58 |
| KRAS |
| Low
expression | − | 112.45 |
| High
expression | 32.00 |
57.23 |
| Recurrence or
metastasis |
| No | − | 143.04 |
|
Yes | 16.00 |
20.39 |
| Clinical stage |
|
I–II | – | 126.16 |
|
III | 37.00 |
83.59 |
| IV | 9.00 |
8.00 |
| Age |
|
<50 | – |
96.33 |
|
≥50 | – |
92.62 |
| Smoking |
| No | – |
88.32 |
|
Yes | – |
98.21 |
| Drinking |
| No | – |
99.42 |
|
Yes | – |
86.05 |
 | Table IX.Univariate analysis results for
prognosis of patients with laryngocarcinoma using the Kaplan-Meier
method. |
Table IX.
Univariate analysis results for
prognosis of patients with laryngocarcinoma using the Kaplan-Meier
method.
| Variables | χ2 | P-value |
|---|
| EGFR | 4.261 | 0.039 |
| Cyclin D1 | 6.219 | 0.013 |
| KRAS | 5.916 | 0.015 |
| Recurrence or
metastasis | 53.047 | <0.001 |
| Clinical stage | 45.233 | <0.001 |
| Age | 0.166 | 0.684 |
| Smoking | 0.168 | 0.682 |
| Drinking | 0.212 | 0.645 |
Association between the clinical
features in laryngocarcinoma and prognosis
Survival analysis of patients with laryngocarcinoma
indicated that the prognosis was associated with the treatment
response and clinical stage (log rank value, 53.047 and 45.233; all
P<0.05). The prognosis of patients with laryngocarcinoma was not
significantly associated with age, smoking and drinking (log rank
value, 0.166, 0.168 and 0.212, respectively; Table IX and Fig. 2; P=0.684, P=0.682 and P=0.645,
respectively; Table IX and Fig. 2).
Multivariate analysis of prognosis
using the Cox proportional hazards model
A total of eight variables, including age, clinical
stage, recurrence or metastasis after treatment, smoking and
drinking status, and EGFR, cyclin D1 and KRAS expression levels,
were selected for the Cox regression model for multivariate
analysis (Table X). The results
indicated that treatment response was significantly associated with
patient prognosis (P=0.001). Furthermore, clinical stage was
significantly associated with patient prognosis, with P=0.001 for
TNM I–II, P=0.017 for TNM III and P<0.001 for TNM IV.
 | Table X.Multivariate analysis results for
prognosis of patients with laryngocarcinoma using the Cox
regression model. |
Table X.
Multivariate analysis results for
prognosis of patients with laryngocarcinoma using the Cox
regression model.
|
|
|
|
|
|
|
| 95.0% CI for
Exp(B) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | B | SE | Wald | df | P-value | Exp(B) | Lower | Upper |
|---|
| EGFR | −1.424 | 1.116 | 1.628 | 1 | 0.202 |
0.241 |
0.027 |
2.145 |
| Cyclin D1 | −0.177 | 0.829 | 0.045 | 1 | 0.831 |
0.838 |
0.165 |
4.254 |
| KRAS | −0.502 | 0.588 | 0.731 | 1 | 0.393 |
0.605 |
0.191 |
1.914 |
| Recurrence or
metastasis | 6.490 | 1.777 | 13.348 | 1 | 0.000 | 658.792 | 20.257 | 21,424.605 |
| Clinical stage
I–II | – | – | 14.152 | 2 | 0.001 | 1 | – | – |
| Clinical stage
III | 2.774 | 1.157 | 5.746 | 1 | 0.017 |
16.025 |
1.658 |
154.848 |
| Clinical stage
IV | 5.078 | 1.389 | 13.355 | 1 | 0.000 | 160.439 | 10.533 |
2,443.721 |
| Age | −1.383 | 1.007 | 1.885 | 1 | 0.170 |
0.251 |
0.035 |
1.806 |
| Smoking | 0.308 | 0.659 | 0.218 | 1 | 0.640 |
1.361 |
0.374 |
4.950 |
| Drinking | −0.684 | 0.639 | 1.147 | 1 | 0.284 |
0.505 |
0.144 |
1.765 |
Discussion
The results of the current study indicated that the
expression of EGFR, cyclin D1 and KRAS in laryngocarcinoma tissues
was significantly increased compared with the vocal cord polyps,
and the expression of EGFR, cyclin D1 and KRAS was closely
associated with the clinical stage, treatment response and
prognosis. The expression levels of EGFR, cyclin D1 and KRAS in
laryngocarcinoma tissues were positively correlated, indicating
that these proteins may be involved in the occurrence and
development of laryngocarcinoma. Furthermore, it may be
hypothesized that increased expression of EGFR, cyclin D1 and KRAS
promote invasion and metastasis of laryngocarcinoma cells and act
in synergy to promote the occurrence and development of
laryngocarcinoma. In the current study, the clinical stage,
recurrence or metastasis status after treatment, and the expression
levels of EGFR, cyclin D1 and KRAS were the factors affecting the
prognosis of patients. Treatment response and clinical stage were
two independent risk factors affecting the prognosis of
patients.
EGFR is a ligand-mediated multifunctional
transmembrane glycoprotein that activates tyrosine kinase and is
present on the cell membrane of the majority of tissue types with
the exception of the hematopoietic system in the human body
(12). EGFR binds EGF with high
affinity and this interaction exhibits time and temperature
dependence, saturation and reversibility (12). Following activation of the EGFR
tyrosine kinase, cascade amplification of signal transduction is
achieved through the RAS-RAF-MEK-MAPK, PI3K-PKC-IKK, and JAK-STAT
pathways, which deliver extracellular mitotic signals into the
cell, thus regulating normal cell growth, differentiation and cell
cycle and promoting damage repair (13). EGFR is highly expressed in a number
of solid tumors and is closely associated to tumor progression,
apoptosis, angiogenesis and metastasis (14). In vitro and in vivo
experiments have demonstrated that EGFR is an important anticancer
target in the development and progression of laryngocarcinoma
(15). Inhibition of EGFR can limit
the growth of laryngeal squamous cell carcinoma and enhance the
anticancer effect when combined with other drugs (16–18). The
results of the current study indicated that EGFR expression in
laryngocarcinoma was significantly increased compared with the
vocal cord polyp tissues. In addition, increased EGFR expression
was identified in tissues with higher TNM stage. High expression
rates of EGFR were identified in 57.9, 77.3 and 100% of stage I–II,
stage III and stage IV tissues, respectively. Furthermore, the EGFR
expression rates were significantly different between different TNM
stages, indicating that the expression of this protein may be
closely associated with the occurrence, development, malignant
transformation and proliferation of laryngocatcinoma. In addition,
the results the current study indicated that EGFR expression was
increased among patients with recurrence or metastasis following
treatment of laryngocarcinoma compared with patients without
recurrence or metastasis (expression rate, 88.9 vs. 60.7%,
respectively) suggesting that EGFR expression may promote
laryngocarcinoma cell invasion and metastasis.
Cyclin D1 interacts with cyclin-dependent kinase
(CDK)4 or CDK6 to induce cell cycle progression from the G1 to S
phase and promote cell division or transformation (19). Aberrant expression of cyclin D1 may
result in the imbalance of the cell cycle, resulting in
tumorigenesis (20). Previous
studies have confirmed that cyclin D1 is closely associated with
the evolution of malignant tumors (21,22).
Overexpression of cyclin D1 is considered to be of clinical
relevance in LSCC (23).
Downregulation of cyclin D1 can inhibit cell growth and induce
apoptosis in LSCC (24). In
vivo studies using an LSCC xenograft model indicated that tumor
growth can be inhibited by cyclin D1 downregulation (25,26). In
the present study, the expression levels of cyclin D1 were
significantly different in laryngocarcinoma tissues compared with
the vocal cord polyp tissues. Furthermore, higher TNM stages were
associated with increased cyclin D1 expression. The expression
rates in tissues with different TNM stages were 26.3, 68.2 and
80.0%, respectively. Furthermore, the cyclin D1 expression rates
were significantly different between different TNM stages,
indicating that the expression of this protein may be closely
associated with the malignant transformation of laryngeal tissues
and tumor progression. The expression of cyclin D1 was increased
among patients with recurrence or metastasis following treatment of
laryngocarcinoma compared with patients without recurrence or
metastasis (expression rate, 60.0 vs. 29.2%, respectively)
suggesting that cyclin D1 expression may serve a role in
laryngocarcinoma invasion and metastasis.
KRAS is a proto-oncogene mediating oncogenic
transformation via the MAPK signaling pathway dependent on the
activation of Raf serine/threonine specific kinase (27,28). A
number of previous studies demonstrated that the KRAS gene served a
role in the occurrence and development of various tumors (29,30). The
majority of studies have focused on the expression of KRAS in other
tissues, including anal (31), oral
(32), tonsil (33), skin (34) and head and neck squamous cell
carcinoma (35). Although KRAS
mutation has been reported in LSCC tissues and cell lines (36,37),
there is not sufficient evidence of the role of KRAS in LSCC. The
current research indicated that KRAS expression in laryngocarcinoma
tissue was significantly higher compared with the vocal cord
tissue, and higher TNM stages were associated with increased KRAS
expression. The high expression rates of tissues with different TNM
stages were 15.8, 50.0 and 80.0%, respectively. Furthermore, the
KRAS expression rates were significantly different between
different TNM stages, indicating that the expression of this
protein may be closely associated with to the occurrence and
development of laryngocarcinoma. KRAS expression in tissues from
patients with recurrence or metastasis after laryngocarcinoma
treatment was significantly higher compared with patients without
recurrence or metastasis (expression rate, 60.0 vs. 20.8%),
suggesting that KRAS expression may serve an important role in
laryngocarcinoma invasion and metastasis.
The current study indicated that the expression
levels of EGFR, cyclin D1 and KRAS are closely associated with the
clinical stage of laryngocarcinoma and recurrence or metastasis
status after treatment. In addition, previous studies indicated
that EGFR, cyclin D1 and KRAS expression levels were associated
with the occurrence and development of laryngocarcinoma, with high
expression levels promoting invasion and metastasis of
laryngocarcinoma cells (37–39).
The results of the current study indicated that the
expression levels of EGFR, cyclin D1 and KRAS in laryngocarcinoma
tissues increased compared with vocal cord polyp tissues tissues
and were closely associated with the clinical stage and tumor
recurrence or metastasis after treatment. There was a positive
correlation between the expression levels of any two of these
factors in laryngocarcinoma, indicating that these genes may act in
synergy to promote the occurrence and development of
laryngocarcinoma. The following mechanism underlying such synergy
may be hypothesized: Activation of EGFR by ligand binding may
active the Ras/Raf pathway and induce the expression and activation
of cyclin D1 through the MAPK pathway to promote cell cycle
progression into S phase and initiate proliferation. Previous
studies have indicated that there was no significant correlation
among EGFR, cyclin D1 and KRAS expression in laryngocarcinoma
(40,41). The reason may be that EGFR, cyclin D1
and KRAS is only a part of numerous complex cellular signaling
pathways involved in the process of carcinogenesis, and may have
different expression stages in these signaling pathways during the
initiation and progression of laryngocarcinoma (40,42).
In the present study, the Kaplan-Meier method was
used to calculate the mean survival time and survival rate of
patients in each group. The results indicated that there was a
significant difference in survival rate between the EGFR, cyclin D1
and KRAS low and high expression groups. The Kaplan-Meier survival
curves indicated that the survival rates of patients in the EGFR,
cyclin D1 and KRAS high expression groups were significantly lower
compared with patients in the low expression groups. Therefore, the
expression of EGFR, cyclin D1 and KRAS may be associated with the
survival rate of patients with laryngocarcinoma and is one of the
factors affecting the survival and prognosis.
Multivariate analysis of prognosis using the Cox
regression model indicated that the clinical stage of
laryngocarcinoma and treatment response were closely associated
with the prognosis of patients. There were significant differences
in survival rates between patients with different TNM stages, which
suggested that the clinical stage of laryngocarcinoma and treatment
response are independent risk factors for the prognosis of
patients, whereas age, smoking, drinking states and expression of
EGFR, cyclin D1 and KRAS were the relative risk factors and may
influence the prognosis in the presence of other factors.
The major limitation of the current study was that
the male/female ratio was 45:1, which is higher than that in
previously reported studies, although LSCC is more prevalent among
males (43). This limitation is due
to the relatively low case number and recruitment of patients from
a single hospital. Future studies should include patients from
multiple hospitals.
In conclusion, the current study indicated that the
expression levels of EGFR, cyclin D1 and KRAS were closely
associated with the clinical stage of patients and may serve roles
in the recurrence or metastasis after treatment. Therefore,
aberrant expression of these three genes may accelerate the growth
and invasion of laryngocarcinoma cells and promote recurrence or
metastasis, thus influencing the prognosis of patients. Clinically,
patient prognosis may be assessed by detecting the expression of
EGFR, cyclin D1 and KRAS genes, especially EGFR, which could serve
as a novel marker for LSCC. Surgery combined with radiotherapy
and/or chemotherapy, which can be adopted for high-expression and
high-stage patients, combined with specific targeted EGFR, cyclin
D1 or KRAS gene recombination therapy may reduce the residual tumor
size, prevent recurrence or metastasis and improve the prognosis of
patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shantou
Science and Technology Planning Project (grant no. Shanfuke
2011-46-4).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL collected the samples and analyzed the data. GW
and SW performed immunohistochemical assays and evaluated the
results. HL and CL performed statistical analysis. XW designed the
present study and was the major contributor to the writing of this
manuscript. All authors read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Shantou Central Hospital (Shantou, China). Written
informed consent was obtained from all participants.
Patient consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhi L, Wenli W, Pengfei G, Pengcheng C,
Wenxian C, Jiasheng L and Yongzhu S: Laryngotracheal reconstruction
with autogenous rib cartilage graft for complex laryngotracheal
stenosis and/or anterior neck defect. Eur Arch Otorhinolaryngol.
271:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Britt CJ and Gourin CG: Contemporary
management of advanced laryngeal cancer. Laryngoscope Investig
Otolaryngol. 2:307–309. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alterio D, Marvaso G, Maffini F, Gandini
S, Chiocca S, Ferrari A, Preda L, Rocca MC, Lepanto D, Fodor C, et
al: Role of EGFR as prognostic factor in head and neck cancer
patients treated with surgery and postoperative radiotherapy:
Proposal of a new approach behind the EGFR overexpression. Med
Oncol. 34:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho KJ, Jeong SU, Kim SB, Lee SW, Choi SH,
Nam SY and Kim SY: Basaloid squamous cell carcinoma of the head and
neck: Subclassification into basal, ductal and mixed subtypes based
on comparison of clinico-pathologic features and expression of p53,
Cyclin D1, epidermal growth factor receptor, p16 and human
papillomavirus. J Pathol Transl Med. 51:374–380. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paliga A, Onerheim R, Gologan A, Chong G,
Spatz A, Niazi T, Garant A, Macheto D, Alcindor T and Vuong T: EGFR
and K-ras gene mutation status in squamous cell anal carcinoma: A
role for concurrent radiation and EGFR inhibitors? Br J Cancer.
107:1864–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellacosa A, Almadori G, Cavallo S, Cadoni
G, Galli J, Ferrandina G, Scambia G and Neri G: Cyclin D1 gene
amplification in human laryngeal squamous cell carcinomas:
Prognostic significance and clinical implications. Clin Cancer Res.
2:175–180. 1996.PubMed/NCBI
|
|
7
|
Trivedi S, Rosen CA and Ferris RL: Current
understanding of the tumor microenvironment of laryngeal dysplasia
and progression to invasive cancer. Curr Opin Otolaryngol Head Neck
Surg. 24:121–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takes RP, Baatenburg de Jong RJ, Schuuring
E, Hermans J, Vis AA, Litvinov SV and van Krieken JH: Markers for
assessment of nodal metastasis in laryngeal carcinoma. Arch
Otolaryngol Head Neck Surg. 123:412–419. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin DM, Ro JY, Hong WK and Hittelman WN:
Dysregulation of epidermal growth factor receptor expression in
premalignant lesions during head and neck tumorigenesis. Cancer
Res. 54:3153–3159. 1994.PubMed/NCBI
|
|
10
|
Horn F, Henze C and Heidrich K:
Interleukin-6 signal transduction and lymphocyte function.
Immunobiology. 202:151–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gospodarowicz MK, Brierley JD and
Wittekind C: TNM classification of malignant tumours. John Wiley
& Sons. 2017.
|
|
12
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tetsu O, Phuchareon J, Eisele DW, Hangauer
MJ and McCormick F: AKT inactivation causes persistent drug
tolerance to EGFR inhibitors. Pharmacol Res. 102:132–137. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moeini A, Sia D, Bardeesy N, Mazzaferro V
and Llovet JM: Molecular pathogenesis and targeted therapies for
intrahepatic cholangiocarcinoma. Clin Cancer Res. 22:291–300. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Li Y, He X, Dong S, Huang Y, Li
X, Li Y, Jin C, Zhang Y and Wang Y: Photothermolysis mediated by
gold nanorods modified with EGFR monoclonal antibody induces Hep-2
cells apoptosis in vitro and in vivo. Int J Nanomedicine.
9:1931–1946. 2014.PubMed/NCBI
|
|
16
|
Cao S, Xia M, Mao Y, Zhang Q, Donkor PO,
Qiu F and Kang N: Combined oridonin with cetuximab treatment shows
synergistic anticancer effects on laryngeal squamous cell
carcinoma: Involvement of inhibition of EGFR and activation of
reactive oxygen species-mediated JNK pathway. Int J Oncol.
49:2075–2087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang N, Zhang JH, Qiu F, Tashiro S,
Onodera S and Ikejima T: Inhibition of EGFR signaling augments
oridonin-induced apoptosis in human laryngeal cancer cells via
enhancing oxidative stress coincident with activation of both the
intrinsic and extrinsic apoptotic pathways. Cancer Lett.
294:147–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hwang H, Biswas R, Chung PS and Ahn JC:
Modulation of EGFR and ROS induced cytochrome c release by
combination of photodynamic therapy and carboplatin in human
cultured head and neck cancer cells and tumor xenograft in nude
mice. J Photochem Photobiol B. 128:70–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gioacchini FM, Alicandri-Ciufelli M,
Kaleci S, Magliulo G, Presutti L and Re M: The prognostic value of
cyclin D1 expression in head and neck squamous cell carcinoma. Eur
Arch Otorhinolaryngol. 273:801–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pletneva MA, Andea A, Palanisamy N, Betz
BL, Carskadon S, Wang M, Patel RM, Fullen DR and Harms PW: Clear
cell melanoma: A cutaneous clear cell malignancy. Arch Pathol Lab
Med. 138:1328–1336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casimiro MC, Velasco-Velazquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pignataro L, Pruneri G, Carboni N,
Capaccio P, Cesana BM, Neri A and Buffa R: Clinical relevance of
cyclin D1 protein overexpression in laryngeal squamous cell
carcinoma. J Clin Oncol. 16:3069–3077. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao Y, Wang J, Lu J, Liu Y, Wang Y, Gao Y
and Jin D: Down-regulation of cyclin D1 by small interfering RNA
inhibits cell growth and induces apoptosis of laryngeal squamous
cell carcinoma. Am J Otolaryngol. 32:541–546. 2011.PubMed/NCBI
|
|
25
|
Feng J, Sun Q, Wu T, Lu J, Qu L, Sun Y,
Tian L, Zhang B, Li D and Liu M: Upregulation of ATF-3 is
correlated with prognosis and proliferation of laryngeal cancer by
regulating Cyclin D1 expression. Int J Clin Exp Pathol.
6:2064–2070. 2013.PubMed/NCBI
|
|
26
|
Li MH, Tian LL, Ren H, Chen XX, Wang Y, Ge
JC, Wu SL, Sun Y, Liu M and Xiao H: MicroRNA-101 is a potential
prognostic indicator of laryngeal squamous cell carcinoma and
modulates CDK8. J Transl Med. 13:2712015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akkiprik M, Celikel CA, Düşünceli F,
Sönmez O, Güllüoğlu BM, Sav A and Ozer A: Relationship between
overexpression of ras p21 oncoprotein and K-ras codon 12 and 13
mutations in Turkish colorectal cancer patients. Turk J
Gastroenterol. 19:22–27. 2008.PubMed/NCBI
|
|
28
|
Haigis KM, Kendall KR, Wang Y, Cheung A,
Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A,
Sebolt-Leopold J, Shannon KM, et al: Differential effects of
oncogenic K-Ras and N-Ras on proliferation, differentiation and
tumor progression in the colon. Nat Genet. 40:600–608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nussinov R, Tsai CJ and Jang H: A new view
of pathway-driven drug resistance in tumor proliferation. Trends
Pharmacol Sci. 38:427–437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asati V, Mahapatra DK and Bharti SK: K-Ras
and its inhibitors towards personalized cancer treatment:
Pharmacological and structural perspectives. Eur J Med Chem.
125:299–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zampino MG, Magni E, Sonzogni A and Renne
G: K-ras status in squamous cell anal carcinoma (SCC): It's time
for target-oriented treatment? Cancer Chemother Pharmacol.
65:197–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin KH, Bae SD, Hong HS, Kim RH, Kang MK
and Park NH: miR-181a shows tumor suppressive effect against oral
squamous cell carcinoma cells by downregulating K-ras. Biochem
Biophys Res Commun. 404:896–902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Damme N, Deron P, Van Roy N, Demetter
P, Bols A, Van Dorpe J, Baert F, Van Laethem JL, Speleman F,
Pauwels P and Peeters M: Epidermal growth factor receptor and K-RAS
status in two cohorts of squamous cell carcinomas. BMC Cancer.
10:1892010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van der Schroeff JG, Evers LM, Boot AJ and
Bos JL: Ras oncogene mutations in basal cell carcinomas and
squamous cell carcinomas of human skin. J Invest Dermatol.
94:423–425. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoa M, Davis SL, Ames SJ and Spanjaard RA:
Amplification of wild-type K-ras promotes growth of head and neck
squamous cell carcinoma. Cancer Res. 62:7154–7156. 2002.PubMed/NCBI
|
|
36
|
Chen X, Kong W, Cai G, Zhang S and Zhang
D: The expressions of K-ras in human laryngeal squamous cell
carcinoma cell lines (Hep-2) and its significance. Lin Chuang Er Bi
Yan Hou Ke Za Zhi. 19:417–419. 2005.(In Chinese). PubMed/NCBI
|
|
37
|
Ruíz-Godoy RLM, Garcia-Cuellar CM, Herrera
González NE, Suchil BL, Pérez-Cárdenas E, Sácnchez-Pérez Y,
Suárez-Roa ML and Meneses A: Mutational analysis of K-ras and Ras
protein expression in larynx squamous cell carcinoma. J Exp Clin
Cancer Res. 25:73–78. 2006.PubMed/NCBI
|
|
38
|
Shang C, Guo Y, Fu S, Fu W and Sun K:
SH3GL2 gene participates in MEK-ERK signal pathway partly by
regulating EGFR in the laryngeal carcinoma cell line Hep2. Med Sci
Monit. 16:BR168–BR173. 2010.PubMed/NCBI
|
|
39
|
Zhang B, Liu W, Li L, Lu J, Liu M, Sun Y
and Jin D: KAI1/CD82 and CyclinD1 as biomarkers of invasion,
metastasis and prognosis of laryngeal squamous cell carcinoma. Int
J Clin Exp Pathol. 6:1060–1067. 2013.PubMed/NCBI
|
|
40
|
Chrysovergis A, Gorgoulis VG, Giotakis I,
Tsiambas E, Karameris A, Kittas C and Kyroudi A: Simultaneous over
activation of EGFR, telomerase (h TERT) and cyclin D1 correlates
with advanced disease in larynx squamous cell carcinoma: A tissue
microarray analysis. Med Oncol. 28:871–877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramnani DM, Wistuba II, Behrens C, Gazdar
AF, Sobin LH and Albores-Saavedra J: K-ras and p53 mutations in the
pathogenesis of classical and goblet cell carcinoids of the
appendix. Cancer. 86:14–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Papadimitrakopoulou V, Izzo JG, Liu DD,
Myers J, Ceron TL, Lewin J, William WN Jr, Atwell A, Lee JJ,
Gillenwater A, et al: Cyclin D1 and cancer development in laryngeal
premalignancy patients. Cancer Prev Res (Phila). 2:14–21. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|