Introduction
As the most common histologic subtype of lung
cancer, lung adenocarcinoma is the leading cause of cancer
mortality worldwide (1,2). Molecular targeted drugs have been
demonstrated to improve quality of life and therapeutic effects in
patients with lung adenocarcinoma whose tumors exhibit driver
oncogenes, including epidermal growth factor receptor mutations,
anaplastic lymphoma kinase gene rearrangements or ROS
proto-oncogene 1 gene fusion (2,3).
However, most lung adenocarcinomas lack an identifiable activated
oncogene and remain to be treated with conventional chemotherapy
(2,3).
Endoplasmic reticulum protein 29 (ERp29), a putative
chaperone protein, is located in the endoplasmic reticulum
(4). Structurally, ERp29 consists of
an N-terminal domain, a flexible loop and a C-terminal domain
(4). ERp29 is upregulated on
exposure to radiation, homocysteine or dopamine (5–7). ERp29
is abnormally expressed in several types of tumors, including
breast cancer, colorectal cancer, basal skin carcinoma and
gallbladder adenocarcinoma (8–11).
Furthermore, ERp29 expression is associated with the pathological
grade, TNM stage, lymph node metastasis, recurrence and prognosis
of patients with cancer (8–11). It has also been revealed that ERp29
overexpression enhances breast cancer cell chemoresistance to
doxorubicin and nasopharyngeal carcinoma cell radioresistance
(9,12).
A previous study has revealed that ERp29 is
significantly overexpressed in 75 patients with lung adenocarcinoma
and of ERp29 inhibition enhances gemcitabine chemosensitivity
(13). Therefore, treatment with
gemcitabine in combination with ERp29 expression inhibition may
promote therapeutic effects in lung adenocarcinoma. However,
underlying mechanisms of this action are yet to be elucidated. It
has been revealed that ERp29 is involved in the regulation of heat
shock protein 27 (HSP) (5,14). In addition, as a small HSP, HSP27 is
associated with gemcitabine chemotherapeutic sensitivity (15,16).
Therefore, the current study hypothesized that ERp29 may affect the
chemosensitivity of lung cancer cells to gemcitabine by regulating
HSP27. In the present study, ERp29 and HSP27 expression was
assessed following lung adenocarcinoma cell treatment with
gemcitabine. Furthermore, effects of combined treatment with
gemcitabine and ERp29 small interfering (si)RNA on cell apoptosis,
cell cycle and HSP27 expression were examined in the present
study.
Materials and methods
Cell lines and cell culture
A549 and SPC-A1 human lung adenocarcinoma cells
(Type Culture Collection of the Chinese Academy of Science,
Shanghai, China) were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and maintained at 37°C in a 5%
CO2-humidified incubator.
Western blot analysis
Total protein was extracted from lung adenocarcinoma
cells using radio immunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). Total protein was
quantified using a bicinchoninic acid assay and 30–60 µg
protein/lane was separated via SDS-PAGE on a 12% gel using a
mini-gel apparatus (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The separated proteins were subsequently transferred onto
polyvinylidene difluoride membranes and for 1.5 h at room
temperature with 5% non-fat dry milk in Tris-buffered saline with
Tween-20 (TBST). The membranes were incubated with primary
antibodies against ERp29 (1:500; cat. no. ab176573; Abcam,
Cambridge, UK), HSP27 (1:1,000; cat. no. 2402), phosphorylated
(p)-HSP27 (Ser82; 1:1,000; cat. no. 9709) (both from Cell Signaling
Technology, Inc., Danvers, MA, USA) and α-tubulin antibodies
(1:3,000; cat. no. T5168; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) overnight at 4°C. Membranes were washed with TBST.
Following primary incubation, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit IgG (1:1,000; cat.
no. 7074) and anti-mouse IgG (1:1,000; cat. no. 7076; both from
Cell Signaling Technology, Inc.) secondary antibodies for 1 h at
room temperature. Protein bands were visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology).
Protein expression was quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). Following treatment with
various doses (0.5, 5 and 50 µM) of gemcitabine (Selleck Chemicals,
Houston, TX, USA) for 24 h, ERp29 expression in lung adenocarcinoma
cells was detected. HSP27 and p-HSP27 expression levels were
detected following treatment with 5 µM gemcitabine for 4, 8 and 24
h in A549 and SPC-A1 cells.
siRNA transfection
ERp29 siRNA, HSP27 siRNA and scrambled siRNA were
designed and synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). The following gene-specific siRNA duplexes:
ERP29-siRNA sense, 5′-GUGAGUCCCUUGUGGAAUATT-3′ and antisense,
5′-UAUUCCACAAGGGACUCACTT-3′ and HSP27-siRNA sense,
5′-ACCUGUGUGUUCUUUUGAUTT-3′ and antisense,
5′-AUCAAAAGAACACACAGGUTT-3′. Cells were transfected with 40 nM
siRNA using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Thereafter, cells were harvested for western blot analysis
following 72-h transfection.
Detection of apoptosis
Cell apoptosis was analyzed using the Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA). A total of 1×105
cells were collected by centrifugation at 170 × g for 5 min at room
temperature. The cells were suspended in 100 µl of binding buffer
and subsequently stained with 5 µl Annexin V-FITC and 5 µl
propidium iodide (PI) in the dark for 15 min at room temperature.
Apoptotic cells were analyzed using a BD FACSCalibur flow cytometer
(BD Biosciences) and FlowJo software (version 7.6.1; TreeStar,
Inc., Ashland, OR, USA). Apoptotic cells were defined as Annexin
V-positive cells.
Cell cycle assay
A cell cycle kit (Beyotime Institute of
Biotechnology) was used to determine the percentage of cells in
G1, S and G2 phases of the cell cycle. Lung
adenocarcinoma cells were divided into 4 groups: The control group
(transfected with control siRNA), the ERp29 siRNA group
(transfected with ERp29 siRNA), the gemcitabine group (transfected
with control siRNA and treated with gemcitabine) and the
combination group (transfected with ERp29 siRNA and treated with
gemcitabine). Single-cell suspensions were fixed using 70% ethanol
for 24 h at 4°C. Cells were subsequently washed with phosphate
buffered saline, stained with 0.5 ml PI for 30 min at room
temperature and analyzed using a BD FACS Calibur flow cytometer and
Cell Quest software (version 5.1; BD Biosciences).
Cytotoxicity assay
Gemcitabine cytotoxicity was quantified using a Cell
Counting kit-8 (CCK-8) assay (7Sea Biotech, Shanghai, China). Cells
were seeded in 96-well plates at a density of 3×103 A549
cells/well or 3.5×103 SPC-A1 cells/well. Following 48-h
treatment with gemcitabine (0.6 µM for A549 or 6 µM for SPC-A1
cells) at room temperature, cells were incubated with 10 µl CCK-8
solution for 1 h at room temperature. The optical density (OD) was
measured at 450 nm using a Multiskan Go Microplate
spectrophotometer (Thermo Fisher Scientific, Inc.). The cell growth
inhibition rate of gemcitabine was calculated as follows:
(1-OD450 of treated cells/OD450 of untreated
cells) ×100%.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean
± standard error of the mean. Differences between two groups were
assessed using Student's t-test. Multiple comparisons between
groups were analyzed using one-way analysis of variance followed by
Fisher's least significant difference post-hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
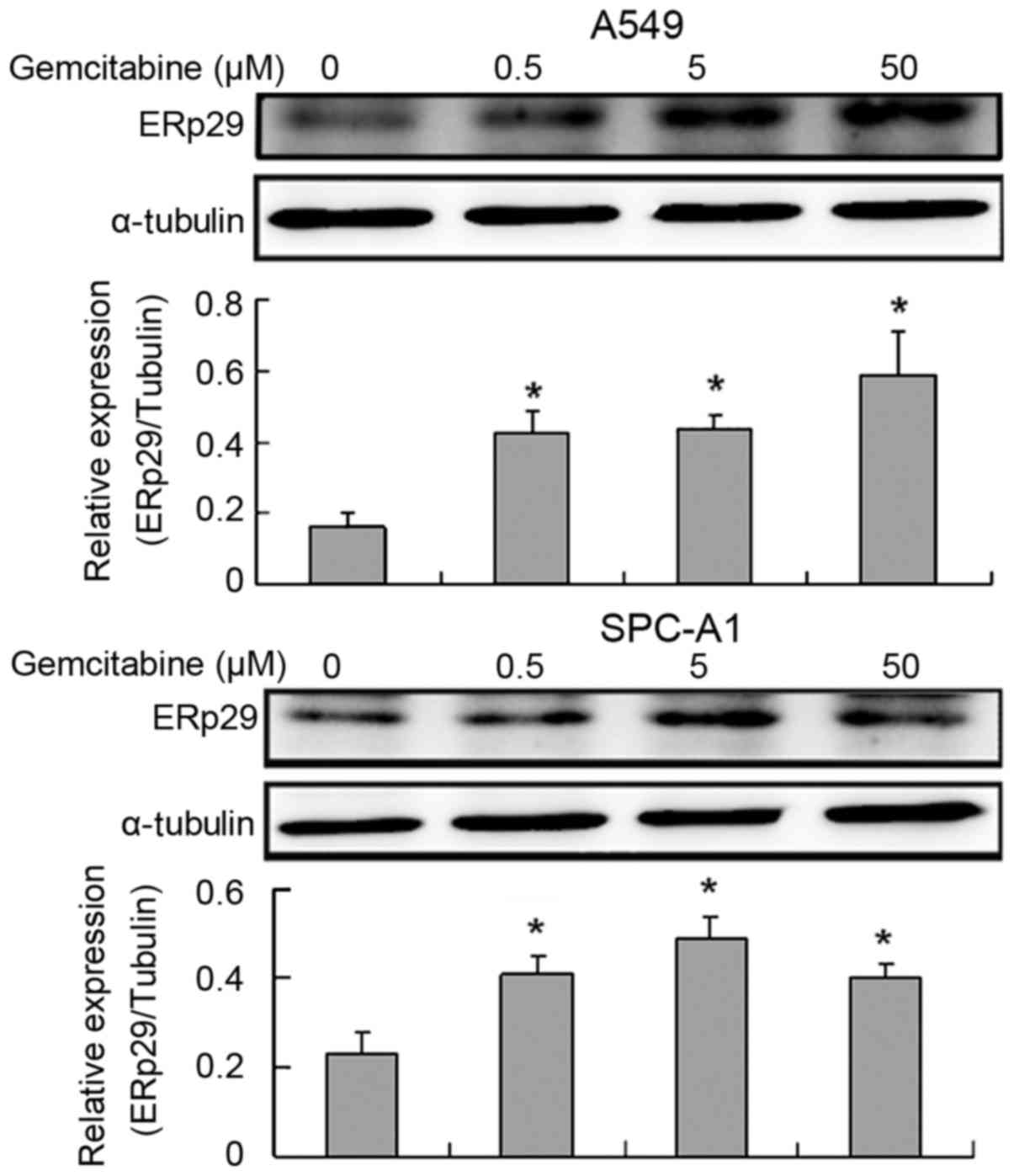
Exposure of lung adenocarcinoma cells
to gemcitabine increases ERp29 expression
As presented in Fig.
1, ERp29 expression was significantly increased on exposure to
gemcitabine (P<0.05). Erp29 expression was significantly
increased at 50 µM in A459 cells and 5 µM in SPC-A1 cells,
respectively.
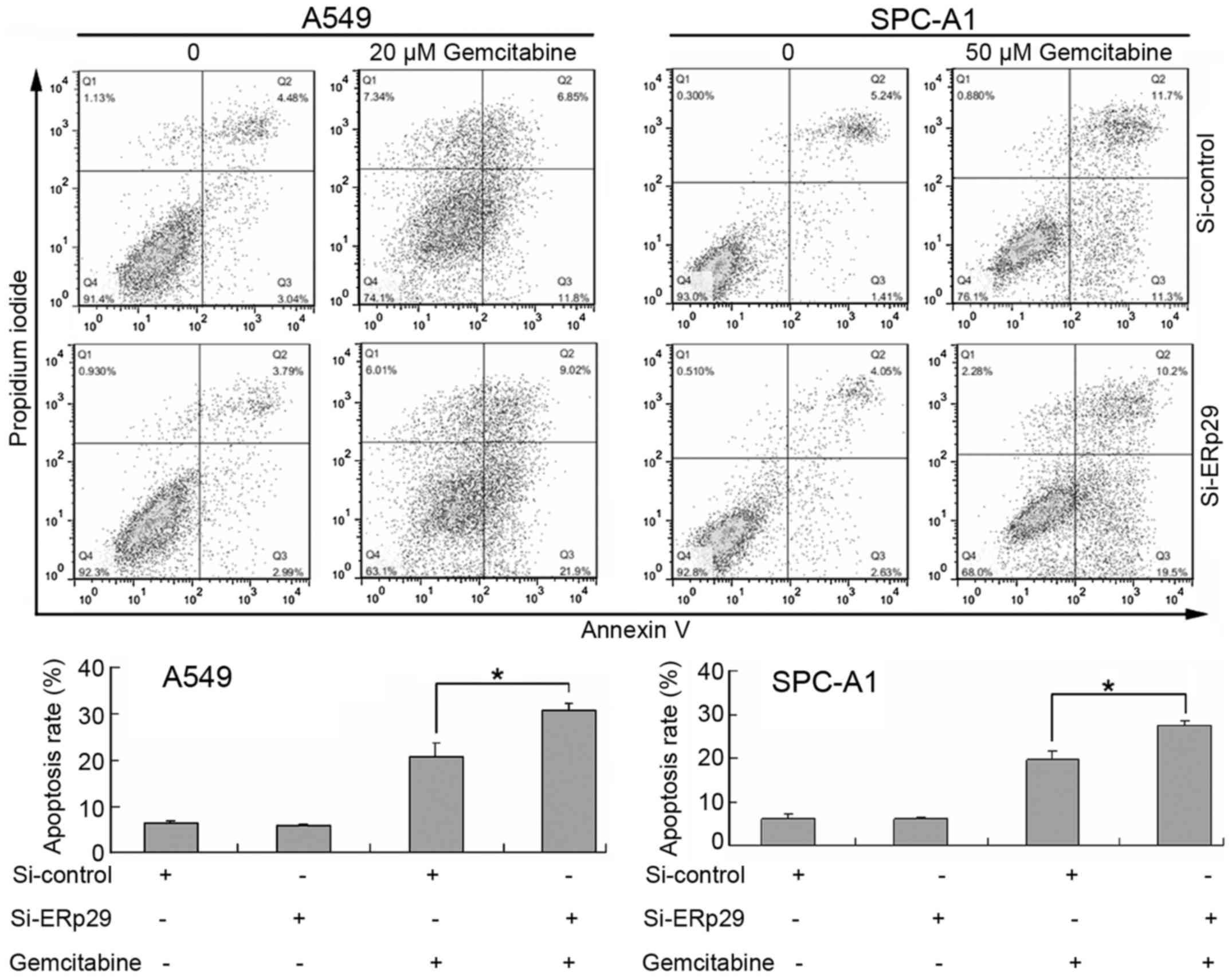
Effects of gemcitabine and ERp29 siRNA
on lung adenocarcinoma cell apoptosis
Following treatment with 20 µM gemcitabine, the
apoptotic rate of A549 cells increased from 6.43±0.55 in the
control group to 20.90±2.83% in the control + 20 µM gemcitabine
group (Fig. 2). Treatment with
gemcitabine in combination with ERp29 siRNA significantly increased
the apoptotic rate to 30.80±1.41% compared with gemcitabine
treatment alone (Fig. 2). Following
treatment with 50 µM gemcitabine, the SPC-A1 cell apoptotic rate
was increased from 6.10±1.12 in the control group to 19.82±1.76% in
the control + 50 µM gemcitabine group. Treatment with gemcitabine
in combination with ERp29 siRNA significantly increased the
apoptotic rate to 27.53±1.11% compared with gemcitabine treatment
alone (Fig. 2). The apoptotic rate
of A549 and SPC-A1 cells in the Si-control and ERp29 siRNA group
were not significantly different.
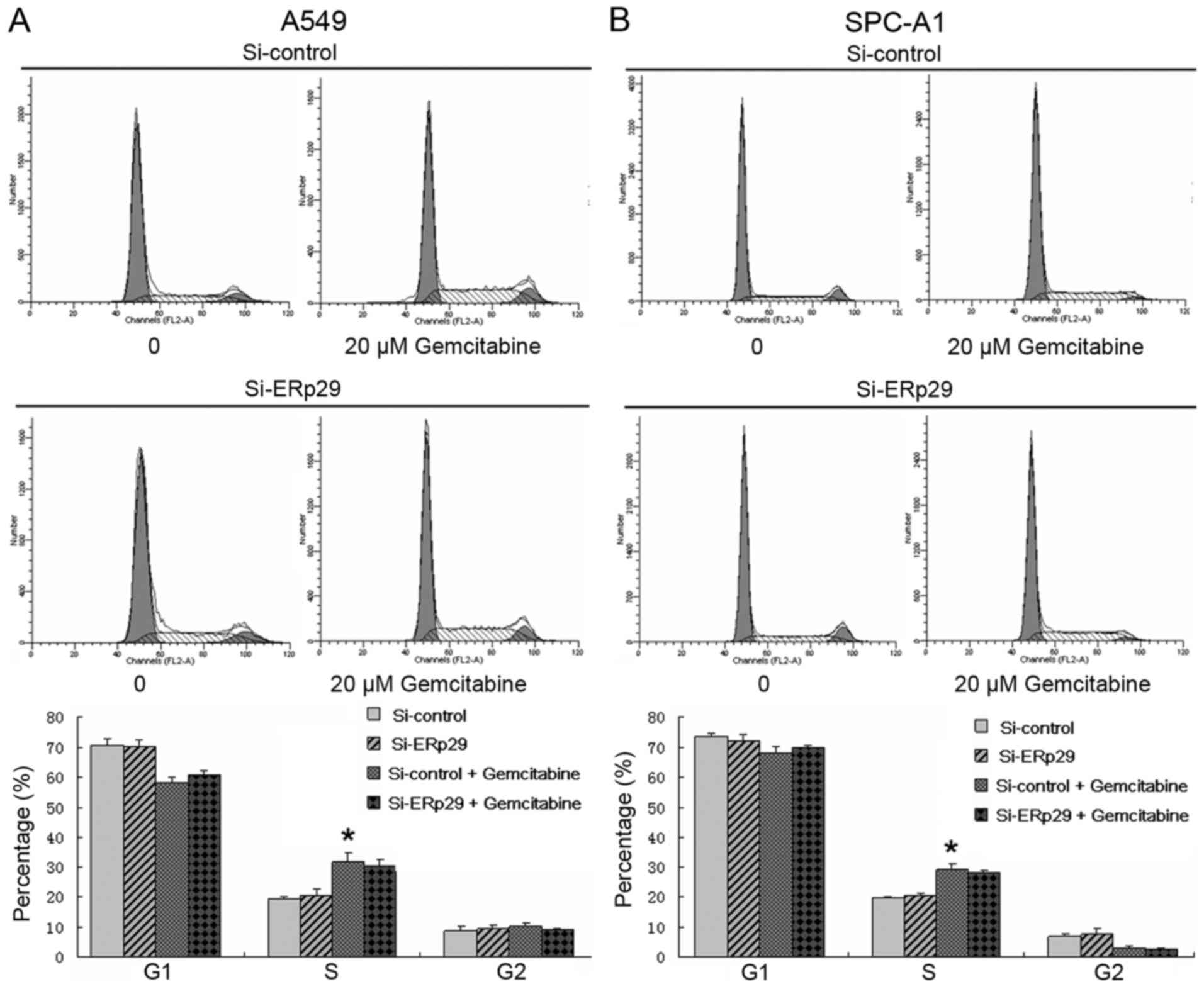
Effects of gemcitabine and ERp29 siRNA
on the cell cycle of lung adenocarcinoma cells
Lung adenocarcinoma cells in the gemcitabine and
combination groups were treated with 20 µM gemcitabine. As
presented in Fig. 3, the percentage
of gemcitabine-treated cells in the S phase was significantly
increased compared with untreated cells in the S phase in the A549
Si-control group (31.80±2.81 vs. 19.12±1.00%; P<0.05). Similar
results were obtained in SPC-A1 cells (29.12±1.84 vs. 19.78±0.39%;
P<0.05). The percentage of gemcitabine-treated cells in the S
phase was increased compared with untreated cells in the S phase in
the A549 Si-ERp29 group and similar results were obtained in SPC-A1
cells. There were no significant differences in the level of
G1 and G2 cell cycle arrest observed in the
ERp29 siRNA or Si-control groups.
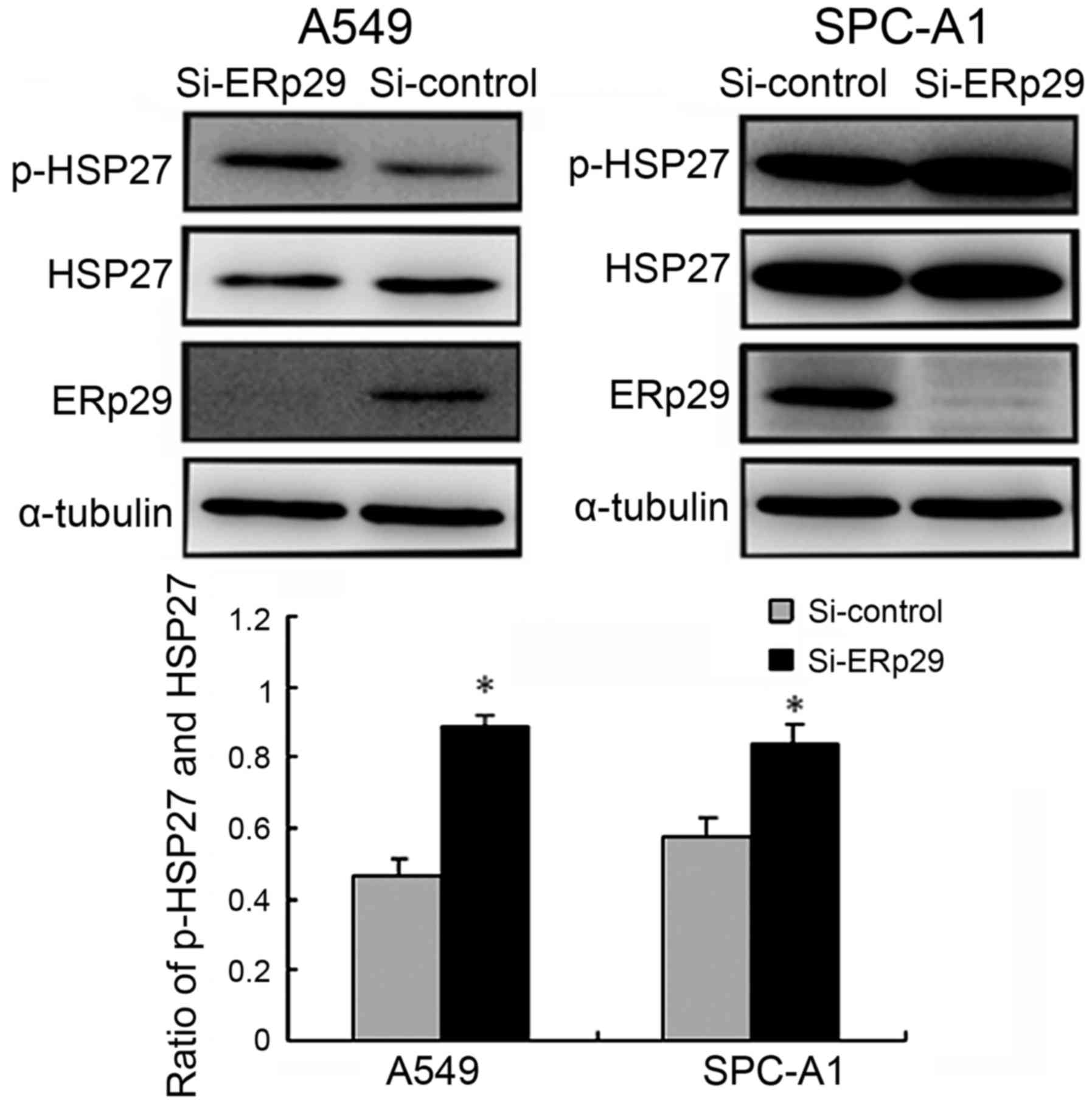
Effects of ERp29 siRNA on HSP27
expression in lung adenocarcinoma cells
Lung adenocarcinoma cell lines were transfected with
ERp29 or control siRNA. At 72 h following transfection, ERp29,
HSP27 and p-HSP27 levels were detected using western blotting. The
downregulation of ERp29 significantly increased the ratio of
p-HSP27 to total HSP27 (P<0.05; Fig.
4).
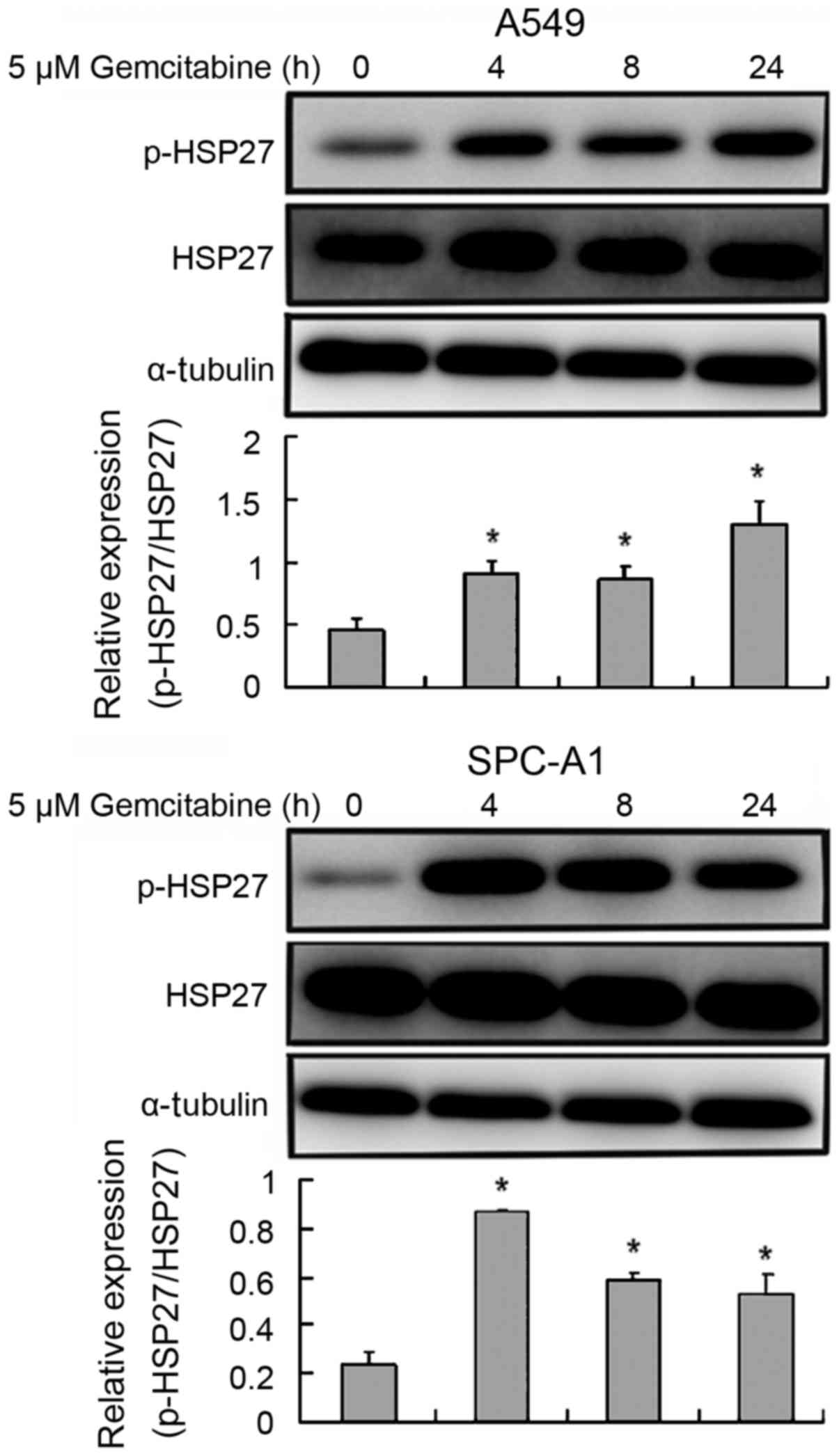
Effects of gemcitabine on HSP27
expression in lung adenocarcinoma cells
Phosphorylation of HSP27 was significantly increased
in gemcitabine-treated cells compared with the 0 h group
(P<0.05; Fig. 5). However,
gemcitabine-induced HSP27 phosphorylation was not found to be
time-dependent.
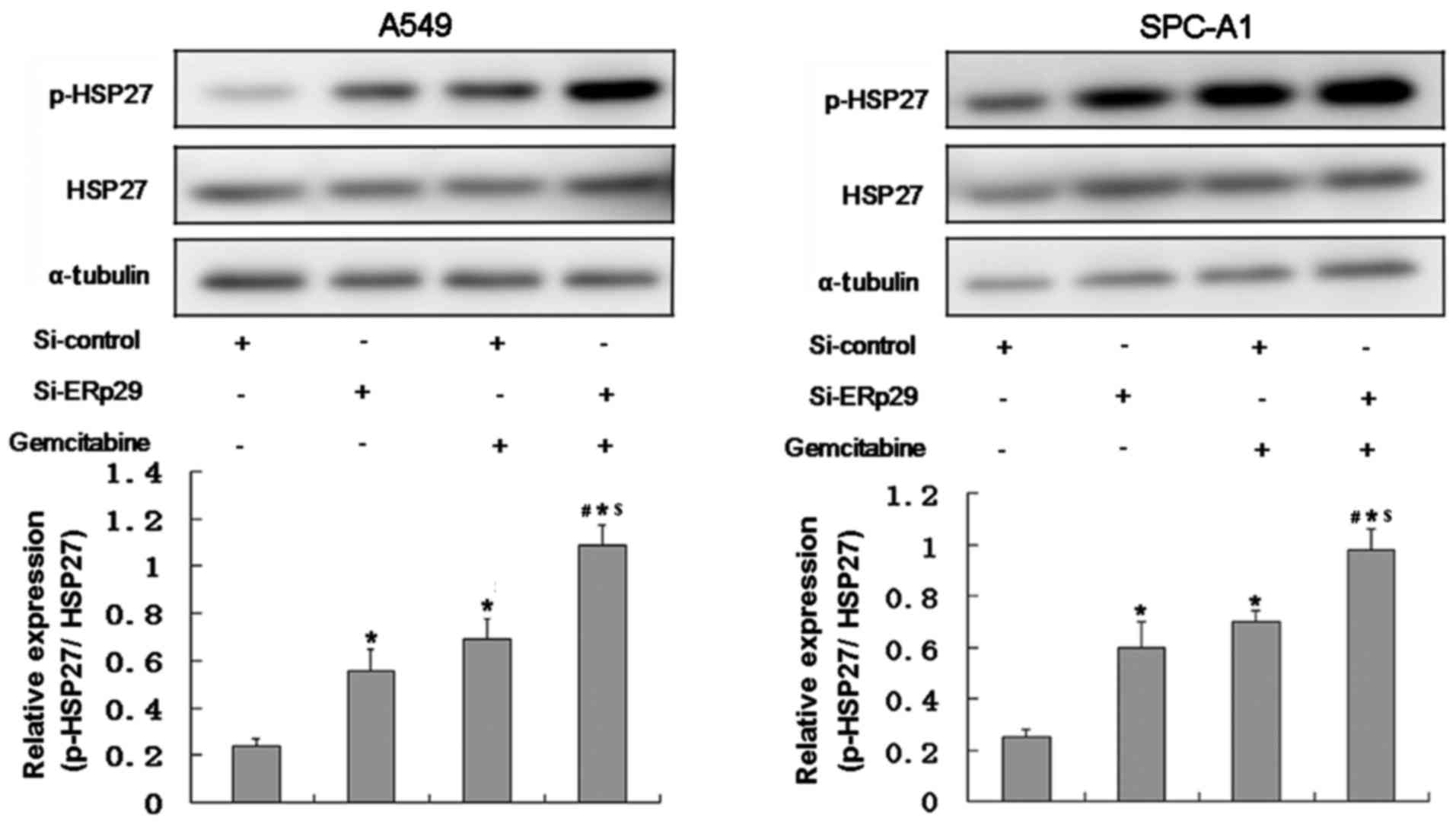
Effects of gemcitabine in combination
with ERp29 siRNA on HSP27 expression
Lung adenocarcinoma cells in the gemcitabine and
combination groups were treated with 5 µM gemcitabine.
Phosphorylation of HSP27 in the combination group was significantly
higher compared with the other three groups (P<0.05; Fig. 6).
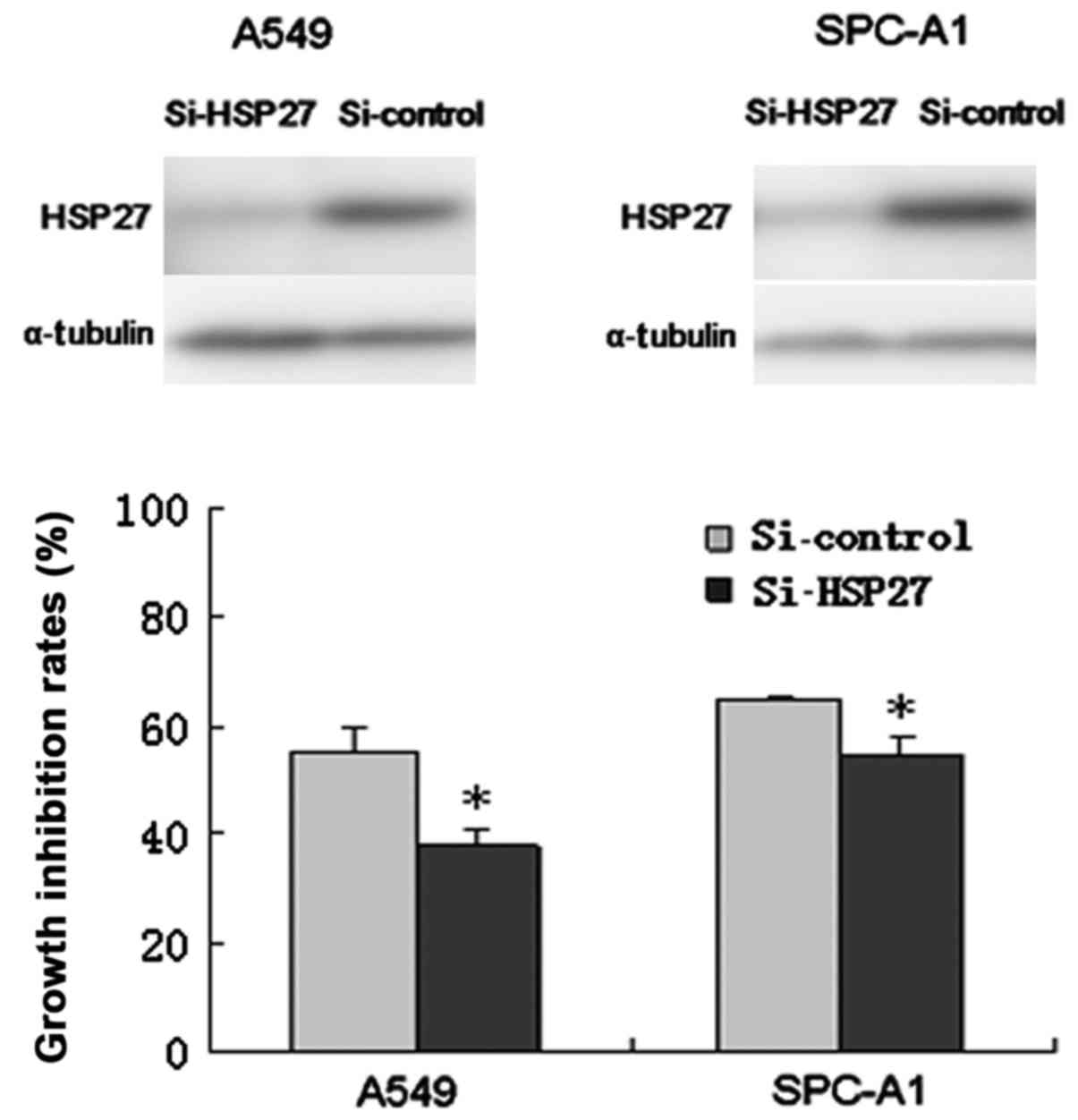
Effects of HSP27 siRNA on lung
adenocarcinoma cell chemosensitivity to gemcitabine
Lung adenocarcinoma cells were transfected with
HSP27 siRNA or control siRNA. As presented in Fig. 7, HSP27 expression was effectively
reduced in A549 and SPC-A1 cells transfected with HSP27 siRNA
compared with the control RNA. The downregulation of HSP27
expression in A549 cells significantly decreased the cell growth
inhibition rate of gemcitabine compared with the control
(37.79±2.99 vs. 55.63±4.28%; P<0.05). Furthermore, the cell
growth inhibition rate of gemcitabine in HSP27-inhibiting SPC-A1
cells was significantly lower than that of the control (54.82±3.15
vs. 64.80±0.62%; P<0.05).
Discussion
The present study investigated the role of ERp29 in
lung adenocarcinoma cell chemosensitivity to gemcitabine. The
results demonstrated that ERp29 expression in A549 and SPC-A1 cells
was increased on exposure to gemcitabine. Furthermore, ERp29
downregulation significantly increased apoptosis induced by
gemcitabine. It was further demonstrated that gemcitabine and ERp29
siRNA synergistically increased HSP27 phosphorylation and HSP27
downregulation significantly reduced chemosensitivity to
gemcitabine.
A previous study revealed that ERp29 was
overexpressed in patients with lung adenocarcinoma and ERp29
inhibition significantly enhanced the cytotoxic effects of
gemcitabine (13). However, the
underlying mechanism of this effect is poorly understood. Zhang
et al (17) demonstrated that
ERp29 overexpression in cortical neurons following axotomy prevents
the reduction of neurite length and neuron number, and protects
from apoptosis. Furthermore, a previous study revealed that ERp29
is upregulated on exposure to radiation and ERp29 expression
protects cells from damage caused by ionizing radiation (5). In the present study, lung
adenocarcinoma cells were exposed to gemcitabine and ERp29
expression was significantly increased. This may be due to certain
stimuli in the tumor microenvironment causing ERp29 upregulation,
which may serve a cytoprotective role (9,17). In
the current study, lung cancer cells were treated with gemcitabine
for 24 h and the analysis of time-dependent effects of gemcitabine
on ERp29 expression requires further examination.
Cell apoptosis is one of the main mechanisms of
antineoplastic activity employed by chemotherapeutic agents
(18). Gemcitabine exerts cytotoxic
effects primarily by inducing tumor cell apoptosis via blockage of
DNA synthesis and repair (19,20). In
the present study, A549 and SPC-A1 apoptotic rates were increased
following treatment with gemcitabine and the combined application
of gemcitabine and ERp29 siRNA synergistically increased apoptotic
rates further. Zhang and Putti (9)
demonstrated that ERp29 downregulation significantly increased
doxorubicin-induced apoptosis and ERp29 overexpression decreased
cell apoptosis. Additionally, ERp29 inhibition enhances cell
apoptosis induced by ionizing radiation and cigarette smoke extract
via caspase-3 and −7 expression (5,12,21,22).
Gemcitabine is a cell cycle-specific drug that acts
primarily in the S phase (23,24). In
the present study, A549 cells were treated with 20 µM gemcitabine
for 48 h. The percentage of cells in the S phase of the gemcitabine
group was significantly higher than that of the control group.
However, the combined application of gemcitabine and ERp29 siRNA
did not alter the effect of gemcitabine. Few studies have assessed
the role of ERp29 in cell cycle control and the exact mechanism
requires further investigation (5,9).
HSPs can be divided into 5 families: HSP110, HSP90,
HSP70, HSP60 and small HSP (25).
HSP27, a small HSP with a molecular weight of 27 kDa, consists of
205 amino acid residues (26). The
C-terminus of HSP27 contains a highly conserved α-crystallin domain
and the N-terminus comprises a WDPF domain and a PSRLFDQXFGEXLL
sequence (26). In the present
study, ERp29 downregulation and treatment with gemcitabine combined
with ERp29 siRNA were revealed to significantly increase HSP27
phosphorylation. Nakashima et al (27) have revealed that HSP27
phosphorylation is increased following pancreatic cancer cell
treatment with gemcitabine. Similar results were observed in the
current study. The phosphorylation of HSP27 is primarily catalyzed
by mitogen-activated protein kinase-activated protein kinase
(MAPKAPK)-2, MAPKAPK-3 and MAPKAPK-5, protein kinase (PK) A, PKB
and PKC (28,29). Furthermore, MAPKAPK2 activation
enhances damaging effects of gemcitabine on DNA and inhibits DNA
repair (30). However, the
association between ERp29 and HSP27 requires to be further
elucidated.
In the current study, HSP27 downregulation
significantly reduced chemosensitivity to gemcitabine in A549 and
SPC-A1 cells. This finding was consistent with a report by Schäfer
et al (15), who revealed
that HSP27 expression inhibition in AsPC-1 pancreatic cancer cells
attenuated gemcitabine cytotoxicity. HSP27 downregulation has been
demonstrated to inhibit apoptosis by regulating caspase-3, B-cell
lymphoma/leukemia-2 (Bcl-2), Bcl-2 associated X protein and
poly-ADP-ribose polymerase (PARP) (31,32). Guo
et al (16) revealed that the
combination of gemcitabine and HSP27 overexpression synergistically
increased apoptosis in pancreatic cancer cells and increased PARP
expression and caspase-3, −8 and −9 cleavage.
In summary, ERp29 expression was upregulated on
exposure to gemcitabine and increased ERp29 expression protected
lung adenocarcinoma cells from cytotoxic effects of gemcitabine. It
was further revealed that ERp29 inhibition increases apoptotic
rates induced by gemcitabine, which is one of the main mechanisms
of its antitumor effect (18,33).
ERp29 may therefore affect lung cancer cell chemosensitivity to
gemcitabine by regulating HSP27 phosphorylation. However, further
studies, including in vivo research, are required to verify
these results.
ERp29 is a novel target, inhibition of ERp29
expression may be used to enhance the therapeutic effect of lung
adenocarcinoma treatment with gemcitabine.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WY and GQ designed the study. WY, ZL, TT and JD
performed the experiment. XZ, HW and XL analyzed the data and
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bender E: Epidemiology: The dominant
malignancy. Nature. 513:S2–S3. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network:
Author correction comprehensive molecular profiling of lung
adenocarcinoma. Nature. 559:E122018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berge EM and Doebele RC: Targeted
therapies in non-small cell lung cancer: Emerging oncogene targets
following the success of epidermal growth factor receptor. Semin
Oncol. 41:110–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mkrtchian S and Sandalova T: ERp29, an
unusual redox-inactive member of the thioredoxin family. Antioxid
Redox Signal. 8:325–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Wang M, Yang Y, Wang Y, Pang X,
Su Y, Wang J, Ai G and Zou Z: ERp29 is a radiation-responsive gene
in IEC-6 cell. J Radiat Res. 49:587–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hung YC, Wang PW, Pan TL, Bazylak G and
Leu YL: Proteomic screening of antioxidant effects exhibited by
radix salvia miltiorrhiza aqueous extract in cultured rat aortic
smooth muscle cells under homocysteine treatment. J Ethnopharmacol.
124:463–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dukes AA, Van Laar VS, Cascio M and
Hastings TG: Changes in endoplasmic reticulum stress proteins and
aldolase A in cells exposed to dopamine. J Neurochem. 106:333–346.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheretis C, Dietrich F, Chatzistamou I,
Politi K, Angelidou E, Kiaris H, Mkrtchian S and Koutselini H:
Expression of ERp29, an endoplasmic reticulum secretion factor in
basal-cell carcinoma. Am J Dermatopathol. 28:410–412. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang D and Putti TC: Over-expression of
ERp29 attenuates doxorubicin-induced cell apoptosis through
up-regulation of Hsp27 in breast cancer cells. Exp Cell Res.
316:3522–3531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan LW, Liu DC and Yang ZL: Correlation
of S1P1 and ERp29 expression to progression, metastasis, and poor
prognosis of gallbladder adenocarcinoma. Hepatobiliary Pancreat Dis
Int. 12:189–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng YJ, Tang N, Liu C, Zhang JY, An SL,
Peng YL, Ma LL, Li GQ, Jiang Q, Hu CT, et al: CLIC4, ERp29, and
Smac/DIABLO derived from metastatic cancer stem-like cells stratify
prognostic risks of colorectal cancer. Clin Cancer Res.
20:3809–3817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi L, Wu P, Zhang X, Qiu Y, Jiang W, Huang
D, Liu Y, Tan P and Tian Y: Inhibiting ERp29 expression enhances
radiosensitivity in human nasopharyngeal carcinoma cell lines. Med
Oncol. 29:721–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye W, Zhang R, Hu Y, Xu X and Ying K:
Increased expression of endoplasmic reticulum protein 29 in lung
adenocarcinoma is associated with chemosensitivity to gemcitabine.
Anticancer Drugs. 26:612–619. 2015.PubMed/NCBI
|
|
14
|
Wu P, Zhang H, Qi L, Tang Q, Tang Y, Xie
Z, Lv Y, Zhao S and Jiang W: Identification of ERp29 as a biomarker
for predicting nasopharyngeal carcinoma response to radiotherapy.
Oncol Rep. 27:987–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schäfer C, Seeliger H, Bader DC, Assmann
G, Buchner D, Guo Y, Ziesch A, Palagyi A, Ochs S, Laubender RP, et
al: Heat shock protein 27 as a prognostic and predictive biomarker
in pancreatic ductal adenocarcinoma. J Cell Mol Med. 16:1776–1791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Ziesch A, Hocke S, Kampmann E, Ochs
S, De Toni EN, Göke B and Gallmeier E: Overexpression of heat shock
protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic
cancer cells through S-phase arrest and apoptosis. J Cell Mol Med.
19:340–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YH, Belegu V, Zou Y, Wang F, Qian
BJ, Liu R, Dai P, Zhao W, Gao FB, Wang L, et al: Endoplasmic
reticulum protein 29 protects axotomized neurons from apoptosis and
promotes neuronal regeneration associated with erk signal. Mol
Neurobiol. 52:522–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moysan E, Bastiat G and Benoit JP:
Gemcitabine versus modified gemcitabine: A review of several
promising chemical modifications. Mol Pharm. 10:430–444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou L, Qi L, Jiang L, Zhou P, Ma J, Xu X
and Li P: Antitumor activity of gemcitabine can be potentiated in
pancreatic cancer through modulation of TLR4/NF-κB signaling by
6-shogaol. AAPS J. 16:246–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pauwels B, Korst AE, Lardon F and
Vermorken JB: Combined modality therapy of gemcitabine and
radiation. Oncologist. 10:34–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang C, Wang JJ, Jing G, Li J, Jin C, Yu
Q, Falkowski MW and Zhang SX: Erp29 attenuates cigarette smoke
extract-induced endoplasmic reticulum stress and mitigates tight
junction damage in retinal pigment epithelial cells. Invest
Ophthalmol Vis Sci. 56:6196–6207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu R, Zhao W, Zhao Q, Liu SJ, Liu J, He
M, Xu Y, Wang W, Liu W, Xia QJ, et al: Endoplasmic reticulum
protein 29 protects cortical neurons from apoptosis and promoting
corticospinal tract regeneration to improve neural behavior via
caspase and erk signal in rats with spinal cord transection. Mol
Neurobiol. 50:1035–1048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morgan MA, Parsels LA, Parsels JD,
Mesiwala AK, Maybaum J and Lawrence TS: Role of checkpoint kinase 1
in preventing premature mitosis in response to gemcitabine. Cancer
Res. 65:6835–6842. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luk PP, Galettis P and Links M: ERK
phosphorylation predicts synergism between gemcitabine and the
epidermal growth factor receptor inhibitor AG1478. Lung Cancer.
73:274–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calderwood SK and Gong J: Heat shock
proteins promote cancer: It's a protection racket. Trends Biochem
Sci. 41:311–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taylor RP and Benjamin IJ: Small heat
shock proteins: A new classification scheme in mammals. J Mol Cell
Cardio. 38:433–444. 2005. View Article : Google Scholar
|
|
27
|
Nakashima M, Adachi S, Yasuda I, Yamauchi
T, Kawaguchi J, Itani M, Yoshioka T, Matsushima-Nishiwaki R, Hirose
Y, Kozawa O, et al: Phosphorylation status of heat shock protein 27
plays a key role in gemcitabine-induced apoptosis of pancreatic
cancer cells. Cancer Lett. 313:218–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zoubeidi A and Gleave M: Small heat shock
proteins in cancer therapy and prognosis. Int J Biochem Cell Bio.
44:1646–1656. 2012. View Article : Google Scholar
|
|
29
|
Kostenko S and Moens U: Heat shock protein
27 phosphorylation: Kinases, phosphatases, functions and pathology.
Cell Mol Life Sci. 66:3289–3307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Köpper F, Bierwirth C, Schön M, Kunze M,
Elvers I, Kranz D, Saini P, Menon MB, Walter D, Sørensen CS, et al:
Damage-induced DNA replication stalling relies on MAPK-activated
protein kinase 2 activity. Proc Natl Acad Sci USA. 110:16856–16861.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Evans J, Ko Y, Mata W, Saquib M, Eldridge
J, Cohen-Gadol A, Leaver HA, Wang S and Rizzo MT: Arachidonic acid
induces brain endothelial cell apoptosis via p38-MAPK and
intracellular calcium signaling. Microvasc Res. 98:145–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim J, Jung H, Lim W, Kim S, Ko Y, Karna S
and Kim O, Choi Y, Choi H and Kim O: Down-regulation of heat-shock
protein 27-induced resistance to photodynamic therapy in oral
cancer cells. J Oral Pathol Med. 42:9–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Li D, Zheng X, Wang E and Wang J:
Selective induction of apoptosis: Promising therapy in pancreatic
cancer. Curr Pharm Des. 19:2259–2268. 2013. View Article : Google Scholar : PubMed/NCBI
|