Introduction
Psoriasis is a chronic immunologically mediated
dermatosis, affecting 2–3% of the general population (1–3).
Multiple mechanisms are involved in its pathogenesis, such as the
hyper-reactivity of T-lymphocytes and dendritic cells, excessive
inflammatory cytokine synthesis, accelerated epidermal turnover,
epidermal hyperproliferation, reduced keratinocyte differentiation,
overexpression of angiogenesis and oxidative stress (4–14). The
extent of skin involvement is variable, ranging from several
psoriatic plaques to generalized forms. The disease evolves with
periods of exacerbation and remission (15,16).
It seems that severe psoriasis is an independent
risk factor for chronic renal disease (17,18). The
mechanisms that mediate kidney failure in patients with psoriasis
are controversial. In a retrospective study, it was estimated that
patients with psoriasis develop chronic renal disease at a higher
percentage compared to controls (5 vs. 2%). Moreover, the risk of
kidney disease was higher in the young patients (18). The treatment should be adapted to
meet the individual needs of the patients with psoriasis.
Non-pharmacological interventions (diet, cessation of smoking and
alcohol intake, weight loss, physical exercise) may improve the
response to therapy. Potentially nephrotoxic drugs should be used
with caution and renal function should be periodically monitored in
patients with psoriasis in order to minimize the risk of adverse
renal events (13,15,19,20).
In medical literature, there are few studies on the
relationship between uremic toxins and the decline of renal
function in patients with psoriasis vulgaris. Recent data suggest
the role of several serum and urinary markers in the early
detection and monitoring of renal disease. The urinary levels of
creatinine, albumin, uric acid (UA) and the estimated glomerular
filtration rate (eGFR) can be evaluated, as well as cystatin C,
neutrophil gelatinase-associated lipocalin, kidney injury molecule
1, cytokines and chemokines (21).
The progressive increase in the level of uremic toxins exerts a
negative impact on kidney function (22–25).
Purine derivatives pertain to the class of low
molecular weight uremic toxins, which frequently accumulate in the
body. Low molecular weight uremic toxins are water-soluble
compounds with a molecular weight below 0.5 kDa, and consequently
are easily removed by dialysis, and do not exert harmful effects on
the body (26,27). The most important uremic toxins
classified as purine derivatives are: Products resulted from the
degradation of purines (adenosine, inosine, xanthine, guanosine,
hypoxanthine, guanine, UA), products of guanosine triphosphate
catabolism (neopteri n) and oxidative DNA base damage product
(8-hydroxy-deoxy-guanosine) (28–30). The
metabolic pathways of purine catabolism imply several phases
(31).
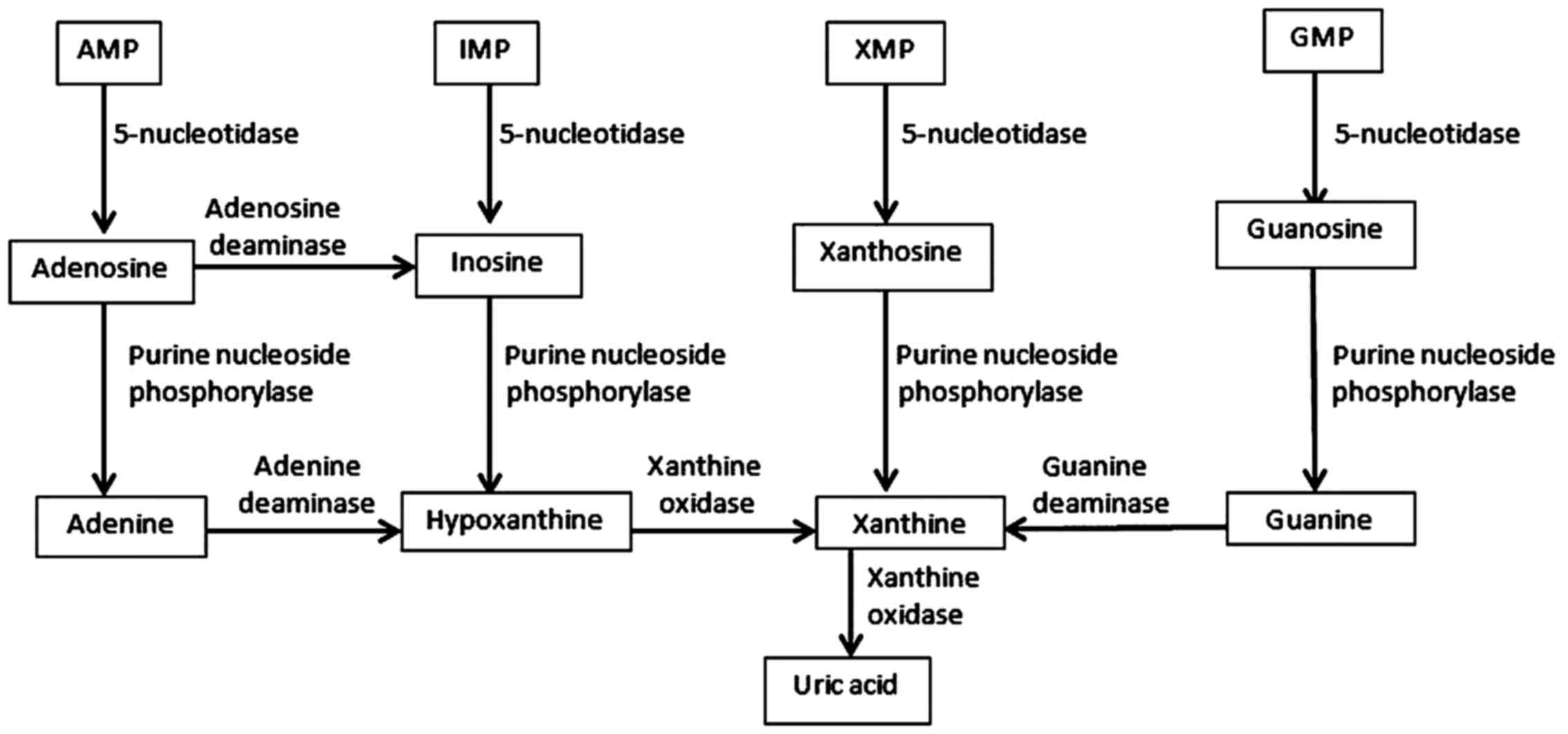
The first step involves the transformation of purine
mononucleotides (AMP-adenosine monophosphate, IMP-inosine
monophosphate, XMP-xanthine monophosphate and GMP-guanine
monophosphate) into purine nucleosides (adenosine, inosine,
xanthosine and guanosine), a reaction catalyzed by 5-nucleotidase
(E.C.3.1.3.5). Subsequently, conversion of purine nucleosides
occurs which frees purine bases (adenine, hypoxanthine, xanthine
and guanine), a reaction catalyzed by purine nucleoside
phosphorylase (E.C.2.2.2.1). The next step involves the conversion
of adenine to hypoxanthine (a reaction catalyzed by adenine
deaminase - E.C.3.5.4.4) and then to xanthine (a reaction catalyzed
by xanthine oxidase - E.C.1.17.3.2). Guanine is converted to
xanthine by guanine deaminase. The final product of purine
metabolism is UA, obtained through the enzymatic oxidation of
xanthine (a reaction catalyzed by xanthine oxidase) (Fig. 1).
In psoriasis patients, the severity of the skin
disease, assessed by the Psoriasis Area Severity Index (PASI), has
been analyzed in relation to the prevalence of renal dysfunctions
(3,16,18,32,33),
purine catabolism (4) and the
oxidative stress level (5,6,34).
Alteration of purine degradation may be associated with the
extension of psoriasis lesions, stimulation of epidermal
proliferation and increased DNA peroxidation (6,35–38).
These reactions produce a wide range of reactive
oxidants: hydrogen peroxide (H2O2),
superoxide anion (O2•−), hydroxyl radical
(OH•), nitric oxide (NO•), peroxynitrite
(ONOO−), and carbonate radical (CO3•−).
Reactive oxygen species and reactive nitrogen species, formed under
the action of xanthine oxidase, act on proteins, lipids and nucleic
acids, causing damage and cell toxicity (39–42).
8-Hydroxy-deoxyguanosine, a representative metabolite for purine
derivatives toxins (43), is a
useful indicator for early diagnosis and management of patients
with psoriasis (6).
Based on these considerations, we aim to establish
characteristic patterns of related-purine derivatives that could
represent specific serological markers for the identification of
those patients with psoriasis vulgaris at a higher risk of
developing renal dysfunctions. In this context, the serum profile
of related-purine derivatives was analyzed in psoriasis patients in
correlation with the severity of the skin disease and markers of
renal impairment. The biomarkers carried out in this study, grouped
under the name of related-purine derivatives, include UA (the final
product of purine catabolism), adenosine deaminase (ADA, an enzyme
which converts irreversibly adenosine and deoxyadenosine to inosine
and deoxy-inosine), xanthine oxidase (XO, which catalyzes the
oxidation of hypoxanthine to xanthine as well as the oxidation of
xanthine to UA) and 8-hydroxy-deoxy-guanosine (8-OHdG, a biomarker
of oxidative DNA damage). Renal function was assessed through the
serum creatinine level, urinary determinations of eGFR (ml/min/1.73
mp), albumin/creatinine ratio (ACR, mg/g) and UA-creatinine ratio
(UACR, mg/mg).
Materials and methods
Study participants
All the study participants provided consent to the
use of their biological samples in research studies. The Ethics
Committee of ‘Victor Babes’ Clinical Hospital for Infectious
Diseases (Bucharest, Romania) approved the study protocol.
A prospective study was conducted on 45 patients
with psoriasis vulgaris and 45 control cases, monitored over a
5-year period. Inclusion criteria for the study were: Adults,
normolipidemic, normoponderal, with a balanced diet. Exclusion
criteria for the study were: Cardiovascular disease, metabolic
syndrome, diabetes, anemia, urinary tract infections,
nephrolithiasis, leukemia, Lesch-Nyhan syndrome, Wilson's disease,
viral hepatitis, sickle-cell disease, chronic kidney disease,
xanthinuria, lead toxicity, treatment with nephrotoxic drugs, folic
acid deficiency, pregnant women and breastfeeding women.
The severity of psoriasis was assessed using the
PASI score, which assesses the severity of three clinical signs
(erythema, thickness, scaling) on a scale from 0 to 4 and the
percentage of the skin area involved. The PASI score interpretation
was as follows: <7, mild chronic plaque-type psoriasis; 7–12,
moderate chronic plaque-type psoriasis; >12, severe chronic
plaque-type psoriasis (41).
Changes of glomerular permeability were detected by
determining eGFR, ACR and UACR. To assess alterations of renal
function, ACR and UACR were determined in spontaneous urine
samples. The status of related-purine derivatives was evaluated by
quantification of the serum levels of UA (mg/dl), adenosine
deaminase (UI/mg protein), XO (UI/mg protein) and 8-OHdG
(ng/ml).
Biological samples were a spontaneous urine sample,
preferably first morning urine collected in sterile,
preservative-free containers. Urine specimens were centrifuged at
3,000 × g for 10 min at room temperature 20°C. The supernatant was
used for measurement of the biological parameters. Venous blood
samples (7 ml) were collected in a vacutainer without an
anticoagulant and centrifuged at 6,000 × g for 10 min at 20°C. The
supernatant was used for biochemical determinations.
Laboratory methods
The determination of creatinine was performed using
the colorimetric technique. The method is based on the reaction
between creatinine and picric acid in alkaline medium. The
absorbance measured at a wavelength of 492 nm is directly
proportional to the amount of creatinine in the sample.
The determination of albuminuria was performed via
turbidimetric immunoassay using polyclonal antibodies against human
albumin. The determination of UA was performed by the colorimetric
technique, using the reaction catalyzed by uricase. The
concentration of quinonimine resulted from the reaction was
determined by measuring the absorbance at a wavelength of 546 nm.
The determination of xanthine-oxidase was performed using a
spectrophotometer (HumaStar 300; HUMAN Gesellschaft für Biochemica
und Diagnostica mbH, Germany, Wiesbaden). Absorbance of the
obtained coloured complex was read at a wavelength of 570 nm. The
determination of adenosine deaminase was performed via
spectrophotometry and the evaluation of the resulting product was
measured at a wavelength of 550 nm.
The quantitative determination of hs 8-OHdG (highly
sensitive oxidative DNA adduct 8-OHdG) was produced in serum using
an enzyme-linked immunosorbent assay (ELISA). The principle of the
method is based on the ability of DNA oxidation products to
interact with 3,3,5,5-tetramethylbenzidine. The method uses
DNA-specific monoclonal antibodies, which cross-react with the
oxidative degradation products of DNA (8-hydroxy-guanine,
8-hydroxy-guanosine). The final product of the reaction is
colorimetrable via microplate reader (Tecan Austria GmbH, Grodig,
Austria) at a wavelength of 450 nm.
Statistical analysis
A comparison of the obtained results between the
groups for quantitative variables was performed using the t-test.
The correlations between variables were determined by linear
regression. The relationship between pairs of two parameters was
assessed by Pearson's correlation coefficient (r). We chose a
significance level (p) of 0.05 (5%) and a confidence interval of
95% for hypothesis testing.
Results
Patient characteristics
We performed a prospective observational study on 45
patients with psoriasis vulgaris (duration of psoriasis 6.4±3.1
years, PASI 10.4±5.3) and 45 healthy volunteers, who met the
inclusion criteria (Table I).
 | Table I.Characteristics of study
participants. |
Table I.
Characteristics of study
participants.
| Items | Psoriasis
vulgaris | Control | P-value |
|---|
| Age (years) | 40.3±11.3 | 39.4±8.3 | 0.763 |
| Male/female
ratio | 24/21 | 25/20 | 0.923 |
| Smokers/non-smokers
ratio | 11/34 | 9/36 | 0.755 |
| BMI
(kg/m2) | 20.4±1.3 | 19.8±1.1 | 0.899 |
| Systolic blood
pressure (mmHg) | 117.3±0.7 | 115.5±0.5 | 0.677 |
| Diastolic blood
pressure (mmHg) | 72.3±0.2 | 73.5±0.4 | 0.546 |
Serum determinations
The serum concentration of UA was 5.4±1.8 mg/dl in
patients with psoriasis vulgaris and 5.1±0.4 mg/dl in the control
group, with statistically insignificant variations between the two
groups. There was an important difference between the activity of
ADA in patients with psoriasis vulgaris and controls (0.29±0.12
UI/mg protein vs. 0.14±0.08 UI/mg protein, P=0.052). The activity
of XO was also significantly increased in psoriasis patients versus
controls (0.42±0.21 UI/mg protein vs. 0.22±0.11 UI/mg protein,
P=0.011). The serum level of 8-OHdG in patients with psoriasis
vulgaris was significantly higher compared to controls (8.3±4.7
ng/ml vs. 3.1±0.05 ng/ml, P=0.002) (Table II).
 | Table II.Serum determinations in psoriasis
patients and controls. |
Table II.
Serum determinations in psoriasis
patients and controls.
| Items | Psoriasis
vulgaris | Control | P-value |
|---|
| Glucose
(mg/dl) | 88.2±5.3 | 81.4±5.1 | 0.654 |
| ASAT (U/l) | 18.4±3.5 | 21.8±6.3 | 0.341 |
| ALAT (U/l) | 16.0±7.2 | 17.3±4.3 | 0.549 |
| Cholesterol
(mg/dl) | 155.3±11.5 | 146.3±12.6 | 0.388 |
| Tryglicerides
(mg/dl) | 82.5±5.3 | 76.9±10.4 | 0.426 |
| Urea (mg/dl) | 39.3±4.5 | 30.4±5.3 | 0.088 |
| CRP (mg/dl) | 0.37±0.24 | 0.19±0.19 | 0.072 |
| Creatinine
(mg/dl) | 0.88±0.12 | 0.73±0.07 | 0.068 |
| UA (mg/dl) | 5.4±1,8 | 5.1±0.4 | 0.066 |
| ADA (U/mg
protein) | 0.29±0.12 | 0.14±0.08 | 0.052 |
| XO (U/mg
protein) | 0.42±0.21 | 0.22±0.11 | 0.011 |
| 8-OHdG (ng/ml) | 8.3±4.7 | 3.1±0.05 | 0.002 |
Urinary determinations
Since the creatinine excretion is a relatively
constant variable, the determination of urinary creatinine may be
valuable in estimating the renal function. Therefore, the urinary
creatinine concentration can be used as a reference parameter for
albuminuria and UA. Statistically significant variations were
observed for ACR and UACR between patients with psoriasis vulgaris
and controls (Table III).
 | Table III.Urinary determinations in psoriasis
patients and controls. |
Table III.
Urinary determinations in psoriasis
patients and controls.
| Items | Psoriasis
vulgaris | Control | P-value |
|---|
| eGFR (ml/min/1.73
mp) | 95.6±7.4 | 102.1±5.6 | 0.127 |
| ACR (mg/g) | 19.3±11.8 | 7.8±5.2 | 0.050 |
| UACR (mg/mg) | 0.39±0.17 | 0.27±0.11 | 0.048 |
Correlation of serum levels of
related-purine derivatives with the severity of psoriasis
Compared to the controls, UA, ADA, XO and 8-OHdG
levels were significantly higher in patients with psoriasis with
PASI >12 (P<0.05) (Table
IV).
 | Table IV.Serum related-purine derivatives
levels in psoriasis patients and controls. |
Table IV.
Serum related-purine derivatives
levels in psoriasis patients and controls.
|
|
| Psoriasis vulgaris
PASI score |
|---|
|
|
|
|
|---|
| Items | Control | <7 | 7–12 | >12 |
|---|
| ADA (UI/mg
protein) | 0.14±0.08 | 0.20±0.04 | 0.24±0.06 | 0.38±0.11 |
| XO (UI/mg
protein) | 0.22±0.11 | 0.29±0.07 | 0.39±0.12 | 0.53±0.23 |
| UA (mg/dl) | 5.1±0.4 | 3.7±0.6 | 5.1±1.1 | 5.7±1.9 |
| 8-OHdG (ng/ml) | 3.1±0.5 | 5.2±2.1 | 7.9±2.2 | 12.4±7.3 |
Positive correlations between ADA, XO, UA and PASI
>12 (r=0.498, P=0.004; r=0.601, P<0.001 and r=0.421, P=0.017)
were obtained. There was a strong positive correlation between the
serum levels of 8-OHdG and PASI (r=0.406, P=0.008 for PASI ranging
from 7 to 12, r=0.782, P=0.000 for PASI >12) (Table V).
 | Table V.Statistical correlations between
serum related-purine derivatives and PASI score. |
Table V.
Statistical correlations between
serum related-purine derivatives and PASI score.
|
| <7 | 7–12 | >12 |
|---|
|
|
|
|
|
|---|
| Items | r | p | r | p | r | p |
|---|
| ADA | 0.094 | 0.353 | 0.137 | 0.062 | 0.498 | 0.004 |
| XO | 0.103 | 0.281 | 0.095 | 0.413 | 0.601 | <0.001 |
| UA | 0.087 | 0.988 | 0.105 | 0.243 | 0.421 | 0.017 |
| 8-OHdG | 0.122 | 0.078 | 0.406 | 0.008 | 0.782 | <0.001 |
Correlations between serum
related-purine derivatives and markers of renal impairment
Serum markers of related-purine derivatives in
patients with psoriasis were associated with markers of renal
impairment. Positive correlations between 8-OHdG and ACR (r=0.452,
P=0.028), between ADA, XO, UA, 8-OHdG (r=0.297 and P=0.032; r=0.031
and P=0.002; r=0.431 and P=0.027; r=0.508 and P<0.001) and UACR.
Negative correlations between UA, 8-OHdG and eGFR (r=−0.301 and
P=0.036; r=−0.384 and P=0.002) were registered (Table VI).
 | Table VI.Statistical correlations between
serum related-purine derivatives and markers of renal impairment in
patients with psoriasis. |
Table VI.
Statistical correlations between
serum related-purine derivatives and markers of renal impairment in
patients with psoriasis.
|
| ACR | UACR | eGFR |
|---|
|
|
|
|
|
|---|
| Items | r | p | r | p | r | p |
|---|
| ADA | 0.111 | 0.564 | 0.297 | 0.032 | −0.088 | 0.512 |
| XO | 0.076 | 0.102 | 0.301 | 0.002 | −0.302 | 0.121 |
| UA | 0.341 | 0.098 | 0.431 | 0.027 | −0.301 | 0.036 |
| 8-OHdG | 0.452 | 0.028 | 0.508 | <0.001 | −0.384 | 0.002 |
Discussion
In the human body, the progressive increase in the
level of uremic toxins exerts a negative impact on all organs,
tissues and systems, causing acute and chronic renal dysfunctions,
cardiovascular, respiratory and hepatic diseases, atherosclerosis,
fibrosis or metabolic alterations (22–25). The
presence of UA derivatives is associated with the induction of
specific dysfunctions and the normalization of UA levels results in
the resolution of the clinical manifestations (26,27,44–46). In
terms of chemical structure, uremic toxins are purine, pyrimidine,
methylamine, phenyl or indole derivatives, guanidine, polyols,
ribonucleosides, peptides, cytokines, advanced glycation end
products, advanced lipoxidation end products and reactive carbonyl
compounds (26,27,46).
The role played by purine degradation in the
pathogenesis of psoriasis is an exciting research topic. The
results of the present study on the status of related-purine
derivatives in patients with psoriasis indicate changes of the
serum level of UA and 8-OHdG and alteration of the enzymatic
activities of adenosine deaminase and xanthine oxidase depending on
the clinical severity of psoriasis and the stage of renal chronic
disease. These results draw attention to the cumulative toxic
effect of purine derivatives on the kidney.
In the present study, we obtained slightly increased
levels of UA, without statistical significance, in patients with
psoriasis versus controls. A significant variation in serum UA
levels correlated with the severity of psoriasis. In patients with
severe psoriasis, with a PASI score higher than 12, a significantly
increased level of UA was obtained compared for both controls and
the group of patients with mild or moderate psoriasis. This was
also supported by the positive relationship established between
serum UA levels and PASI score and between serum UA levels and the
results of the tests assessing the renal function in patients with
psoriasis.
Stimulation of epidermal proliferation and increased
DNA damage may be associated with hyperuricemia, and the normal UA
levels could be explained by the selection criteria of patients
[normal body mass index (BMI), lack of inflammation, balanced
nutritional status]. In addition, the results of this study show
that the risk of kidney disease is more evident in patients with
severe psoriasis. Our findings are consistent with several reports
that have emphasized harmful effects of UA on kidney, the target
organ of hyperuricemia (26–28). Data related to the correlation
between serum UA levels and the severity of psoriasis are
inconsistent. In some studies, elevated serum values were obtained
in patients with psoriasis versus controls (38), whereas other studies reported normal
UA levels in psoriasis (35,37). Positive correlations between UA
levels and the following parameters have been reported: PASI score,
cutaneous extension of psoriatic lesions, and BMI (35).
Elevated levels of serum UA promote endothelial
dysfunction and renal lesions by decreasing the availability of
nitrogen monoxide and inducing oxidative stress. UA-induced
endothelial damage could be caused by the reduction in
intracellular ATP due to the inactivation of aconitase-2 and enoyl
CoA-hydratase-1, decreased mitochondrial DNA/nuclear DNA ratio,
increased mitochondrial calcium, resulting in the alteration of
membrane potential and generation of reactive oxygen species
(47). Consequently, endothelial
dysfunction can be associated with systemic manifestations
(arteriosclerosis, insulin resistance) and renal damage (hypoxia,
inflammation, glomerulosclerosis, tubulointerstitial fibrosis)
(47,48).
In this study, we revealed that elevated UA levels
observed in patients with severe psoriasis were associated with
increased xanthine oxidase activity. The serum levels of xanthine
oxidase were negatively correlated with eGFR and UACR in urine. The
relationship between xanthine oxidase and renal impairment can be
explained by the activation of renin-angiotensin system,
preglomerular arteriolopathy, induction of oxidative stress and
inflammation, and endothelial dysfunctions (49). The role of xanthine oxidase in the
terminal differentiation of keratinocytes may be explained by
localization of the enzyme in the granular layer of the epidermis
(50). The stimulation of the
inflammatory process in human keratinocytes by irradiation with UV
rays was correlated with the overexpression of xanthine oxidase and
increased production of superoxide (51).
Adenosine is another endogenous purine nucleoside,
possibly involved in the pathogenesis of psoriasis. It is thought
that adenosine may exert anti-inflammatory and immunomodulatory
effects through specific receptors expressed on endothelial cells,
leukocytes, mast cells, macrophages, dendritic cells and
consequently may limit the extension of psoriatic lesions.
Anti-inflammatory effects of adenosine may be achieved by
increasing intracellular cAMP levels, modulation of apoptosis,
reduction in cytokine synthesis, leucocyte recruitment and immune
function regulation (52). The
catabolism and bioavailability of adenosine may be modulated by
adenosine deaminase (53). In our
study, higher serum adenosine deaminase levels were obtained in
patients with psoriasis compared to the controls. In addition, the
activity of adenosine deaminase was associated with the severity of
the disease. In patients with mild or moderate psoriasis, an
enhanced adenosine deaminase activity was observed, but no
correlation with PASI score was revealed. In patients with severe
psoriasis significantly elevated levels of adenosine deaminase were
determined compared to those with mild and moderate psoriasis and
control group. There was also a strong positive correlation between
adenosine deaminase activity and PASI score in patients with severe
psoriasis. These findings support the hypothesis that adenosine
deaminase could be validated by further studies as a useful
indicator in monitoring patients with psoriasis, assessing the
response to therapy and predicting the prognosis of the disease
(52,54,55). In
previous studies on patients with psoriasis, adenosine deaminase
activity was found to decline after treatment with PUVA,
cyclosporine, etanercept, and psoralen, reconfirming the ability of
this enzyme to be associated with T-cell activation (55,56). The
increase in adenosine deaminase activity could become a predictive
factor for identifying patients with psoriasis at risk of
developing relapses prior to the occurrence of clinical
manifestations.
An accelerated purine catabolism stimulates the
production of free oxygen radicals. The accumulation of reactive
oxygen species is associated with changes of DNA structure
(oxidation, methylation, single and double strand breaks,
cross-links to protein, deletions or translocations) (57,58). In
our study, a significant increase in serum levels of 8-OHdG was
found in patients with psoriasis versus the controls. The strong
correlation between serum concentration of 8-OHdG and PASI score in
patients with severe psoriasis and the correlation between 8-OHdG
levels and markers of renal impairment suggest that 8-OHdG could
favour the onset and/or development of renal disease in patients
with psoriasis. In medical literature, there are limited data on
the role of 8-OHdG in progressive renal fibrosis (59,60),
hypertension associated with proteinuria (61), chronic renal failure (62), diabetes associated with proteinuria
(63,64), bladder cancer (65), renal cancer (66) and urothelial carcinoma (67).
Psoriatic arthritis is an important condition
associated with psoriasis (68).
Regarding urate-lowering drugs, the study by Namazi suggested the
beneficial role of allopurinol in the treatment of psoriasis given
its ability to neutralize free radicals and inhibit both the
secretion of tumour necrosis factor alpha and expression of
intercellular adhesion molecule-1 (69). The study by Tsuruta et al
concluded that hyperuricemia may be considered an independent risk
factor for psoriatic arthritis (70). In addition, findings showed that
patients with psoriasis and psoriatic arthritis had an important
risk of gout (71).
Taken together, our data and those of the
aforementioned studies suggest that severe psoriasis is a risk
factor for the development of renal disease. In patients with
psoriasis, renal and urinary tract abnormalities have been
reported, such as IgA nephropathy, secondary renal amyloidosis,
proliferative membranous glomerulonephritis, proliferative
mesangial glomerulonephritis, focal proliferative
glomerulonephritis, nephrolithiasis and recurrent urinary tract
infections (32,72). The relationship between psoriasis and
renal disease can be explained by three main mechanisms:
Immune-mediated renal damage, drug-related renal damage, and
chronic-renal damage (33).
In summary, psoriasis vulgaris can be regarded as a
cascade of events that starts from inflammation, oxidative stress
and a series of comorbidities. Our study indicates that renal
impairment is a frequent condition in patients with psoriasis
vulgaris. The related-purine derivatives may be specific
serological markers for identifying those patients with psoriasis
vulgaris at a high risk of developing renal dysfunctions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
IN, MT, CM and SRG conceived the study, identified
and reviewed the literature. CIM, MIM and MIS collected the data.
CDE and CE analyzed and interpreted the data. All authors equally
contributed to writing the manuscript, and CDE and CE edited and
revised the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Victor Babes’ Clinical Hospital for Infectious Diseases
(Bucharest, Romania). All the participants gave their consent to
the use of their biological samples in research studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davidovici BB, Sattar N, Prinz J, Puig L,
Emery P, Barker JN, van de Kerkhof P, Ståhle M, Nestle FO,
Girolomoni G and Krueger JG: Psoriasis and systemic inflammatory
diseases: Potential mechanistic links between skin disease and
co-morbid conditions. J Invest Dermatol. 130:1785–1796. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kölliker Frers RA, Bisoendial RJ, Montoya
SF, Kerzkerg E, Castilla R, Tak PP, Milei J and Capani F: Psoriasis
and cardiovascular risk: Immune-mediated crosstalk between
metabolic, vascular and autoimmune inflammation. IJC Metab Endocr.
6:43–54. 2015. View Article : Google Scholar
|
|
3
|
Sârbu MI, Georgescu SR, Tampa M, Sârbu AE
and Simionescu O: Biological therapies in psoriasis - revisited.
Rom J Intern Med. 56:75–84. 2018.PubMed/NCBI
|
|
4
|
Murari K, Ray AS and Lodha RS: Adenosine
deaminase: A potential biomarker for evaluating the severity of
psoriasis. Int J Pharma Bio Sci. 6:629–634. 2015.
|
|
5
|
Shree UGB, Vishal B, Shindhu M, Shenoy MM
and Shenoy C: Advanced oxidation protein product in psoriasis and
its correlation with disease severity. Int J Sci Stud. 2:156–159.
2015.
|
|
6
|
Basavaraj KH, Vasu Devaraju P and Rao KS:
Studies on serum 8-hydroxy guanosine (8-OHdG) as reliable biomarker
for psoriasis. J Eur Acad Dermatol Venereol. 27:655–657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nwabudike LC and Tatu AL: Reply to Happle
R. And al. Koebners sheep in Wolf's clothing: Does the isotopic
response exist as a distinct phenomenon? J Eur Acad Dermatol
Venereol. 32:336–337. 2018.
|
|
8
|
Tampa M, Sarbu MI, Mitran MI, Mitran CI,
Matei C and Georgescu SR: The pathophysiological mechanisms and the
quest for biomarkers in psoriasis, a stress-related skin disease.
Dis Markers. 2018:58236842018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55:1191–1196. 2014.PubMed/NCBI
|
|
10
|
Batani A, Brănișteanu DE, Ilie MA, Boda D,
Ianosi S, Ianosi G and Caruntu C: Assessment of dermal papillary
and microvascular parameters in psoriasis vulgaris using in vivo
reflectance confocal microscopy. Exp Ther Med. 15:1241–1246.
2018.PubMed/NCBI
|
|
11
|
Negrei C, Arsene AL, Toderescu CD, Boda D
and Ilie M: Acitretin treatment in psoriasis may influence the cell
membrane fluidity. Farmacia. 60:767–772. 2012.
|
|
12
|
Nicolae I, Ene CD, Schipor S, Tampa M,
Matei C and Georgescu SR: Dopamine-chemical mediator in atopic
dermatitis. Rev Chim. 64:1201–1206. 2013.
|
|
13
|
Negrei C, Ginghină O, Căruntu C, Burcea
Dragomiroiu GT, Jinescu GE and Boda DA: Investigation relevance of
methotrexate polyglutamates in biological systems by high
performance liquid chromatography. Rev Chim. 66:766–768. 2015.
|
|
14
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015. View Article : Google Scholar
|
|
15
|
Gisondi P, Galvan A, Idolazzi L and
Girolomoni G: Management of moderate to severe psoriasis in
patients with metabolic comorbidities. Front Med (Lausanne).
2:12015.PubMed/NCBI
|
|
16
|
Sarbu MI, Tampa M, Matei C, Mitran CI,
Mitran MI, Pituru S, Pop CS, Saramet G and Georgescu SR: Infliximab
biosimilar versus methotrexate for the treatment of moderate to
severe psoriasis. Farmacia. 65:962–967. 2017.
|
|
17
|
Wan J, Wang S, Haynes K, Denburg MR, Shin
DB and Gelfand JM: Risk of moderate to advanced kidney disease in
patients with psoriasis: Population based cohort study. BMJ. Oct
15–2013.(Epub ahead of print). doi:
https://doi.org/10.1136/bmj.f5961. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chi CC, Wang J, Chen YF, Wang SH, Chen FL
and Tung TH: Risk of incident chronic kidney disease and end-stage
renal disease in patients with psoriasis: A nationwide
population-based cohort study. J Dermatol Sci. 78:232–238. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Negrei C, Caruntu C, Ginghina O,
Dragomiroiu GT, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim.
66:607–610. 2015.
|
|
20
|
Sârbu MI, Tampa M, Mitran MI, Mitran CI,
Limbău AM and Georgescu SR: Adverse reactions of biological
therapies in patients with psoriasis. J Mind Med Sci. 4:4–12. 2017.
View Article : Google Scholar
|
|
21
|
Lisowska-Myjak B: Serum and urinary
biomarkers of acute kidney injury. Blood Purif. 29:357–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu HJ, Yen CH, Wu IW, Hsu KH, Chen CK,
Sun CY, Chou CC, Chen CY, Tsai CJ, Wu MS, et al: The association of
uremic toxins and inflammation in hemodialysis patients. PLoS One.
9:e1026912014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu G, Tu W and Qin S: The relationship
between deiodinase activity and inflammatory responses under the
stimulation of uremic toxins. J Transl Med. 12:2392014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Duranton F, Cohen G, De Smet R, Rodriguez
M, Jankowski J, Vanholder R and Argiles A: European Uremic Toxin
Work Group: Normal and pathologic concentrations of uremic toxins.
J Am Soc Nephrol. 23:1258–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boueiz A, Damarla M and Hassoun PM:
Xanthine oxidoreductase in respiratory and cardiovascular
disorders. Am J Physiol Lung Cell Mol Physiol. 294:830–840. 2008.
View Article : Google Scholar
|
|
26
|
Lisowska-Myjak B and Skarżyńska E: Role of
uremic compounds in organ injury. J Nephrol Ther.
5:10002052015.
|
|
27
|
Lisowska-Myjak B: Uremic toxins and their
effects on multiple organ systems. Nephron Clin Pract. 128:303–311.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia JF, Liang QL, Hu P, Wang YM, Li P and
Luo GA: Correlations of six related purine metabolites and diabetic
nephropathy in Chinese type 2 diabetic patients. Clin Biochem.
42:215–220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia J, Wang Z and Zhang F: Association
between related purine metabolites and diabetic retinopathy in type
2 diabetic patients. Int J Endocrinol. Feb 13–2014.(Epub ahead of
print). doi: 10.1155/2014/651050. View Article : Google Scholar
|
|
30
|
Avci E, Cakir E, Cevher SC, Yaman H,
Agilli M and Bilgi C: Determination of oxidative stress and
cellular inflammation in patients with diabetic nephropathy and
non-diabetic nephropathy being administered hemodialysis treatment
due to chronic renal failure. Ren Fail. 36:767–773. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishikawa T, Aw W and Kaneko K: Metabolic
interactions of purine derivatives with human ABC transporter
ABCG2: Genetic testing to assess gout risk. Pharmaceuticals
(Basel). 6:1347–1360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dervisoglu E, Akturk AS, Yildiz K, Kiran R
and Yilmaz A: The spectrum of renal abnormalities in patients with
psoriasis. Int Urol Nephrol. 44:509–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Visconti L, Leonardi G, Buemi M, Santoro
D, Cernaro V, Ricciardi CA, Lacquaniti A and Coppolino G: Kidney
disease and psoriasis: Novel evidences beyond old concepts. Clin
Rheumatol. 35:297–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boda D, Negrei C, Nicolescu F and Bălălău
CR: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
35
|
Kwon HH, Kwon IH, Choi JW and Youn JI:
Cross-sectional study on the correlation of serum UA with disease
severity in Korean patients with psoriasis. Clin Exp Dermatol.
36:473–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Christophers E and Mrowietz U: Psoriasis.
Fitzpatrick's dermatology in general medicine. Freedberg IM, Eisen
AS, Wolff K, Austen KF, Goldsmith LA and Katz SI: 1. 6th.
McGraw-Hill; New York, NY: pp. 407–427. 2003
|
|
37
|
Ataseven A, Kesli R, Kurtipek GS and
Ozturk P: Assessment of lipocalin 2, clusterin, soluble tumor
necrosis factor receptor-1, interleukin-6, homocysteine, and UA
levels in patients with psoriasis. Dis Markers. 2014.5417092014.
doi: 10.1155/2014/541709. PubMed/NCBI
|
|
38
|
Gisondi P, Targher G, Cagalli A and
Girolomoni G: Hyperuricemia in patients with chronic plaque
psoriasis. J Am Acad Dermatol. 70:127–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ciragil P, Kurutas EB and Miraloglu M: New
markers: Urine xanthine oxidase and myeloperoxidase in the early
detection of urinary tract infection. Dis Markers. 2014.2693622014.
doi: org/10.1155/2014/269362. PubMed/NCBI
|
|
40
|
Vorbach C, Harrison R and Capecchi MR:
Xanthine oxidoreductase is central to the evolution and function of
the innate immune system. Trends Immunol. 24:512–517. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tampa M, Nicolae I, Ene CD, Sarbu I, Matei
C and Georgescu SR: Vitamin C and thiobarbitUA reactive substances
(Tbars) in psoriasis vulgaris related to psoriasis area severity
index (Pasi). Rev Chim. 68:43–47. 2017.
|
|
42
|
Matei C, Tampa M, Caruntu C, Ion RM,
Georgescu SR, Dumitrascu GR, Constantin C and Neagu M: Protein
microarray for complex apoptosis monitoring of dysplastic oral
keratinocytes in experimental photodynamic therapy. Biol Res.
47:332014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dinu LU, Ene CD, Nicolae IL, Tampa M,
Matei CL and Georgescu SR: The serum levels of
8-hidroxy-deoxyguanosine under the chemicals influence. Rev Chim.
65:1319–1326. 2014.
|
|
44
|
Barreto FC, Stinghen AE, de Oliveira RB,
Franco AT, Moreno AN, Barreto DV, Pecoits-Filho R, Drüeke TB and
Massy ZA: The quest for a better understanding of chronic kidney
disease complications: An update on uremic toxins. J Bras Nefrol.
36:221–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Glorieux G and Tattersall J: Uraemic
toxins and new methods to control their accumulation: Game changers
for the concept of dialysis adequacy. Clin Kidney J. 8:353–362.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Semchyshyn HM: Reactive carbonyl species
in vivo. Generation and dual biological effects.
ScientificWorldJournal 2014. 4178422014.doi:
10.1155/2014/417842.
|
|
47
|
Sánchez-Lozada LG, Lanaspa MA,
Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D,
Nakagawa T, Yu MA, Kang DH and Johnson RJ: UA-induced endothelial
dysfunction is associated with mitochondrial alterations and
decreased intracellular ATP concentrations. Nephron, Exp Nephrol.
121:71–78. 2012. View Article : Google Scholar
|
|
48
|
Davidovici BB, Sattar N, Prinz J, Puig L,
Emery P, Barker JN, van de Kerkhof P, Ståhle M, Nestle FO,
Girolomoni G, et al: Psoriasis and systemic inflammatory diseases:
Potential mechanistic links between skin disease and co-morbid
conditions. J Invest Dermatol. 130:1785–1796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim IY, Lee DW, Lee SB and Kwak IS: The
role of UA in kidney fibrosis: Experimental evidences for the
causal relationship. BioMed Res Int. 2014.6387322014. doi:
10.1155/2014/638732. PubMed/NCBI
|
|
50
|
Reiners JJ Jr and Rupp T: Conversion of
xanthine dehydrogenase to xanthine oxidase occurs during
keratinocyte differentiation: Modulation by
12-O-tetradecanoylphorbol-13-acetate. J Invest Dermatol.
93:132–135. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deliconstantinos G, Villiotou V and
Stavrides JC: Alterations of nitric oxide synthase and xanthine
oxidase activities of human keratinocytes by ultraviolet B
radiation. Potential role for peroxynitrite in skin inflammation.
Biochem Pharmacol. 51:1727–1738. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Festugato M: Adenosine: An endogenous
mediator in the pathogenesis of psoriasis. An Bras Dermatol.
90:862–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Maiuolo J, Oppedisano F, Gratteri S,
Muscoli C and Mollace V: Regulation of UA metabolism and excretion.
Int J Cardiol. 213:8–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Coimbra S and Santos-Silva A: Biomarkers
of psoriasis severity and therapy monitoring. World J Dermatol.
3:15–27. 2014. View Article : Google Scholar
|
|
55
|
Yıldırım FE, Karaduman A, Pinar A and
Aksoy Y: CD26/ dipeptidyl-peptidase IV and adenosine deaminase
serum levels in psoriatic patients treated with cyclosporine,
etanercept, and psoralen plus ultraviolet A phototherapy. Int J
Dermatol. 50:948–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bukulmez G, Akan T and Ciliv G: Serum
adenosine deaminase levels in patients with psoriasis: A
prospective case-control study. Eur J Dermatol. 10:274–276.
2000.PubMed/NCBI
|
|
57
|
Nicolae I, ENE CD, Georgescu SR, Tampa M,
Matei C and Ceausu E: Effects of UV radiation and oxidative DNA
adduct 8-hydroxy-2′-deoxiguanosine on the skin diseases. Rev Chim.
65:1036–1041. 2014.
|
|
58
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Remuzzi G and Bertani T: Pathophysiology
of progressive nephropathies. N Engl J Med. 339:1448–1456. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsai JP, Liou JH, Yeh KT, Tai HC, Cheng YW
and Chang HR: Intensity of cytosol expression of 8-OHdG in normal
renal tubules is associated with the severity of renal fibrosis.
Swiss Med Wkly. 141:w132682011.PubMed/NCBI
|
|
61
|
Dincer Y, Sekercioglu N, Pekpak M, Gunes
KN and Akcay T: Assessment of DNA oxidation and antioxidant
activity in hypertensive patients with chronic kidney disease. Ren
Fail. 30:1006–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Domenici FA, Vannucchi MT, Jordão AA Jr,
Meirelles MS and Vannucchi H: DNA oxidative damage in patients with
dialysis treatment. Ren Fail. 27:689–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shah SV, Baliga R, Rajapurkar M and
Fonseca VA: Oxidants in chronic kidney disease. J Am Soc Nephrol.
18:16–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY
and Lim SJ: Effects of sildenafil on oxidative and inflammatory
injuries of the kidney in streptozotocin-induced diabetic rats. Am
J Nephrol. 29:274–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Akçay T, Saygili I, Andican G and Yalçin
V: Increased formation of 8-hydroxy-2′-deoxyguanosine in peripheral
blood leukocytes in bladder cancer. Urol Int. 71:271–274. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Miyake H, Hara I, Kamidono S and Eto H:
Prognostic significance of oxidative DNA damage evaluated by
8-hydroxy-2′-deoxyguanosine in patients undergoing radical
nephrectomy for renal cell carcinoma. Urology. 64:1057–1061. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chang CH, Yang CM and Yang AH: Renal
diagnosis of chronic hemodialysis patients with urinary tract
transitional cell carcinoma in Taiwan. Cancer. 109:1487–1492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tatu AL and Nwabudike LC:
Metoprolol-associated onset of psoriatic arthropathy. Am J Ther.
24:e370–e371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Namazi MR: Cannabinoids, loratadine and
allopurinol as novel additions to the antipsoriatic ammunition. J
Eur Acad Dermatol Venereol. 19:319–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tsuruta N, Imafuku S and Narisawa Y:
Hyperuricemia is an independent risk factor for psoriatic arthritis
in psoriatic patients. J Dermatol. 44:1349–1352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Merola JF, Wu S, Han J, Choi HK and
Qureshi AA: Psoriasis, psoriatic arthritis and risk of gout in US
men and women. Ann Rheum Dis. 74:1495–1500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Singh NP, Prakash A, Kubba S, Ganguli A,
Singh AK, Sikdar S, Agarwal SK, Dinda AK and Grover C: Psoriatic
nephropathy - does an entity exist? Ren Fail. 27:123–127. 2005.
View Article : Google Scholar : PubMed/NCBI
|