Introduction
Polycystic ovary syndrome (PCOS) is mainly
characterized by polycystic changes and endocrine disorders in
multiple follicular atresia in the ovaries (1). PCOS patients are mainly women of
childbearing age and this disease seriously endangers women's
physical and mental health. More than 40% of patients with
anovulatory infertility have PCOS (2). PCOS can lead to hormonal abnormalities,
which are characterized by increase in serum androgens and insulin
(INS) and significant reduction in gonadotropins. These hormone
disorders can reduce menstrual blood volume or even lead to
menopause, hormone obesity, acne, infertility, INS resistant
diabetes (3).
Peroxisome proliferator-activated receptor γ (PPARγ)
activates gene transcription and regulates the expression of
downstream target genes. Its ligand is called peroxisome
proliferator. PPARγ activates peroxisomes after binding to
peroxisome proliferator and exerts a series of biological effects
(4,5). PPARγ exists in various tissues such as
fat, small intestine, and ovarian tissues, and plays an important
physiological role in the process of cell growth, production of
steroid hormones, remodeling of tissues, and metabolism of glucose
and lipids (6,7). PPARγ is downregulated by luteinizing
hormone (LH). In recent years, many studies have shown that
androgens may also regulate PPARγ expression. Activated PPARγ
regulates the secretion of sex hormones and regulate the
occurrence, growth, and excretion of follicles (8). Levels and proportions of sex hormones
in the body affect ovarian microenvironment, and a good ovarian
microenvironment is the basis for the development of high-quality
follicles and ovarian growth (9).
Aromatase enzymes in ovarian granulosa cells catalyze the formation
of androstenedione, testosterone, estradiol (E2) and estrone
(10). Keller et al (11) found significant upregulation of PPARγ
expression in prenatal androgenic (PA) female monkeys.
Rosiglitazone (RGZ) is an agonist of PPARγ, and its main efficacy
is to increase INS sensitivity and reduce INS resistance (12).
In this study, the mechanism of PCOS ovarian
function abnormality was explored by detecting the expression of
PPARγ mRNA in PCOS and the expression of PPARγ mRNA under different
concentrations of testosterone, INS and RGZ.
Materials and methods
Materials
Research subjects
Five patients with PCOS who were treated in
Affiliated Hospital of Jining Medical University (Jining, China)
from June 2016 to December 2017 and 30 normal controls with
non-PCOS who received conventional in vitro fertilization
embryo transfer were selected. The study was approved by the Ethics
Committee of Affiliated Hospital of Jining Medical University, and
each patient signed an informed consent.
Inclusion criteria
All PCOS patients met the revised PCOS diagnostic
criteria of the American Society of Reproductive Medicine meeting
in Rotterdam, The Netherlands, with normal fasting blood glucose
and normal spousal semen. PCOS diagnostic criteria: B-scan
ultrasonography shows no less than 12 small follicles with a volume
of 2–9 mm or large follicles with a volume greater than 10 ml in
unilateral or bilateral ovaries; level of serum testosterone is not
less than 2.8 nmol/l or androstenedione is not less than 5.3 nmol/l
on the 3rd day of menstruation, suggesting the presence of
hyperandrogenism; The number of ovulations are reduced or there is
non-ovulation. Patients who met two of these criteria were
diagnosed as PCOS patients. Females with normal menstrual regular
ovulation and normal endocrine function showing no abnormal changes
in the abdominal cavity were diagnosed as non-PCOS subjects.
Exclusion criteria
Patients with thyroid glands, adrenal glands and
other endocrine disorders, severe hypertension and diabetes,
abdominal cavity, pelvic tuberculosis, immune factors inducing
premature infertility and previous history of ovarian surgery were
excluded.
Methods
Reagents and materials
PBS buffer (Wuhan Procell life science &
Technology Co., Ltd., Wuhan, China); Percoll separation solution
(volume fraction 50%; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China); collagenase I (Shanghai Cosroma Biotech
Co., Ltd., Shanghai, China); M199 medium (10% FBS + 100 U/ml
penicillin + 100 µg/ml streptomycin; Wuhan Procell Life Science
& Technology Co., Ltd., Wuhan, China); testosterone (Shandong
XiYa Chemical Industry Co., Ltd., Shandong, China); INS (Beijing
Kuer Chemical Technology Co., Ltd., Beijing, China); RGZ
(SinoStandards, Chengdu, China). TRIzol reagent and PowerUp
SYBR™-Green Master Mix kit [Thermo Fisher Scientific (China) Inc.,
Beijing, China]; reproductive hormone detection kits (Beijing
Keruimei Technology Co., Ltd., Beijing, China). Primers used were
all synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
Collection of follicular fluid and
extraction of granular cells
Ovarian granulosa cells were collected from 5 cases
of PCOS patients (observation group) and 30 cases of normal people
(5 cases were used as control group). Puncture fluid of follicles
with a diameter of 18 mm or more was collected. Collected
follicular fluid should be clear and free from any menstrual blood
contamination. The remaining follicular fluid after collecting eggs
was centrifuged at 1,200 × g for 10 min at 4°C, and the supernatant
was discarded. The pellet was washed 3 times with 1:1 PBS buffer
and resuspended in PBS. An equal volume of Percoll cell separation
solution was added and centrifuged at 1,200 × g for 30 min at 4°C.
After centrifugation, the liquid was divided into 4 layers. From
top to bottom, the 4 layers were supernatant layer (light yellow),
granule cell layer (white circle flocculent), separation liquid
layer (colorless and transparent), and red blood cell layer,
respectively. Granulosa cell layer was aspirated into ammonium
chloride solution and vortexed at 37°C. The pellet was retained
after centrifugation. Equal volume of 1 g/l collagenase I was mixed
with the pellet, followed by incubation at 37°C for 10 min. After
filtering with a 300 mesh sieve, the mixture was centrifuged at
1,080 × g for 8 min at room temperature and the supernatant was
discarded.
Detection of hormones in observation
group and control group
According to ABC ellesa method, 3 ml of fasting
venous blood was collected from each participant in observation
group and control group in strict accordance with the instructions
of the kit.
Culture and treatment of granular
cells
A total of 25 cases of human ovarian granulosa cells
in the control group were cultured. After washing with PBS 3 times,
cells were resuspended in M199 medium and were inoculated into
6-well plates with 10–20 cells in 1 ml medium for each well. Cells
were cultured in an incubator (37°C, 5% CO2). Different
concentration of testosterone, INS and RGZ treatment solution was
prepared with LM199, and the concentrations of each group were T1
group, 10−3 mol/ml and T2 group, 10−2 mol/ml
(13); INS1 group, 10−5
mol/ml and INS2 group, 10−4 mol/ml; RGZ1 group,
10−3 mol/ml and RGZ2 group, 10−4 mol/ml
(14). After discarding the old
culture solution, 1 ml of different treatment solution was added
into each well, and 1 ml of LM199 medium was used as blank control.
This experiment was performed in triplicate.
RT-qPCR detection of PPARγ mRNA
expression
After cell culture had continued for 24 h, cells
were washed with PBS three times, and 1 ml of TRIzol was added. The
mixture was allowed to stand for 3 min at room temperature and
mixed by pipetting. cDNA was obtained by reverse transcription
using a reverse transcription kit (Beyotime Institute of
Biotechnology, Shanghai, China) in a 20 µl reaction system
according to the following conditions: 37°C for 45 min and 95°C for
5 min. PCR reactions conditions were: 94°C for 5 min, followed by
35 cycles of 56°C for 45 sec and 72°C for 1 min, and then 72°C for
10 min. GAPDH was used as an endogenous control and the primer
sequences are 5′-AGAGATGCCATTCTGGCC-3′ (forward) and
5′-GTGGAGTAGAAATGCTGGAGA-3′ (reverse). Upstream and downstream
primers for PPARγ are: 5′-CAGGAGCAGAGCAAAGA-3′ and
5′-TGGTGAAGACGCCAGTGGA-3′, respectively. 2−ΔCq method
was used to statistically process RT-qPCR results (15).
Statistical analysis
SPSS19.0 [AsiaAnalytics (formerly SPSS China),
Shanghai, China] statistical package was used for all statistical
analysis. Measured data were expressed as mean ± standard
deviation. Comparisons between two groups based on the distribution
characteristics were performed using two independent samples
t-test, and comparisons among multiple groups were performed using
ANOVA analysis and LSD test, as a post hoc test. Enumeration data
were processed using χ2 test. The test significance level is
α=0.05.
Results
Basic clinical data of PCOS patients
and controls
There was no significant difference in age, sex,
BMI, infertility time and menstrual cycle between 5 PCOS patients
and 30 normal controls (P>0.05). Regarding basal endocrinology,
levels of LH and testosterone in patients with PCOS were
significantly higher than those in the healthy subjects
(P<0.01), but there were no significant differences in other
three endocrine hormones (P>0.05) (Table I).
 | Table I.Basic clinical data of PCOS patients
and controls. |
Table I.
Basic clinical data of PCOS patients
and controls.
| Variables | PCOS patients
(n=5) | Normal people
(n=30) | T/χ2 | P-value |
|---|
| Age (years) | 28.97±2.76 | 29.73±3.62 | 0.54 | 0.61 |
| Menstrual
cycle (cases) |
|
| 0.867 | 0.33 |
| Menstrual
period (n, %) | 1 (20.00) | 6 (20.00) |
|
|
|
Follicular phase (n, %) | 2 (40.00) | 10 (33.33) |
|
|
| Luteal phase (n,
%) | 2 (40.00) | 14 (46.67) |
|
|
| BMI
(kg/m2) | 23.75±2.14 | 22.87±1.97 | 0.86 | 0.43 |
| Infertility time
(years) | 5.24±1.97 | 4.37±2.56 | 0.87 | 0.41 |
| FSH (pg/ml) | 5.76±2.11 | 5.37±1.87 | 0.39 | 0.71 |
| LH (pg/ml) | 11.76±2.66 | 5.62±1.47 | 5.04 | <0.001 |
| T (ng/ml) | 1.01±0.36 | 0.32±0.18 | 4.20 | 0.01 |
| E2 (pg/ml) | 30.82±4.82 | 31.77±4.49 | 0.42 | 0.70 |
| PRL (ng/ml) | 17.79±6.10 | 19.11±5.95 | 0.45 | 0.67 |
Expression of PPARγ mRNA in the
observation and control groups
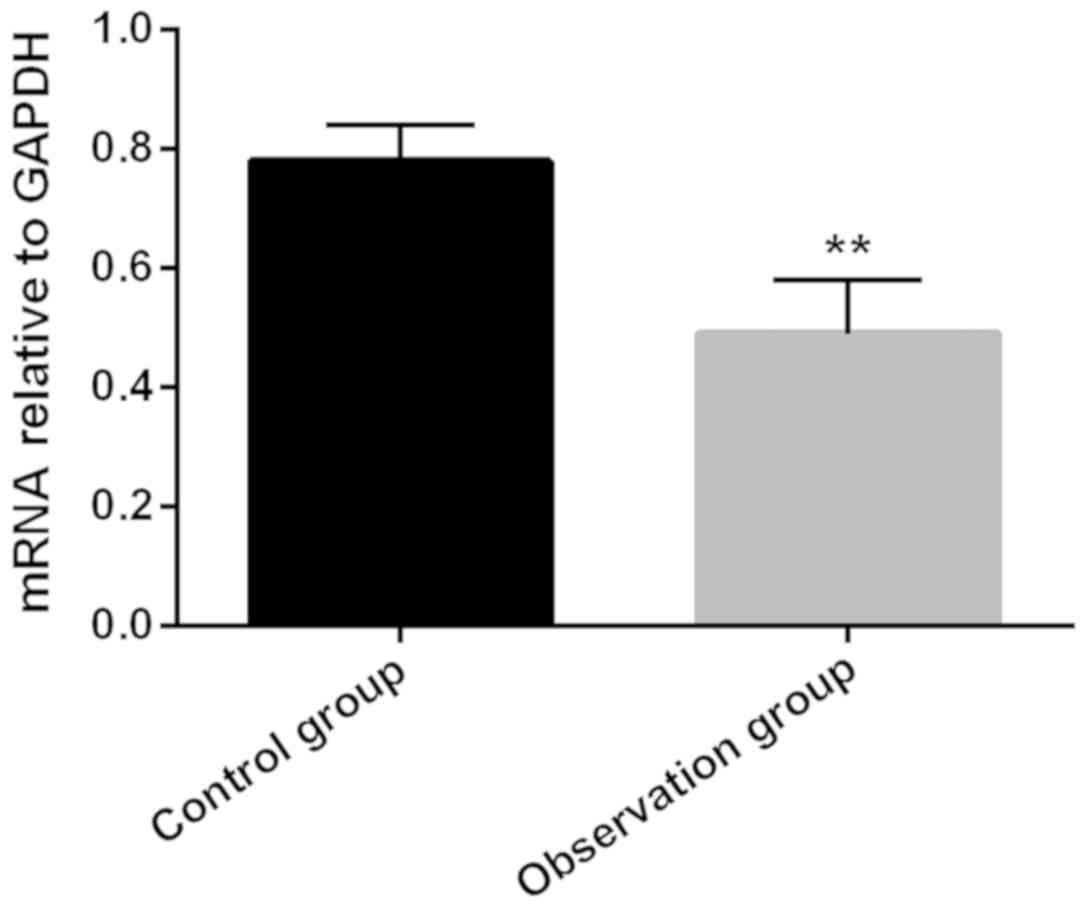
Relative expression level of PPARγ mRNA in ovarian
granulosa cells in the observation group was 0.49±0.09, which was
significantly lower than that in the control group (0.78±0.06;
P<0.05) (Fig. 1).
Expression of PPARγ mRNA in granular
cells under the treatment of different concentrations of
testosterone
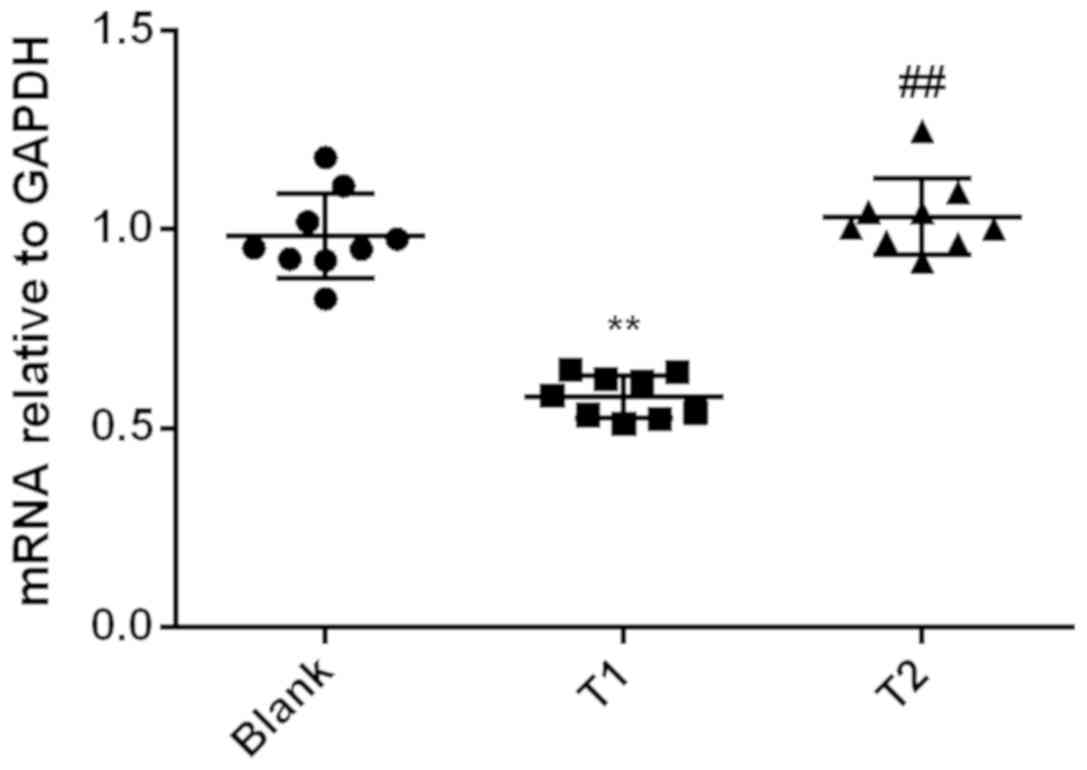
Relative expression level of PPARγ mRNA in group T1
(0.58±0.01) was significantly lower than that in the control group
(0.98±0.04) and T2 (1.03±0.03; P<0.01). However, there was no
significant difference between T2 group and the control group
(P>0.05) (Fig. 2).
Expression of PPARγ mRNA in granular
cells under the treatment of different concentrations of INS
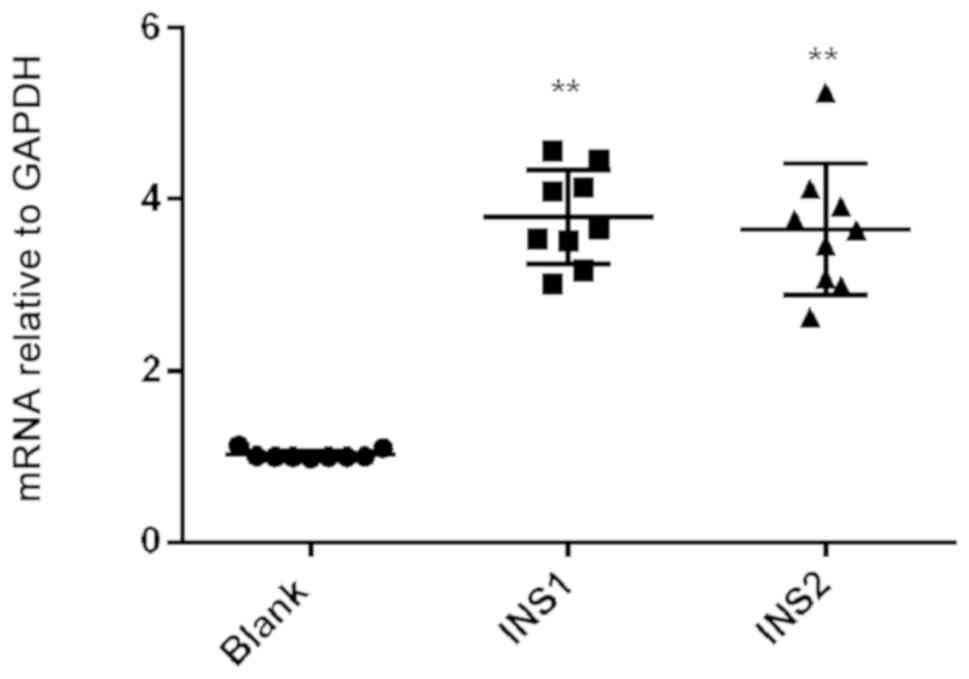
Relative expression of PPARγ mRNA in INS1 group
(3.79±0.19) and INS2 group (3.65±0.26) was significantly higher
than that in the control group (1.02±0.02; P<0.01). However,
there were no significant differences in PPARγ mRNA expression
level between the INS1 group and INS2 group (P>0.05) (Fig. 3).
Expression of PPARγ mRNA in granular
cells under the treatment of different concentrations of RGZ
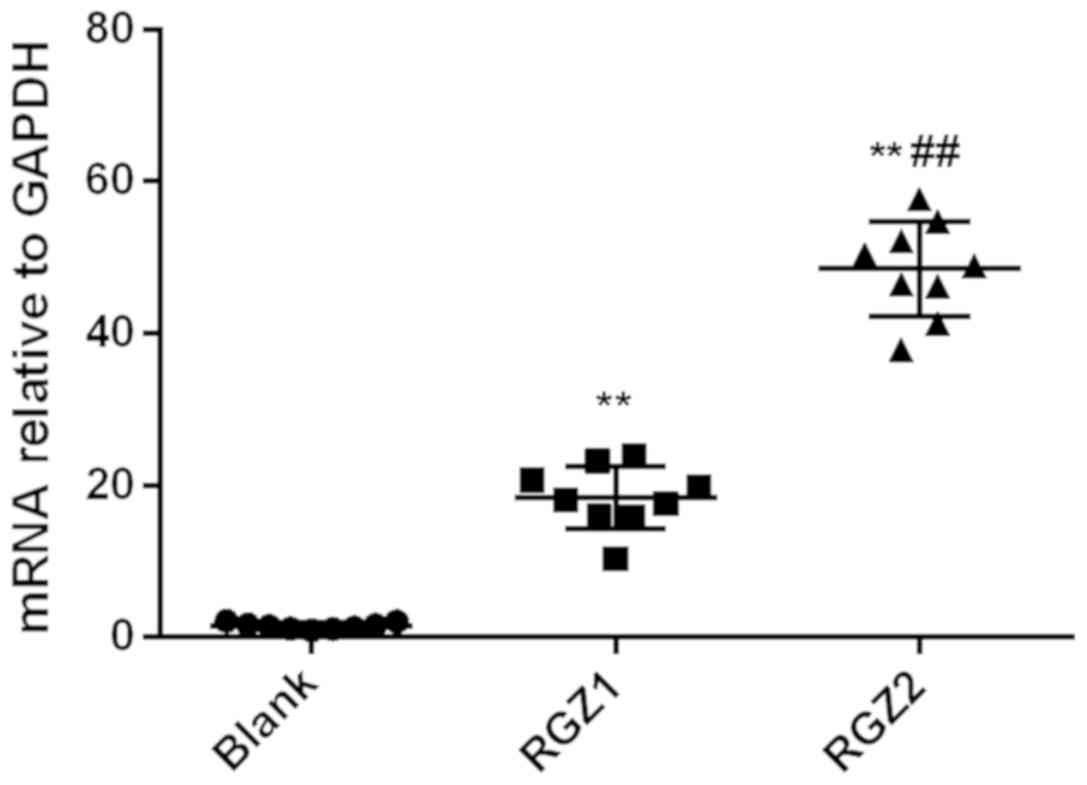
Relative expression of PPARγ mRNA in the RGZ1 group
(18.40±1.39) and RGZ2 group (45.47±2.09) was significantly higher
than that in the control group (1.52±0.15; P<0.01). Relative
expression of PARγ mRNA in the RGZ2 group was significantly higher
than that in the RGZ1 group (P<0.01) (Fig. 4).
Discussion
PCOS as one of the most common reproductive system
diseases among women during childbearing age can easily lead to
anovulatory infertility (16). PCOS
is mainly manifested as INS resistance and hyperandrogenism.
Malignant elevation of androgens inhibits the process of follicle
predominance, resulting in sparse or no-ovulation and less
menstruation or amenorrhea (17).
Peroxidase is one of the major factors in regulating sex hormones
and regulates the production, development, and excretion of
follicles (18). In this study, we
examined the expression of PPARγ mRNA in PCOS and the expression of
PPARγ mRNA under the treatment of different concentrations of
testosterone, INS, and RGZ to explore the possible mechanism of
PCOS ovarian dysfunction.
Levels of LH and testosterone in patients with PCOS
were significantly higher than those in 30 normal subjects
(P<0.01), and there was no significant difference in levels of
FSH, E2, and PRL (P>0.05). Compared with normal people, PCOS
patients have high levels of testosterone, suggesting the existing
of high level androgen in serum of PCOS patients. Multiple
follicular atresia in the ovary leads to polycystic changes in the
ovary. Excessive androgens beyond the normal range convert to
estrone, which in turn negatively affects the
hypothalamus-pituitary-gonadal axis and reduces pituitary
gonadotropism. This effect can reduce the threshold of pituitary
response to gonadotropin-releasing hormone, promote the secretion
of LH, promote the production of more androgens, and the excess
androgen will aggravate the ovulation disorder and a vicious circle
is formed (19,20). Since PPARγ is downregulated by LH
(21), high levels of LH may suggest
downregulated PPARγ mRNA expression in PCOS patients. Therefore, we
detected the expression of PPARγ mRNA in the observation group and
the control group and found that relative expression level of PPARγ
mRNA in the observation group was significantly lower than that in
the control group (P<0.01). Therefore, there may be a certain
regulatory relationship between testosterone and PPARγ mRNA
expression and PCOS. Subsequently we examined the expression of
PPARγ mRNA under different concentrations of testosterone, INS, and
RGZ. Our study found that the relative expression level of PPARγ
mRNA in T1 group was significantly lower than that in the control
group and T2 group (P<0.01); however, there was no significant
difference between T2 group and the control group (P>0.05),
indicating that a certain concentration of testosterone can inhibit
PPARγ mRNA expression, as expected, excess testosterone may
downregulate PPARγ by stimulating LH secretion. However, when the
concentration is too high, this inhibitory effect is abolished.
This may be because excessively high testosterone activates the
negative feedback regulation mechanism of the
hypothalamic-pituitary-gonadal axis or reduces the expression of
gonadotropin by other means, so as to reduce the secretion of LH.
Expression level of PPARγ mRNA in group INS1 and INS2 was higher
than that in the control group (P<0.01). However, there was no
significant difference in expression level of PPARγ mRNA between
INS1 and INS2 (P>0.05), indicating that INS has the ability to
promote the expression of PPARγ. Two main characteristics of PCOS
patients are hyperinsulinemia and hyperandrogenism, and the
expression of PPARγ is downregulated, indicating that the
inhibitory effect of androgen in PCOS patients is greater than that
of INS. Relative expression level of PPARγ mRNA in RGZ1 group and
RGZ2 group was significantly higher than that in the control group
(P<0.01). Relative expression level of PPARγ mRNA in RGZ2 group
was significantly higher than that in RGZ1 group (P<0.01),
indicating that RGZ could induce PPARγ expression by acting as an
agonist of PPARγ. Münzker et al (22) also found that serum testosterone and
LH levels were significantly higher in PCOS patients than in normal
subjects. Kauffman et al (23) found that hormone levels were abnormal
in rat model of PCOS and LH and testoserone levels were
significantly elevated.
Our study has some shortcomings. Due to the limited
resorces, the small sample size may affect the results.
Hypothalamus-pituitary-gonadal axis has a complex regulatory
mechanism. This study found that testosterone can affect the
expression of PPARγ possibly by regulating LH. INS can act on PPARγ
to induce its expression. PPARγ itself can also affect the
secretion of hormones (5,24). Therefore, there may be a complex
system of feedback and negative feedback regulation between
testosterone, INS, and PPARγ, and the specific regulatory pathways
and signaling pathways remain to be further studied. Single-factor
control variable method was used to investigate the effects of
different concentrations of testosterone, INS, and RGZ on PPARγ
mRNA expression. However, this is not an applicable method. For
example, in patients with PCOS, there are high levels of androgens
and INS. Therefore, the combinations of different factors should
also be included.
In conclusion, proper concentrations of testosterone
can reduce the content of PPARγ mRNA in ovarian granulosa cells,
while PPARγ mRNA expression can be induced by a certain
concentration of RGZ and INS. Expression of PPARγ mRNA is abnormal
in PCOS, which may be involved in the pathogenesis of this
disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC drafted the manuscript. JC and GM were mainly
devoted to collecting and interpreting the general data. JC and TY
were responsible for hormone detection and PCR. All authors read
and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Jining Medical University (Jining, China).
Signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blauschmidt S, Greither T, Lampe K, Köller
S, Kaltwaßer P and Behre HM: Dipeptidyl peptidase 4 serum activity
and concentration are increased in women with polycystic ovary
syndrome. Clin Endocrinol (Oxf). 87:741–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarkar K, Mandal RK, Mandal A and Sarkar
S: Least centre distance based MAXNET architecture to obtain
threshold for brain tumor edema segmentation from FLAIR MRI. Int J
Comp Sci Engin. 5:112–120. 2017.
|
|
3
|
Okamoto H, Hamada M, Sakamoto E, Wakita A,
Nakamura S, Kato T, Abe Y, Takahashi K, Igaki H and Itami J:
Log-file analysis of accuracy of beam localization for brain tumor
treatment by CyberKnife. Pract Radiat Oncol. 6:e361–e367. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murakami M, Tognini P, Liu Y, Eckel-Mahan
KL, Baldi P and Sassone-Corsi P: Gut microbiota directs
PPARγ-driven reprogramming of the liver circadian clock by
nutritional challenge. EMBO Rep. 17:1292–1303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dasgupta S, Sirisha P, Neelaveni K,
Anuradha K, Sudhakar G and Reddy BM: Polymorphisms in the IRS-1 and
PPAR-γ genes and their association with polycystic ovary syndrome
among South Indian women. Gene. 503:140–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng X, Yu W, Li X, Zhou F, Zhang W, Shen
Q, Li J, Zhang C and Shen P: Apigenin, a modulator of PPARγ,
attenuates HFD-induced NAFLD by regulating hepatocyte lipid
metabolism and oxidative stress via Nrf2 activation. Biochem
Pharmacol. 136:136–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez MO, Sharma S, Kim S, Rickert E,
Hsueh K, Hwang V, Olefsky JM and Webster NJG: Obese neuronal PPARγ
knock-out mice are leptin sensitive but show impaired glucose
tolerance and fertility. Endocrinology. 158:121–133.
2017.PubMed/NCBI
|
|
8
|
Angela M, Endo Y, Asou HK, Yamamoto T,
Tumes DJ, Tokuyama H, Yokote K and Nakayama T: Fatty acid metabolic
reprogramming via mTOR-mediated inductions of PPARγ directs early
activation of T cells. Nat Commun. 7:136832016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krishnan A and Muthusami S: Hormonal
alterations in PCOS and its influence on bone metabolism. J
Endocrinol. 232:R99–R113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dasgupta S and Reddy BM: The role of
epistasis in the etiology of Polycystic Ovary Syndrome among Indian
women: SNP-SNP and SNP-environment interactions. Ann Hum Genet.
77:288–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keller E, Chazenbalk GD, Aguilera P,
Madrigal V, Grogan T, Elashoff D, Dumesic DA and Abbott DH:
Impaired preadipocyte differentiation into adipocytes in
subcutaneous abdominal adipose of PCOS-like female rhesus monkeys.
Endocrinology. 155:2696–2703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao F, Yu Y, Feng L, Li J, Zhang M, Lan X,
Yan X, Liu Y, Guan F, Zhang M, et al: Adipogenic miR-27a in adipose
tissue upregulates macrophage activation via inhibiting PPARγ of
insulin resistance induced by high-fat diet-associated obesity. Exp
Cell Res. 355:105–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Y and Wang CL: Effects of testosterone
on PPARγ and P450 arom expression in polycystic ovary syndrome
patients and related mechanisms. Eur Rev Med Pharmacol Sci.
22:1549–1553. 2018.PubMed/NCBI
|
|
14
|
Hu WH, Chen L, Tong J, Zhao CY, Mao S and
Qiao J: Expression of PPARγ mRNA in granulosa cells and its
correlation with clinical characteristics of polycystic ovary
syndrome. Beijing Da Xue Xue Bao Yi Xue Ban. 45:859–863. 2013.(In
Chinese). PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakuramachi M, Igaki H, Ikemura M,
Yamashita H, Okuma K, Sekiya N, Hayakawa Y, Sakumi A, Takahashi W,
Hasegawa H, et al: Detection of residual metastatic tumor in the
brain following Gamma Knife radiosurgery using a single or a series
of magnetic resonance imaging scans: An autopsy study. Oncol Lett.
14:2033–2040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang LL, Xie JK, Cui JQ, Wei D, Yin BL,
Zhang YN, Chen YH, Han X, Wang Q and Zhang CL: Promoter methylation
of yes-associated protein (YAP1) gene in polycystic ovary syndrome.
Medicine (Baltimore). 96:e57682017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vissenberg R, Manders VD, Mastenbroek S,
Fliers E, Afink GB, Ris-Stalpers C, Goddijn M and Bisschop PH:
Pathophysiological aspects of thyroid hormone disorders/thyroid
peroxidase autoantibodies and reproduction. Hum Reprod Update.
21:378–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phumyu N, Boonanuntanasarn S, Jangprai A,
Yoshizaki G and Na-Nakorn U: Pubertal effects of
17α-methyltestosterone on GH-IGF-related genes of the
hypothalamic-pituitary-liver gonadal axis and other biological
parameters in male, female and sex-reversed Nile tilapia. Gen Comp
Endocrinol. 177:278–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ebrahimi F, Schuetz P, Mueller B, Urwyler
SA, Donath MY and Christ-Crain M: Effects of IL-1[beta] on the
hypothalamic-pituitary-gonadal axis in men with obesity and
metabolic syndrome - A randomized, double-blind, placebo-controlled
trial. In: 19th European Congress of Endocrinology ECE 2017,
Lisbon, Portugal. Endocrine Abst. 49:2017.
|
|
21
|
Dunning KR, Anastasi MR, Zhang VJ, Russell
DL and Robker RL: Regulation of fatty acid oxidation in mouse
cumulus-oocyte complexes during maturation and modulation by PPAR
agonists. PLoS One. 9:e873272014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Münzker J, Hofer D, Trummer C, Ulbing M,
Harger A, Pieber T, Owen L, Keevil B, Brabant G, Lerchbaum E, et
al: Testosterone to dihydrotestosterone ratio as a new biomarker
for an adverse metabolic phenotype in the polycystic ovary
syndrome. J Clin Endocrinol Metab. 100:653–660. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kauffman AS, Thackray VG, Ryan GE, Tolson
KP, Glidewell-Kenney CA, Semaan SJ, Poling MC, Iwata N, Breen KM,
Duleba AJ, et al: A novel letrozole model recapitulates both the
reproductive and metabolic phenotypes of polycystic ovary syndrome
in female mice. Biol Reprod. 93:692015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbosa G, Sá LBPC, Rocha DRTW and Arbex
AK: Polycystic ovary syndrome (PCOS) and fertility. Open J Endocr
Metab Dis. 6:58–65. 2016. View Article : Google Scholar
|