Introduction
Hypertension is a common disorder that affects ~25%
of the adult population (1).
Hypertension induces abnormal blood dynamics and sugar and fat
metabolic disorders, and also exerts negative effects on target
organs such as the heart, brain and kidney. It has also been
demonstrated that arterial hypertension accounts for 9.7 million
fatalities annually, which is >50% of the 17 million deaths
resulting from various cardiovascular diseases (2). Epidemiological and interventional
studies have demonstrated that the interactions between multiple
factors, environmental as well as genetic, may lead to the
development of high blood pressure (3,4), and
have revealed an evident association between high-salt dietary
intake and hypertension (3).
A high-salt diet is known to be associated with
increased arterial pressure; hence, high dietary salt intake is
considered to be the main cause of cardiovascular, cerebrovascular
and renal morbidity and mortality. Furthermore, progress in
agriculture and farming has given rise to a constant growth in high
salt consumption over the past several centuries (5). With the development of modern food
processing and increasing popularity of high-salt fast food in
recent decades, our daily intake of salt is markedly higher than
what is required to maintain the normal physiological functions of
sodium. Thus, high-salt diet is a major risk factor for the
pathogenesis and progression of hypertension in modern society
(6). Accumulating evidence indicates
that the mechanism of high salt-induced hypertension may include
expanded blood volume, increased blood flow and, ultimately,
hypertension (7). Furthermore, as
numerous studies indicate, high dietary salt intake poses a threat
to target organs, increasing the risk of early morbidity and
mortality, in addition to its impact on blood pressure, and
clinical data also confirm that multiple factors likely contribute
to high salt-induced hypertension (2). However, their exact interplay has not
been fully elucidated, indicating that there may be additional
factors underlying high salt-induced hypertension and
cardiovascular remodeling; thus, further investigation is
required.
Endothelial dysfunction (ED), which is considered to
be implicated in the pathogenesis and progression of hypertensive
heart disease (8), is typically
manifested by reduced nitric oxide (NO) bioavailability, and its
underlying mechanism may involve loss of endothelial nitric oxide
synthase (eNOS) and cofactor tetrahydrobiopterin (BH4), hence
leading to uncoupling of eNOS, due to which the enzyme produces
superoxide anion rather than NO, further aggravating oxidative
stress (OS) and inducing vascular pathogenesis (9). Roeleveld et al (10) previously presented evidence that
endothelium-dependent dilation due to acetylcholine (ACh) in aortic
segments from animals on high-salt diet was terminated by the
inhibition of NOS with an L-arginine analog; however, these
responses were only slightly inhibited in rats on a low-salt diet.
More recent studies further demonstrated that the endothelial
capacity for agonist-induced NO release was inhibited following
high-salt intake for only 3 days (11,12),
which indicates that defects in the response to or production of NO
may be implicated in salt-sensitive hypertension. In addition to
decreasing NO bioavailability, ED also augments the release of
angiotensin II (Ang II) and endothelin-1 (ET-1), accompanied by
increasing superoxide anion (O2•−) production
via the angiotensin type 1-ETA/NADPH oxidase pathway (13–15),
which further aggravates ED and OS. Hypertension has been recently
viewed as a low-grade inflammatory disease, in which adaptive
immunity is a critical mediator, with the components of both the
innate and adaptive immune system contributing to its pathogenesis
(16). Accumulation of macrophages
and T cells has been observed in the perivascular fat, heart and
kidney of hypertensive patients, as well as in hypertensive
experimental animals (17). Notably,
numerous studies (18–20) have explored the effect of Ang II on
inducing inflammation in hypertension and other diseases,
demonstrating the potential role of increased Ang II levels in
several disorders and the effect of Ang II on promoting OS,
resulting in inflammation, vascular dysfunction and target organ
damage.
Traditional Chinese Medicine (TCM) has been used in
China for >2,000 years and it has been one of the most enduring
and widely tested alternative medicines worldwide. Due to its
general acceptance and satisfactory efficacy, TCM has been
attracting increasing scientific interest (21). Xin-Ji-Er-Kang (XJEK), which is a
topical Chinese herbal medicine, is a representative TCM compound
and is derived from 14 herbal medicines, including Panax
ginseng C.A.Mey., Astragalus mongholicus Bunge,
Ophiopogon japonicus (Thunb.) Ker-Gawl. and Polygonatum
odoratum (Mill.) Druce. Clinical studies and basic research
have both indicated the therapeutic effect of XJEK on coronary
heart disease and experimental hypertension via decreasing OS,
enhancing antioxidant activity and protecting the endothelium
(8,22).
The aim of the present study was to determine
whether the chronic intake of XJEK ameliorates hypertension induced
by a high-salt diet and elucidate the underlying mechanism,
focusing on the involvement of ED and inflammation.
Materials and methods
Animals
A total of 40 Kunming male mice (age, 7–8 weeks;
weight 20±2 g) were obtained from Shanghai SLAC Laboratory Animal
Co., Ltd. [Shanghai, China; certificate no. SCXK (HU) 2012-0002]
and kept in the animal facility of Anhui Medical University (Hefei,
China). All animals were handled strictly according to the rules
and regulations outlined in the National Institutes of Health Guide
for the Care and Use of Laboratory Animals (NIH Publications no.
8023, revision 1978 (23), and
approval was granted from the Animal Care and Use Committee of
Anhui Medical University. The mice were kept under standard
conditions (temperature, 24°C; humidity, 55±5%) with a 12-h
dark/light cycle in solid-bottomed polypropylene cages and had
access to commercial mouse chew and tap water ad libitum.
After allowing the mice to acclimatize for 3 days, hypertension was
induced by high-salt intake, as described previously (24).
Chemicals
XJEK, which is composed of 14 diversified medicinal
herbs, as previously reported (8),
was purchased from Nanjing Pharmaceutical Hefei Pharmacy Chain Co.,
Ltd. (Hefei, China), and irbesartan was purchased from Jiangsu
Hengrui Medicine Co., Ltd. (Lianyungang, China; H20000513).
Induction of hypertension in mice and
experimental design
Mice were randomized into four groups (n=10 per
group) and the hypertension model was successfully induced
(confirmed by measuring systolic blood pressure ≥130 mmHg) by a
high-salt (8% NaCl) diet for 8 weeks. The groups were as follows:
i) Control group: 10 mice were fed a standard diet alone for 8
weeks; ii) model group: 10 mice were fed a diet containing 8% NaCl
for 8 weeks and were intragastrically administered distilled water
for the last 4 weeks; iii) XJEK + high-salt-treated group: 10 mice
were fed a diet containing 8% NaCl for 8 weeks and received
intragastric administration of XJEK 7.5 g/kg/day for the last 4
weeks; and iv) irbesartan + high-salt-treated group: 10 mice were
fed a diet containing 8% NaCl for 8 weeks with intragastric
administration of irbesartan 5 mg/kg/day for the last 4 weeks.
Measurement of systolic blood pressure
(SBP)
SBP was measured using the tail-cuff method
(ALC-NIBP; Shanghai Alcott Biotech Co., Ltd., Shanghai China). The
basal blood pressure of each group was recorded prior to the
experiment and then once weekly during the following 8 weeks; all
measurements were handled by the same person at the same time of
day. Prior to measurement, the mice were kept in a warm environment
(27°C) for 30 min for the assessment of tail artery pulsations and
a steady pulse level. The mean number of SBP measurements was
20.
Hemodynamics and cardiac remodeling
index
The mice were anesthetized with 10% chloral hydrate
(300 mg/kg, intraperitoneal; cat. no. CC3431-250G; Beijing Cool
Laibo Technology Co., Ltd., Beijing, China), the right carotid
artery was cannulated with a catheter (Transonic Scisense, Inc.,
London, ON, Canada) connected to an admittance control unit, and
then the catheter was advanced along the right coronary artery and
inserted into the left ventricle. The signals were registered on a
four-channel acquisition system (BL420S; Chengdu Taimeng Software
Co. Ltd., Chengdu, China). The admittance catheters were soaked for
30 min in Alconox prior to insertion into the common carotid artery
according to the instructions of the manufacturer (Transonic
Scisense, Inc.) The left ventricular systolic pressure (LVSP), left
ventricular end-diastolic pressure (LVEDP) and the increase rate of
the left ventricular pressure (±dp/dtmax)
were recorded accordingly.
Blood and tissue sampling
Chloral hydrate (300 mg/kg) was given to mice
anesthetized by intraperitoneal injection. Blood (~1 ml) was
collected from the heart into anticoagulant-coated tubes and
immediately centrifuged at 4,000 × g for 10 min at 4°C, and the
serum was preserved at −80°C for future analysis. Next, the mice
were sacrificed via exsanguination. The thoracic cavity was opened
to expose the still-beating heart. Hearts and aortic tissue were
then harvested. The hearts were rinsed in ice-cold 0.9% NaCl
solution, photographed, blotted and weighed, and the heart weight
index [heart weight (HW)/body weight (BW)] was calculated. Heart
samples were divided into two parts, each of which was ~5 mm thick.
Aortic samples (4–5 mm in length) were placed in Krebs solution
(composition detailed below) for further use.
Isolated vascular ring
experiments
The thoracic aorta was excised immediately following
the opening of the thoracic cavity. Transverse rings (4–5 mm in
length) were cut, followed by removal of loose connective tissues,
and all vessel rings were suspended in organ baths containing Krebs
solution of the following composition (mM): NaCl, 118; KCl, 4.75;
NaHCO3, 25; MgSO4, 1.2; CaCl2, 2;
KH2PO4, 1.2; and glucose, 11. Krebs solution
was maintained at 37±1°C and injected with 95% O2:5%
CO2 (pH 7.4). This experiment had been previously
performed for mouse aortic strips, and 0.5 g of tension was
determined as optimal for this type of tissue (25). The rings underwent equilibration from
60–90 min, during which time warm Krebs solution was used to
stretch and wash the tissues every 15 min. The
concentration-relaxation response curves to ACh (10-9-10-6M) were
constructed for intact rings that were precontracted by 10-5M
phenylephrine. Relaxant responses to ACh were expressed as a
percentage of precontraction induced by phenylephrine.
Histological and morphological
analyses of the heart and thoracic aorta
The heart apex was fixed for 48 h at 27°C by
immersion in neutral 10% buffered formalin for histological
analysis. Hematoxylin-eosin (HE; 27°C for 4 h) and Van Gieson
staining (27°C for 4 h) were applied to observe the prepared 5-µm
paraffin sections. Subsequently, the myocyte cross-sectional area
(CSA), perivascular collagen area (PVCA) and collagen volume
fraction (CVF) were quantitatively analyzed with ImageJ software
(1.8.0; National Institutes of Health, Bethesda, MD, USA) in Leica
inverted optical electron microscope (magnification, ×400; Leica DM
IL, DC 300; Leica Microsystems GmbH, Wetzlar, Germany). The
thoracic aortas were removed from mice, cleaned and fixed (27°C for
48 h) in neutral 10% buffered formalin. Paraffin-embedded thoracic
aorta samples were cut in 5-µm sections, dewaxed and stained (27°C
for 4 h) with HE; then, the total aortic area (TAA), luminal area
(LA), CSA, aortic radius (AR), luminal radius (L) and media
thickness (M) of the aorta were assessed with Image-Pro Plus (6.0;
Media Cybernetics, Inc., Rockville, MD, USA) and the proportion of
M/L was calculated as previously described (25).
Measurement of serum NO, superoxide
dismutase (SOD), malondialdehyde (MDA), ET-1, brain natriuretic
peptide (BNP), Ang II and aldosterone content
NO activity was determined according to the method
described by Veltkamp et al (26), in which most NO was instantly
converted to nitrite (NO2−) and nitrate
(NO3−) due to its instability in
physiological solutions. The levels of
NO2−/NO3− in the serum
were measured with the application of an NO assay kit (A013-2;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
according to the manufacturer's instructions. Under the effect of
aspergillus nitrite reductase, nitrate briefly reverted to nitrite,
and the Griess reagent was applied for measurement of the total
nitrite. Spectrophotometry at 540 nm was employed to detect
absorbance. ELISA was applied to assess the contents of ET-1 (H093,
Nanjing Jiancheng Bioengineering Institute), Ang II (H185; Nanjing
Jiancheng Bioengineering Institute), BNP (H166; Nanjing Jiancheng
Bioengineering Institute) and aldosterone (H188; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
instructions.
The thiobarbituric acid reactive substances assay
was adopted to detect the MDA content, strictly following the MDA
assay kit's (TBA method) instructions (A003-1; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) and the absorbance was
measured at a wavelength of 532 nm. Furthermore, the absorbance at
550 nm was detected using a SOD assay kit (A001-3; Nanjing
Jiancheng Bioengineering Institute), to determine SOD activity.
Measurement of eNOS activity levels in
the serum and cardiac tissue
eNOS activity in the serum and cardiac tissue was
evaluated using ELISA (H195; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's instructions.
Immunohistochemistry for eNOS,
interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-10
Immunohistochemical staining was performed by
applying the Ultra Sensitive S-P kit produced by Wuhan Boster
Biological Technology, Ltd. (Wuhan, China) in accordance with the
manufacturer's instructions. Formalin (10%) was used for fixation
at 24°C for 48 h, and the 5 µm paraffin sections were observed.
Deparaffinization of sections was performed in 10 mM sodium citrate
buffer (pH 6.0), followed by microwave treatment for 10 min twice.
The sections were then incubated with endogenous peroxidase
blocking solution (3% hydrogen peroxide; AR1108; Whuan Boster
Biological Technology Ltd., Wuhan, China) for 10 min at room
temperature. Primary rabbit polyclonal antibodies against eNOS
(1:100; ab76198, Abcam, Cambridge, UK) and polyclonal antibodies
against IL-1β (1:200; 12242; Cell Signaling Technology, Inc.,
Danvers, MA, USA), TNF-α (1:200; ab6671; Abcam) and IL-10 (1:200;
20850-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) were added
and incubated at 4°C for 18 h. The sections were washed three times
using PBS, and then incubated (37°C) with biotin-conjugated
anti-rabbit (1:100; SPN-9001; OriGene Technologies, Inc.,
Rockville, MD, USA) and anti-mouse (1:100, SPN-9002, OriGene
Technologies, Inc.) secondary antibody for 10 min. The sections
were again washed three times with PBS, followed by incubation with
streptavidin-peroxidase for 10 min at 37°C and another three washes
with PBS. Following haematoxylin counterstaining (27°C) for 10 min,
the sections were incubated (27°C) in diaminobenzidine for 5 min.
At the same time, the negative group was constructed where one
slice was selected from each group and was treated as
aforementioned, but with PBS instead of antibodies. Leica inverted
optical electron microscope (magnification, ×400; Leica DM IL, DC
300; Leica Microsystems GmbH, Wetzlar, Germany) was used to capture
images.
Western blotting for IL-1β, TNF-α and
IL-10
Frozen heart tissue specimens (20 mg) were added to
990 µl radioimmunoprecipitation assay lysis buffer (CW2334S;
Beijing ComWin Biotech Co., Ltd., Beijing, China) and 10 µl
protease inhibitor (CW2383S; Beijing ComWin Biotech Co., Ltd.). The
tissues were ground and the tissue homogenate was centrifuged at
12,000 × g for 15 min at 4°C. The supernatant was collected and
protein concentration was determined using a BCA Protein Assay kit
(P0010; Beyotime Institute of Biotechnology, Haimen, China). Equal
amounts (10 µl) of loaded proteins were separated by 10% SDS-PAGE
and transferred to polyvinylidene difluoride membranes, which were
then blocked at room temperature for 1 h with 5% dry skimmed milk
in Tris-buffered saline containing 1% Tween-20 (TBST). The
membranes were incubated with antibodies against glucocorticoid
receptor, IL-1β (1:1,000), TNF-α (1:500), IL-10 (1:1,000) and GAPDH
(1:10,000) overnight at 4°C. GAPDH antibodies (ab181602) were
purchased from Abcam. Membranes were washed with TBST three times,
5 min each. IL-1β, TNF-α and IL-10 were incubated (at 27°C) with
anti-rabbit immunoglobulin (Ig)G secondary antibody conjugated to
horseradish peroxidase (HRP) for 1 h (1:10,000; ab7090; Abcam), and
eNOS and GAPDH were incubated (at 27°C) with anti-mouse IgG
secondary antibody conjugated to HRP for 1 h (1:10,000; ab97040;
Abcam). Following extensive washing, the protein bands were
detected by enhanced chemiluminescence reagents (ECL kit; GE
Healthcare Life Sciences, Little Chalfont, UK). The Chemi Q4800
mini Imaging System (Bioshine, Shanghai, China) was used to
visualize protein bands, and densitometry analysis was performed
with ImageJ software. The density of each immunoreactive band was
normalized to the density of its corresponding band of GAPDH.
Statistical analysis
In the present study, all data are from at least
three independent experiments and are expressed as the mean ±
standard deviation. Data were analyzed by one-way analysis of
variance to test the significance of differences between the
control and drug-treated groups. Comparisons between multiple
groups was performed using the Scheffe post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
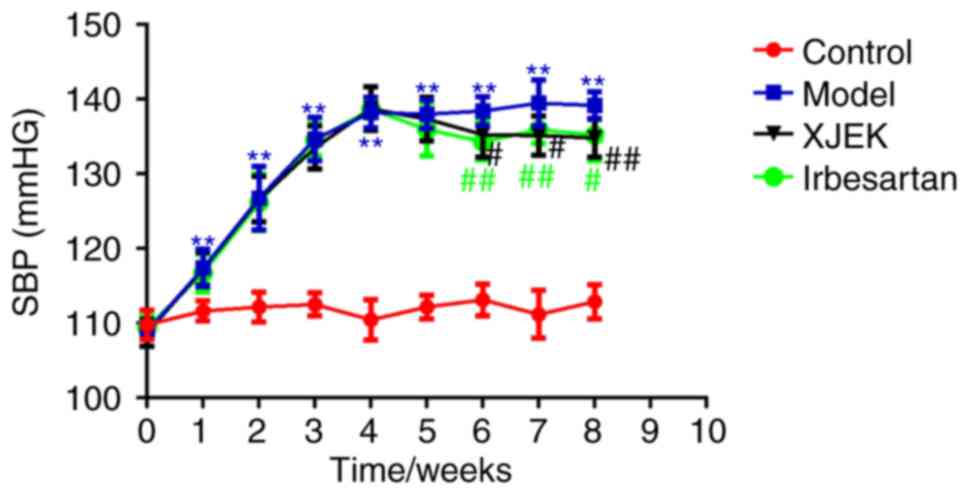
Effects of XJEK on SBP in high
salt-induced hypertensive mice
Time-related changes in SBP among the four groups
are presented in Fig. 1. No marked
difference was observed in SBP among experimental groups at
baseline. The high daily intake of salt for 8 weeks induced a
marked elevation in SBP (139.17±1.80 mmHg) in the model group
compared with in the control group (112.89±2.31 mmHg; P<0.01),
whereas administration of XJEK for the last 4 weeks markedly
decreased SBP, compared with the model (134.81±2.53; P<0.01).
The SBP in hypertensive mice on irbesartan treatment was also
downregulated compared with in the model group (135.25±3.54;
P<0.01).
Effects of XJEK on hemodynamic
parameters in high salt-induced hypertensive mice
In all groups, in vivo left ventricular
function was evaluated at the end of the 8 weeks. As indicated in
Table I, the systolic cardiac
parameters in the model group, such as LVSP
+dp/dtmax, and diastolic cardiac
parameters (−dp/dtmax), were all raised in the
model group compared with the control, which was reversed by XJEK
treatment. Similar results were achieved in the positive control
group with irbesartan treatment.
 | Table I.Effects of XJEK on cardiac function
in high-salt induced hypertensive mice. |
Table I.
Effects of XJEK on cardiac function
in high-salt induced hypertensive mice.
| Group | LVSP (mmHg) | LVEDP (mmHg) |
+dp/dtmax (mmHg/sec) |
-dp/dtmax (mmHg/sec) |
|---|
| Control | 83.13±8.59 | −3.78±5.85 |
4,147.56±715.84 |
−3,178.7±673.53 |
| Model |
102.05±20.96a | −2.63±5.31 |
5,315.25±1,122.92a |
−4,343.32±1,049.13a |
| XJEK | 88.52±8.30 | −1.26±3.90 |
4,275.33±474.32b |
−3,497.60±479.50b |
| Irbesartan | 96.84±14.87 | −3.59±6.82 |
4,958.59±922.37 |
−4,250.01±756.12 |
Effects of XJEK on cardiac remodeling
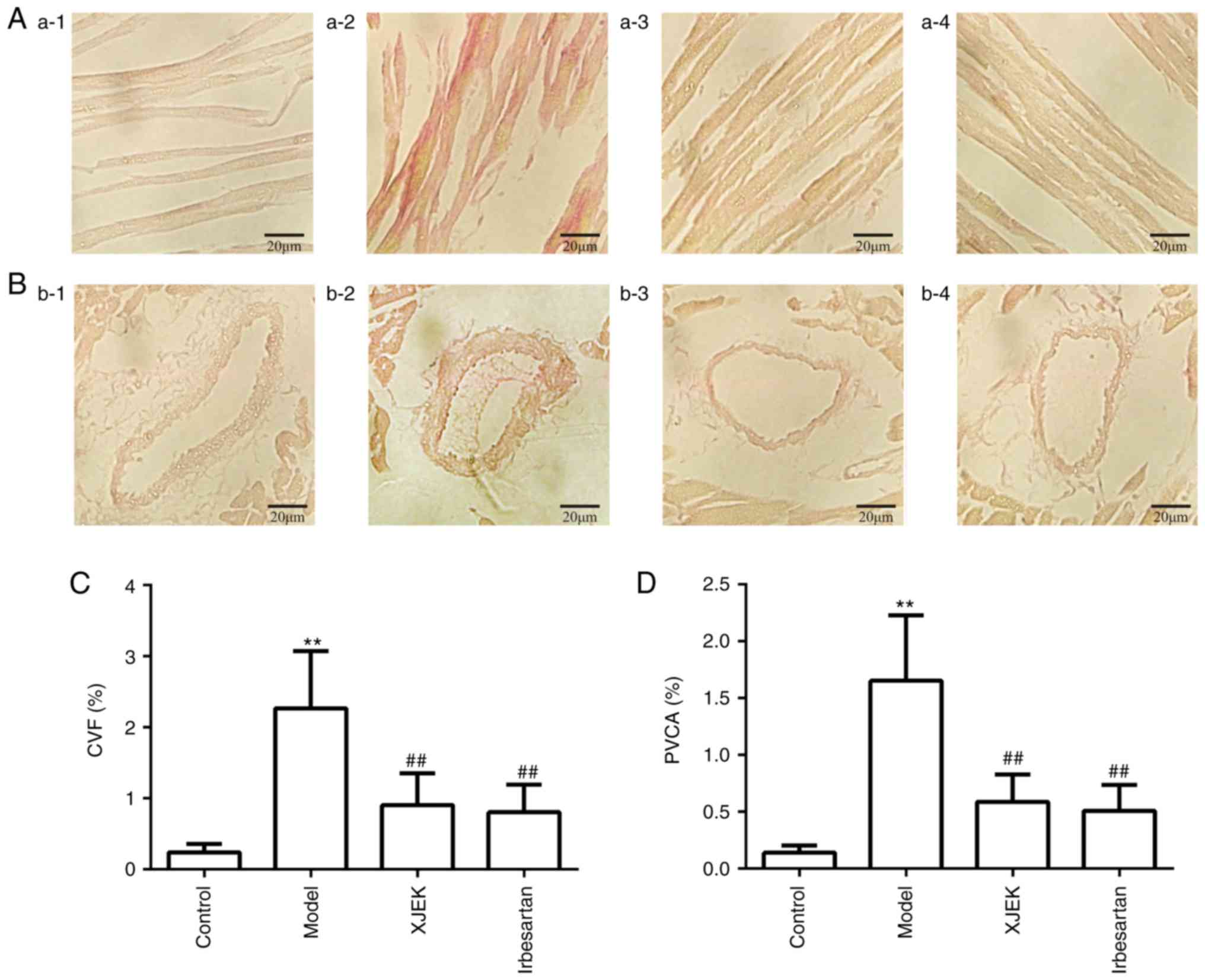
in high salt-induced hypertensive mice
The histological study of the hearts of the
experimentally-induced hypertensive mice in the model group
demonstrated that myocyte CSA, and the levels of CVF was all
markedly increased compared with those in the control group
(P<0.01; Fig. 2A-C). Treatment
with XJEK for 4 weeks reversed these pathological changes
(P<0.01), as did irbesartan in the positive control group
(P<0.05). Compared with in the control group, the HW/BW ratio
was increased in the model group, reflecting cardiac hypertrophy
(P<0.01; Fig. 2D).
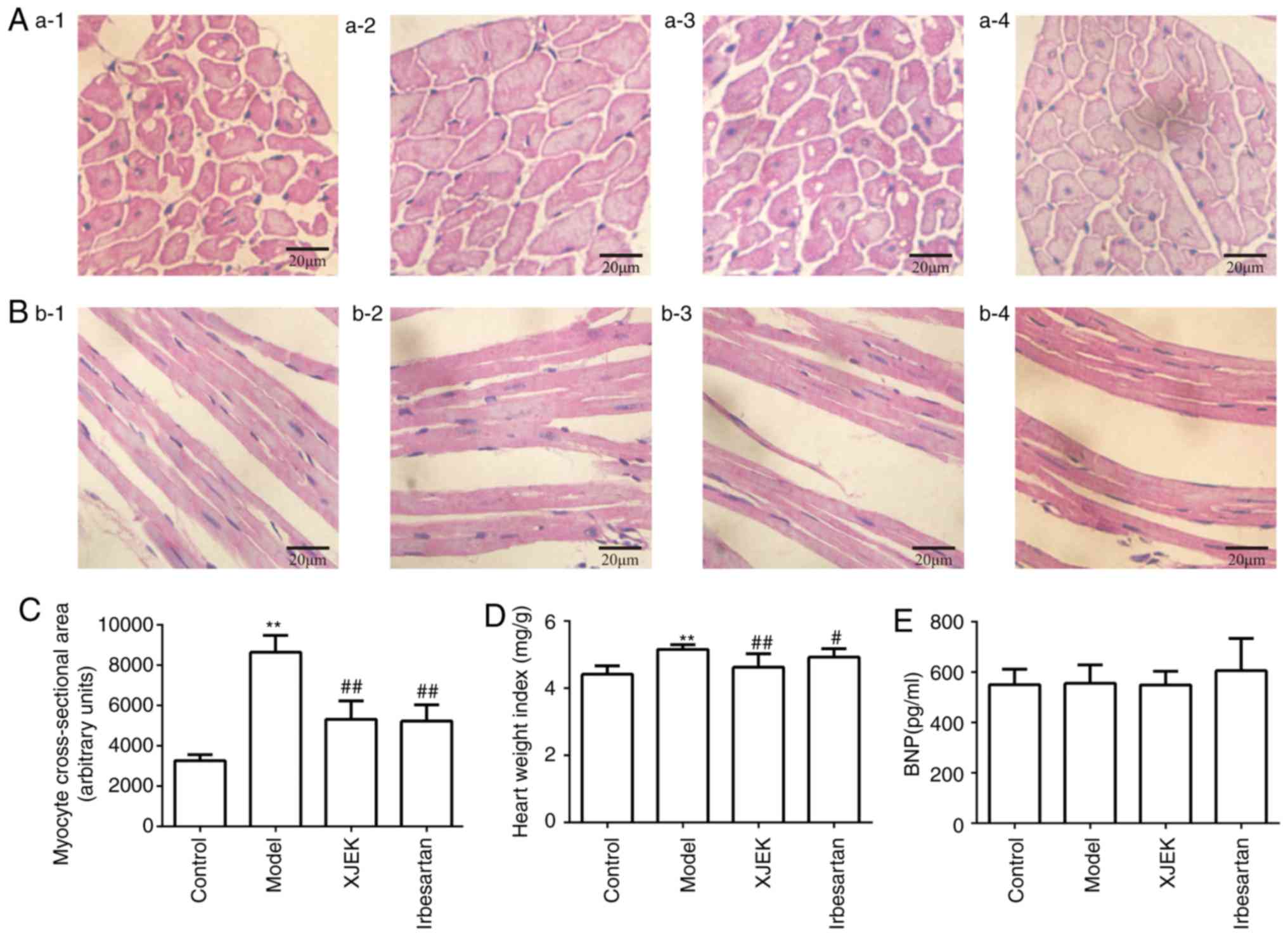 | Figure 2.Effects of XJEK on HW/BW, myocyte CSA
in high-salt induced hypertensive mice. (A) Representative figure
of myocyte cross-section (HE staining, ×400). (B) Representative
figure of myocyte long axis (HE staining, ×400). 1: Control group;
2: Model group; 3: XJEK + high-salt-treated group; 4: Irbesartan +
high-salt-treated group. (C) Quantified results of myocyte CSA. (D)
Quantified results of HW/BW. (E) Plasma BNP content in high-salt
induced hypertensive mice. Data are presented as the mean ±
standard deviation, n=10. **P<0.01 vs. control group;
#P<0.05, ##P<0.01 vs. model group. XJEK, Xin-Ji-Er-Kang;
HW/BW, heart weight/body weight; CSA, cross-sectional area; HE,
hematoxylin and eosin; BNP, brain natriuretic peptide. |
BNP may be associated with cardiovascular disorders,
even at levels far below contemporary thresholds for diagnosing
heart failure. The present study revealed no significant difference
in the level of BNP among the four experimental groups, which may
be attributed to the cardiac compensatory phase, which protects
against high-salt diet-induced damage (Fig. 2E). The level of PVCA in the model
group was significantly higher than that in the control group, but
was reversed in the XJEK group (P<0.01; Fig. 3).
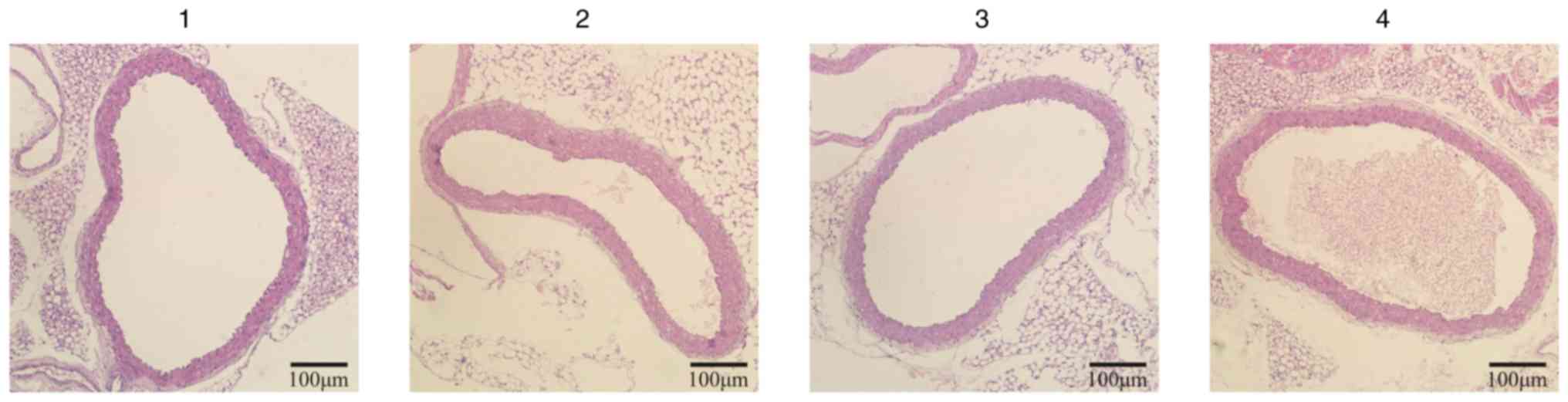
Effects of XJEK on aortic remodeling
in high salt-induced hypertensive mice
The vascular remodeling of the upper thoracic aorta
in mice on a high-salt diet was evaluated at the end of the 8th
week. The TAA, LA, CSA, CSA/TAA, AR, L and M values and the M/L
ratio of the aorta in high salt-induced hypertensive mice were
elevated compared with those in control mice, which was effectively
reversed by XJEK and irbesartan intervention over the last 4 weeks
(Fig. 4; Table II).
 | Table II.Effects of XJEK on aorta remodeling
in high-salt induced hypertensive mice. |
Table II.
Effects of XJEK on aorta remodeling
in high-salt induced hypertensive mice.
| Group | TAA (×103 µm2) | LA (×103 µm2) | CSA (×103 µm2) | CSA/TAA (%) | AR (µm) | L (µm) | M (µm) | M/L (%) |
|---|
| Control | 431.4±120.5 | 290.0±106.3 | 141.3±26.0 | 34.2±7.2 | 367.5±53.2 | 299.4±57.1 | 68.0±10.3 | 23.7±6.9 |
| Model |
680.5±100.7b |
433.7±52.3a |
246.8±64.8b | 35.9±4.7 |
464.5±34.9b |
371.0±23.2a |
93.4±19.6a | 25.2±4.8 |
| XJEK |
455.0±71.6d |
304.4±78.9d |
150.6±18.0d | 33.9±7.0 |
379.7±29.7d |
309.3±39.3d |
70.4±12.3c | 23.4±6.4 |
| Irbesartan |
461.6±85.7d |
312.4±70.1d |
149.3±21.6d | 32.7±3.8 |
381.9±36.9d |
313.6±36.7d |
68.3±6.7c | 22.1±3.4 |
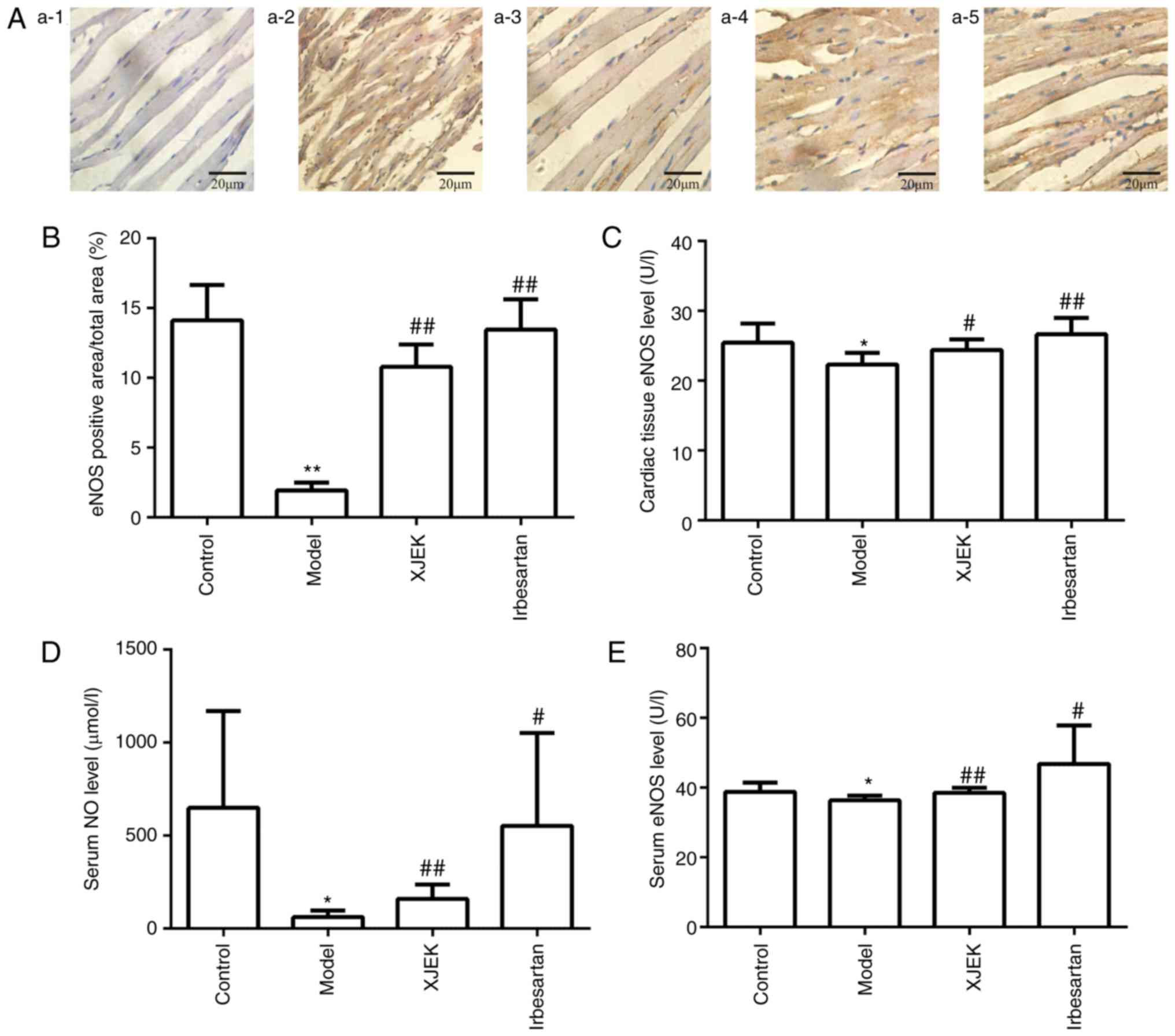
Effects of XJEK on ED in high
salt-induced hypertensive mice
NO content and eNOS activity in the cardiac tissue
and serum of model group mice were markedly decreased compared with
those in the control group (P<0.05), whereas mice treated with
XJEK or irbesartan exhibited increased NO and eNOS levels compared
with in the model group (P<0.05; Fig.
5).
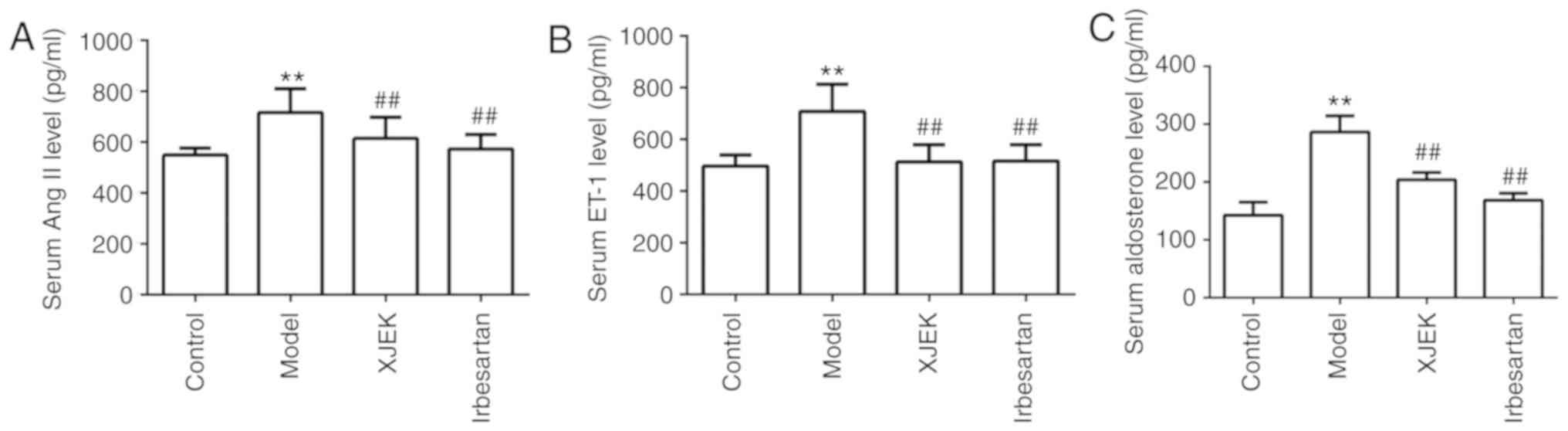
The Ang II, ET-1 and aldosterone levels were
detected 8 weeks later. The serum Ang II, ET-1 and aldosterone
content was increased in hypertensive mice compared with that in
the control group (P<0.01), but was markedly reduced following
XJEK and irbesartan intervention (P<0.01; Fig. 6).
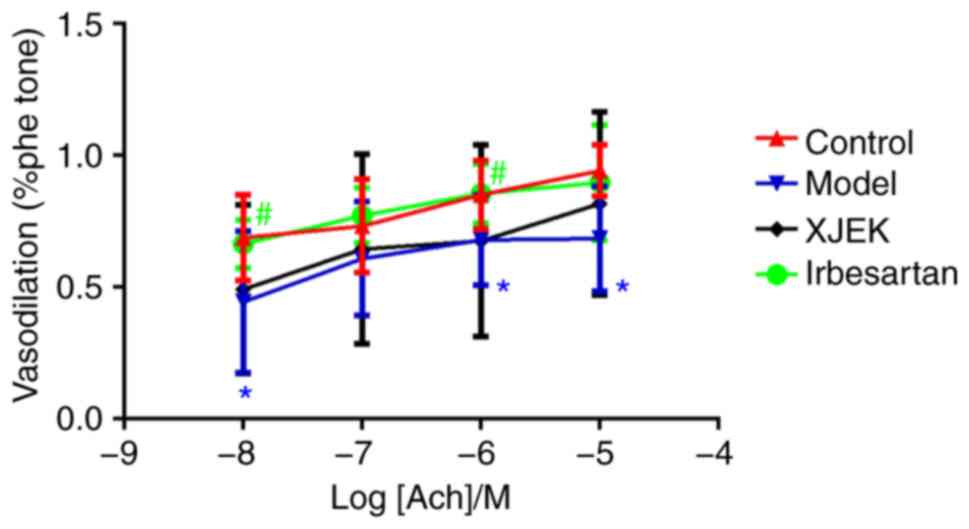
Compared with those of the control group, the aortic
rings of mice on a high-salt diet exhibited drastically decreased
endothelium-dependent vasodilator responses to ACh in aortic
segments stimulated by phenylephrine (Fig. 7). Compared with the model group, XJEK
treatment markedly improved the vasodilation induced by ACh in the
aortic rings of high salt-induced hypertensive mice, indicating
that high salt-induced hypertension may lead to increased ED,
whereas 4-week experimental therapy with XJEK ameliorated the ED
induced by high-salt intake.
Effects of XJEK on inflammatory
cytokine expression in high salt-induced hypertensive mice
The expression of IL-1β and TNF-α in cardiac tissues
was markedly increased in the high-salt group compared with control
group mice (P<0.01), while the increase in expression was
inhibited by XJEK and irbesartan intervention (P<0.01; Fig. 8A and B). In high salt-induced
hypertensive mice, the expression of IL-10 in cardiac tissues was
reduced significantly compared with controls (P<0.01), but was
then markedly increased with XJEK and irbesartan treatment compared
with the model group (P<0.01; Fig.
8C). The immunohistochemical results of IL-1 β, TNF-α and IL-10
are presented in Figs. 8D-F.
Furthermore, the results of IL-1β, TNF-α and IL-10 western blotting
were consistent with those of immunohistochemistry (P<0.01;
Fig. 8G-L).
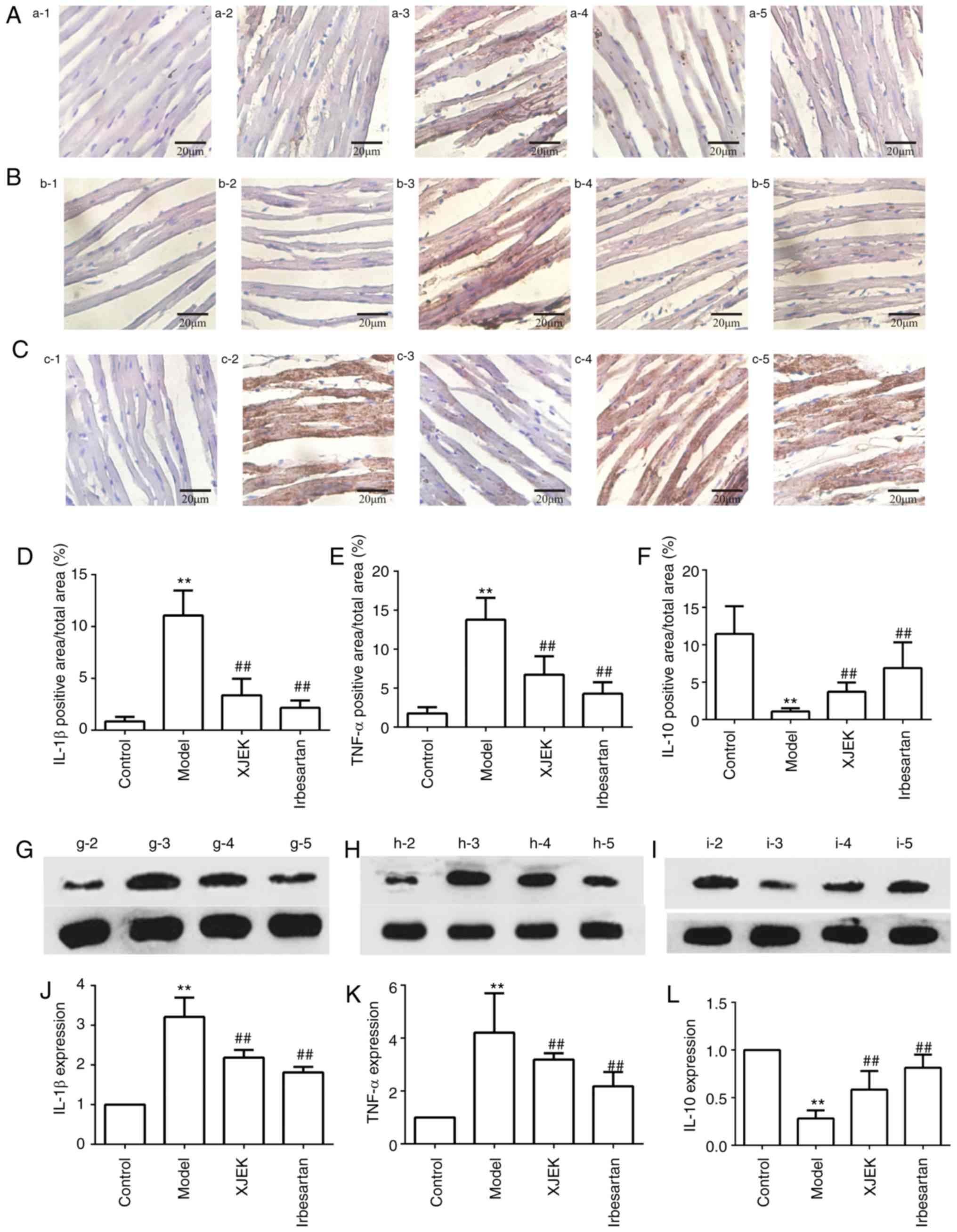 | Figure 8.Effects of XJEK on IL-1β, TNF-α and
IL-10 in high-salt induced hypertensive mice. (A) Representative
images of IL-1β immunohistological staining in heart
(magnification, ×400). (B) Representative images of TNF-α
immunohistological staining in heart (magnification, ×400). (C)
Representative images of IL-10 immunohistological staining in heart
(magnification, ×400). Quantified results of (D) IL-1β, (E) TNF-α
and (F) IL-10 immunohistological staining (n=10). (G-I)
Representative figures of (G) IL-1β, (H) TNF-α and (I) IL-10
protein via western blotting. Quantitative analyses of (J) IL-1β,
(K) TNF-α and (L) IL-10 protein. 1: Negative group; 2: Control
group; 3: Model group; 4: XJEK + high-salt-treated group; 5:
Irbesartan + high-salt-treated group. Data are presented as the
mean ± standard deviation. **P<0.01 vs. control group;
##P<0.01 vs. model group. XJEK, Xin-Ji-Er-Kang; IL, interleukin;
TNF, tumor necrosis factor. |
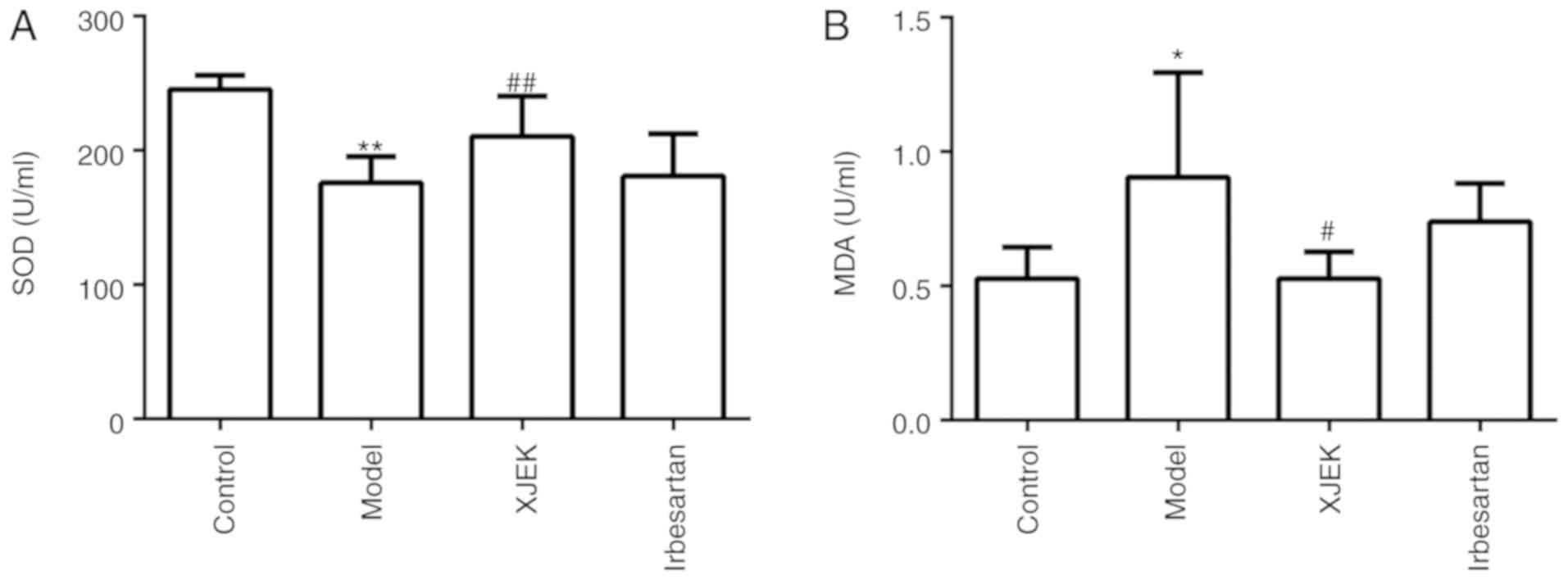
Effect of XJEK on serum SOD activity
and MDA content in high salt-induced hypertensive mice
Compared with the control group, the SOD level was
markedly decreased in the model group (P<0.01), as presented in
Fig. 9A. By contrast, the serum MDA
level was observably increased in the model group compared with
that in the control group (P<0.05; Fig. 9B). XJEK administration during the
last 4 weeks markedly decreased MDA content (P<0.05) and
enhanced serum SOD activity (P<0.01).
Discussion
Hypertension is the most common type of
cardiovascular disease and the leading cause of mortality and
disability worldwide (27).
Experimental observations have demonstrated that a long-term
high-salt diet may induce hypertension and renal injury in normal
Sprague-Dawley rats and it was also found to be associated with ED
(1,28,29). In
accordance with previous reports, the present study demonstrated
that a high-salt diet led to hypertension and cardiovascular
remodeling in mice, manifested by the deterioration of cardiac
hemodynamics, HW/BW index, cardiomyocyte CSA and longitudinal
diameter, aortic wall thickness, TAA and media thickness. In
addition, obvious ED, OS and inflammatory status were observed in
mice, clearly reflecting the association of high-salt diet with
hypertension. Of note, treatment with XJEK markedly alleviated
these pathophysiological changes. The dose of XJEK for the present
study was based on the clinical dose, and was converted for mice
according to pharmacology conversion formula (30). Furthermore, the dose of XJEK was
considered based on our previous report (31,32) and
with minor modifications according to different animal models.
The endothelium has long been viewed as a protective
biocompatible barrier between tissues and circulating blood, and it
is also a key regulator of vascular homeostasis, due to the fact
that it not only acts as a barrier, but serves as an active signal
transducer that circulates influency, modifying the vessel wall
phenotype (33). ED is a systemic
pathological condition resulting from an imbalance between
endothelium-dependent vasoconstriction and vasodilation,
specifically associated with reduced NO production, downregulation
of eNOS expression and reduced endothelium-dependent vasodilator
response (34–36), upregulation of ET-1, and increased
reactive oxygen species, cytokine and chemokine production
(37). It has been established that
environmental factors, such as high salt intake, contribute to
vascular remodeling, ultimately leading to BP elevation and
cardiovascular diseases, which often co-exist with a lack of NO
(28) and overexpression of ET-1
(38). Normally, the
renin-angiotensin II system (RAS) can effectively regulate arterial
BP and Na+ excretion, a process in which the peptide
hormone Ang II serves an important role (39). Salt restriction may stimulate Ang II
formation, while the high dietary salt intake suppresses it
(20). However, the dysfunction of
RAS and unusually increased Ang II levels account for numerous
pathological conditions (39).
Previous studies have demonstrated a correlation between ET-1 and
Ang II, i.e., ET-1 enhances the activity of the
angiotensin-converting enzyme, promoting the conversion of Ang I to
Ang II, which then boosts the activity of the endothelin-converting
enzyme (40,41). Amiri et al (38) demonstrated that ET-1 overexpression
associated with high salt consumption promoted ED and vascular
remodeling of resistance vessels, and contributed to an increase in
BP, mediated in part by endothelin type A and type B receptors.
Abnormal elevation of Ang II and ET-1 stimulates
O2•− production by NADPH oxidase, and
elevated O2•− levels disturb normal signal
transduction mechanisms in endothelial and vascular smooth muscle
cells, and induce the production of more O2•−
by decreasing vascular levels of BH4, an important co-factor for NO
production (42). The interaction
between O2•− and NO may lead to increased
peroxynitrite (ONOO−) formation, which in turn results
in further increase in O2•−. This accelerates
the oxidation of the NOS co-factor BH4 by ONOO− and
leads to eNOS uncoupling, during which the catalytic activity of
eNOS generates additional O2•− instead of NO,
leading to a vicious cycle of oxidative stress, which is a process
referred to as eNOS uncoupling (43). The present study demonstrated that
salt loading in mice led to significant ED, manifested by decreased
serum NO availability and eNOS activity, increasing serum Ang II
and ET-1 content, and leading to endothelium-dependent vasorelaxant
dysfunction and an obvious OS status. Treatment with XJEK for 4
weeks improved high salt-induced ED, as was reflected by the
amelioration of NO-dependent artery relaxation and restoration of
the balance between vasodilation (NO and eNOS) and vasoconstriction
(Ang II and ET-1) factors. Under normal conditions, increased
dietary salt intake suppresses plasma renin activity, leading to
suppression of circulating Ang II levels and reduced plasma levels
of aldosterone, resulting in reduced reabsorption of Na+
by the renal tubules to facilitate Na+ excretion and
maintain blood volume. Ang II also stimulates the secretion of
aldosterone in the adrenal cortex, causing retention of water and
sodium in the body and increasing BP (44). In the present study, the aldosterone
content was increased in the serum of model mice, while it was
significantly decreased in the serum of mice treated with XJEK.
Furthermore, Ang II accounts for the downregulation of MDA content
and the upregulation of SOD activity in the serum, ameliorating OS
status.
It has been indicated previously that the immune
system and inflammatory response are also crucial for the
pathogenesis of hypertension, in which the innate and adaptive
immune cells transmigrate and accumulate in the interstitium of
affected tissues, where they trigger cytokine release and
activation, causing remodeling as well as impaired cardiovascular
function and target organ injury (45). Cytokines represent a diverse group of
various soluble short-acting molecules and may be divided into two
main subgroups, namely pro-inflammatory cytokines (including TNF-α
and IL-1β), which are produced predominantly by activated
macrophages and anti-inflammatory cytokines (e.g., IL-10) that
suppress the inflammatory response (46).
TNF-α, produced by a variety of cells, acts on its
receptors and activates multiple signal pathways, such as death and
survival pathways, and NADPH oxidase activation. NADPH oxidase
produces superoxide, which immediately responds to NO, generating
the strong oxidant peroxynitrite. Furthermore, TNFα impedes the
eNOS promoter and leads to the destabilization of eNOS mRNA,
consequently decreasing eNOS protein levels and the ability of the
endothelium to yield NO (47,48).
Aortic ring experimentation has demonstrated that TNF-α reduces
endothelium-dependent vasodilatation. Cytokine IL-10, as a noted
key product of regulatory T cells (Tregs), exerts anti-inflammatory
and, thus, protective effects in hypertension (49). It has also been demonstrated that
boosted Treg function ameliorates Ang II and aldosterone-induced
hypertension, cardiac fibrosis, coronary inflammation, electrical
remodeling and impaired endothelial-dependent vasodilatation, which
are possibly mediated at least partially by IL-10 release from
Tregs (50). Previous studies
(32,51) have confirmed the key role of the
balance between pro- and anti-inflammatory cytokines in
cardiovascular remodeling and ED that often accompany hypertension,
and restoring of this balance is crucial in the prevention of
cardiovascular damage. Based on these findings, the present study
demonstrated the presence of an immunological imbalance exists in
the high salt-induced hypertensive mouse model, reflected by an
increase in TNF-α and IL-1β and a decrease in IL-10 levels.
Furthermore, treatment with XJEK restored the balance between pro-
and anti-inflammatory status.
In conclusion, the present study demonstrated that
the endothelial function in mice with hypertension induced by a
high-salt diet resulted in cardiovascular remodeling and OS, which
was reversed by XJEK or irbesartan treatment. Although XJEK
possesses similar protective properties to those of irbesartan,
XJEK is more effective in reducing blood pressure, and it possibly
serves a role in attenuating vascular OS and inflammation and
improving ACh-induced vasorelaxation and ED. Hence, it may be
concluded that XJEK was able to suppress hypertension-induced
damage in a high salt-induced hypertensive mouse model by
ameliorating ED, and the underlying mechanisms may include
restoring the balance between vasodilation and vasoconstriction
factors, immunological balance and downregulating OS. Further
research is required to fully elucidate the possible mechanisms and
potential therapeutic implications of these findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81373774)
and Anhui Medical University Foundation for Middle-aged and Young
Scientist Leaders of Disciplines in Science (grant no. 201324).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GH and PC performed the experiments
SG and AS supervised the design of the entire
experiment and collaborated in the discussion of the results. GH
wrote and revised the manuscript. LD, LW and JH helped analyze the
experimental data. YZ, GC and MC interpreted the data and revised
the manuscript. All authors read and approved the fnal version of
the manuscript for publication.
Ethics approval and consent to
participate
All the animals were handled strictly according to
the rules and regulations outlined in the National Institutes of
Health Guide for the Care and Use of Laboratory Animals (NIH
Publications no. 8023, revision 1978), and approval was granted
from the Animal Care and Use Committee of Anhui Medical University
(Hefei, China). In this study, no peritonitis was observed in
animals following intraperitoneal injection of 10% chloral hydrate
and no other abnormal symptoms were found.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glover M, Zuber AM and O'Shaughnessy KM:
Hypertension, dietary salt intake, and the role of the
thiazide-sensitive sodium chloride transporter NCCT. Cardiovasc
Ther. 29:68–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd-Jones D, Adams R, Carnethon M, De
Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund
K, et al: Heart disease and stroke statistics-2009 update: A report
from the American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Circulation. 119:480–486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asghar M, Tayebati SK, Lokhandwala MF and
Hussain T: Potential dopamine-1 receptor stimulation in
hypertension management. Curr Hypertens Rep. 13:294–302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basson J, Simino J and Rao DC: Between
candidate genes and whole genomes: Time for alternative approaches
in blood pressure genetics. Curr Hypertens Rep. 14:46–61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lev-Ran A and Porta M: Salt and
hypertension: A phylogenetic perspective. Diabetes Metab Res Rev.
21:118–131. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drenjančević-Perić I, Jelaković B, Lombard
JH, Kunert MP, Kibel A and Gros M: High-salt diet and hypertension:
Focus on the renin-angiotensin system. Kidney Blood Press Res.
34:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oloyo AK, Sofola OA and Yakubu MA:
Orchidectomy attenuates high-salt diet-induced increases in blood
pressure, renovascular resistance, and hind limb vascular
dysfunction: Role of testosterone. Clin Exp Pharmacol Physiol.
43:825–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo K, Lan CZ, Yu TT, Huang LL, Wang XH,
Pan C and Gao S: Effects of Xin-Ji-Er-Kang formula on 2K1C-induced
hypertension and cardiovascular remodeling in rats. J
Ethnopharmacol. 155:1227–1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boegehold MA, Drenjancevic I and Lombard
JH: Salt, Angiotensin II, superoxide, and endothelial function.
Compr Physiol. 6:215–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roeleveld RJ, Vonk-Noordegraaf A, Marcus
JT, Bronzwaer JG, Marques KM, Postmus PE and Boonstra A: Effects of
epoprostenol on right ventricular hypertrophy and dilatation in
pulmonary hypertension. Chest. 125:572–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu J, Drenjancevic-Peric I, McEwen S,
Friesema J, Schulta D, Yu M, Roman RJ and Lombard JH: Role of
superoxide and angiotensin II suppression in salt-induced changes
in endothelial Ca2+ signaling and NO production in rat aorta. Am J
Physiol Heart Circ Physiol. 291:H929–H938. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu J, Mori T, Huang T and Lombard JH:
Effect of high-salt diet on NO release and superoxide production in
rat aorta. Am J Physiol Heart Circ Physiol. 286:H575–H583. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Fink GD, Watts SW, Northcott CA,
Galligan JJ, Pagano PJ and Chen AF: Endothelin-1 increases vascular
superoxide via endothelin(A)-NADPH oxidase pathway in low-renin
hypertension. Circulation. 107:1053–1058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pagano PJ, Clark JK, Cifuentes-Pagano ME,
Clark SM, Callis GM and Quinn MT: Localization of a constitutively
active, phagocyte-like NADPH oxidase in rabbit aortic adventitia:
Enhancement by angiotensin II. Proc Natl Acad Sci USA.
94:14483–14488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rajagopalan S, Kurz S, Münzel T, Tarpey M,
Freeman BA, Griendling KK and Harrison DG: Angiotensin II-mediated
hypertension in the rat increases vascular superoxide production
via membrane NADH/NADPH oxidase activation. Contribution to
alterations of vasomotor tone. J Clin Invest. 97:1916–1923. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Batista PR, Palacios R, Martin A,
Hernanz R, Médici CT, Silva MA, Rossi EM, Aguado A, Vassallo DV,
Salaices M and Alonso MJ: Toll-like receptor 4 upregulation by
angiotensin II contributes to hypertension and vascular dysfunction
through reactive oxygen species production. PLoS One.
9:e1040202014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harrison DG, Marvar PJ and Titze JM:
Vascular inflammatory cells in hypertension. Front Physiol.
3:1282012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsusaka T and Ichikawa I: Biological
functions of angiotensin and its receptors. Annu Rev Physiol.
59:395–412. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LW, Qin KM, Zhu YH, Cai H, Li WD and
Cai BC: Research status and prospect of primary processing of
traditional Chinese medicinal materials. Zhongguo Zhong Yao Za Zhi.
40:602–606. 2015.(In Chinese). PubMed/NCBI
|
|
22
|
Wang QM, Chen GL, Wang YJ, Wang HS, Gao MH
and Gong YZ: An experimental study on inhibitory effect of
xinjierkang granules on virus myocarditis. Zhongguo Zhong Yao Za
Zhi. 25:293–296. 2000.(In Chinese). PubMed/NCBI
|
|
23
|
U.S Department of Health Education &
Welfare. Guide for the care and use of laboratory animals (revised
edition). 1978.
|
|
24
|
Preuss HG, Knapka JJ, MacArthy P, Yousufi
AK, Sabnis SG and Antonovych TT: High sucrose diets increase blood
pressure of both salt-sensitive and salt-resistant rats. Am J
Hypertens. 5:585–591. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu TT, Guo K, Chen HC, Lan CZ, Wang J,
Huang LL, Wang XH, Zhang Z and Gao S: Effects of traditional
Chinese medicine Xin-Ji-Er-Kang formula on 2K1C hypertensive rats:
Role of oxidative stress and endothelial dysfunction. BMC
Complement Altern Med. 13:1732013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Veltkamp R, Rajapakse N, Robins G, Puskar
M, Shimizu K and Busija D: Transient focal ischemia increases
endothelial nitric oxide synthase in cerebral blood vessels.
Stroke. 33:2704–2710. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma S, Wang Q, Zhang Y, Yang D, Li D, Tang
B and Yang Y: Transgenic overexpression of uncoupling protein 2
attenuates salt-induced vascular dysfunction by inhibition of
oxidative stress. Am J Hypertens. 27:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Channon KM: Tetrahydrobiopterin: A
vascular redox target to improve endothelial function. Curr Vasc
Pharmacol. 10:705–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu JW, Bailey AP, Tan W, Shparago M and
Young E: Long-term high salt diet causes hypertension and decreases
renal expression of vascular endothelial growth factor in
sprague-dawley rats. J Am Soc Hypertens. 2:275–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jian W, Enze C and Lizhi L: Xin Ji Er Kang
in Coronary Heart Disease. J NanJing Univ Trad Chin Med.
14:201–212. 1998.(in Chinese).
|
|
31
|
Gao S, Wang XH, Huang LL, Yu TT, Du SM,
Guo YW, Jia Y and Wang J: Effects of a compound Chinese medicine
Xinji' erkang on isoproterenol-induced ventricular remodeling in
mice. Zhong Xi Yi Jie He Xue Bao. 10:330–336. 2012.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu J, Zhang YX, Wang L, Ding L, Huang GY,
Cai GW and Gao S: Protective effects of Xinji'erkang on myocardial
infarction induced cardiac injury in mice. BMC Complement Altern
Med. 17:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kazmi RS, Boyce S and Lwaleed BA:
Homeostasis of hemostasis: The role of endothelium. Semin Thromb
Hemost. 41:549–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bunbupha S, Pakdeechote P, Kukongviriyapan
U, Prachaney P and Kukongviriyapan V: Asiatic acid reduces blood
pressure by enhancing nitric oxide bioavailability with modulation
of eNOS and p47phox expression in L-NAME-induced hypertensive rats.
Phytother Res. 28:1506–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fu JY, Qian LB, Zhu LG, Liang HT, Tan YN,
Lu HT, Lu JF, Wang HP and Xia Q: Betulinic acid ameliorates
endothelium-dependent relaxation in L-NAME-induced hypertensive
rats by reducing oxidative stress. Eur J Pharm Sci. 44:385–391.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zatz R and Baylis C: Chronic nitric oxide
inhibition model six years on. Hypertension. 32:958–964. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shao Y, Cheng Z, Li X, Chernaya V, Wang H
and Yang XF: Immunosuppressive/anti-inflammatory cytokines directly
and indirectly inhibit endothelial dysfunction-a novel mechanism
for maintaining vascular function. J Hematol Oncol. 7:802014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amiri F, Ko EA, Javeshghani D, Reudelhuber
TL and Schiffrin EL: Deleterious combined effects of salt-loading
and endothelial cell restricted endothelin-1 overexpression on
blood pressure and vascular function in mice. J Hypertens.
28:1243–1251. 2010.PubMed/NCBI
|
|
39
|
O'Donnell M, Mente A, Rangarajan S,
McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, et
al: Urinary sodium and potassium excretion, mortality, and
cardiovascular events. N Engl J Med. 371:612–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lagerqvist EL, Finnin BA, Pouton CW and
Haynes JM: Endothelin-1 and angiotensin II modulate rate and
contraction amplitude in a subpopulation of mouse embryonic stem
cell-derived cardiomyocyte-containing bodies. Stem Cell Res.
6:23–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai IJ, Croft KD, Puddey IB, Beilin LJ
and Barden A: 20-Hydroxyeicosatetraenoic acid synthesis is
increased in human neutrophils and platelets by angiotensin II and
endothelin-1. Am J Physiol Heart Circ Physiol. 300:H1194–H1200.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nurkiewicz TR, Wu G, Li P and Boegehold
MA: Decreased arteriolar tetrahydrobiopterin is linked to
superoxide generation from nitric oxide synthase in mice fed high
salt. Microcirculation. 17:147–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Channon KM: Tetrahydrobiopterin: Regulator
of endothelial nitric oxide synthase in vascular disease. Trends
Cardiovasc Med. 14:323–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Campbell DJ: Do intravenous and
subcutaneous angiotensin II increase blood pressure by different
mechanisms? Clin Exp Pharmacol Physiol. 40:560–570. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Androulakis ES, Tousoulis D, Papageorgiou
N, Tsioufis C, Kallikazaros I and Stefanadis C: Essential
hypertension: Is there a role for inflammatory mechanisms? Cardiol
Rev. 17:216–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sprague AH and Khalil RA: Inflammatory
cytokines in vascular dysfunction and vascular disease. Biochem
Pharmacol. 78:539–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alonso J, Sánchez de Miguel L, Montón M,
Casado S and López-Farré A: Endothelial cytosolic proteins bind to
the 3′ untranslated region of endothelial nitric oxide synthase
mRNA: Regulation by tumor necrosis factor alpha. Mol Cell Biol.
17:5719–5726. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Neumann P, Gertzberg N and Johnson A:
TNF-alpha induces a decrease in eNOS promoter activity. Am J
Physiol Lung Cell Mol Physiol. 286:L452–L459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ren B and She Q: Study on the association
between IL-1β, IL-8 and IL-10 gene polymorphisms and risk of
coronary artery disease. Int J Clin Exp Med. 8:7937–7943.
2015.PubMed/NCBI
|
|
50
|
Kasal DA, Barhoumi T, Li MW, Yamamoto N,
Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P and Schiffrin
EL: T regulatory lymphocytes prevent aldosterone-induced vascular
injury. Hypertension. 59:324–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schiffrin EL: Peroxisome
proliferator-activated receptors and cardiovascular remodeling. Am
J Physiol Heart Circ Physiol. 288:H1037–H1043. 2005. View Article : Google Scholar : PubMed/NCBI
|