Introduction
It is hypothesized that the rise of colorectal
cancer (CRC) incidence and mortality rate in China is due to the
prevalence of the westernized lifestyle, obesity and physical
inactivity (1,2). However, the mechanisms underlying CRC
tumorigenesis have not been fully understood until now (3). Importantly, the lack of efficient
biomarkers for CRC diagnosis and treatment has resulted in tumors
reaching an advanced tumor stage prior to diagnosis and
consequently a worse overall survival (4). Therefore, the identification of novel
biomarkers will help us to improve the survival quality of CRC
patients.
microRNAs (miR/miRNAs) are a family of non-coding
RNAs that can regulate target genes expression through
3′-untranslated region (UTR), 5′-UTR or open reading frame binding
(5–7). Extensive studies have demonstrated that
miRNA expression is dysregulated in cancer, functioning as either
tumor suppressors or oncogenes (8,9). miR-761
was demonstrated to be closely associated with the initiation and
progression of several cancer types (10–12). For
instance, Zhou et al (10)
demonstrated that miR-761 overexpression could impact the function
of mitochondrial through targeting Mitofusin-2 and resulted in
impaired tumor growth and metastasis. The oncogenic role of miR-761
was also validated in non-small cell lung cancer (NSCLC) by Yan
et al (11). They
demonstrated miR-761 targets inhibitor of growth family, member 4
and tissue inhibitor of metalloproteinase 2 to promote NSCLC
progression and metastasis (11). On
the contrary, Shi et al (12)
reported that miR-761 expression was significantly downregulated in
ovarian cancer tissues compared with in their paired noncancerous
tissues. Functional assays revealed that miR-761 suppressed ovarian
cancer proliferation and invasion by targeting MSI1 (12). In particular, Cao et al
(13) demonstrated miR-761 regulates
chemo-sensitive CRC cells by targeting forkhead box protein M1.
However, more effort is required to fully elucidate the function of
miR-761 in CRC.
In this present study, Rab3D was identified as a
direct target of miR-761 as it contains a binding sequence in its
3′-UTR. The biological role of miR-761 in regulating CRC cell
proliferation and migration was also investigated. It was also
demonstrated that miR-761 expression was significantly
downregulated in CRC and could regulate cell proliferation and
migration through targeting Rab3D. Identification this connection
will provide novel therapeutic approaches for CRC.
Materials and methods
Clinical specimens
A total of 113 pairs of CRC tissues and adjacent
normal tissues were obtained from CRC patients who underwent
treatment between May 2010 and December 2012 at Cancer Hospital of
China Medical University, Liaoning Cancer Hospital and Institute
(Shenyang, China). All collected tissues were snap-frozen and
stored at −80°C until RNA or protein extraction. None of these
patients have ever received any anticancer treatments prior to
surgery. Furthermore, the protocol was approved by the Research
Ethics Committee of Cancer Hospital of China Medical University,
Liaoning Cancer Hospital and Institute and written informed consent
was obtained from all patients. The clinicopathological features of
these enrolled patients were summarized in Table I.
 | Table I.Correlations of miR-761 and
clinicopathological features of CRC patients. |
Table I.
Correlations of miR-761 and
clinicopathological features of CRC patients.
| Clinicopathological
features | No. | Low miR-761
(n=58) | High miR-761
(n=55) | P-value |
|---|
| Age (years) |
|
|
| ns |
|
>60 | 53 | 30 | 23 |
|
|
<60 | 60 | 28 | 32 |
|
| Sex |
|
|
| ns |
| Male | 51 | 27 | 24 |
|
|
Female | 62 | 31 | 31 |
|
| Tumor size (cm) |
|
|
| 0.023 |
|
>5 | 64 | 34 | 30 |
|
|
<5 | 49 | 24 | 25 |
|
| Tumor stage |
|
|
| 0.016 |
| I–II | 48 | 22 | 26 |
|
| III | 65 | 36 | 29 |
|
Cell culture and transfection
Human normal colon epithelial cell line (FHC) and
CRC cell lines (HT29, SW480 and SW620) were purchased from the
American Type Culture Collection (Manassas, VA, USA). CRC cells
were cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) was used to culture the FHC cells. These cells
were maintained in a 37°C humidified atmosphere containing 5%
CO2.
Transfection was conducted using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, cells were cultured until about 70% confluence. miR-761
mimic (miR-mimic) for miR-761 upregulation, negative control (NC)
miRNA and pCMV3-Rab3D plasmid for Rab3D overexpression was mixed
with Lipofectamine® 2000 and incubated with cell
suspensions. Following 48-h transfection, cells were collected and
used for subsequent experimentation. miRNAs were purchased from
Shanghai GenePharma Co., Ltd., (Shanghai, China). pCMV3-Rab3D
plasmid was purchased from Sino Biological Inc., (Beijing,
China).
Cell proliferation assay
Cell proliferation was measured by Cell Counting
kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocol. Briefly, cells were
seeded at a density of 5×103 cells/well and incubated
for 0, 24, 48 and 72 h. Following this the CCK-8 reagent was added
to each well and further incubated for 4 h. Absorbance was detected
at 450 nm using an ELISA plate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Wound healing assay
Cells were plated in 6-well plates and allowed to
grow until ~90% confluence. A pipette tip (200 µl) was used to
create wound in cell surface following serum starvation for 24 h.
Images was captured 0 and 24 h after the wound was created using a
CX31 light microscope (Olympus Corporation, Tokyo, Japan).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from tissues and cells using
TRIzol® reagent (Beyotime Institute of Biotechnology),
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). The expression level of
miR-761 was assessed by qPCR using SYBR® Premix Ex
Taq™ kit (Takara Biotechnology Co., Ltd.) on an ABI 7500
real-time PCR System (Applied Biosystems, Foster City, CA, USA).
The following primer pairs were used for qPCR: miR-761 forward,
5′-ACAGCAGGCACAGAC-3′ and reverse, 5′-GAGCAGGCTGGAGAA-3′; U6 small
nuclear (sn)RNA forward, 5′-CTGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The following thermocyclinf conditions
were used for the qPCR: Initial denaturation at 95°C for 10 min; 40
cycles of 95°C for 30 sec and 58°C for 30 sec. miR-761 expression
levels were quantified using the 2−ΔΔCq method and
normalized to the internal reference gene U6 (14).
Western blot analysis
Radioimmunoprecipitation assay protein lysis buffer
(Beyotime Institute of Biotechnology) was used to extract total
protein from cells and tissues. Total protein concentration was
analyzed by bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology) and 50 µg protein/lane was separated
via SDS-PAGE on a 10% gel. The separated proteins were transferred
onto polyvinlyidene difluoride membranes (Beyotime Institute of
Biotechnology) and blocked for 1 h at room temperature with 5%
fat-free milk blocking. The membranes were incubated with primary
antibodies (rabbit anti-Rab3D; 1:1,000; cat. no. ab128997; Abcam,
Cambridge, MA, USA and rabbit anti-GAPDH; 1:1,000; cat. no. 5174,
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C. Following washing, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (1:10,000; cat. no. 7074; Cell Signaling Technology, Inc.)
for 1 h at room temperature. Protein bands were developed using the
BeyoECL Plus kit (Beyotime Institute of Biotechnology).
Luciferase activity reporter
assay
TargetScan (www.targetscan.org/vert_72) was used to predict target
genes of miR-761. Rab3D was identified as a potential target of
miR-761 with a putative miR-761 binding site in the 3′-UTR.
Wild-type (wt) and mutant (mut) Rab3D 3′-UTR was cloned into
pMIR-Report (Promega Corporation, Madison, WI, USA). For luciferase
activity analysis, cells were seeded into 24-well plates at a
density of 5×103 cells/well and co-transfected with wt
or mut Rab3D construct along with miR-mimic or NC using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were collected at 48 h following
transfection for luciferase activities measurement using a Dual
Luciferase Assay system (Promega Corporation). Firefly luciferase
activity was normalized to Renilla luciferase activity.
Statistical analysis
Data are presented as the mean ± standard deviation
from at least three independent experiments. Difference in groups
was analyzed using Student's t-test or one-way analysis of variance
and Tukey test at GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). Kaplan-Meier method and log-rank test was used to
calculate overall survival. Correlation of miR-761 and Rab3D was
analyzed using Spearman's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-761 is downregulated
in CRC tissues and cell lines
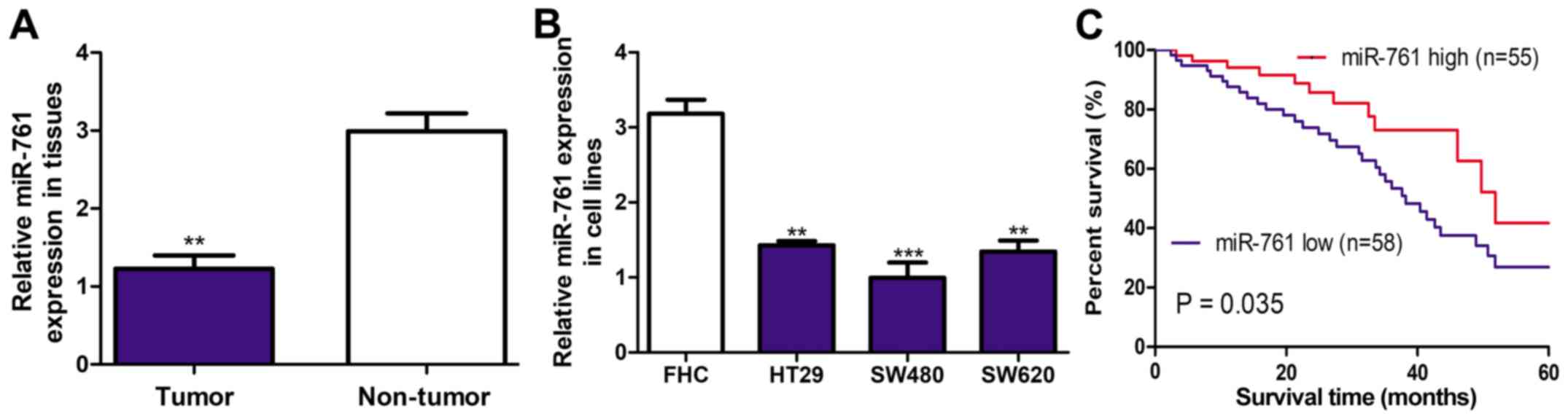
It was demonstrated that miR-761 expression level
was significantly downregulated in CRC tissues compared with the
adjacent normal tissues using RT-qPCR (P<0.01 Fig. 1A). Furthermore, miR-761 expression
level in CRC cell lines was examined and demonstrated that miR-761
expression was significantly downregulated in CRC cell lines
compared with the FHC cell line (P<0.01; Fig. 1B). Furthermore, miR-761 expression
was the lowest in the SW480 cell line out of the three CRC cell
lines investigated (Fig. 1B).
Therefore, SW480 cell line was selected for further functional
assays.
Downregulation of miR-761 is
correlated with poor 5-year overall survival
The association of miR-761 expression and 5-year
overall survival was further investigated to evaluate the clinical
significance of miR-761 expression in CRC progression. The median
expression of miR-761 level was used to subdivide into miR-761 low
(n=58) or high (n=55) expression groups. It was demonstrated that
miR-761 low expression was correlated with worse 5-year overall
survival (P=0.035; Fig. 1C). The
analysis of association between miR-761 and clinicopathological
features demonstrated that low miR-761 expression was correlated
with tumor size (P=0.023) and tumor stage (P=0.016; Table I). However, no significant
association between miR-761 expression and age or sex was observed
(Table I).
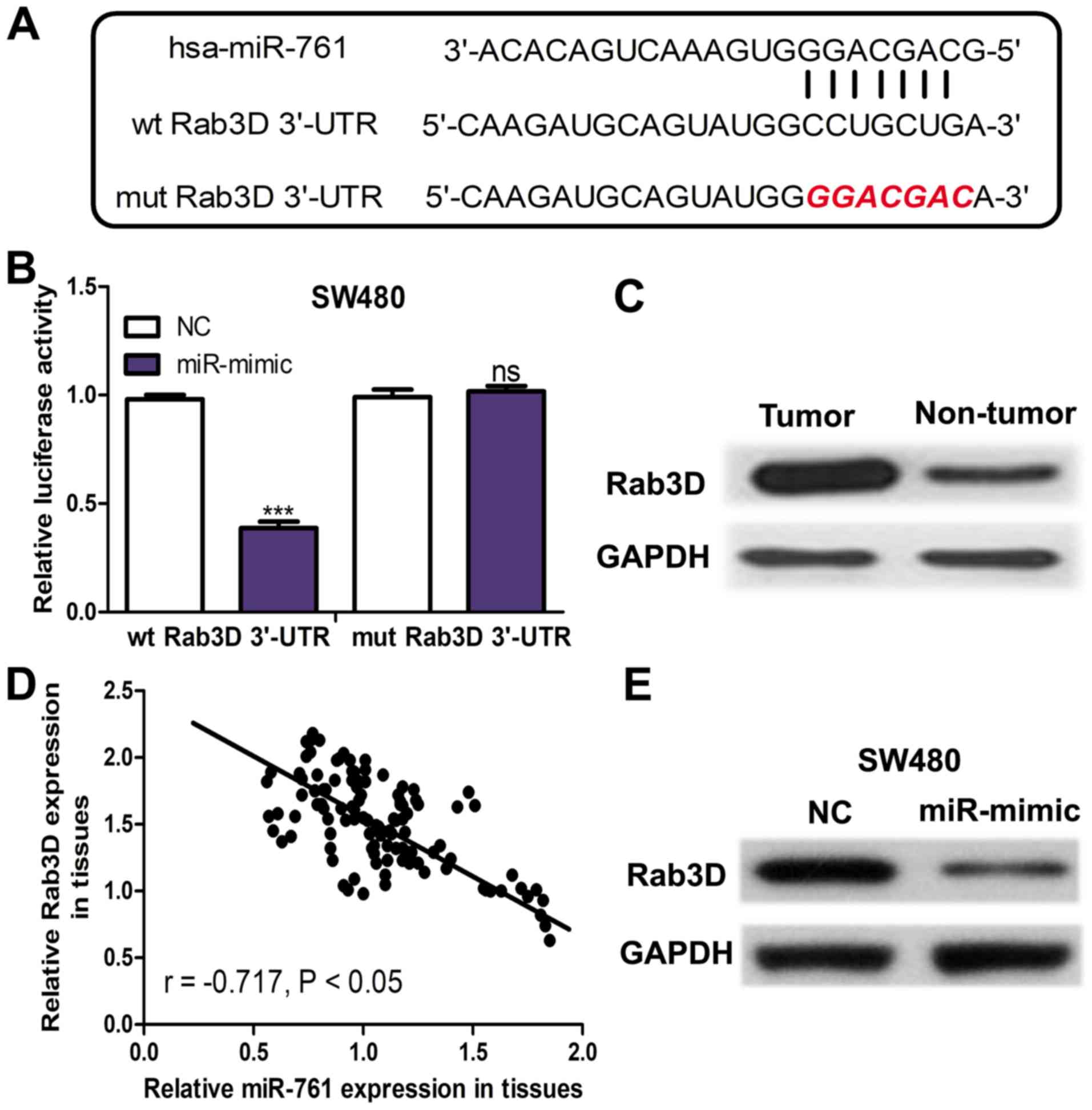
Rab3D is a direct target of
miR-761
TargetScan was used to predict potential targets of
miR-761. The binding site between miR-761 and Rab3D 3′-UTR was
presented in Fig. 2A. The Luciferase
activity reporter assay revealed that miR-mimic significantly
decreased the luciferase activity of wt Rab3D 3′-UTR (P<0.001)
but not mut Rab3D 3′-UTR (Fig. 2B).
The protein expression of Rab3D was measured in the collected
tissues and it was demonstrated that Rab3D expression was
upregulated in CRC tissues compared with in the adjacent normal
tissues (Fig. 2C). Furthermore, an
inverse correlation between miR-761 and Rab3D expression was
identified in CRC tissues (Fig. 2D).
Furthermore, miR-761 expression in miRNAs transfected SW480 cell
line was detected. It was demonstrated that Rab3D expression was
downregulated in the SW480 cell line transfected with miR-mimic
compared with the NC (Fig. 2E).
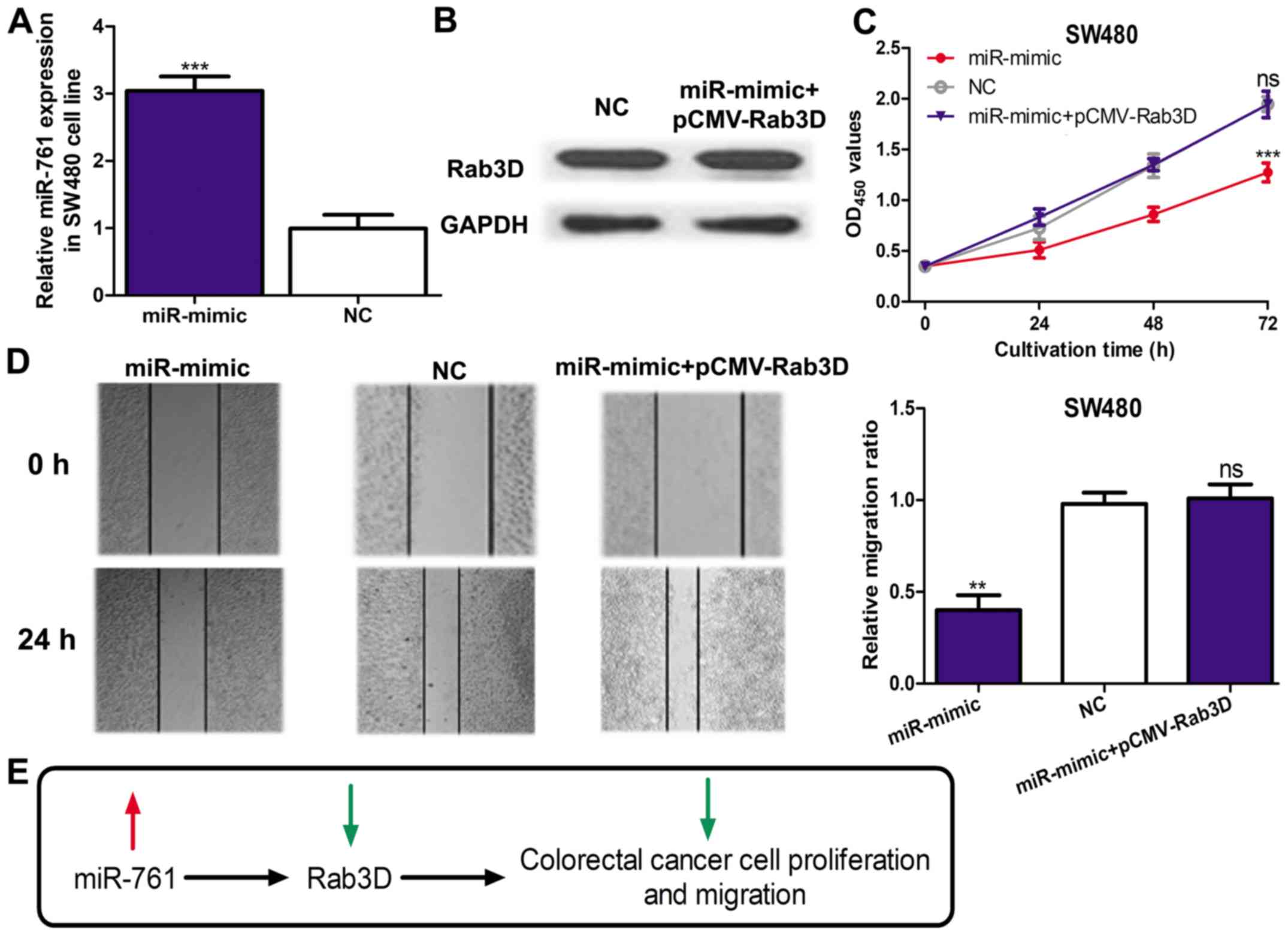
miR-761 regulates cell proliferation
and migration through targeting Rab3D
To investigate whether Rab3D was a mediator for the
biological role of miR-761, an miR-mimic and pCMV3-Rab3D was
co-transfected into the SW480 cell line. miR-761 expression was
significantly elevated by the miR-mimic (P<0.001; Fig. 3A). In addition, Rab3D expression in
the miR-mimic and pCMV-Rab3D co-transfected cells was similar to
that in the NC transfected cell, which suggested pCMV-Rab3D could
efficiently abolish the effect of the miR-mimic on Rab3D expression
(Fig. 3B). A cell proliferation
assay revealed that miR-mimic could significantly downregulate
SW480 cell proliferation (P<0.001) but this effect can be
partially reversed by pCMV-Rab3D (Fig.
3C). Similarly, the cell migration assay also demonstrated that
the inhibition effect of miR-mimic on cell migration could be
reversed by pCMV-Rab3D (Fig. 3D).
These results confirmed overexpression of miR-761 inhibited the
proliferation and migratory abilities of CRC through regulating the
expression of Rab3D (Fig. 3E).
Discussion
CRC development has been reported to be accompanied
by numerous genetic and epigenetic alterations (4). However, the molecular mechanisms
underlying CRC pathogenesis are still not fully understood and
therefore the therapeutic methods for treating CRC remain limited
(3,4). Therefore, the identification of novel
biomarkers to facilitate the development of novel therapeutic
methods for CRC is in urgent demand.
miR-761 was aberrantly expressed in several human
types of cancer including hepatocellular carcinoma, NSCLC and
ovarian cancer (10–12). miR-761 was also capable of regulating
cell behaviors through regulating numerous target genes (10–12). For
example, it was reported that miR-761 regulated the sensitivity of
CRC cell to chemotherapy but little is known regarding the function
of miR-761 in mediating cell behaviors (13). In addition, the current study
demonstrated that miR-761 expression was downregulated in CRC
tissues compared with in matched normal tissues. Furthermore, low
miR-761 expression was a predictor for advancer tumor stage, large
tumor size and even poorer 5-year overall survival of CRC patients.
Collectively, miR-761 expression may serve as a prognostic
predictor for CRC.
miRNAs are important regulators for tumorigenesis
and it was proved that miRNAs have the potential to be used as
novel therapeutic methods for cancer (10–13,15).
Rab3D, one member of the Rab GTPase family, serves an oncogenic
role in several cancer types including osteosarcoma, esophageal
squamous cell carcinoma and CRC (16–18).
Evidence demonstrated that Rab3D is a key player in regulating
tumor metastasis, which suggested that silencing Rab3D expression
may be a way to restrain cancer metastasis (19). However, the molecule that regulates
Rad3D expression in the progression of cancer is poorly understood.
In the present study, it was demonstrated Rab3D was a direct target
of miR-761 by bioinformatics analysis and luciferase activity
reporter assay. Importantly, the present study demonstrated the
inverse correlation between miR-761 and Rab3D expression in CRC
tissues. In vitro functional assays revealed overexpression
of miR-761 inhibited CRC cell proliferation and migration. In
addition, it was demonstrated that overexpression of Rab3D
attenuated the suppressive effects of miR-mimic on CRC cell
proliferation and migration.
In conclusion, the present study demonstrated that
miR-761 which was downregulated in CRC inhibited CRC cell
proliferation and migration partially through regulating Rab3D. The
present study also demonstrate that low miR-761 was correlated with
worse 5-year overall survival of CRC patients. Furthermore, the
present study provides a novel insight into the mechanism regarding
CRC progression and a promising future for miR-761/Rab3D
axis-oriented treatment of CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YR, GS, PJ and QM participated in the study design,
carried out experiments, collected and analyzed the data and
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Cancer Hospital of China Medical University, Liaoning
Cancer Hospital and Institute.
Patient consent for publication
Written informed consent was obtained from all
patients prior to enrollment in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goss PE, Strasser-Weippl K, Lee-Bychkovsky
BL, Fan L, Li J, Chavarri-Guerra Y, Liedke PE, Pramesh CS,
Badovinac-Crnjevic T, Sheikine Y, et al: Challenges to effective
cancer control in China, India, and Russia. Lancet Oncol.
15:489–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varghese C and Shin HR: Strengthening
cancer control in China. Lancet Oncol. 15:484–485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chakradhar S: Colorectal cancer: 5 big
questions. Nature. 521:S162015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brody H: Colorectal cancer. Nature.
521:S12015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohtsuka M, Ling H, Doki Y, Mori M and
Calin GA: MicroRNA processing and human cancer. J Clin Med.
4:1651–1667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melo SA and Esteller M: Dysregulation of
microRNAs in cancer: Playing with fire. FEBS Lett. 585:2087–2099.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moretti F, Thermann R and Hentze MW:
Mechanism of translational regulation by miR-2 from sites in the 5′
untranslated region or the open reading frame. RNA. 16:2493–2502.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visone R and Croce CM: MiRNAs and cancer.
Am J Pathol. 174:1131–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Leva G and Croce CM: miRNA profiling of
cancer. Curr Opin Genet Dev. 23:3–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Zhang L, Zheng B, Yan Y, Zhang Y,
Xie H, Zhou L, Zheng S and Wang W: MicroRNA-761 is upregulated in
hepatocellular carcinoma and regulates tumorigenesis by targeting
Mitofusin-2. Cancer Sci. 107:424–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan A, Yang C, Chen Z, Li C and Cai L:
MiR-761 promotes progression and metastasis of non-small cell lung
cancer by targeting ING4 and TIMP2. Cell Physiol Biochem. 37:55–66.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi C and Zhang Z: miR-761 inhibits tumor
progression by targeting MSI1 in ovarian carcinoma. Tumour Biol.
37:5437–5443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao S, Lin L, Xia X and Wu H: MicroRNA-761
promotes the sensitivity of colorectal cancer cells to
5-Fluorouracil through targeting FOXM1. Oncotarget. 9:321–331.
2017.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiashi W, Chuang Q, Zhenjun Z, Guangbin W,
Bin L and Ming H: MicroRNA-506-3p inhibits osteosarcoma cell
proliferation and metastasis by suppressing RAB3D expression. Aging
(Albany NY). 10:1294–1305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Kong RR and Sun LZ: Silencing of
Rab3D suppresses the proliferation and invasion of esophageal
squamous cell carcinoma cells. Biomed Pharmacother. 91:402–407.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Y, Ye GY, Qin SL, Mu YF, Zhang L, Qi
Y, Qiu YE, Yu MH and Zhong M: High expression of Rab3D predicts
poor prognosis and associates with tumor progression in colorectal
cancer. Int J Biochem Cell Biol. 75:53–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Liu W, Lu X, Fu Y, Li L and Luo Y:
High expression of small GTPase Rab3D promotes cancer progression
and metastasis. Oncotarget. 6:11125–11138. 2015.PubMed/NCBI
|