Introduction
Statistics provided by the American Cancer Society
revealed that 1,735,350 new cancer cases and 609,640 cancer deaths
are projected to occur in the USA in 2018 (1). Cervical cancer is the fourth most
common and deadly cancer that affects women worldwide, after
breast, colorectal and lung cancer. An estimated 70% of global
cervical cancer cases occur in underdeveloped areas and developing
countries (2). However, patients
with cervical cancer should not be subject to high rates of
mortality even in developing countries. Therefore, it is desirable
to decrease the mortality rate of cervical cancer. According to The
National Central Cancer Registry of China, there were estimated to
be 4.29 million new cases of cancer and 2.81 million
cancer-associated mortalities in 2015 in China (3). For women, two of the 10 most common
cancers are gynecologic cancers, with breast cancer (268,600 new
cases) being the most prevalent and cervical cancer (98,900 new
cases) being the seventh most common cancer in China, which is the
largest formerly developing country (3). Therefore, research to identify novel
therapeutic and preventative treatments for cervical cancer is
underway in China (3).
Human papillomavirus (HPV) infection is one of the
major risk factors for cervical cancer worldwide (4). High-risk HPV, including types 16, 18,
31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68, is the major
cause of cervical cancer. It has been reported that different
cervical cancer cell lines were initiated by different HPV
subtypes. Cervical cancer cell lines with different HPV
statuses/types, including HeLa (HPV16/18+), SiHa (HPV16+), CaSki
(HPV16+) and C33A (HPV-), are commonly used to study cervical
cancer (5,6). The HeLa cell line, which was derived
from cervical cancer patient Henrietta Lacks at Johns Hopkins
Hospital in 1951, is most commonly used. Tissue specimens harvested
during her diagnosis and treatment were the first successfully
isolated and cultured cervical cancer cells. The HeLa cells were
later confirmed to be HPV16/18+. In the case of one drug,
dehydrocostus lactone, which has been identified to inhibit the
proliferation and invasion of HeLa cell lines, this effect was
associated with a time- and dose-dependent reduction in Akt
phosphorylation. These inhibitory effects were enhanced when
treatment was combined with specific phosphatidylinositide-3 kinase
(PI3K)/Akt inhibitors, suggesting that the drugs may act via
pathways including including PI3K/Akt (7). In the present study, the potential
inhibitory effects of epigallocatechin-3-gallate (EGCG) on the
proliferation of cervical cancer cells were assessed.
MicroRNAs (miRNAs/miRs) are small non-coding RNA
molecules that regulate the expression of protein-coding target
genes in eukaryotes (8). It has been
reported that miRNAs are important regulatory factors involved in
numerous crucial biological processes, including metabolism,
migration, invasion, apoptosis and cell proliferation (9). However, a number of studies have
indicated that epigenetic alterations also have a key role in
carcinogenesis and metastasis. An increasing number of studies have
focused on non-coding RNAs, including miRNAs and long non-coding
RNAs (10,11). miRNAs may be used as biomarkers to
assess disease progression or characteristics and may therefore
have an application in the treatment of various cancer types
(12). Furthermore, aberrant DNA
methylation and histone modification in cervical cancer have drawn
attention and have been extensively studied. An increasing number
of studies have focused on non-coding RNAs, including miRNAs and
long non-coding RNAs in particular. miRNAs may be used as
biomarkers to assess disease progression or characteristics, and
may therefore have an application in the treatment of various
cancer types (13). It has been
recognized that genetic mutation is a factor in the development of
cervical carcinoma (14). In recent
years, a number of studies have focused on investigating the
molecular basis and mechanisms of epigenetic modifications in
cervical cancer, including the role of DNA methylation and histone
modification, and the diagnostic, prognostic and therapeutic
potentials of miRNAs (15,16).
Previous studies have reported that certain miRNAs
are downregulated in cervical cancer, while others are upregulated
(17,18). It has been confirmed that miR-203
expression is typically downregulated in cervical cancer cells and
tumor tissue (19). A recent study
by our group demonstrated that miR-125b suppresses tumor growth by
inhibiting PI3K/Akt/mammalian target of rapamycin (mTOR) activity,
and may thus be an effective therapeutic target for cancer
treatment (20). In addition, a
microarray analysis indicated that miR-210 was upregulated in
cervical carcinoma, while miR-29a was downregulated in grade II–III
HPV16+ cervical intraepithelial neoplasia tumors (21). Green tea, a popular drink in China,
has been thought to have beneficial health effects for thousands of
years. It is mentioned in ‘Hua yang guo zhi’, a document written in
the Eastern Jin Dynasty (317–420 AD). Tea has been demonstrated to
inhibit tumor growth in vitro and in vivo (22,23).
Cervical carcinoma is commonly initiated by differential high-risk
subtypes of human papillomavirus, while the genesis of the majority
of tumors may be suppressed by green tea, green tea extract and
green tea polyphenols, particularly EGCG (24). The aim of the current study was to
reveal the mechanism by which EGCG inhibits the proliferation of
cervical cancer cell lines and the regulation of miRNA expression
in different HPV subtypes. The current study demonstrated that EGCG
may become a novel substance to prevent and treat cervical
cancer.
Materials and methods
Cell culture
The human cervical cancer cell lines HeLa
(HPV16/18+), SiHa (HPV16+), CaSki (HPV16+) and C33A (HPV-) were
provided by the Cell Bank of the Shanghai Institute of Cell
Biology, Chinese Academy of Sciences (Shanghai, China). Cells were
electroporated with engineered HPV types 16 and 18 and grown in a
1:1 (v:v) mixture of Dulbecco's modified Eagle's medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and Ham's F12
nutrient mix (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan)
containing 5% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C in a humidified atmosphere
containing 5% CO2.
Green tea compound
EGCG (Sigma-Aldrich; Merck KGaA) was dissolved in
double distilled H2O at a concentration of 5 mg/ml to
prepare a stock solution. The solution was stored in an atmosphere
of N2 at −80°C and diluted compound solutions were
freshly prepared prior to the experiments.
MTT assay
The viability of HeLa cells was measured using an
MTT-based Cell Growth Determination kit (Sigma-Aldrich; Merck
KGaA). Cells were seeded into 96-well flat-bottomed plates (10,000
cells in 100 µl/well), incubated overnight at 4°C and treated with
different concentrations of EGCG (100, 80, 60, 40, 20 and 10 µg/ml)
in triplicate for 0, 24 and 48 h, respectively. Cell culture medium
with DMSO (final concentration, 0.1%; Invitrogen; Thermo Fisher
Scientific, Inc.) was used as the negative controls. After drug
treatment, 10 µl MTT solution was added to each well and the plates
were incubated at 37°C for another 2 h. The supernatant was
subsequently removed and DMSO was added to each well to dissolve
the formazan. The absorbance/optical density (OD) was read at 492
nm using a Thermo MK3 spectrophotometric microplate reader (Thermo
Fisher Scientific, Inc.). The inhibition rate of HeLa cells by EGCG
was calculated using the following formula: Inhibition rate
(%)=(1-ODdrug exposure/ODcontrol) ×100%. The
half inhibition concentration (IC50) value of EGCG in inhibiting
the growth of HeLa cells was measured by the MTT assay. Values were
determined from the inhibition rate vs. drug concentration graphs
using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla,
CA, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis of miRNAs
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), complementary (c)DNA
was synthesized by stem-loop RT-qPCR using the Two Step Stemaim-it
miR qRT-PCR Quantitation kit (SYBR Green; cat. no. LM-0101A;
Novland Biopharma, Shanghai China). qPCR analyses of Homo
sapiens (hsa)-miR-210, hsa-miR-29a, hsa-miR-203 and
hsa-miR-125b was performed with an MX3000P Detection System
(Agilent Technologies, Inc., Santa Clara, CA, USA) following the
manufacturer's protocol. U6 was used as the endogenous control,
with the expression of targeted miRNAs normalized to that of U6.
cDNA was synthesized from 2 µg of total RNA. RT was performed using
the following conditions: 30 min at 16°C, 30 min at 42°C and 5 min
at 85°C, followed by a hold at 4°C. All RT reactions, including
no-template controls and RT minus controls, were run in duplicate.
The PCR amplifications using the Two Step Stemaim-it miR qRT-PCR
Quantitation kit (SYBR Green) were performed in a 96-well plate
with the following thermocycling conditions: 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. All
reactions were run in triplicate. The threshold cycle (Cq) is
defined as the fractional cycle number at which the fluorescence
passes the fixed threshold. The Cq values were calculated
automatically and the quantity of expressed miRNA was calculated
using the following formulae: ∆Cq=Cq(target)-Cq(reference: U6),
ΔΔCq=Cqtest miRNA-Cqcontrol and Relative
Expression Quantity of miRNA=2−ΔΔCq (25,26).
Primers used in the present study were as follows: U6 small nuclear
RNA forward, 5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
3′-GGAACGCTTCACGAATTTG-5′; hsa-miR-210 forward,
5′-GTGCAGGGTCCGAGGT-3′ and reverse, 3′-TGTGCGTGTGACAGCGGC-5′;
hsa-miR-29a forward, 5′-GTGCAGGGTCCGAGGT-3′ and reverse,
3′-CGTAGCACCATCTGAAATCGG-5′; hsa-miR-203 forward,
5′-GTGCAGGGTCCGAGGT-3′ and reverse, 3′-CGCGTGAAATGTTTAGGACC-5′
hsa-miR-125b forward, 5′-GTGCAGGGTCCGAGGT-3′ and reverse,
3′-CGTCCCTGAGACCCTAACTTGT-5′.
Statistical analysis
Values are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). Differences were analyzed by one-way
analysis of variance followed by the Sidak test for multiple
comparisons and multiple linear regression analysis was performed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EGCG inhibits HeLa cell
proliferation
Growth retardation of HeLa cells was observed by
using an MTT assay. Compared with that in the control group, the
growth of HeLa cells was inhibited by EGCG at concentrations
ranging from 10 to 100 µg/ml and treatment times of 24 and 48 h.
Prior to drug the administration, the cells exhibited a normal
growth behavior the inhibition rate was also considered to be the
lowest (0%; Fig. 1). The cell growth
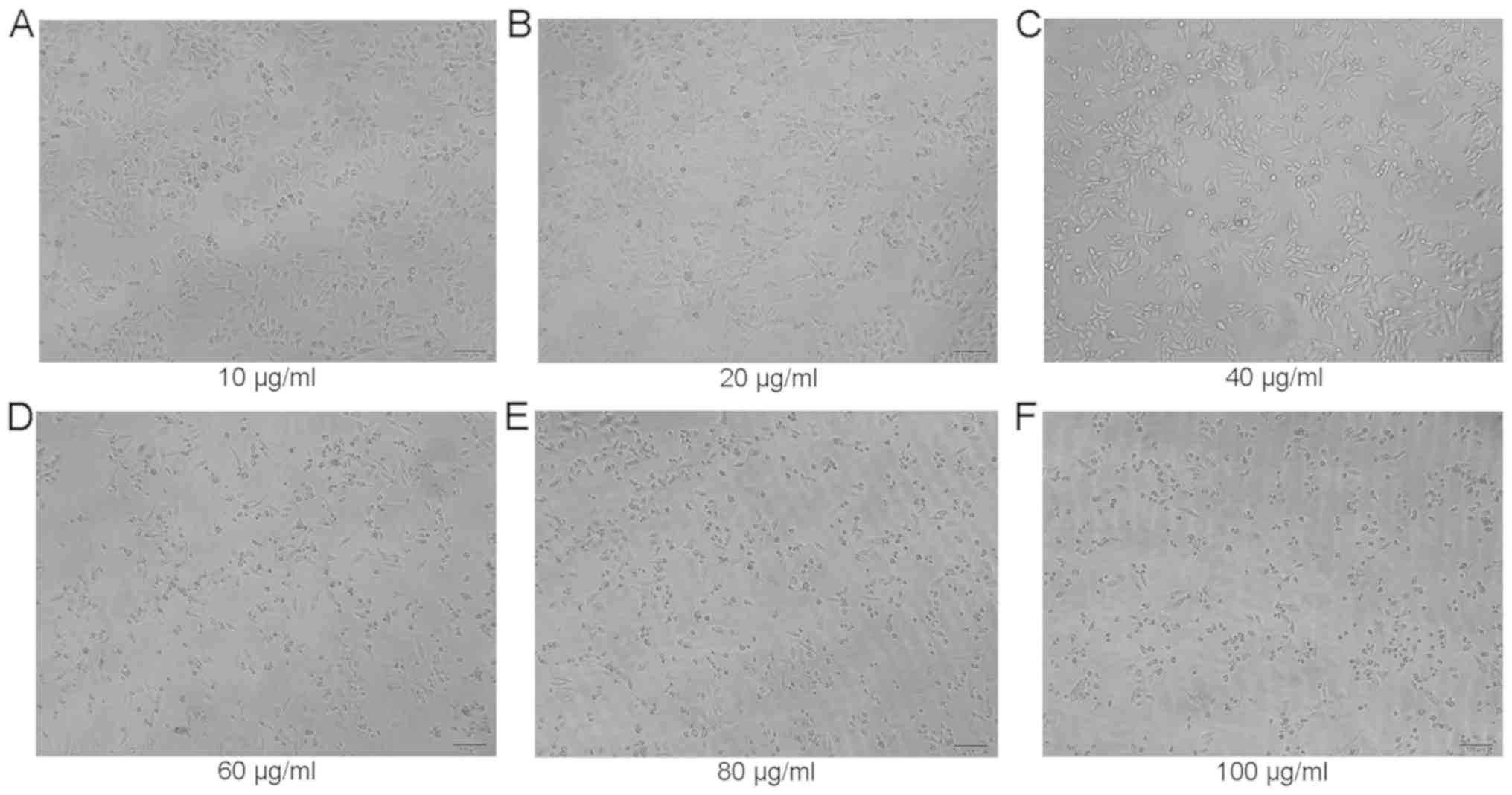
was also inhibited to a certain extent after 24 h of incubation
with EGCG (Fig. 2A-F), and cell
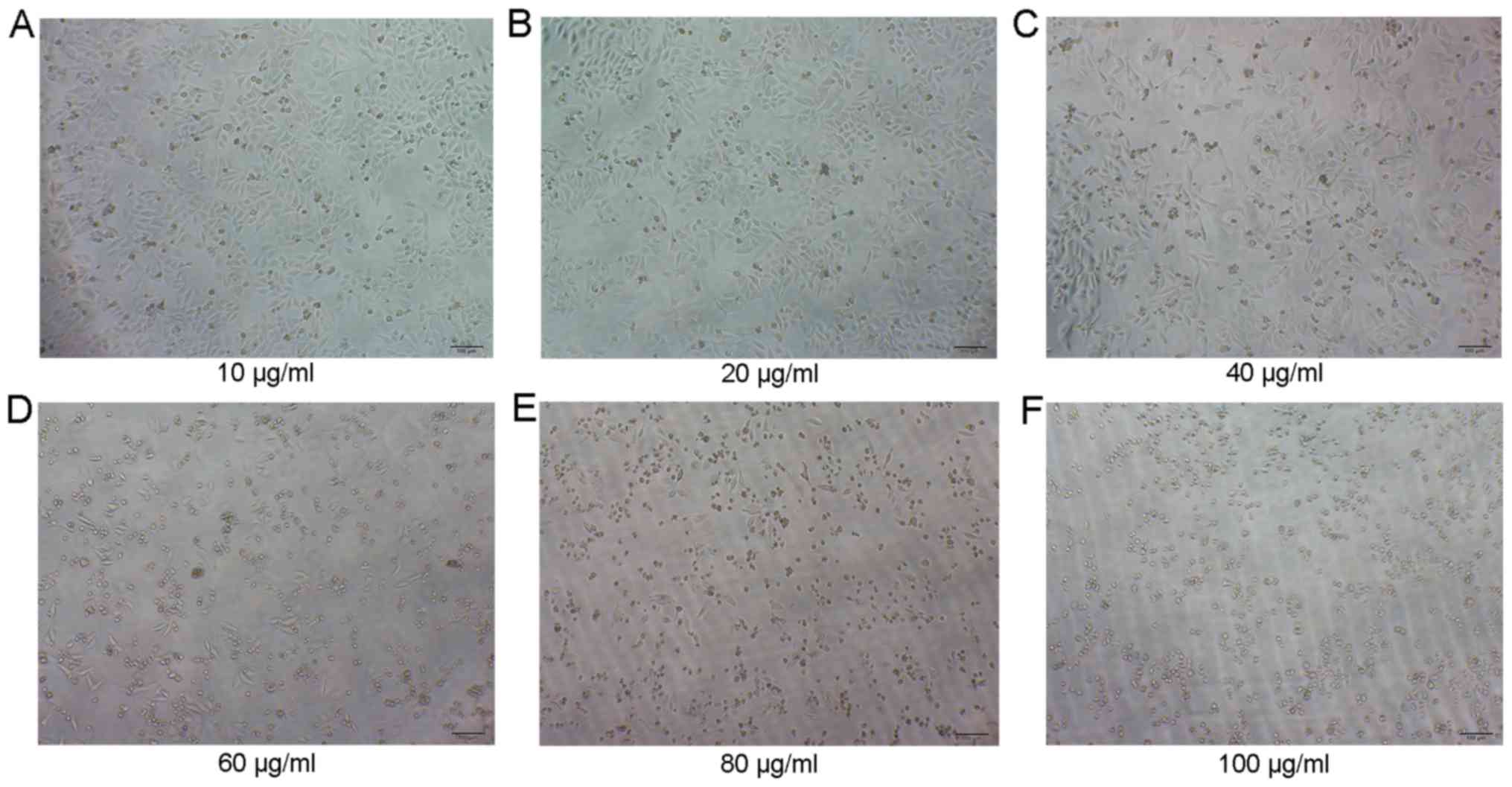
growth was significantly inhibited after 48 h (Fig. 3A-F), and the inhibition was
positively associated with the drug concentration. The cell growth
inhibition by EGCG exhibited time-dependent (P<0.01) and
dose-dependent (P<0.01) characteristics according to GraphPad
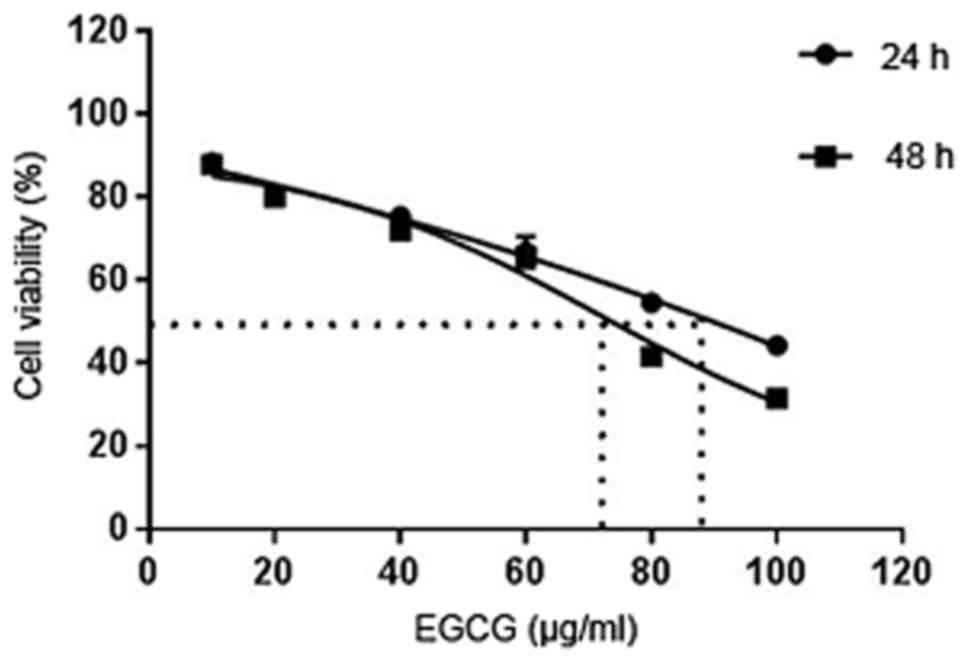
Prism 6 analysis. The IC50 at 24 and 48 h of treatment
was 90.74 and 72.74 µg/ml, respectively (Fig. 4).
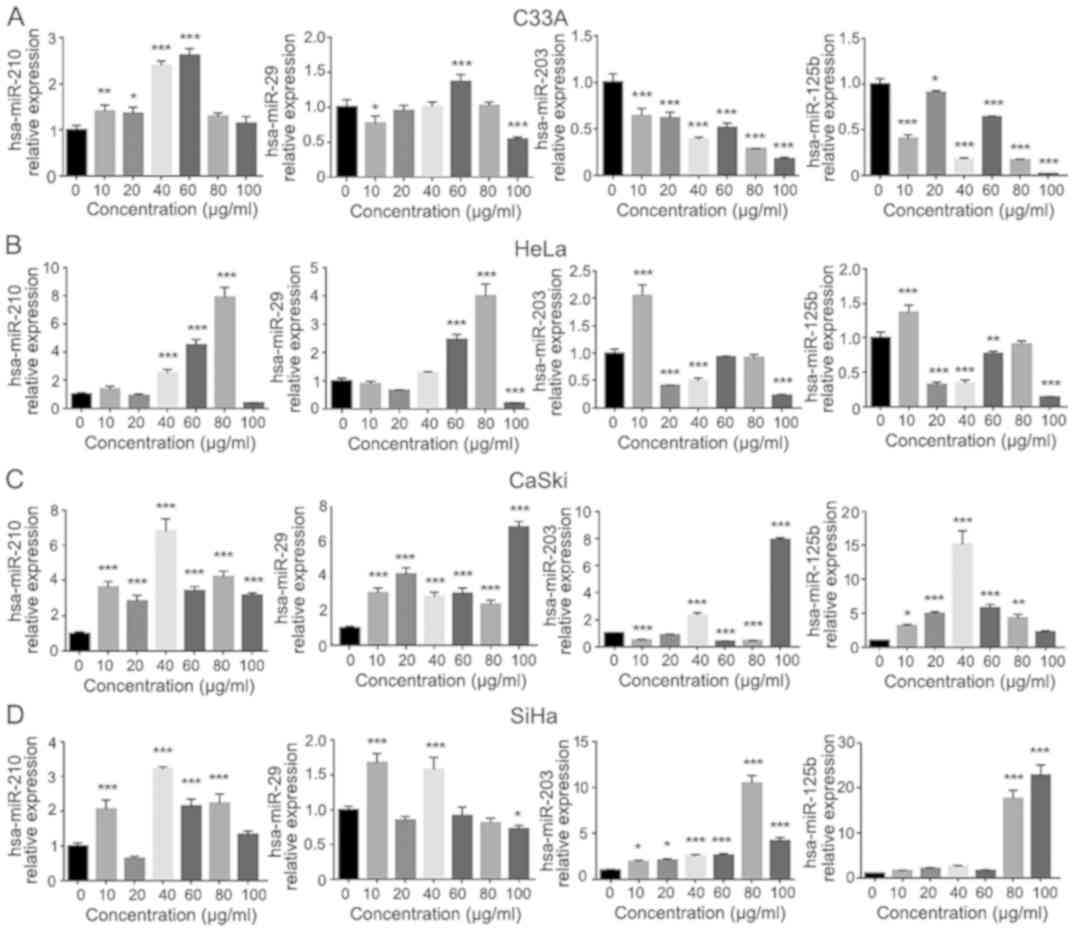
EGCG treatment affects the expression
of hsa-miR-210, hsa-miR-29a, hsa-miR-203 and hsa-miR-125b in
cervical carcinoma cell lines
The results revealed that EGCG treatment regulated
the expression of various miRNAs in different cervical carcinoma
cell lines. Significant results are compared with the control
cells. miR-203 (all P<0.0001) and miR-125b (P<0.01–0.0001)
were significantly downregulated by EGCG treatment, while miR-210
was significantly upregulated by EGCG especially at 40 and 60 µg/ml
(both P<0.0001; Fig. 5A). miR-29
did not follow a trend in C33A cells. In HeLa cells, miR-210 was
significantly upregulated by 40, 60 and 80 µg/ml EGCG, miR-29 was
significantly upregulated by 60 and 80 µg/ml EGCG (all P<0.0001;
Fig. 5B). miR-203 was significantly
downregulated by 20, 40 and 100 µg/ml EGCG (all P<0.0001) and
was significantly upregulated by 10 µg/ml EGCG (all P<0.0001),
therefore the expression of miR-203 did not follow a trend in HeLa
cells. The expression of miR-125b was significantly downregulated
by 20, 40, 60 and 100 µg/ml EGCG (P<0.001–0.0001), however it
was significantly upregulated at 10 µg/ml EGCG (P<0.0001).
miR-210 and miR-29 was significantly upregulated at
all concentrations in CaSki cells (all P<0.0001; Fig. 5C). miR-203 was also significantly
upregulated at 40 and 100 µg/ml, but significantly downregulated at
10, 60 and 80 µg/ml EGCG (all P<0.0001). miR-125b expression was
significantly upregulated with 10–80 µg/ml EGCG, especially at 40
µg/ml (P<0.0001) in CaSki cells. In SiHa cells, miR-210 was
significantly upregulated by 10, 40, 60 and 80 µg/ml EGCG (all
P<0.0001), while miR-29 was significantly upregulated at 10 and
40 µg/ml (both P<0.0001; Fig.
5D). miR-203 was significantly upregulated at all
concentrations (P<0.001–0.0001) and miR-125b was significantly
upregulated with 80 and 100 µg/ml (both P<0.0001).
Discussion
The latest data from Globocan 2018 clarified that
the cancer incidence rate and mortality rate in China is the
highest in the world (27). Among
the 18 million new cancer cases and 9.6 million cancer-associated
mortality worldwide, 3.804 million and 2.296 million were in China,
respectively (28). According to a
recent report from the Chinese authoritative official news agency
Xinhuanet cervical cancer is the second most common cancer among
women, following breast cancer, with 530,000 new cases reported
worldwide every year (29). China
has very high incidence and mortality rates, with ~98,900 new cases
reported in 2015 and 30,500 mortalities. In other words, at least
three Chinese women succumbed to cervical cancer every hour
(30). Thus, the Chinese government
has made efforts towards the prevention and treatment of cervical
cancer, for example HPV E6/E7 mRNA testing has been applied to
assess its potential as a predictive biomarker (31) and the recombinant human interferon
α2a vaginal suppository has been used for the treatment of cervical
cancer (32). Even so, a more cost
effective method remains necessary. The regular consumption of
vegetables and fruit reduces the risk of various cancer types, and
the consumption of tea, particularly green tea, is associated with
a reduced cancer risk (33,34). Cervical cancer is no exception as
stated previously (22). Studies
have demonstrated that natural Chinese medicine monomers and their
derivatives can induce cancer cell apoptosis (35), autophagy (36) or regulatory signaling pathways
(37) by regulating miRNAs. In
addition, the monomers and their derivatives may also affect the
sensitivity of chemotherapy drugs (38).
Shanghai, one of the most developed and wealthy
cities in China, is located in the Yangtze River Delta and is the
major production area of green tea. Therefore, the present study
attempted to seek a way to improve the national conditions in the
field of cervical cancer and HPV infection, to ultimately provide
an economical and appropriate treatment.
Cervical cancer is characterized by epigenetic
modifications, including DNA methylation, aberrant expression of
non-coding RNAs and histone modification, which occur throughout
the process of cancer progression and carcinogenesis A previous
study has reported aberrant regulation of miRNAs in gynecological
cancers; for instance, hsa-miR-126, hsa-miR-143 and hsa-miR-145
were downregulated, while hsa-miR-15b, hsa-miR-16, hsa-miR-146a and
hsa-miR-155 were upregulated (39,40). The
potential effect of green tea catechins has been revealed by a
number of previous studies. EGCG is the major active component of
green tea and it has been reported to block carcinogenesis by
affecting a wide array of signal transduction pathways, including
mitogen-activated protein kinase, PI3K/Akt, Janus kinase/signal
transducer and activator of transcription, mTOR, epithelial growth
factor and Notch/Wnt in vitro. EGCG has potential as an
effective treatment for cancer intervention and prevention due to
its anti-tumor bioavailability, safety and cost-effectiveness. EGCG
is also able to regulate vascular endothelial growth factor, matrix
metalloproteinases, urine plasminogen activator, insulin-like
growth factor-1, EGF receptor and cyclin-dependent kinases (CDKs),
as well as inhibit the activity of telomerase, degrade DNA methyl
transferases, and inhibit cancer cell growth, proliferation,
metastasis and angiogenesis (41,42). It
has been postulated that cyclin d1-CDK4/6 complexes (D-type cyclins
forming complexes through interaction with CDKs) are mainly
responsible for driving the cell cycle transition from G1 to S
phase (43). Catechin hydrate was
reaveled to demonstrate inhibitory activity on SiHa cell
proliferation by mediating apoptosis compared with cells without
treatment (44). A recent study
indicated that catechinmetabolites EGCM7, and EGC-M9, produced from
EGCG, inhibit the proliferation of the HeLa (HPV16/18+) cell lines;
however, the inhibitory mechanisms of catechin metabolites,
produced by intestinal microbiota against cervical cancer cells,
have not been clarified (45).
However, the mechanism underlying cell cycle arrest and apoptosis
induction mediated by EGCG remains to be elucidated.
Previous studies have shown that miR-203
upregulation has been identified in colon (46) and ovarian (47) cancers, whereas miR-203 downregulation
was observed in mucosa-associated hepatocellular carcinoma
(48), central nervous system tumors
(49) and lymphoid tissue lymphoma
(50). In cervical cells, ectopic
expression of hsa-miR-203 decreased their rates of proliferation
and anchorage-independent growth (51). miR-125b was downregulated in ovarian
cancer, oral cancer, cervical cancer and hepatic carcinoma
(52). Furthermore, miR-125b can
increase the chemosensitivity and decrease the proliferation of
breast cancer cells; the mechanism for this was be identical to
that reported in previous studies that investigated apoptosis
through the HAX-1 signaling pathway (53). In gallbladder cancer, miR-125b-5p
enhances chemosensitivity by downregulating Bcl-2 (54). A previous study by our group,
miRNA-125b was demonstrated to regulate the PI3K/Akt/mTOR pathway
by targeting phosphatidylinositol-4,5-bisphosphate 3-kinase
catalytic subunit δ (PIK3CD), thus reducing HeLa cell apoptosis via
suppression of B-cell lymphoma 2 (19). Another previous study by our group
from 2013 established that miR-203 is downregulated in cervical
cancer; in addition, it was demonstrated that miR-203 suppresses
cervical cancer cell proliferation and angiogenesis by targeting
VEGFA (20). The results of the
present study demonstrate that EGCG inhibits the proliferation of
cervical cancer cell lines via a mechanism involving regulation by
miRNAs. HeLa cells were treated with EGCG and a time- and
dose-dependent effect on cell proliferation was observed. In
addition, miR-203 and miR-125b were downregulated by EGCG treatment
in C33A cells, while miR-210 was upregulated; the same result was
observed in HeLa cells. miR-210, miR-29 and miR-125b were all
upregulated in CaSki cells, while miR-210, miR-203 and miR-125b
were upregulated to different expression levels in SiHa cells.
According to ancient Chinese literature, green tea
has been used in China for ~3,000 years. It is the largest producer
of tea worldwide, with an annual output of ~2.58 million tons in
2017, including 1.44 million tons of green tea. High-quality
varieties are concentrated in east China. EGCG and EGC are highly
bioactive compared with other tea catechins, and their mechanisms
of action include roles in angiogenesis. EGCG has the potential to
inhibit HPV and treat cervical cancer possibly by regulating the
expression of associated miRNAs, so it may be considered an
effective treatment drug to the cervical cancer in the future.
Further investigation using these agents and research regarding the
factors affecting the diagnosis and treatment of cervical cancer
may yield a novel, cost-effective and safe drug with good efficacy
for the treatment of cervical cancer.
Acknowledgements
The authors would like to thank Dr. Huafei Zou
(Biology Department of California Institute for Biomedical
Research, San Diego, CA, USA) for proofreading of the manuscript
and providing helpful comments.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed in the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and LM designed experiments; QY, WaZ, PY, QG and
JW performed the experiments; and YH analyzed the experimental
results. ML analyzed the sequencing data and developed analysis
tools. LM assisted with the supervision of the research. YZ and YH
wrote the manuscript. WeZ performed a final check and gave approval
of the version of the manuscript to be submitted.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang X, Tang H and Chen T: Epidemiology
of gynecologic cancers in China. J Gynecol Oncol. 29:e72018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Cheng X, Ye J, Xu X, Hong Y, Sui
L, You Z and Xie X: Distribution of human papilloma virus genotype
prevalence in invasive cervical carcinomas and precancerous lesions
in the Yangtze River Delta area, China. BMC Cancer. 18:4872018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Depuydt CE, Beert J, Bosmans E and
Salembier G: Human Papillomavirus (HPV) virion induced cancer and
subfertility, two sides of the same coin. Facts Views Vis Obgyn.
8:211–222. 2016.PubMed/NCBI
|
|
7
|
Jiang E, Sun X, Kang H, Sun L, An W, Yao Y
and Hu X: Dehydrocostus lactone inhibits proliferation,
antiapoptosis, and invasion of cervical cancer cells through
PI3K/Akt signaling pathway. Int J Gynecol Cancer. 25:1179–1186.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
micrornas. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romano G, Veneziano D, Acunzo M and Croce
CM: Small non-coding RNA and cancer. Carcinogenesis. 38:485–491.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorenzi L, Avila Cobos F, Decock A,
Everaert C, Helsmoortel H, Lefever S, Verboom K, Volders PJ,
Speleman F, Vandesompele J and Mestdagh P: Long non-coding RNA
expression profiling in cancer: Challenges and opportunities. Genes
Chromosomes Cancer. 2018 Nov 21;Doi: 10.1002/gcc.22709. (Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soto D, Song C and McLaughlin-Drubin ME:
Epigenetic alterations in human papillomavirus-associated cancers.
Viruses. 9:E2482017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eskander RN: The epigenetic landscape in
the treatment of gynecologic malignancies. Am Soc Clin Oncol Educ
Book. 480–487. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pardini B, De Maria D, Francavilla A, Di
Gaetano C, Ronco G and Naccarati A: MicroRNAs as markers of
progression in cervical cancer: A systematic review. BMC Cancer.
18:6962018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laengsri V, Kerdpin U, Plabplueng C,
Treeratanapiboon L and Nuchnoi P: Cervical cancer markers:
Epigenetics and microRNAs. Lab Med. 49:97–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Li B and Xie X: The roles and
clinical significance of microRNAs in cervical cancer. Histol
Histopathol. 31:131–139. 2016.PubMed/NCBI
|
|
18
|
Granados-López AJ, Ruiz-Carrillo JL,
Servín-González LS, Martínez-Rodríguez JL, Reyes-Estrada CA,
Gutiérrez-Hernández R and López JA: Use of mature miRNA strand
selection in miRNAs families in cervical cancer development. Int J
Mol Sci. 18:E4072017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui F, Li X, Zhu X, Huang L, Huang Y, Mao
C, Yan Q, Zhu J, Zhao W and Shi H: MiR-125b inhibits tumor growth
and promotes apoptosis of cervical cancer cells by targeting
phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol
Biochem. 30:1310–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y,
Zhu J, Cui F, Zhao W and Shi H: miR-203 suppresses tumor growth and
angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol
Biochem. 32:64–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Granados López AJ and López JA: Multistep
model of cervical cancer: Participation of miRNAs and coding genes.
Int J Mol Sci. 15:15700–15733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YQ, Lu JL, Liang YR and Li QS:
Suppressive effects of EGCG on cervical cancer. Molecules.
23:E23342018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou IC, Amarnani S, Chong MT and Bishayee
A: Green tea and the risk of gastric cancer: Epidemiological
evidence. World J Gastroenterol. 19:3713–3722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson R, Bryant S and Huntley AL: Green
tea and green tea catechin extracts: An overview of the clinical
evidence. Maturitas. 73:280–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Danese E, Minicozzi AM, Benati M, Paviati
E, Lima-Oliveira G, Gusella M, Pasini F, Salvagno GL, Montagnana M
and Lippi G: Reference miRNAs for colorectal cancer: Analysis and
verification of current data. Sci Rep. 7:84132017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global Cancer Statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 2018 Oct 23;Doi: 10.1002/ijc.31937.
View Article : Google Scholar
|
|
29
|
Quan Y and Yi Q: Across China: HPV vaccine
becomes available for women under 45 in Shanghai. xinhuanet.
03–Aug;2018.http://www.xinhuanet.com/english/2018-03/08/c_137025464.htm
|
|
30
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang L, Zhu Y, Bai Y, Zhang X and Ren C:
The clinical application of HPV E6/E7 mRNA testing in triaging
women with atypical squamous cells of undetermined significance or
low-grade squamous intra-epithelial lesion Pap smear: A
meta-analysis. J Cancer Res Ther. 13:613–620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Terenzi F, Saikia P and Sen GC:
Interferon-inducible protein P56, inhibits HPV DNA replication by
binding to the viral protein E1. EMBO J. 27:3311–3321. 2015.
View Article : Google Scholar
|
|
33
|
Shirakami Y and Shimizu M: Possible
mechanisms of green tea and its constituents against cancer.
Molecules. 23:E22842018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bettuzzi S, Brausi M, Rizzi F, Castagnetti
G, Peracchia G and Corti A: Chemoprevention of human prostate
cancer by oral administration of green tea catechins in volunteers
with high-grade prostate intraepithelial neoplasia: A preliminary
report from a one-year proof-of-principle study. Cancer Res.
66:1234–1240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lewinska A, Adamczyk-Grochala J,
Kwasniewicz E, Deregowska A and Wnuk M: Ursolic acid-mediated
changes in glycolytic pathway promote cytotoxic autophagy and
apoptosis in phenotypically different breast cancer cells.
Apoptosis. 22:800–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu L, Liu T, Xiao Y, Li X, Zhu Y, Zhao Y,
Bao J and Wu C: Polygonatum odoratum lectin induces apoptosis and
autophagy by regulation of microRNA-1290 and microRNA-15a-3p in
human lung adenocarcinoma A549 cells. Int J Biol Macromol.
85:217–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar VB, Yuan TC, Liou JW, Yang CJ, Sung
PJ and Weng CF: Antroquinonol inhibits NSCLC proliferation by
altering PI3K/mTOR proteins and miRNA expression profiles. Mutat
Res. 707:42–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou DH, Wang X and Feng Q: EGCG enhances
the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC
A549 cells. Nutr Cancer. 66:636–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lorincz AT: Virtues and weaknesses of DNA
methylation as a test for cervical cancer prevention. Acta Cytol.
60:501–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Srivastava SK, Ahmad A, Zubair H, Miree O,
Singh S, Rocconi RP, Scalici J and Singh AP: MicroRNAs in
gynecological cancers: Small molecules with big implications.
Cancer Lett. 407:123–138. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh BN, Shankar S and Srivastava PK:
Green tea catechin, epigallocat-echin-3-gallate (EGCG): Mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang F, Chang Z, Fan Q and Wang L:
Epigallocatechin 3 gallate inhibitsthe proliferation and migration
of human ovarian carcinoma cells by modulating p38 kinase and
matrix metalloproteinase-2. Mol Med Rep. 9:1085–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang D, Al-Hendy M, Richard-Davis G,
Montgomery-Rice V, Rajaratnam V and Al-Hendy A: Anti proliferative
and proapoptotic effects of epigallocatechin gallate on human
leiomyoma cells. Fertil Steril. 94:1887–1893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Al-Hazzani AA and Alshatwi AA: Catechin
hydrate inhibits proliferation and mediates apoptosis of SiHa human
cervical cancer cells. Food Chem Toxicol. 49:3281–3286. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hara-Terawaki A, Takagaki A, Kobayashi H
and Nanjo F: Inhibitory activity of catechin metabolites produced
by intestinal microbiota on proliferation of HeLa cells. Biol Pharm
Bull. 40:1331–1335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gaur A, Jewell DA, Liang Y, Ridzon D,
Moore JH, Chen C, Ambros VR and Israel MA: Characterization of
microRNA expression levels and their biological correlates in human
cancer cell lines. Cancer Res. 67:2456–2468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Craig VJ, Cogliatti SB, Rehrauer H,
Wündisch T and Müller A: Epigenetic silencing of microRNA-203
dysregulates ABL1 expression and drives Helicobacter-associated
gastric lymphomagenesis. Cancer Res. 71:3616–3624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Banzhaf-Strathmann J and Edbauer D: Good
guy or bad guy: The opposing roles of microRNA 125b in cancer. Cell
Commun Signal. 12:302014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jiménez-Wences H, Peralta-Zaragoza O and
Fernández-Tilapa G: Human papilloma virus, DNA methylation and
microRNA expression in cervical cancer (Review). Oncol Rep.
31:2467–2476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu G, Zhao X, Wang J, Lv L, Wang C, Feng
L, Shen L and Ren W: miR-125b regulates the drug-resistance of
breast cancer cells to doxorubicin by targeting HAX-1. Oncol Lett.
15:1621–1629. 2018.PubMed/NCBI
|
|
54
|
Yang D, Zhan M, Chen T, Chen W, Zhang Y,
Xu S, Yan J, Huang Q and Wang J: miR-125b-5p enhances chemotherapy
sensitivity to cisplatin by down-regulating Bcl-2 in gallbladder
cancer. Sci Rep. 7:431092017. View Article : Google Scholar : PubMed/NCBI
|