Introduction
Hematological malignancy of acute leukemia is highly
heterogeneous. At present, there are no standardized chemotherapy
regimens for acute lymphoblastic leukemia (ALL) bone marrow
microenvironment (1), with specific
three dimensional (3D) structure, can support the growth of
leukemia cells as well as protect them from chemotherapy drug
induced death (2–4). 3D scaffold needs some properties to
better mimic the bone marrow environment, such as adequate pore
size, interconnected pores and high surface area, which will
facilitate cells penetration and the formation of cellular
associations (5). 3D scaffold
provides greater physiological relevance in comparison with
conventional 2D culture (6). The
shortcoming of 2D culture is getting increasingly obvious by
studies showing that cell behavior and gene expression are
significantly influenced by the physical and mechanical properties
of their microenvironment (7). Of
the drug discoveries based on 2D culture 95% fail to provide
clinical benefit (8). In this study,
we compared the effects of 3D and 2D culture on drug resistance of
leukemic cells. Polycaprolactone (PCL) is widely used as a 3D
scaffold material for its good biocompatibility and
biodegradability (9,10). For cell culture experiments, the
scaffold with a fiber size of 300 nm in diameter and a pore size of
300 nm diameter was used. PCL scaffold increased cell survival and
drug resistance toward chemotherapy drug Ara-C and daunorubicin
(DNR). Importantly, we found that the molecular mechanisms of cell
survival and drug resistance observed in the scaffold were similar
to those reported in vivo. Moreover, we explored how to
reverse the drug resistance in 3D culture. The developed,
molecularly characterized PCL scaffold could serve as a
drug-testing platform for chemotherapies against drug-resistant
cells.
Materials and methods
Materials
Cell counting kit-8 (CCK-8) and polyvinylidene
fluoride (PVDF) membrane were purchased from Beyotime Biotechnology
(Shanghai, China). BCA protein assay kit, protease inhibitor
cocktail and RIPA lysis buffer were purchased from CWBiotech Co.
Ltd. (Beijing, China). Rat-tail type I collagen were purchased from
Solarbio Science & Technology Co., Ltd. (Beijing, China).
Ara-C, DNR and discoidin domain receptor 1 (DDR1)-IN-1 were
purchased from MedChemExpress USA (New Jersey, USA), RPMI-1640
media was purchased from Hyclone (GE Healthcare Life Sciences,
Logan, UT, USA). Fetal bovine serum (FBS) was purchased from
Biological Industries (Beit Haemek, Israel). CD2-PC5 (cat. no.
A07745), CD3-ECD (cat. no. A07748), CD4-FITC (cat. no. A07751),
CD8-PE (cat. no. A07756), CD34-ECD (cat. no. B49202), (cat. no.
B36294), all purchased from Beckman coulter, Inc. (Brea, CA, USA).
Stat3 (cat. no. 9139), DDR1 (no. 5583), p-STAT3 (no. 9145), GAPDH
(cat. no. 5174s), primary antibodies, goat anti-mouse (cat. no.
7076s) and rabbit HRP-conjugated secondary antibody (cat. no.
14708s) were purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA). PCL scaffolds were purchased from 3D Biotek LLC
(New Jersey, USA).
The study was approved by the Ethics Committee of
Qingdao University (Qingdao, China).
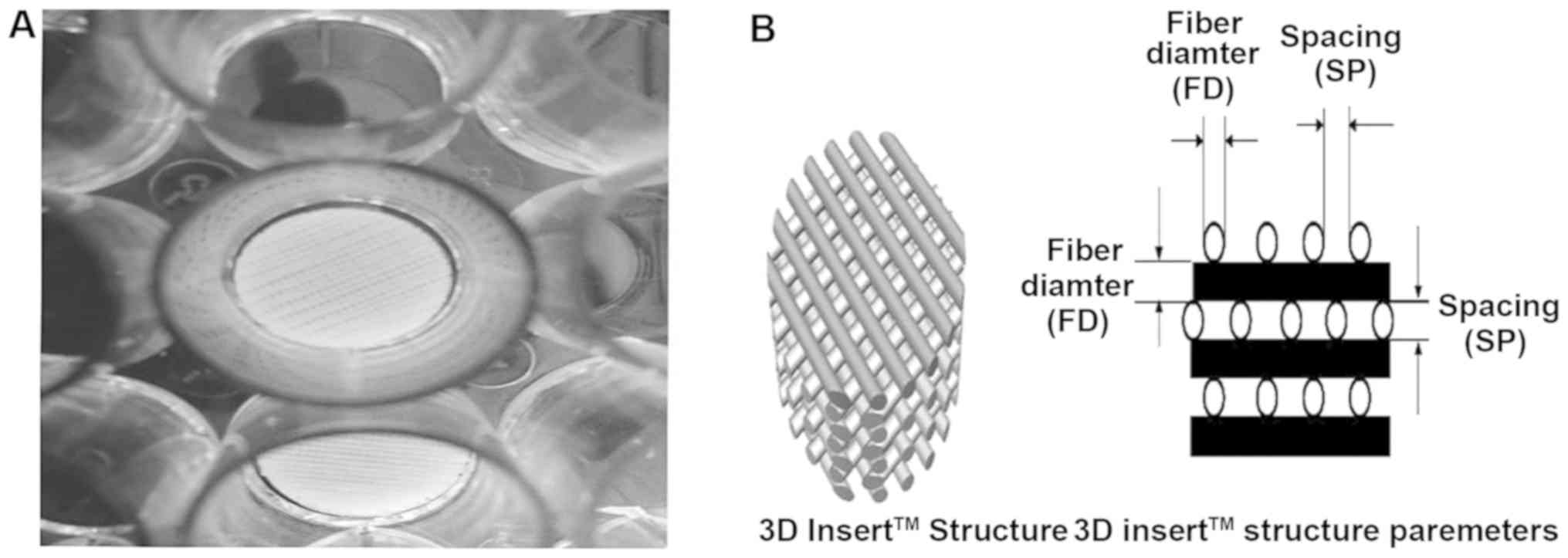
Scaffold coating
Collagen, a major extracellular matrix (ECM)
component, is coated to scaffold to make cell adhere better and as
a ligand of DDR1 (11). DDR1
functions as the unique receptor tyrosine kinase (RTK) which can be
activated by collagen (12,13). PCL scaffolds were transferred into
the 24-well plates and coated with collagen type I in different
concentrations (10, 20, 40, 80, 160 and 320 µg/ml). Then the PCL
scaffold was dried in biosafety hood overnight.
Cell culture
The acute T leukemia cell line Jurkat, Clone E6-1
(ATCC® TIB-152™) was cultured in RPMI-1640 supplemented
with 10% FBS and 1% penicillin/streptomycin. The cells were
maintained at 37°C with 5% CO2 and 95% air (13). For experiments on scaffolds, the
seeding density is different from 2.5×105 to
5×105 cells/ml according to experiments and the seeding
method was in strict accordance to the 3D Insert™ cell seeding
protocol.
Cell proliferation and viability
Cell seeding efficiency was determined 4 h after
seeding into scaffold by counting the number of cells remaining in
the media (14). Cell
proliferation/viability was assessed for 6, 24, 48, 96, 144 and 168
h using CCK-8 reagent. Jurkat cells were seeded at a density of
2.5×105 cells/ml and incubated with CCK-8 reagent for 2
h at 37°C. A total of 100 µl solution was taken for recording the
optical density after incubation. Absorbance at 450 nm was measured
using the Tecan Safire 2 multifunctional microplate reader.
Scanning electron microscopy
(SEM)
In order to visualize the growth of the cells in the
scaffold, the scaffold was examined by SEM (JSM-840; JEOL, Ltd.,
Tokyo, Japan) following cultivation with the Jurkat cells for 72
and 144 h. Briefly, the scaffold was treated with 3% glutaral for 4
h. Then, the scaffold was washed twice with PBS, following 30, 50,
70, 80 and 100% ethanol for dehydration. Then the carbon dioxide
critical point dryer (XD-1; Eiko, Japan) was used to dry the
scaffold. Prior to SEM analysis, samples were coated with gold for
120 sec using auto fine coater (IB-3; EiKo, Japan).
Flow cytometry analysis
Flow cytometry analysis was performed to analyze
whether the cell phenotype changes after cultivation in scaffold
for one week. The expression of CD2, CD3, CD4, CD8, CD34 and CD45
were analyzed. Briefly, nonadherent cells were removed by PBS
washing, then the cells were digested from the scaffold by
collagenase treatment. After digestion, cells were harvested by
gently pipetting the cell suspension up and down five times within
the scaffold. Then the supernatant was centrifuged at 100 × g for 5
min to pellet the cells. The cells pellet was resuspended in PBS
and incubated with antibodies for 15 min at 37°C. Flow cytometry
analysis was performed using FC500 (Beckman Coulter, Inc., Brea,
CA, USA). Isotypes were used as a control.
Western blot analysis
The cells were lysed with RIPA buffer containing
phosphatase and protease inhibitor cocktail. Protein was extracted
from cells using an ultrasonicator for two cycles, 5 sec each time
with a 10-second interval. After incubation for 15 min on ice, the
lysate was centrifuged at 15,493 × g for 20 min. After
centrifugation, the supernatant was collected and quantified with
BCA protein assay. Total protein (20 µg) was separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to PVDF membrane. After blocking with 5%
non-fat milk for 1 h at room temperature, the membranes were probed
with primary antibody against DDR1, STAT3, p-STAT3 and GAPDH
overnight at 4°C, followed by incubation with HRP-conjugated
secondary antibody. The immunoreactive bands were visualized using
Fusion FX7 detection reagents (Vilber Lourmat, Marne-la-Vallée,
France). Autoradiograms were scanned and the labeled bands were
quantified using Image J analysis software (National Institutes of
Health, Bethesda, MD, USA).
Chemoresistance of cells cultured on
scaffolds
3D culture platforms are capable of mimicking
microenvironments in vivo, which provide greater
physiological relevance in comparison to conventional 2D cultures
(15–18). Ara-C (1 µM) and DNR (500 nM) were
used in our experiments according to the clinical application
5×105/ml Jurkat cells were seeded into the tissue
culture plate systems (TCPS), uncoated PCL scaffold (UPS) and
collagen type I coated polycaprolactone scaffold (CTCPS) for 24 h.
Nonadherent cells were removed along with the medium and new medium
with or without drugs was added. For TCPS, cells were taken out
with medium and centrifuged. After removing the medium, the cells
were seeded into wells and medium with or without drugs was added.
The medium was replaced every 48 h. Das et al found that
inhibition of DDR1 expression significantly enhanced
chemosensitivity of breast cancer cells to genotoxic drugs
(19). Thus, DDR1 signaling provides
a new target for therapeutic intervention to leukemia cells.
DDR1-IN-1, an orally bioavailable DDR1 inhibitor (20,21), was
used in our experiments.
Statistical analysis
All experimental data were analyzed by Student's
t-test. All experiments were done three times. Data are presented
as mean ± standard error. P<0.05 was considered to indicate a
statistically significant difference. All tests were completed
using PRISM 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
PCL scaffold coating with ECM
proteins
Collagen type I is one of the main bone marrow ECM
proteins and also the ligand of DDR1 (22). In order to mimic the bone marrow
microenvironment and further study the function of DDR1, PCL
scaffold coating with collagen type I was used in our experiments
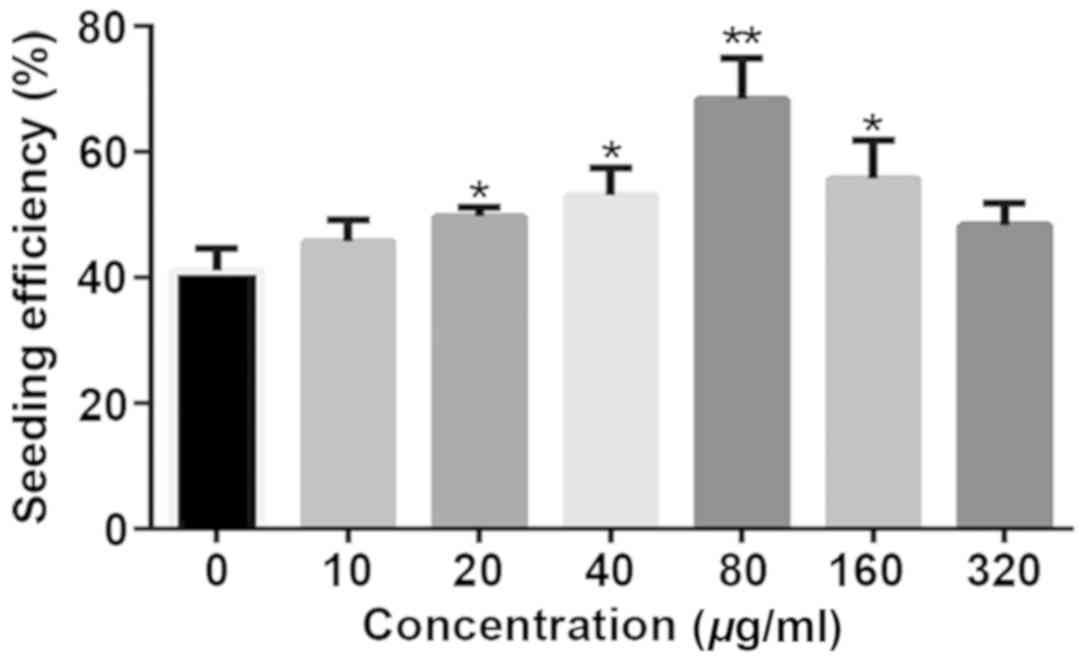
(Fig. 1). The effect of different
concentrations of collagen type I on the cell seeding and cell
proliferation was improved compared to UPS. Indeed, collagen type I
coating enhanced the seeding efficiency. The seeding efficiency
increased gradually at low concentration of collagen type I, but
decreased at high concentration (160 or 320 µg/ml) when compared
with 80 µg/ml collagen type I coated PCL scaffolds (Fig. 2).
Cell proliferation on different
scaffolds
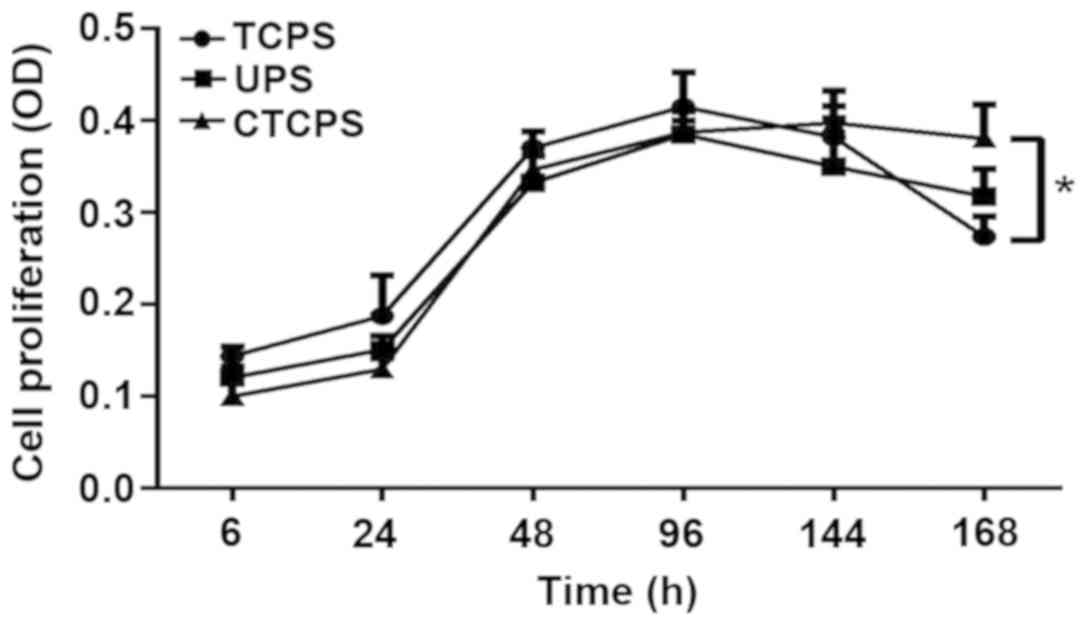
To investigate the effect of PCL scaffold on cell
proliferation, we compared proliferation of Jurkat cells on TCPS,
UPS and 80 µg/ml CTCPS for 168 h. CCK-8 reagent was used to analyze
the extent of cell proliferation/viability. Different growth
kinetics were observed for Jurkat cells cultured on different
conditions. Coating the PCL scaffold with collagen type I enhanced
cell proliferation compared with the TCPS and UPS, especially for a
longer time culture. For the initial 96 h, the TCPS revealed the
maximum cell proliferation/viability compared with the UPS and
CTCPS. However, with the prolongation of the cell culturing, the
CTCPS showed better cell proliferation than the UPS. Moreover,
CTCPS can maintain cell proliferation for 168 h. After 166 h, the
TCPS revealed a significant deterioration in cell proliferation
(Fig. 3).
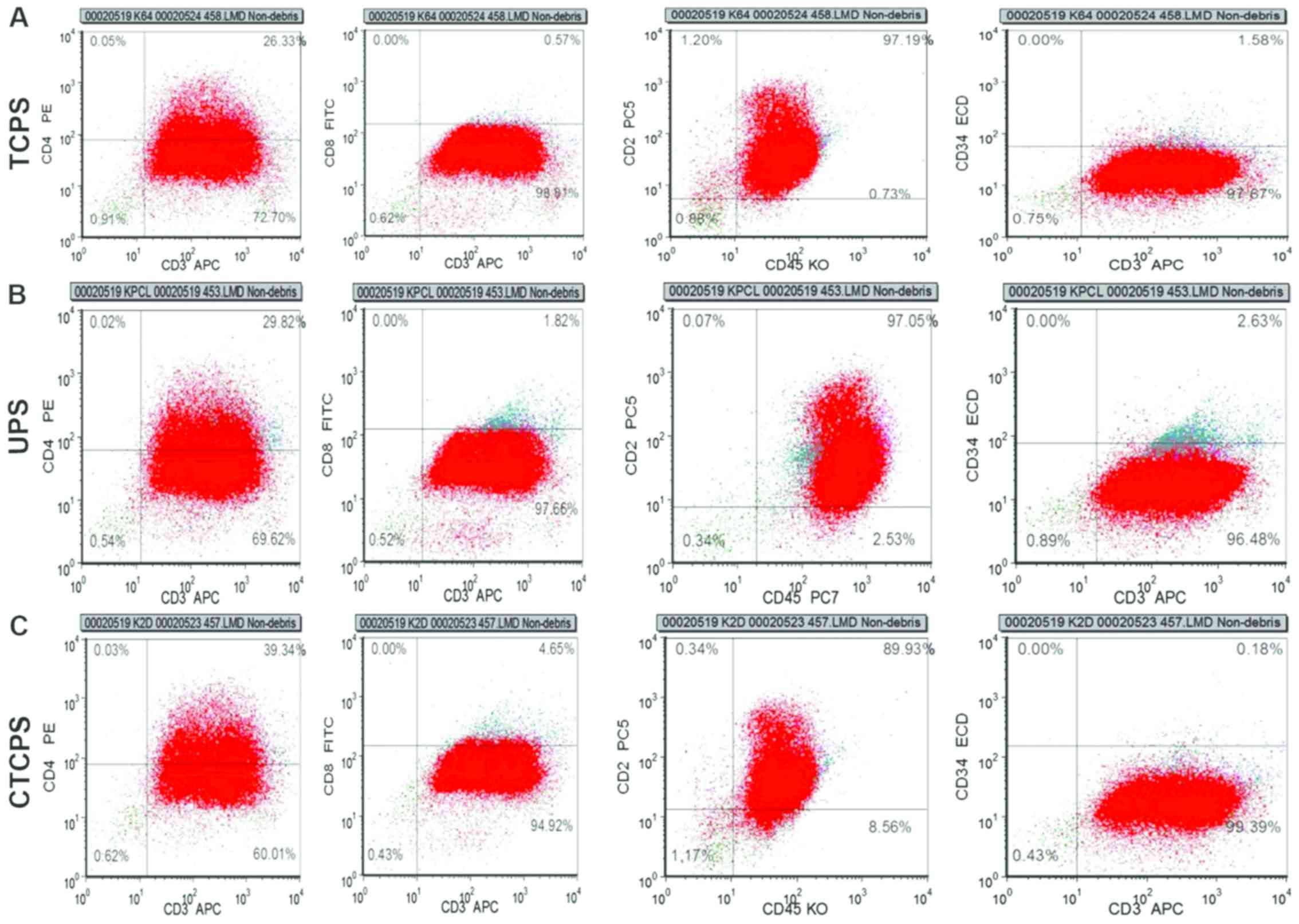
Cellular phenotype analysis
The cell proliferation/viability result showed that
80 µg/ml CTCPS was able to support cell survival and proliferation
better. It is very important for cells to maintain the initial
phenotype and not differentiate with the prolongation of the cell
culture. The expression of CD2, CD3, CD4, CD8, CD34, CD45 in Jurkat
cells cultured in different conditions such as UPS and 80 µg/ml
CTCPS were analyzed at 168 h. The results showed that the
percentage of cells with the phenotype of
CD2+CD3+CD4+dimCD8−CD34−CD45+dim
was maintained for 168 h of culture on the scaffolds compared to
that cultured on TCPS (Fig. 4).
Similarly, the phenotype of Jurkat cell on the CTCPS and UPS showed
no difference in comparison to the TCPS for 168 h.
Sensitivity of drugs on Jurkat cells
at TCPS, UPS and CTCPS
The 80 µg/ml CTCPS was more suitable for cell
proliferation and growth for a longer period without changing cell
phenotype. So we used 80 µg/ml CTCPS for drug testing applications.
Jurkat cells were treated with Ara-C and DNR or their combination
(Fig. 5). CCK-8 reagent was used to
test cell cytotoxicity of Jurkat cells cultured on TCPS, UPS and 80
µg/ml CTCPS. Jurkat cells revealed high sensitivity to Ara-C
cultured on TCPS. Cell proliferation reduced to 89.58±3.26,
62.86±6.84 and 53.36±3.37% for 48, 144 and 168 h respectively on
TCPS, according to the CCK-8 results (Fig. 5A). Cells cultured on 80 µg/ml CTCPS
showed resistance to drug treatment, as shown by the result that
cell proliferation was 103.98±5.3, 88.69±2.19, 77.11±3.47% at 48,
144 and 168 h. Moreover, the drug sensitivity of cells cultured on
UPS was between TCPS and CTCPS, with the relative percentage of
87.98±3.74, 73.28±5.5 and 63.65±0.81% at 48, 144 and 168 h. Cells
cultured under the three conditions showed more sensitivity to DNR
or Ara-C + DNR combination than Ara-C alone. A significant death of
Jurkat cells was observed in TCPS (57.67±8.02, 42±4.58%) when
compared to CTCPS (78±5.57, 57.33±5.03%) in the presence of DNR for
96 and 168 h (Fig. 5B; *P<0.05
vs. TCPS). A similar trend of combination use of Ara-C and DNR was
obtained in TCPS (24.67±4.73%) and UPS cultures (39±7.55%) in
comparison to 80 µg/ml CTCPS (46.33±4.04%) for 168 h (Fig. 5C; **P<0.01 vs. TCPS). In general,
the cells cultured on 80 µg/ml CTCPS showed drug resistance for a
longer culture period compared with TCPS and UPS.
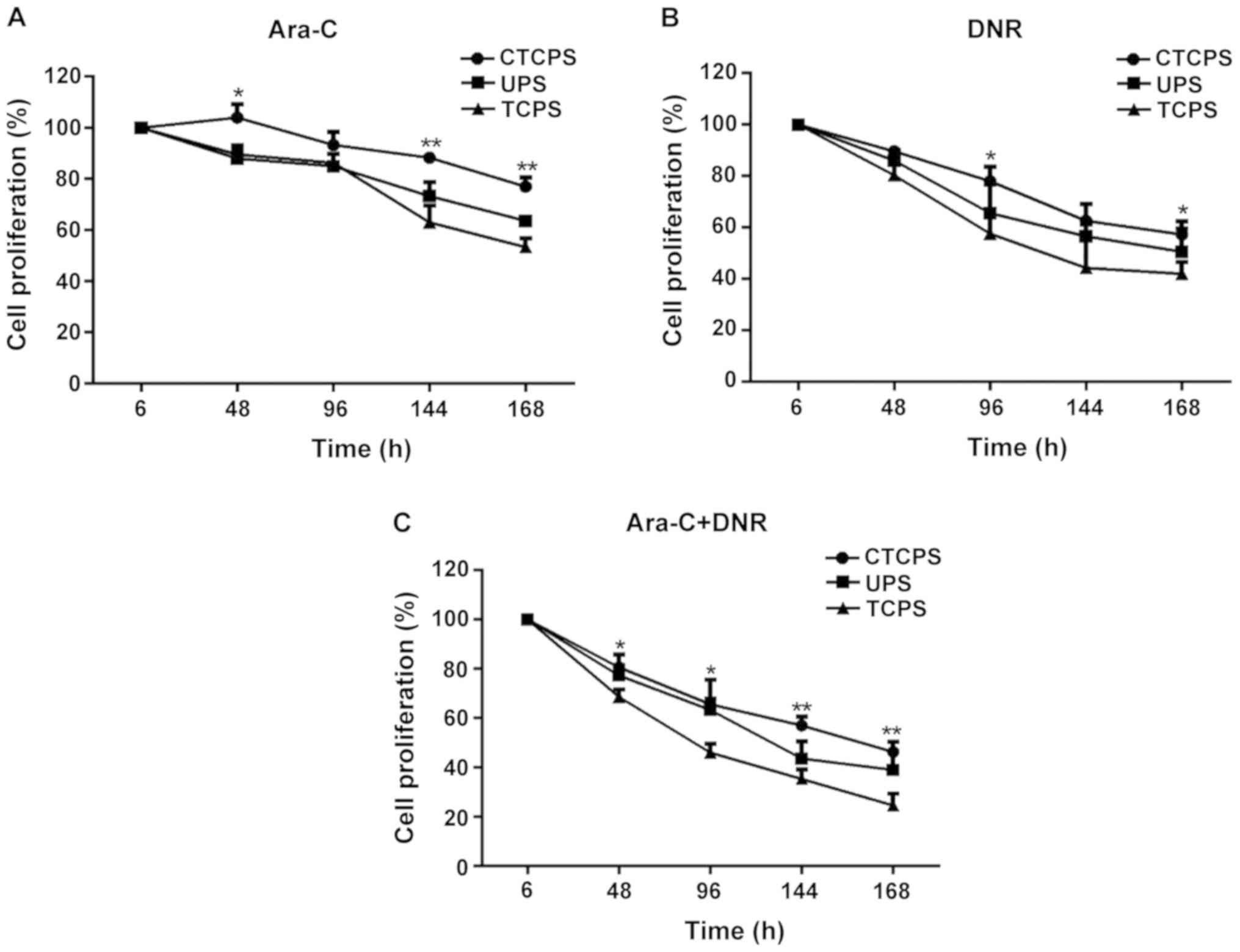 | Figure 5.Comparison of sensitivity of Jurkat
cell to (A) Ara-C, (B) DNR, and (C) combination of both on a TCPS,
UPS, and 80 µg/ml (CTCPS). A total of 80 µg/ml CTCPS was different
from TCPS and *P<0.05, **P<0.01 vs. TCPS at different
time-points in Ara-C, DNR, and combination of both. Ara-C,
cytarabine; DNR, daunorubicin; TCPS, tissue culture plate system;
UPS, uncoated PCL scaffold; CTCPS, collagen type I coated PCL
scaffold. |
DDR1 and p-STAT3 mediated cell
chemoresistance in CTCPS
We have shown that cell cultured on 80 µg/ml CTCPS
revealed chemoresistance compared to TCPS. We further explored the
possible mechanisms that lead to cell chemoresistance. Evidence
suggests that high expression of DDR1 and p-STAT3 is related to
cell proliferation, invasion, migration and chemoresistance of
malignant tumors (23,24). STAT3 is the downstream effector of
DDR1 pathway and STAT3 can be phosphorylated by DDR1 (25,26).
Therefore, we wanted to verify whether the drug resistance to Ara-C
and DNR of cells cultured in 3D scaffold is related to the high
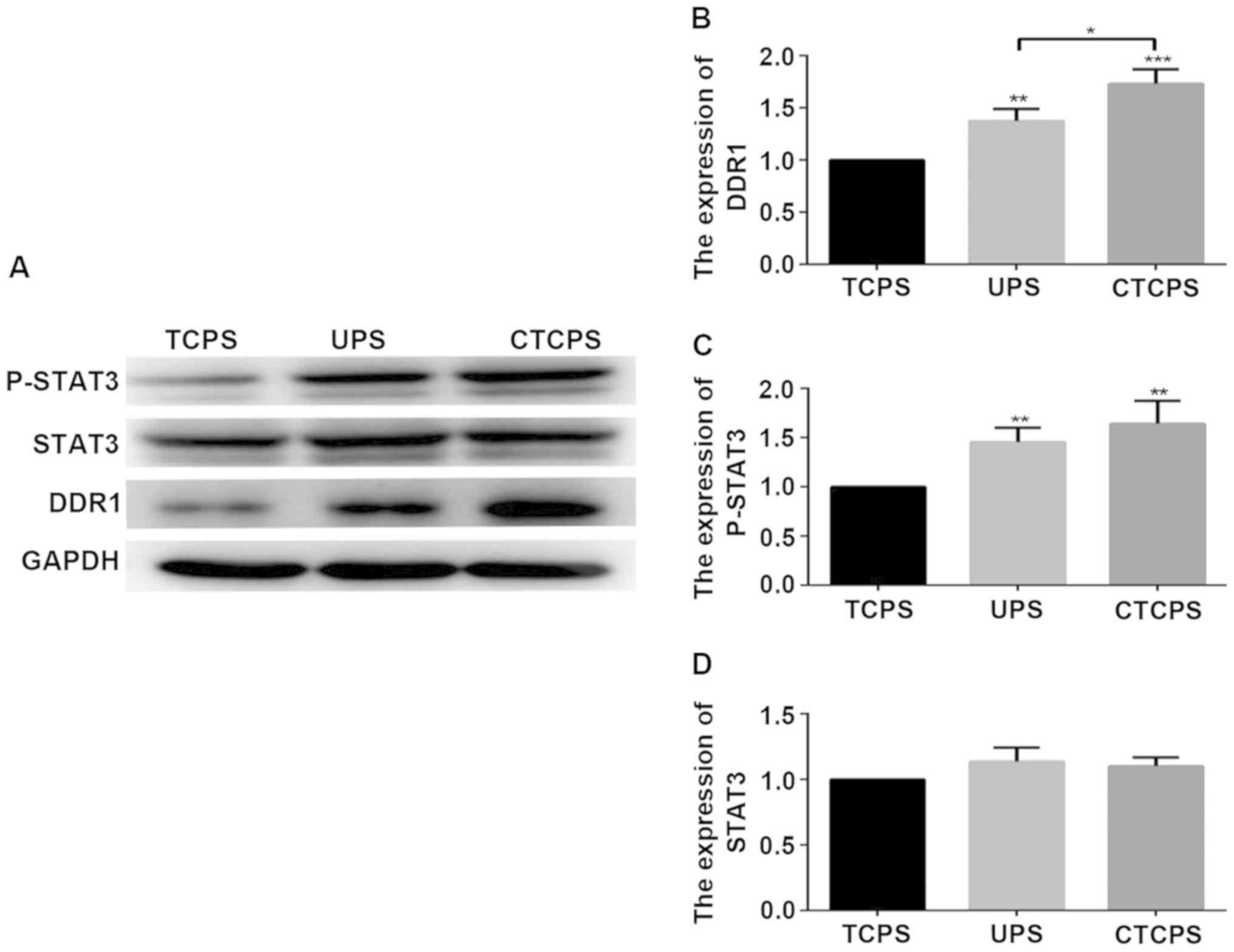
expression of DDR1 and p-STAT3. The levels of DDR1 and p-STAT3 were
highly expressed on the UPS and 80 µg/ml CTCPS compared to the TCPS
(Fig. 6). Next, we chose DDR1-IN-1,
a potent and selective DDR1 receptor tyrosine kinase inhibitor
(21), to further analyze whether
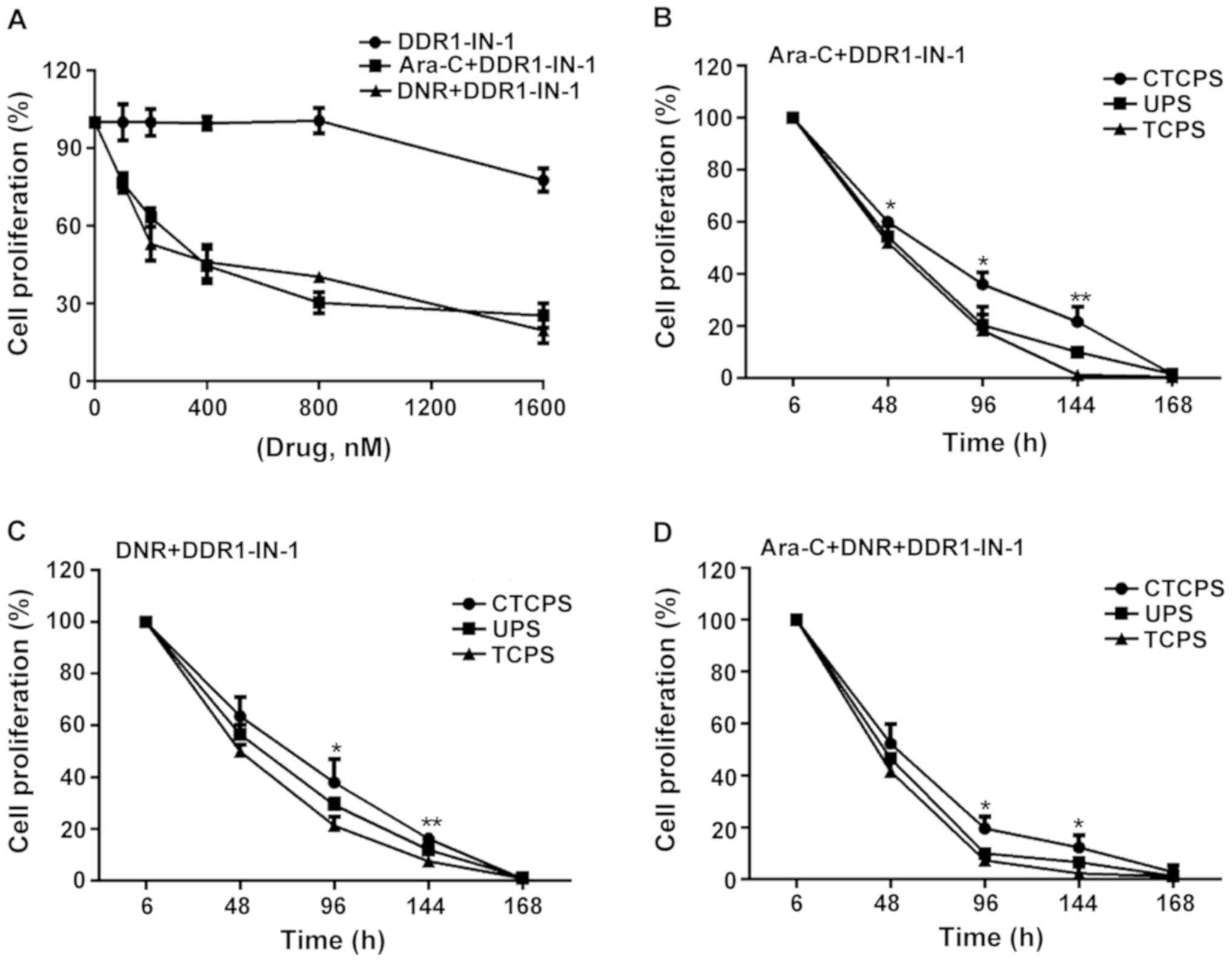
DDR1 inhibition would reverse the chemoresistance. The
concentration of DDR1-IN-1 used was determined in Jurkat cells
cultured in TCPS (Fig. 7A).
IC50 of 335 nM with DNR was chosen for further
experiments. DDR1-IN-1 (1 µM) which is sufficient to inhibit DDR1
autophosphorylation alone did not inhibit cell proliferation after
incubation for 48 h, whereas Ara-C (Fig.
7B) and DNR (Fig. 7C) singly or
in combination (Fig. 7D), along with
DDR1-IN-1 induced significant cell death, wherein almost all the
cells were wiped out in contrast with DDR1-IN-1 alone at 144 h.
Discussion
ALL is still a disease that cannot be completely
cured by chemotherapy. Hematopoietic stem cell transplantation is
the only way to cure leukemia. However, the high cost, easy
relapse, severe GVHD side effects and technical limitations make
hematopoietic stem cell transplantation hard to carry out for most
leukemia patients in China (27). In
recent years, the research and development of targeted drugs has
become a hot spot (28,29). The prognosis of many hematological
malignancies has greatly improved because of the new drugs
(30). Drug testing in vitro
needs an environment similar to the niche in vivo where
those cells reside (31). Although
researchers are trying to establish cell microenvironments in
vitro which is closer to the bone marrow niche in vivo,
the progress is unsatisfactory. Since leukemia cells reside in the
bone marrow niche, where leukemia cells escape from the
chemotherapy drugs (32,33). Compared with traditional 2D culture,
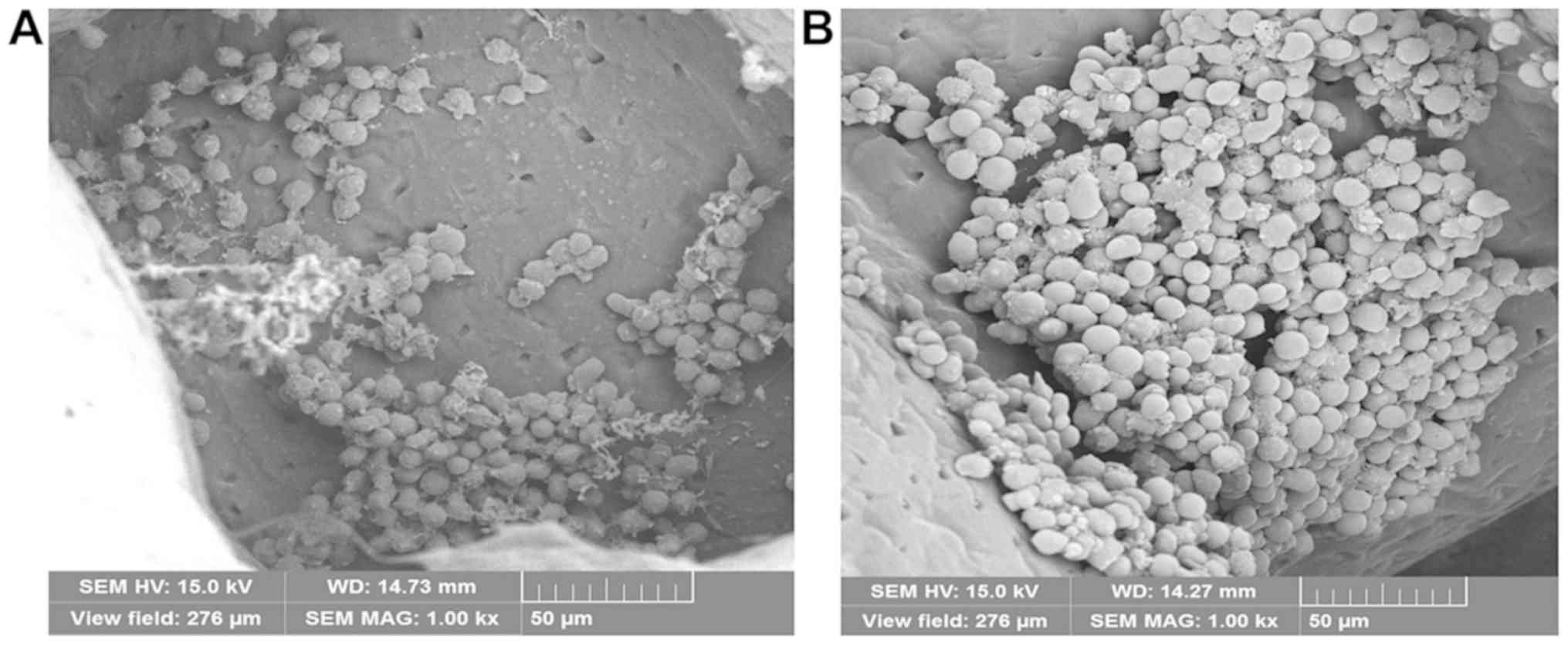
3D Insert™ PCL scaffold is a lot more like in vivo
environment. Cells expanded on the scaffolds have more
physiological-like morphology and function (Figs. 8 and 9). In order to mimic bone marrow
microenvironment, the scaffolds are coated with collagen. The
results showed that the concentration of 80 µg/ml CTCPS is the
maximum cell seeding efficiency, whereas the high concentration
(160, 320 µg/ml) leads to a decrease of seeding efficiency. This
may be attributed to the quick gel formation rate of high
concentration of collagen type I, which blocks the infiltration and
migration of Jurkat cells.
The initial cell proliferation lag on scaffolds
compared to that in TCPS could be attributed to the limited cells
bound to the surface and the scaffold niche at the time of seeding
(in order to reduce the number of cell culture medium replaced, we
seeded 2×104 cells per well on scaffold), the cells also
need an adaptive process for new culture environments. That is the
possible reason why the proliferation of cells for 48 and 96 h on
TCPS was higher than that on UPS and CTCPS. With the prolongation
of culture time, more cells adhere to the scaffold surface, divide
and migrate into the scaffold and get adapted to the new culture
environments. Moreover, this scenario was lacking in the TCPS; that
is the possible reason why the cell proliferation was significantly
reduced by 144 and 168 h. With the increasing number of cells, the
decrease of cell surface area for collagen type I adsorption is
inevitable, leading to less area for cell attachment. Thereby G0/G1
cells decrease and non-adhered cells actively divide, overpopulate
and die, indicating that CTCPS are more suitable than TCPS and UPS
for longer time culture (14,34).
We further explored whether different culture
conditions lead to changes in cell phenotype. The result showed
that despite culturing Jurkat cells for 168 h, the original
phenotype of Jurkat cells was retained the same on the 80 µg/ml
CTCPS and UPS compared to TCPS. That is an important characteristic
in studies of leukemia cells. It has been reported that DDR1
activates STAT3 signaling during tumor progression in human breast
cancer metastasis (25).
Overexpression of discoidin DDR1 enhanced the cisplatin resistance
of ovarian cancer cells (35), but
in ALL, the expression and effect of DDR1 on the drug-resistance is
not yet clear. The result showed that the expression levels of
DDR1, P-STAT3 in Jurkat cells cultured in the CTCPS were higher
than those of cells cultured in TCPS significantly suggesting that
the increased expression of DDR1 activated by collagen type I may
be the possible reason for the cell survival. The cells grow in a
3D environment may contribute to the expression of DDR1, and may be
the reason why the expression levels of DDR1 in the UPS were higher
than in the TCPS. The treatment of Jurkat cells cultured on CTCPS
with Ara-C, DNR or their combination revealed low sensitivity
compared with TCPS until the 168 h. The conclusion of the
experiment proves that DDR1/STAT3 mediated mechanism may be the
reason of Jurkat cell chemoresistance. We hypothesize that the
collagen type I promote the Jurkat cells to harbor in the 3-D niche
of the scaffold and activate the DDR1 protein as a ligand.
Activation of the DDR1 pathway leads to cell chemoresistance
(19).
To further confirm the mechanism, we use DDR1
receptor tyrosine kinase inhibitor DDR1-IN-1 to block DDR1. By
blocking the function of DDR1, the sensitivity of leukemic cells to
chemotherapeutic drugs is enhanced. These results indicate that the
explored CTCPS could support chemotherapy drug resistance to Ara-C,
DNR or combination and chemoresistance mechanism operates through
the DDR1/STAT3 pathway, recapitulating at least in part the drug
resistance seen in the in vivo condition. Thus 3D
architecture is also important in providing the chemoresistance.
This could be attributed to the similar morphology of the PCL
scaffolds to the bone marrow, as collagen type I helps in cell
chemoresistance by activating DDR1 (36).
Biomimetic structure is an important parameter in
the clinical trial of drugs. Because in some cases the cells were
sensitive to the drug in 2D culture, but were resistant to 3D
culture (37,38). On one hand, we think that the bionic
3D scaffold with the capability of supporting the leukemic cells
for a longer period has immense potential in drug screening. On the
other hand, in response to DDR1 as a drug target, drugs can be
designed for the treatment of leukemia, which include antibodies
against the DDR1 ligand or the receptor itself or a small molecular
chemical inhibitor for the intracellular domain of DDR1 kinase. The
application of 3D cell culture system enables researchers to create
superior in vitro models to obtain more realistic
physiological results from studies in vitro. As a result,
the use of 3D cell culture will decrease the overall therapeutic
and pharmaceutical product development cost and shorten the time to
market.
Acknowledgements
Not applicable.
Funding
The project described was supported by Qingdao City
People's Livelihood Science and technology project
(16-6-2-30-nsh).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG and CZ conceived and designed the study and were
responsible for cell culture. RY, AS, LS, XL, SW, ZS and TL
detected cell proliferation and viability and performed flow
cytometry analysis. SL, YG, JL, XF and WW contributed to western
blot analysis. HZ, ZC, GL and FM analyzed chemoresistance of cells
cultured on scaffolds. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingdao University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nair MS, Mony U, Menon D, Koyakutty M,
Sidharthan N, Pavithran K, Nair SV and Menon KN: Development and
molecular characterization of polymeric micro-nanofibrous scaffold
of a defined 3-D niche for in vitro chemosensitivity analysis
against acute myeloid leukemia cells. Int J Nanomedicine.
10:3603–3622. 2015.PubMed/NCBI
|
|
2
|
Funayama K, Murai F, Shimane M, Nomura H
and Asano S: Adhesion-induced drug resistance in leukemia stem
cells. Pharmacology. 86:79–84. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wojtkowiak JW, Verduzco D, Schramm KJ and
Gillies RJ: Drug resistance and cellular adaptation to tumor acidic
pH microenvironment. Mol Pharm. 8:2032–2038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang JY, Yu P, Chen S, Xing H, Chen Y,
Wang M, Tang K, Tian Z, Rao Q and Wang J: Activation of Rac1 GTPase
promotes leukemia cell chemotherapy resistance, quiescence and
niche interaction. Mol Oncol. 7:907–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumbleton J, Agarwal P, Huang H, Hogrebe
N, Han R, Gooch KJ and He X: The effect of RGD peptide on 2D and
miniaturized 3D culture of HEPM cells, MSCs, and ADSCs with
alginate hydrogel. Cell Mol Bioeng. 9:277–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duval K, Grover H, Han LH, Mou Y, Pegoraro
AF, Fredberg J and Chen Z: Modeling physiological events in 2D vs.
3D cell culture. Physiology (Bethesda). 32:266–277. 2017.PubMed/NCBI
|
|
7
|
Nugraha B, Mohr MA, Ponti A, Emmert MY,
Weibel F, Hoerstrup SP, Moll S, Certa U, Prunotto M and Pantazis P:
Monitoring and manipulating cellular crosstalk during kidney
fibrosis inside a 3D in vitro co-culture. Sci Rep. 7:144902017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou J, Hong Z, Feng F, Chai Y, Zhang Y,
Jiang Q, Hu Y, Wu S, Wu Y, Gao X, et al: A novel chemotherapeutic
sensitivity-testing system based on collagen gel droplet embedded
3D-culture methods for hepatocellular carcinoma. BMC Cancer.
17:7292017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burton TP, Corcoran A and Callanan A: The
effect of electrospun polycaprolactone scaffold morphology on human
kidney epithelial cells. Biomed Mater. 13:0150062017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caicedo-Carvajal CE, Liu Q, Remache Y, Goy
A and Suh KS: Cancer tissue engineering: A novel 3D polystyrene
scaffold for in vitro isolation and amplification of lymphoma
cancer cells from heterogeneous cell mixtures. J Tissue Eng.
2011:3623262011.PubMed/NCBI
|
|
11
|
Hur H, Ham IH, Lee D, Jin H, Aguilera KY,
Oh HJ, Han SU, Kwon JE, Kim YB, Ding K, et al: Discoidin domain
receptor 1 activity drives an aggressive phenotype in gastric
carcinoma. BMC Cancer. 17:872017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moreau V and Saltel F: Type I collagen
fibrils and discoidin domain receptor 1 set invadosomes straight.
Mol Cell Oncol. 2:e10049632015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stępnik M, Spryszyńska S, Gorzkiewicz A
and Ferlińska M: Cytotoxicity of anticancer drugs and PJ-34
(poly(ADP-ribose)polymerase-1 (PARP-1) inhibitor) on HL-60 and
Jurkat cells. Adv Clin Exp Med. 26:379–385. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blanco TM, Mantalaris A, Bismarck A and
Panoskaltsis N: The development of a three-dimensional scaffold for
ex vivo biomimicry of human acute myeloid leukaemia. Biomaterials.
31:2243–2251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao M, Xie J, Piruska A and Huck WTS: 3D
microniches reveal the importance of cell size and shape. Nat
Commun. 8:19622017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell CC, Dankers ACA, Lauschke VM,
Sison-Young R, Jenkins R, Rowe C, Goldring CE, Park K, Regan SL,
Walker T, et al: Comparison of hepatic 2D sandwich cultures and 3D
spheroids for long-term toxicity applications: A multi-center
study. Toxicol Sci. 162:655–666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xue R, Qian Y, Li L, Yao G, Yang L and Sun
Y: Polycaprolactone nanofiber scaffold enhances the osteogenic
differentiation potency of various human tissue-derived mesenchymal
stem cells. Stem Cell Res Ther. 8:1482017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yildirim ED, Besunder R, Pappas D, Allen
F, Güçeri S and Sun W: Accelerated differentiation of osteoblast
cells on polycaprolactone scaffolds driven by a combined effect of
protein coating and plasma modification. Biofabrication.
2:0141092010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Das S, Ongusaha PP, Yang YS, Park JM,
Aaronson SA and Lee SW: Discoidin domain receptor 1 receptor
tyrosine kinase induces cyclooxygenase-2 and promotes
chemoresistance through nuclear factor-kappaB pathway activation.
Cancer Res. 66:8123–8130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Canning P, Tan L, Chu K, Lee SW, Gray NS
and Bullock AN: Structural mechanisms determining inhibition of the
collagen receptor DDR1 by selective and multi-targeted type II
kinase inhibitors. J Mol Biol. 426:2457–2470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HG, Tan L, Weisberg EL, Liu F, Canning
P, Choi HG, Ezell SA, Wu H, Zhao Z, Wang J, et al: Discovery of a
potent and selective DDR1 receptor tyrosine kinase inhibitor. ACS
Chem Biol. 8:2145–2150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y, Wang B, Zhao Y, Xiao Z, Li J, Cui
Y, Han S, Wei J, Chen B, Han J, et al: Collagen scaffold
microenvironments modulate cell lineage commitment for
differentiation of bone marrow cells into regulatory dendritic
cells. Sci Rep. 7:420492017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CH, Chiang MC and Chen YJ: STAT3
mediates resistance to anoikis and promotes invasiveness of
nasopharyngeal cancer cells. Int J Mol Med. 40:1549–1556. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang JC, Zhang Y, He SJ, Li MM, Cai XL,
Wang H, Xu LM and Cao J: TM4SF1 promotes metastasis of pancreatic
cancer via regulating the expression of DDR1. Sci Rep. 7:458952017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao H, Chakraborty G, Zhang Z, Akalay I,
Gadiya M, Gao Y, Sinha S, Hu J, Jiang C, Akram M, et al:
Multi-organ site metastatic reactivation mediated by non-canonical
discoidin domain receptor 1 signaling. Cell. 166:47–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Sun X, Bao Y, Mo J, Du H, Hu J and
Zhang X: E2F1 silencing inhibits migration and invasion of
osteosarcoma cells via regulating DDR1 expression. Int J Oncol.
51:1639–1650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang C and Zhang X: Incidence survey of
leukemia in China. Chin Med Sci J. 6:65–70. 1991.PubMed/NCBI
|
|
28
|
Broxterman HJ and Georgopapadakou NH:
Anticancer therapeutics: ‘Addictive’ targets, multi-targeted drugs,
new drug combinations. Drug Resist Updat. 8:183–197. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Da Costa EM, McInnes G, Beaudry A and
Raynal NJ: DNA methylation-targeted drugs. Cancer J. 23:270–276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jamison C, Nelson D, Eren M, Gauchan D,
Ramaekers R, Norvell M and Copur MS: What is the optimal dose and
schedule for dasatinib in chronic myeloid leukemia: Two case
reports and review of the literature. Oncol Res. 23:1–5. 2015.
View Article : Google Scholar
|
|
31
|
Wang JZ, Zhu YX, Ma HC, Chen SN, Chao JY,
Ruan WD, Wang D, Du FG and Meng YZ: Developing multi-cellular tumor
spheroid model (MCTS) in the chitosan/collagen/alginate (CCA)
fibrous scaffold for anticancer drug screening. Mater Sci Eng C.
62:215–225. 2016. View Article : Google Scholar
|
|
32
|
Poulos MG, Gars EJ, Gutkin MC, Kloss CC,
Ginsberg M, Scandura JM, Rafii S and Butler JM: Activation of the
vascular niche supports leukemic progression and resistance to
chemotherapy. Exp Hematol. 42:976–986.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prieto-Vila M, Takahashi RU, Usuba W,
Kohama I and Ochiya T: Drug resistance driven by cancer stem cells
and their niche. Int J Mol Sci. 18:25742017. View Article : Google Scholar
|
|
34
|
Feng Q, Chai C, Jiang XS, Leong KW and Mao
HQ: Expansion of engrafting human hematopoietic stem/progenitor
cells in three-dimensional scaffolds with surface-immobilized
fibronectin. J Biomed Mater Res A. 78:781–791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng Y, Zhao F, Hui L, Li X, Zhang D, Lin
W, Chen Z and Ning Y: Suppressing miR-199a-3p by promoter
methylation contributes to tumor aggressiveness and cisplatin
resistance of ovarian cancer through promoting DDR1 expression. J
Ovarian Res. 10:502017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carbone A and Gloghini A: Activated DDR1
increases RS cell survival. Blood. 122:4152–4154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim MK, Kim Y, Choo H and Chong Y:
Quercetin-glutamic acid conjugate with a non-hydrolysable linker; a
novel scaffold for multidrug resistance reversal agents through
inhibition of P-glycoprotein. Bioorg Med Chem. 25:1219–1226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Z and Zhao X: A 3D model of ovarian
cancer cell lines on peptide nanofiber scaffold to explore the
cell-scaffold interaction and chemotherapeutic resistance of
anticancer drugs. Int J Nanomedicine. 6:303–310. 2011. View Article : Google Scholar : PubMed/NCBI
|