Introduction
Finding the best course of treatment for coronary
artery disease (CAD) is a recurring challenge in everyday clinical
practice. Coronary revascularization is only justified for
hemodynamically relevant stenosis (1,2). While
coronary angiography can identify a coronary stenosis by
conventional visual assessment, defining the functional hemodynamic
significance of an intermediate stenosis can be difficult.
During cardiac catherization, the functional flow
reserve (FFR) can be measured as the maximum available blood flow
in a stenosed coronary segment. FFR is the current gold standard
for deciding if revascularization is required in angiographically
ambiguous coronary artery stenosis and is recommended by the 2014
ESC/EACTS guidelines on myocardial revascularization and the 2011
ACCF/AHA/SCAI guidelines for percutaneous coronary intervention
(PCI) (3,4).
Despite this recommendation and the alleged
benefits, the use of FFR is still limited. The administration of
vasodilators such as adenosine, which is required to induce maximal
hyperaemia when measuring FFR, can cause side effects (i.e. chest
pain, dyspnoea, AV-blockage) during the procedure. Those side
effects, cost and increased procedural time are preventing FFR from
becoming a standard procedure in day-to-day clinical setting.
A rather new method used to determine the severity
of a coronary stenosis is the instantaneous wave-free ratio (iFR).
By identifying a period of naturally occurring constant peripheral
resistance during diastole, there is no need for vasodilators
(5). Several studies have shown
similar diagnostic accuracy for FFR and iFR in the same coronary
artery (5–7).
Following the described comparable accuracy of FFR
and iFR, the goal of this meta-analysis was to compare the clinical
outcome of patients with CAD in which the stenosis was either
evaluated visually by coronary angiography alone, or by hemodynamic
assessment using FFR or iFR.
Materials and methods
Study design
This meta-analysis was conducted following the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) statement and is based on the review of previously
published articles (8–16). No ethical approval and patient
consent were necessary.
Literature search
In July 2017 PubMed, EMBASE, Cochrane Central
Register of Controlled Trials (CENTRAL), were searched for studies
evaluating the clinical outcomes of FFR and iFR. The keywords were
‘fractional flow reserve’ and ‘myocardial’ or ‘fractional flow
reserve’ or ‘wave-free ratio’ or ‘iFR and coronary’ or ‘FFR and
coronary’ with no other filter. No language restrictions were
applied. We selected studies, which used a comparative analysis to
identify a culprit coronary lesion.
Patient population with inclusion and
exclusion criteria
The following inclusion criteria were applied. The
design was either a randomized clinical trial (RCT) or an
observational study comparing either angiography and FFR guided or
iFR and FFR guided PCI. Participants were adult (18 years and
older) patients with indication for PCI. All data of one-year
clinical outcomes (major adverse cardiac event (MACE), death from
any cause, myocardial infarction (MI) or unplanned
revascularization) could be retrieved from the published full
text.
We applied the following exclusion
criteria
The studies were not conducted on humans (studies on
animals or in vitro systems). The literature presented
results from a sub-study, was a duplicate or did not report
clinical outcomes of angiography or FFR and iFR. All literature,
that contained only diagnostic studies, surveys, reviews, case
reports, comments, or meta-analysis. Three investigators (SB, ACS
and VB) selected studies independently, and disagreements were
resolved by discussion among all authors.
The following data of eligible studies were
documented (Table I): Name of the
study, first author, year of publication, and details of the study
design, characteristics of patients, data of clinical outcomes, and
the studies were sorted by analyzed type of culprit assessment.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| A, Studies comparing
FFR guided PCI and angiography guided PCI |
|---|
|
|---|
| Author, year | Design | Centers
(Countries) | Number of enrolled
patients for each methoda | Indication for
PCI | Exclusion
criteria | Outcomes
reported | Maximum Follow-up
period (months) | (Refs.) |
|---|
| Tonino et al
2009 | Prospective RCT | 20 (N.A.) | 509 (FFR) 496
(Angio) | Multivessel CAD (50%
of the vessel diameter in at least two major epicardial coronary
arteries) | Recent STEMI (<5
days); NSTEMI with peak creatinine kinase >1,000 U/l;
significant left main CAD; previous CABG; cardiogenic shock;
extremely tortuous or calcified coronary arteries; life expectancy
<2 years; contraindication for drug-eluding stents;
pregnancy | Primary endpoint:
MACE Secondary endpoints: Procedure time; amount of contrast agent;
functional CCS class (after 1 year); HRQoL; number of antianginal
medication; individual components of MACE; MACE at 30 days and 6
months; cost-effectiveness | 12 | (8) |
| Puymirat et al
2012 | Retrospective,
nonrandomized | 1 (1) | 222 (FFR) 495
(Angio) | Stable or unstable
Angina in small coronary vessel (<3 mm diameter) | Patients with PCI
treatment in vessels ≥3 mm; bypass graft stenting; STEMI or
non-STEMI; PCI without stenting | Primary endpoint:
MACE Secondary endpoints: Stent thrombosis; periprocedural MI;
bleeding complications (major thrombolysis in MI); use of
transfusion during hospital stay | 60 | (9) |
| Li et al
2013 | Retrospective,
nonrandomized | 1 (1) | 1,090 (FFR) 6,268
(Angio) | Patients referred for
revascularization | STEMI; cardiogenic
shock; referred for CABG | Primary endpoint:
MACE Secondary endpoint: death; MI; repeated revascularization | 84 | (10) |
| Chen et al
2015 | Prospective
RCT | 8 (1) | 160 (FFR) 160
(Angio) | Silent ischemia,
Stable or unstable Angina with a single true coronary bifurcation
lesion (diameter of stenosis ≤50% in both the main vessel and the
side branch, each with a reference diameter of ≥2.5 to ≤4.5
mm) | MI within one
month; left ventricular ejection <30%; previous CABG; a distal
left main coronary artery trifurcation lesion with a no canalized
right coronary artery chronic total occlusion; calcification
requiring rotational atherectomy; planned surgery necessitating
antiplatelet therapy interrupting within 6 months post-PCI; study
drug contraindication or intolerance; estimated glomerular
filtration rate <40 ml/min/1.73 m2: platelet count
<10×109/l; liver dysfunction; pregnancy; expected
life span <1 year | Primary endpoint: 1
year rate of MACE Secondary endpoints: individual MACE (cardiac
death, MI or TVR); stent thrombosis; restenosis | 12 | (11) |
| Layland et
al 2015 | Prospective
RCT | 6 (1) | 176 (FFR) 174
(Angio) | NSTEMI and at least
one risk factor for CAD with invasive management planned or history
of recurrent ischemic symptoms within 5 days | Presence of
ischemic symptoms without medical therapy; hemodynamic instability;
MI with persistent ST elevation; anti-platelet intolerance; planned
non-coronary surgery; history of CABG; coronary disease; life
expectancy <1 year | Primary endpoint:
Difference in patient numbers allocated to medical treatment
between the PCI and the FFR guided group Secondary endpoint:
Feasibility and safety of routine FFR; relationship between FFR and
stenosis severity as assessed by angiography; MACE; hospital
resources; HRQoL | 12 | (12) |
| Park et al
2015 | Prospective
RCT | 6 (1) | 114 (FFR) 115
(Angio) | Intermediate
coronary stenosis in a native coronary artery with a reference
diameter of <2.5 mm | Angiographically
significant left main disease; cardiogenic shock; chronic kidney
disease; a life expectancy <2 years; conductions disturbance
more than first degree AV-block; contraindication to adenosine | Primary endpoint:
MACE (after 2 and 5 years) | 60 | (13) |
| De Backer et
al 2016 | Retrospektive,
nonrandomized | 1 (1) | 695 (FFR) 695
(Angio) | Stable Angina | Coronary stenosis
<50% or >89% | Primary endpoint:
MACE Secondary endpoints: Death; MI; repeated revascularization;
combined endpoint of death and MI | 48 | (14) |
|
| B, Studies
comparing FFR guided PCI and instantaneous wave-free ratio
(iFR®) guided PCI |
|
| Author,
year | Design | Centers
(Countries) | Number of
enrolled patientsb | Indication for
PCI | Exclusion
criteria | Outcomes
reported | Maximum
Follow-up period (months) | (Refs.) |
|
| Davies et al
2017 | Prospective
RCT | 49 (19) | 1,250 (FFR) 1,242
(iFR) | Intermediate
coronary stenosis | Tandem stenosis,
previous CABG, significant left main artery stenosis, total
coronary occlusion, restenosis, hemodynamic instability,
contraindication to adenosine administration or PCI or drug eluting
stent, heavily calcified or tortuous vessels, significant hepatic
or lung disease or malignant disease with unfavorable prognosis,
pregnancy, severe valvular heart disease, recent STEMI, more than
one target vessel | Primary endpoint:
MACE | 12 | (15) |
| Götberg et
al 2017 | Prospective
RCT | 15 (3) | 1,007 (FFR) 1,012
(iFR) | Stable or unstable
Angina, NSTEMI | Previous CABG; life
expectancy <1 year; unstable hemodynamics | Primary endpoint:
MACE Secondary endpoints: MI; death; unplanned revascularization;
chest discomfort during the procedure; TVR; stent thrombosis;
restenosis | 12 | (16) |
Statistical analysis
In case the extracted data was appropriate for
pooled analysis, a meta-analysis was performed. Dichotomous data
was analyzed using the Mantel-Haenszel model and reported as an
odds ratio (OR). Forest plots were used for visualization of the
results.
The heterogeneity of studies was calculated using
the I2 index. An I2 value of 0–25% represents
insignificant heterogeneity; >25-50% low heterogeneity;
>50-75% moderate heterogeneity; and >75% high heterogeneity
(17). All results were calculated
using a random-effects model. If concerns for high heterogeneity
existed, a sensitivity analysis was performed. Funnel plots were
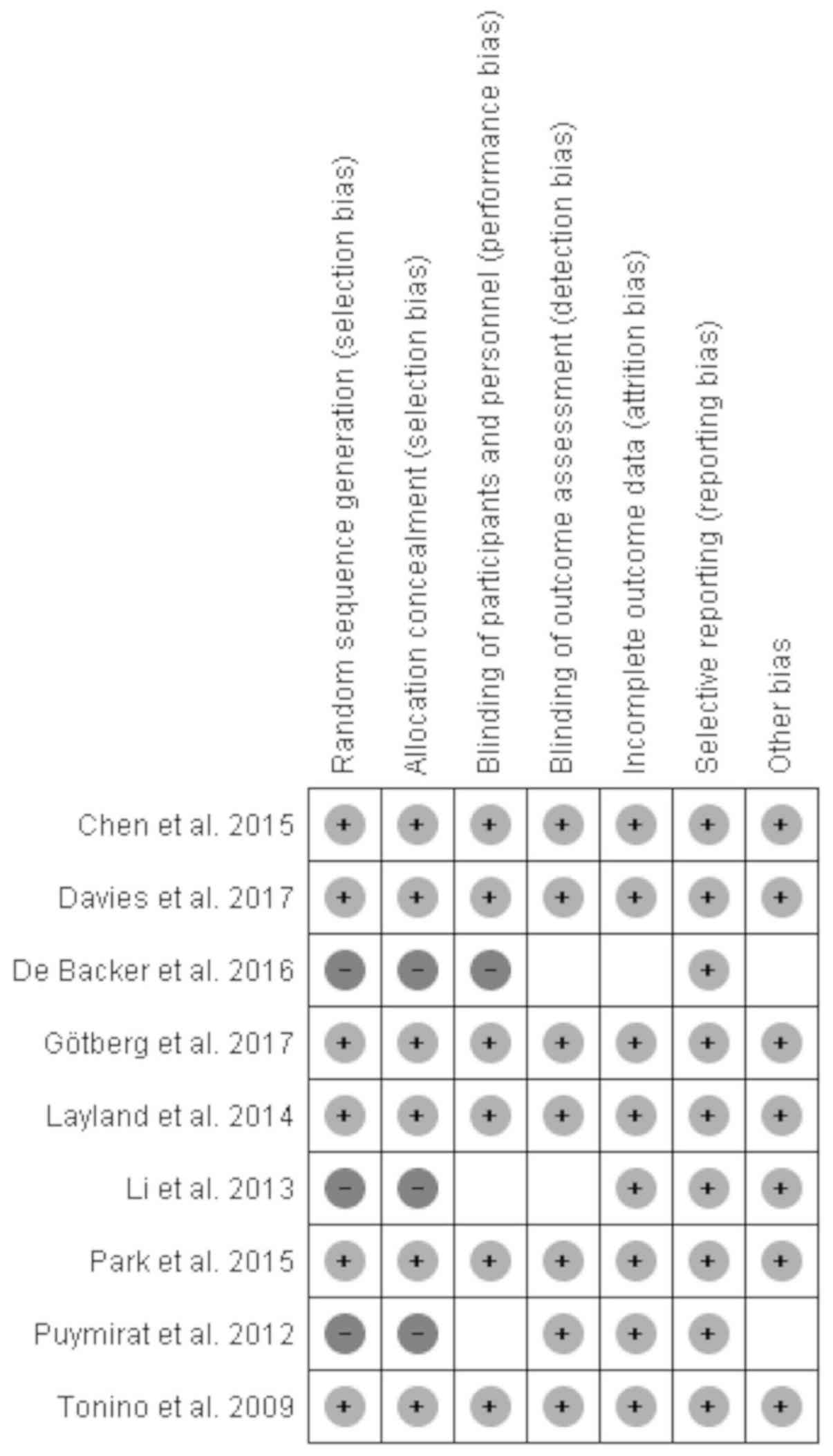
used to visualize publication bias. For other bias, a risk of bias
assessment figure was used (Fig. 1).
The comparison between angiography and iFR was performed with a
network meta-analysis. For meta-analysis calculations, the Review
Manager version 5.3 (The Cochrane Collaboration, The Nordic
Cochrane Centre, Copenhagen, Denmark) and for network meta-analysis
the SAS system release version 9.3 (SAS Institute Inc., Cary, NC,
USA) was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
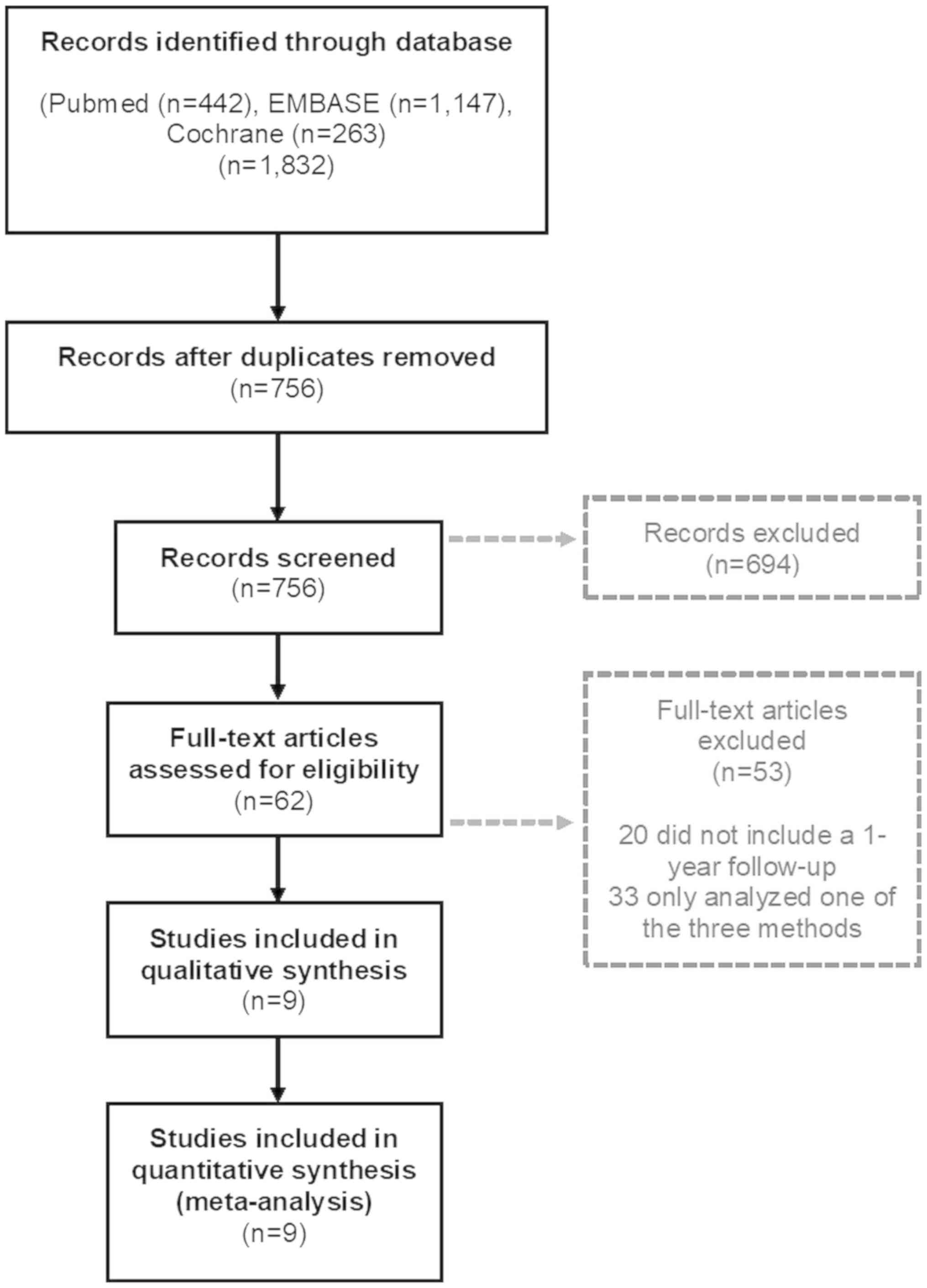
A total of 756 studies were screened, and nine
studies were identified that fulfilled the previously determined
inclusion criteria. A flow chart shows the selection process and
the reasons for exclusion (Fig. 2).
A total of 694 articles were eliminated, since their titles or
abstracts did not fit our inclusion criteria.
The full-texts of 62 articles were assessed and 53
were not included in the quantitative and qualitative analysis
since they did not contain a one-year follow-up or only analyzed a
single method of coronary artery lesion evaluation. In total, the
remaining studies included 15,880 patients with a one-year
follow-up for MACE after PCI. Five studies and 8,403 patients were
used for the analysis of angiography guided PCI, seven studies with
5,223 patients were used to analyze the FFR guided PCI and two
studies with 2,254 patients were used for analysis of the iFR
guided PCI. The exact number of patients for each study can be seen
in Table I.
Overall, the nine studies did not have significant
differences regarding patient baseline characteristics, which can
be seen in Table II. Table III shows the rates for MACE one
year after the intervention as well as the individual components of
MACE, death from any cause, MI and unplanned revascularization.
 | Table II.Patient characteristics of the
included studies. |
Table II.
Patient characteristics of the
included studies.
| A, Studies
comparing FFR guided PCI and angiography guided PCI |
|---|
|
|---|
| Author, year | Method used for
PCI | Patients | Age ± SD | BMI ± SD
(kg/m2) | Male (%) | HTN (%) | Diabetes (%) | HC (%) | Current smoking
(%) | Family history of
CAD (%) | Prior MI (%) | MVD (%) | (Refs.) |
|---|
| Tonino | FFR | 509 |
64.6±10.3 | N.A. | 384 (75.4) | 312 (61.3) | 123 (24.2) | 366 (71.9) | 138 (27.1) | 205
(40.3) | 187
(36.7) | 509 (100) | (8) |
| et al
2009 | Angiography | 496 |
64.2±10.2 | N.A. | 360 (72.6) | 327 (65.9) | 125 (25.2) | 362 (73.0) | 156 (31.5) | 190
(38.3) | 180
(36.3) | 496 (100) |
|
| Puymirat | FFR | 222 | 71.6±9.8 | 26.6±4.3 | 129 (58) | 130 (59) | 58 (26) | 137 (62) | 86 (39) | 77
(35) | N.A. | 38 (17) | (9) |
| et al
2012 | Angiography | 495 |
71.7±10.6 | 27.0±4.4 | 336 (68) | 323 (65) | 163 (33) | 333 (67) | 226 (46) | 143 (29) | N.A. | 46 (9) |
|
| Li | FFR | 1,090 |
65.7±11.3 | 30.4±5.8 | 683 (62.7) | 864 (79.2) | 306 (28.0) | 608 (55.7) | 140 (12.8) | N.A. | 270 (25) | 73 (19.8) | (10) |
| et al
2013 | Angiography | 6,268 |
67.9±11.6 | 30.2±5.9 | 4,416 (70.4) | 4,897 (78.1) | 1,862 (29.7) | 5,119 (81.6) | 836 (13.3) | N.A. | 1,882 (31.0) | 2186 (19) |
|
| Chen | FFR | 160 | 65.2±9.6 | N.A. | 121 (75.6) | 116 (72.5) | 48 (30.0) | 27 (16.9) | 66 (41.3) | N.A. | 12
(7.5) | 112 (69.8) | (11) |
| et al
2015 | Angiography | 160 | 65.4±9.2 | N.A. | 116 (72.5) | 106 (68.3) | 43 (26.9) | 32 (20.0) | 64 (40.0) | N.A. | 19
(11.9) | 110 (68.8) |
|
| Layland | FFR | 176 |
62.3±11.0 | N.A. | 133 (75.6) | 78 (44.3) | 26 (14.8) | 71 (40.3) | 72 (40.9) | N.A. | 22
(12.5) | 51 (29.0) | (12) |
| et al
2015 | Angiography | 174 |
61.6±11.1 | N.A. | 127 (73.0) | 81 (46.6) | 26 (14.9) | 56 (32.2) | 71 (40.8) | N.A. | 24
(13.8) | 55 (31.6) |
|
| Park | FFR | 114 |
62±10 | N.A. | 83 (72.8) | 73 (64) | 30 (26) | 80 (70) | 30 (26) | N.A. | 22
(19) | 72 (63) | (13) |
| et al
2015 | Angiography | 115 |
63±10 | N.A. | 87 (75.7) | 65 (57) | 39 (34) | 78 (68) | 38 (33) | N.A. | 20
(17) | 66 (57) |
|
| De Backer | FFR | 695 |
64.6±10.5 |
28.3±10.6 | 511 (73.5) | 465 (66.9) | 179 (25.8) | 511 (73.5) | 173 (24.9) | 343
(49.4) | 238
(34.2) | 199 (28.7) | (14) |
| et al
2016 | Angiography | 695 |
64.7±10.3 | 27.7±7.9 | 507 (72.9) | 477 (68.6) | 164 (23.6) | 514 (74.0) | 180 (25.9) | 328
(47.2) | 237
(34.1) | 202 (29.1) |
|
|
| B, Studies
comparing FFR guided PCI and Instantaneous Wave-free Ratio
(iFR®) guided PCI |
|
| Author,
year | Method used for
PCI |
Patients | Age ±
SD | BMI
(kg/m2) | Male
(%) | HTN (%) | Diabetes
(%) | HC (%) | Current Smoking
(%) | Family history
of CAD (%) | Prior MI
(%) | MVD (%) | (Refs.) |
|
| Davies | FFR | 1,250 | 65.2±10.6 | N.A. | 929 (74.3) | 884 (70.7) | 376 (30.1) | 792 (63.4) | 262 (21.0) | N.A. | 376 (30.1) | 519 (41.5) | (15) |
| et al
2017 | iFR | 1,242 | 65.5±10.8 | N.A. | 962 (77.5) | 873 (70.3) | 382 (30.8) | 794 (63.9) | 243 (19.6) | N.A. | 358 (28.8) | 505 (40.7) |
|
| Götberg | FFR | 1,007 | 67.4±9.2 | 27.6 ±4.3 | 766 (75.2) | 710 (69.7) | 213 (20.9) | 704 (69.2) | 167 (16.3) | N.A. | 335 (32.9) | 368 (36.1) | (16) |
| et al
2017 | iFR | 1,012 | 67.6±9.6 | 27.6 ±4.3 | 756 (74.2) | 730 (71.6) | 232 (22.8) | 733 (71.9) | 159 (15.6) | N.A. | 337 (33.1) | 364 (35.7) |
|
 | Table III.Primary endpoints at 12 months of the
included studies. |
Table III.
Primary endpoints at 12 months of the
included studies.
| A, Studies
comparing FFR guided PCI and angiography guided PCI |
|---|
|
|---|
| Author, year | Method used for
PCI | Patients | Death from any
cause (%) | Myocardial
infarction (%) | Unplanned
revascularization (%) | MACE (%) | (Refs.) |
|---|
| Tonino et al
2009 | FFR | 509 | 9
(1.8) | 29 (5.7) | 33 (6.5) | 67
(13.2) | (8) |
|
| Angiography | 496 | 15 (3.0) | 43 (8.7) | 47 (9.5) | 91
(18.3) |
|
| Puymirat et
al 2012 | FFR | 222 | 3
(1.4) | N.A. | 10 (4.5) | 13
(5.9) | (9) |
|
| Angiography | 479 | 13
(2.7) | N.A. | 59
(12.3) | 90
(18.8) |
|
| Li et al
2013 | FFR | 1,090 | 120 (11.0) | 135 (18.2) | N.A. | 206
(18.9) | (10) |
|
| Angiography | 6,268 | 690 (11.0) | 826 (13.2) | N.A. | 1,292 (20.6) |
|
| Chen et al
2015 | FFR | 160 | 3
(1.9) | 19
(11.9) | 9
(5.6) | 29
(18.1) | (11) |
|
| Angiography | 160 | 2
(1.3) | 22
(13.8) | 11 (6.9) | 29
(18.1) |
|
| Layland et
al 2015 | FFR | 176 | 5
(2.8) | 11 (6.2) | N.A. | 14
(8.0) | (12) |
|
| Angiography |
| 174 | 3
(1.7) | 15 (8.6) | N.A. |
|
| Park et al
2015 | FFR | 114 | N.A. | N.A. | N.A. | 5
(4.4) | (13) |
|
| Angiography |
| 115 | N.A. | N.A. | N.A. |
|
| De Backer et
al 2016 | FFR | 695 | 110
(15.8) | 217 (31.2) | 254 (36.5) | 255
(36.7) | (14) |
|
| Angiography | 695 | 191
(27.5) | 210 (30.2) | 231 (33.2) | 236
(34.0) |
|
|
| B, Studies
comparing FFR guided PCI and Instantaneous Wave-free Ratio
(iFR®) guided PCI |
|
| Author,
year | Method used for
PCI |
Patients | Death from any
cause (%) | Myocardial
Infarction (%) | Unplanned
Revascularization (%) | MACE
(%) | (Refs.) |
| Davies et al
2017 | FFR | 1,182 | 13
(1.1) | 28 (2.4) | 63 (5.3) | 83
(7.0) | (15) |
|
| iFR | 1,148 | 22
(1.9) | 31 (2.7) | 46 (4.0) | 78
(6.8) |
|
| Götberg et
al 2017 | FFR | 1,007 | 12
(1.2) | 17 (1.7) | 46 (4.6) | 61
(6.1) | (16) |
|
| iFR | 1,012 | 15
(1.5) | 22 (2.2) | 47 (4.6) | 68
(6.7) |
|
Analysis of one-year rates associated
with angiography guided vs. FFR guided PCI
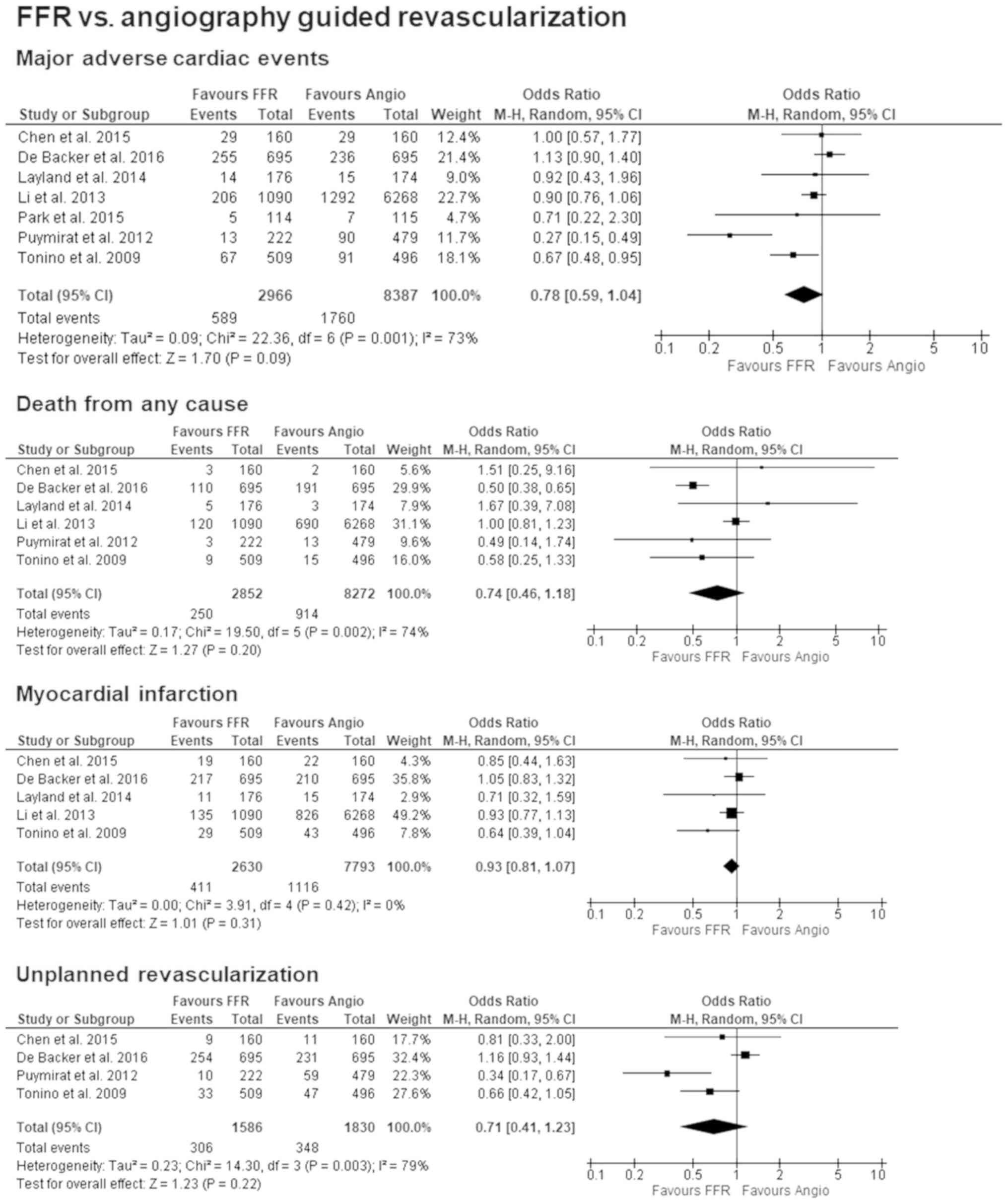
All the included studies reported on the outcomes of
MACE. After one year the results were OR: 0.78 [95% CI: 0.59–1.04];
P=0.09, I2=73% and were supportive of better outcomes
using FFR guided PCI. As for the single components of MACE, data
for death from any cause was available in six of the seven studies
and found a slight tendency towards FFR guided PCI with OR: 0.74
[95% CI: 0.46–1.18]; P=0.20, I2=74%. Five studies
published outcomes for MI and when comparing both methods, the
results were OR: 0.93 [95% CI: 0.81–1.07]; P=0.31,
I2=0%, but showed no significant difference. Unplanned
revascularization was reported in four of the included studies, and
they also leaned in favour of FFR guided PCI with an OR: 0.71 [95%
CI: 0.41–1.23]; P=0.22, I2=79%. Forest plots for all
primary outcomes can be seen in Fig.
3.
When excluding the three retrospective studies
(9,10,14) the
preference for FFR remained, but the homogeneity changed to OR:
0.77 [95% CI: 0.59–1.00]; P=0.05, I2=0% regarding MACE.
When comparing outcomes for MI and excluding the retrospective
studies, three studies remained, and the results stayed within the
same range with an OR: 0.70 [95% CI: 0.50–1.00]; P=0.05,
I2=0%. The assessment for any risk of bias, which is
visualized in Fig. 1, only showed a
high risk for selection and performance bias in those three
retrospective studies.
Analysis of one-year rates associated
with FFR guided vs. iFR guided PCI
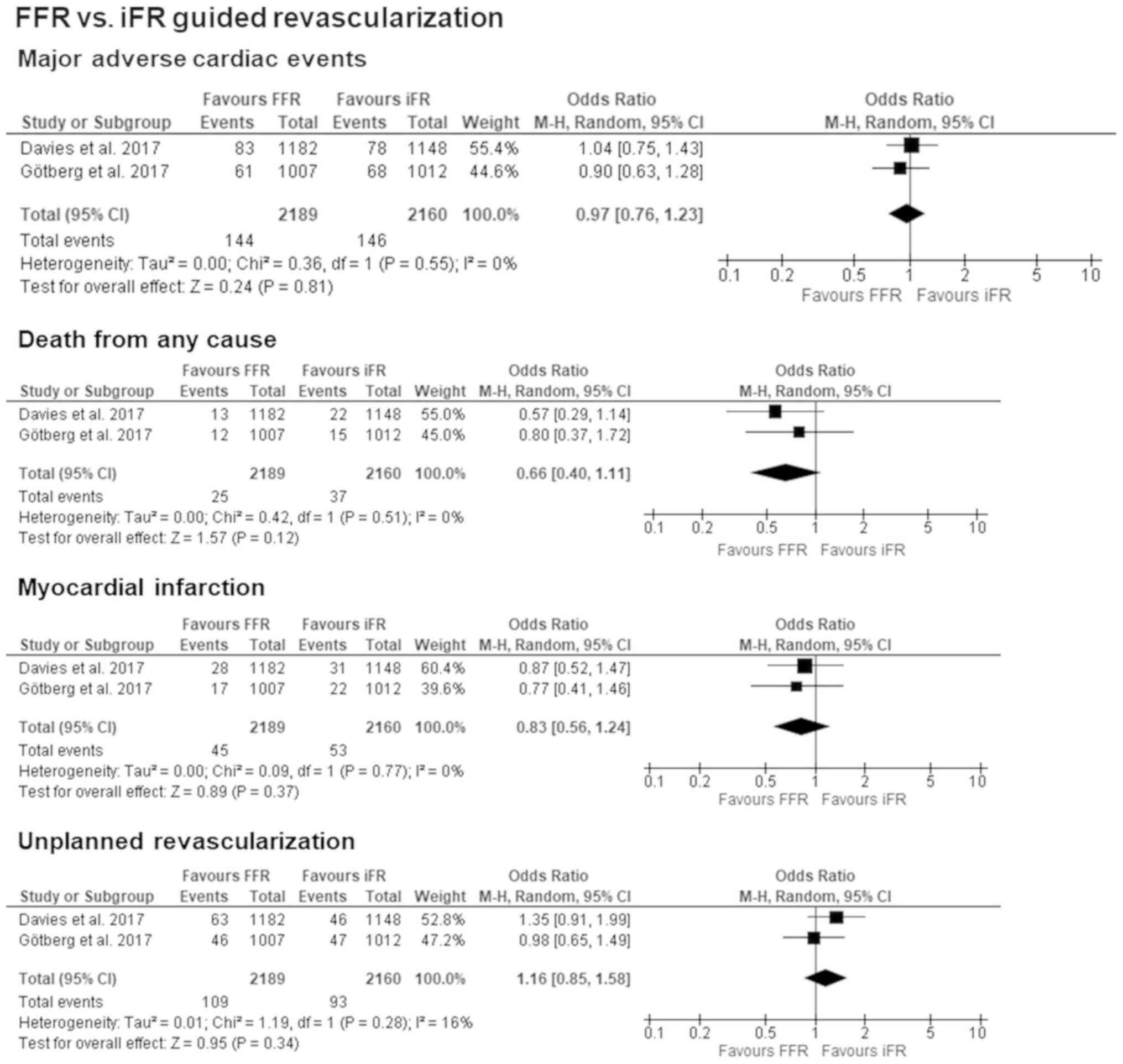
When analyzing the two available studies, there was
no significant difference between FFR and iFR regarding MACE with
OR: 0.97 [95% CI: 0.76–1.23]; P=0.81. Both studies also reported on
the individual components of MACE, and when comparing the two
methods in terms of death from any cause (OR: 0.66 [95% CI:
0.40–1.11]; P=0.12, I2=0%), MI OR: 0.83 [95% CI:
0.56–1.24]; P=0.37, I2=0%, and unplanned
revascularization (OR: 1.16 [95% CI: 0.85–1.58]; P=0.34,
I2=16%), neither reached the level of significance but
showed a tendency towards iFR guided revascularization. The forest
plots for all primary outcomes are presented in Fig. 4.
Network-analysis of one-year rates
associated with angiography guided vs. iFR guided PCI
When conducting a network-analysis to compare
angiography and iFR guided revascularization after one-year, the
result for MACE was OR: 0.80 [95% CI: 0.55–1.17]; P=0.25. Death
from any cause had an OR of: 1.12 [95% CI: 0.56–2.25]; P=0.75 and
MI an OR: 1.12 [95% CI: 0.74–1.71]; P=0.60. The results for
unplanned revascularization were OR: 0.61 [95% CI: 0.33–1.15];
P=0.13.
Discussion
This meta-analysis was conducted to analyze the
clinical outcomes as described above of studies containing
angiography and FFR guided PCI or iFR compared to FFR guided PCI.
When comparing FFR and iFR with angiography outcomes, the main
finding was a tendency towards FFR/iFR in all 4 clinical
endpoints.
With the analysis of MACE having factored in all
seven included studies, one could interpret these results to be the
most convincing. When looking at the Odds Ratio, a tendency towards
FFR becomes clear, and this is supported by the results of the
individual components of MACE. Although the I2 of MACE,
death from any cause and unplanned revascularization were >50%,
we decided not to exclude further studies in order to uphold a
larger number of included patients.
FFR was first tested on its usefulness to determine
the need for revascularization in intermediate coronary stenosis
two decades ago (18), and
thereafter several studies have been carried out to show the safety
of FFR and its superiority over angiography guided PCI. Such
studies include FAME (Fractional Flow Reserve vs. Angiography for
Guiding Percutaneous Coronary Intervention) (8) and DEFER (Deferral vs. performance of
percutaneous coronary intervention of functionally non-significant
coronary stenosis) (1). The later
published FAME 2 study (Fractional Flow Reserve-Guided PCI vs.
Medical Therapy in Stable Coronary Disease) further showed
significantly better outcomes for FFR guided PCI combined with best
medical treatment in comparison to best medical treatment alone
(2) and was stopped prematurely due
to the efficacy of the combined therapy. For additional information
on the clinical outcomes of FFR guided PCI, the FAME 3 study is
looking to compare this method with CABG surgery in patients with
multivessel CAD (19).
In contrast to our findings, a meta-analysis by
Enezate et al (20) showed
preference towards FFR and found a significant difference regarding
MACE and MI at not exactly 1 year but >9 months follow-up (OR:
0.51 [95% CI: 0.37–0.70]; P<0.0001, I2=21% and OR:
0.54 [95% CI: 0.39–0.75]; P=0.0003, I2=17%,
respectively) and also for in-hospital events (OR: 0.63 [95% CI:
0.47–0.86]; P=0.004, I2=75% and OR: 0.53 [95% CI:
0.40–0.70]; P<0.00001, I2=19%, respectively). Another
finding of this study was the lower rate of PCI performed compared
to the total number of lesions when using FFR, showing that not
every visually identified lesion results in a reduction of blood
flow and necessarily needs a PCI.
Furthermore, a meta-analysis by Zhang et al,
which included studies with follow-ups from 9 up to 50.9 months,
supports the superiority of FFR guided PCI (21). They analyzed the combined incidents
of MACE and major adverse cardiac and cerebrovascular events and
found a decreased event rate in FFR guided PCI (OR: 1.71 [95% CI:
1.31–2.23; P<0.001, I2=55%). This preference for FFR
remained when retrospective studies (OR: 1.41 [95% CI: 1.06–1.88;
P=0.02, I2=48%) were excluded. Since this meta-analysis
was carried out in 2015, only one randomized study could be
included, and the incorporation of the latest randomized trials may
alter the results.
In addition, not all studies support the findings of
the FAME study. The DEFER-DES study, which compared the 5-year
outcomes of angiography and FFR guided PCI using drug eluting
stents (DES), did not find any superiority for FFR guided DES
implantation or routine DES implantation regarding the rate of MACE
(11.6±3.0 and 14.2±3.3%, respectively (P=0.55) (13). A meta-analysis including only
prospective studies from 2016 also found no significant difference
for MACE (OR: 0.82 [95% CI: 0.64–1.06]; P=0.13, I2=0%),
mortality or repeat revascularization (22). Only the comparison regarding MI
reached a significant level showing a preference for FFR (OR: 0.67
[95% CI: 0.47–0.96]; P=0.03, I2=0%). Several sensitivity
analyses were conducted, where only the exclusion of the FAME study
generated a change in MI results [OR: 0.81 (95% CI: 0.46–1.43);
P=0.47, I2=0%] and the difference between the two
methods did not remain. Differing from our study, that
meta-analysis did not have the focus on one-year outcomes but
included studies with reported outcomes from 3 months up to 5
years, which might lead to a better discrimination for MACE and
overall survival. The focus on the exact same follow-up point of
time is a new aspect of this meta-analysis and improves the
comparability of the included studies and their individual
results.
Excluding the retrospective studies from this
current analysis prompted a change of results regarding MACE as
well. By doing this, the trend moved stronger towards FFR, and the
heterogeneity moved from high to low. This may indicate the
existence of influencing factors in these three retrospective
studies. With more studies being published in the future, in
another meta-analysis carried out later one may alter the inclusion
criteria and thus reduce the heterogeneity. One way could be to
only include prospective RCT's or to exclude any study which had
NSTEMI as an indication for PCI such as Layland et al
(12).
Another aspect of our meta-analysis was the
difference between iFR guided and FFR guided PCI, since the absence
of inferiority of iFR compared to FFR has been shown in the
iFR-SWEDHEART (14), as well as in
DEFINE-FLAIR (15). Both studies
were published in 2017, after the accuracy of iFR was first
compared to FFR in the ADVISE (ADenosine Vasodilator Independent
Stenosis Evaluation) (5) and the
CLARIFY (Classification Accuracy of Pressure-Only Ratios Against
Indices Using Flow Study) study (6).
The statistical analysis had a high homogeneity throughout and
showed that iFR was not inferior to FFR in all four entities. This
must be seen in the context of iFR-SWEDHEART and DEFINE-FLAIR being
the only multicenter, randomized, blinded trials focusing on FFR
guided and iFR guided PCI, since iFR is a rather new technique. In
addition, both studies reported on the observed discomfort of the
patients during the procedure and showed significant lower numbers
in chest discomfort (P<0.001) when using iFR. With both included
studies showing equal diagnostic results it is not surprising for
the meta-analysis to confirm the absence of inferiority of iFR.
Nevertheless, it is important to validate the results of the
individual trials, especially since to our knowledge a
meta-analysis of iFR has not been performed at this point.
But, in order to compare angiography and iFR guided
revascularization in a more direct way, we also conducted network
meta-analysis. Although this network meta-analysis cannot be
equated to a direct comparison, one can see that iFR is not
inferior to angiography guided revascularization. This result is a
very novel aspect of this paper and should be considered when
talking about the best procedure when performing PCI.
As described above, iFR achieved similar results in
comparison to FFR in similar study conditions. Nevertheless,
further investigations should be conducted on iFR by itself in more
complex situations, but also in a direct comparison to angiography
and other treatment strategies for CAD, such as CABG.
Limitations of this meta-analysis are similar to the
limitations of other meta-analyses. This includes the fact that we
had no access to primary data, and the accuracy of our analysis
depends on the accuracy of the primary sources. This meta-analysis
includes prospective randomized controlled trials as well as
retrospective non-randomized studies. Furthermore, the threshold
for ischemia detection was not defined uniformly between the FFR
studies (some studies used 0.75 and others 0.8). Lastly, it should
be noted that the sample size of some of the studies was small and
the populations for the three interventions all differed in size
(angiography guided PCI included 8,403 patients; FFR guided PCI
only included 5,223) Furthermore, we could only include two iFR
studies in this meta-analysis, since iFR is a relatively new
clinical procedure.
Overall, FFR guided PCI showed superiority in MACE
during one year of follow up rates when comparing with angiography
guided PCI. The high heterogeneity did not remain when excluding
three retrospective studies and even reinforced the preference
towards FFR. iFR guided PCI also did not show inferiority to FFR
guided PCI, and thus one can assume iFR to be superior to solely
angiography guided PCI as well. Because low heterogeneity and the
small number of available studies limits the validity, further
trials should be included in future analyses. A direct comparison
of angiography and iFR may also be advised. When talking about
those further studies, not only longer follow-up periods are needed
to proof better outcomes for iFR and FFR guided coronary
interventions regarding MACE, but also different clinical outcomes
have to analyzed.
Acknowledgements
University Hospital Mannheim is a member of DZHK
(Deutsches Zentrum für Herz-Kreislauf-Forschung, German Centre for
Cardiovascular Research, partner site Heidelberg/Mannheim). The
authors would like to thank Mr. Volker Braun from the Library of
the Medical Faculty of Mannheim of the University of Heidelberg for
helping with the systematic literature review.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SB, MB, IA and DL made substantial contributions to
the design of the study, the acquisition and interpretation of
data, drafting of the manuscript, revision of the manuscript for
important intellectual content, and agree to be accountable for all
aspects of the work in ensuring that questions associated with the
accuracy or integrity of any part of the study are appropriately
investigated and resolved. KSEM, SH, FE, ACS and TB made
substantial contributions to the acquisition and analysis of data,
and drafted the manuscript. All authors gave final approval of the
version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CAD
|
coronary artery disease
|
|
CABG
|
coronary artery bypass graft
|
|
DES
|
drug eluting stents
|
|
FFR
|
fractional flow reserve
|
|
iFR
|
instantaneous wave-free ratio
|
|
MACE
|
major adverse cardiac event
|
|
MI
|
myocardial infarction
|
|
OR
|
odds ratio
|
|
PCI
|
percutaneous coronary intervention
|
|
RCT
|
randomized clinical trial
|
References
|
1
|
Pijls NH, van Schaardenburgh P, Manoharan
G, Boersma E, Bech JW, van't Veer M, Bär F, Hoorntje J, Koolen J,
Wijns W and de Bruyne B: Percutaneous coronary intervention of
functionally nonsignificant stenosis: 5-year follow-up of the DEFER
study. J Am Coll Cardiol. 49:2105–2111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Bruyne B, Pijls NH, Kalesan B, Barbato
E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt
N, et al: Fractional flow reserve-guided PCI versus medical therapy
in stable coronary disease. N Engl J Med. 367:991–1001. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Authors/Task Force members, ; Windecker S,
Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm
C, Head SJ, et al: 2014 ESC/EACTS Guidelines on myocardial
revascularization: The Task Force on Myocardial Revascularization
of the European Society of Cardiology (ESC) and the European
Association for Cardio-Thoracic Surgery (EACTS)Developed with the
special contribution of the European Association of Percutaneous
Cardiovascular Interventions (EAPCI). Eur Heart J. 35:2541–2619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for percutaneous
coronary intervention. A report of the American college of
cardiology foundation/American heart association task force on
practice guidelines and the society for cardiovascular angiography
and interventions. Circulation. 124:e574–e651. 2011.PubMed/NCBI
|
|
5
|
Sen S, Escaned J, Malik IS, Mikhail GW,
Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R, et al:
Development and validation of a new adenosine-independent index of
stenosis severity from coronary wave-intensity analysis: Results of
the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation)
study. J Am Coll Cardiol. 59:1392–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sen S, Asrress KN, Nijjer S, Petraco R,
Malik IS, Foale RA, Mikhail GW, Foin N, Broyd C, Hadjiloizou N, et
al: Diagnostic classification of the instantaneous wave-free ratio
is equivalent to fractional flow reserve and is not improved with
adenosine administration. Results of CLARIFY (Classification
Accuracy of Pressure-Only Ratios Against Indices Using Flow Study).
J Am Coll Cardiol. 61:1409–1420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petraco R, Al-Lamee R, Gotberg M, Sharp A,
Hellig F, Nijjer SS, Echavarria-Pinto M, van de Hoef TP, Sen S,
Tanaka N, et al: Real-time use of instantaneous wave-free ratio:
Results of the ADVISE in-practice: An international, multicenter
evaluation of instantaneous wave-free ratio in clinical practice.
Am Heart J. 168:739–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tonino PA, De Bruyne B, Pijls NH, Siebert
U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrøm T,
Oldroyd KG, et al: Fractional flow reserve versus angiography for
guiding percutaneous coronary intervention. N Engl J Med.
360:213–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puymirat E, Peace A, Mangiacapra F, Conte
M, Ntarladimas Y, Bartunek J, Vanderheyden M, Wijns W, De Bruyne B
and Barbato E: Long-term clinical outcome after fractional flow
reserve-guided percutaneous coronary revascularization in patients
with small-vessel disease. Circ Cardiovasc Interv. 5:62–68. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Elrashidi MY, Flammer AJ, Lennon RJ,
Bell MR, Holmes DR, Bresnahan JF, Rihal CS, Lerman LO and Lerman A:
Long-term outcomes of fractional flow reserve-guided vs.
angiography-guided percutaneous coronary intervention in
contemporary practice. Eur Heart J. 34:1375–1383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen SL, Ye F, Zhang JJ, Xu T, Tian NL,
Liu ZZ, Lin S, Shan SJ, Ge Z, You W, et al: Randomized Comparison
of ffr-guided and angiography-guided provisional stenting of true
coronary bifurcation lesions: The DKCRUSH-VI Trial (Double Kissing
Crush Versus Provisional Stenting Technique for Treatment of
Coronary Bifurcation Lesions VI). JACC Cardiovasc Interv.
8:536–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Layland J, Oldroyd KG, Curzen N, Sood A,
Balachandran K, Das R, Junejo S, Ahmed N, Lee MM, Shaukat A, et al:
Fractional flow reserve vs. angiography in guiding management to
optimize outcomes in non-ST-segment elevation myocardial
infarction: The British Heart Foundation FAMOUS-NSTEMI randomized
trial. Eur Heart J. 36:100–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SH, Jeon KH, Lee JM, Nam CW, Doh JH,
Lee BK, Rha SW, Yoo KD, Jung KT, Cho YS, et al: Long-term clinical
outcomes of fractional flow reserve-guided versus routine
drug-eluting stent implantation in patients with intermediate
coronary stenosis: Five-year clinical outcomes of DEFER-DES trial.
Circ Cardiovasc Interv. 8:e0024422015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Backer O, Biasco L, Lønborg J, Pedersen
F, Holmvang L, Kelbaek H, Arnous S, Saunamäki K, Helqvist S,
Kastrup J, et al: Long-term outcome of FFR-guided PCI for stable
coronary artery disease in daily clinical practice: A propensity
score-matched landmark analysis. EuroIntervention. 11:e1257–e1266.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davies JE, Sen S, Dehbi HM, Al-Lamee R,
Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J,
et al: Use of the instantaneous wave-free ratio or fractional flow
reserve in PCI. N Engl J Med. 376:1824–1834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Götberg M, Christiansen EH, Gudmundsdottir
IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Öhagen P,
Olsson H, Omerovic E, et al: Instantaneous wave-free ratio versus
fractional flow reserve to guide PCI. N Engl J Med. 376:1813–1823.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pijls NH, De Bruyne B, Peels K, Van Der
Voort PH, Bonnier HJ, Bartunek J Koolen JJ and Koolen JJ:
Measurement of fractional flow reserve to assess the functional
severity of coronary-artery stenoses. N Engl J Med. 334:1703–1708.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann FM, De Bruyne B, Pijls NH,
Desai M, Oldroyd KG, Park SJ, Reardon MJ, Wendler O, Woo J, Yeung
AC and Fearon WF: Rationale and design of the Fractional Flow
Reserve versus Angiography for Multivessel Evaluation (FAME) 3
Trial: A comparison of fractional flow reserve-guided percutaneous
coronary intervention and coronary artery bypass graft surgery in
patients with multivessel coronary artery disease. Am Heart J.
170:619–626.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Enezate T, Omran J, Al-Dadah AS, Alpert M,
White CJ, Abu-Fadel M, Aronow H, Cohen M, Aguirre F, Patel M and
Mahmud E: Fractional flow reserve versus angiography guided
percutaneous coronary intervention: An updated systematic review.
Catheter Cardiovasc Interv. Oct 5–2017.(Epub ahead of print).
|
|
21
|
Zhang D, Lv S, Song X, Yuan F, Xu F, Zhang
M, Yan S and Cao X: Fractional flow reserve versus angiography for
guiding percutaneous coronary intervention: A meta-analysis. Heart.
101:455–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bundhun PK, Yanamala CM and Huang F:
Comparing the adverse clinical outcomes associated with fraction
flow reserve-guided versus angiography-guided percutaneous coronary
intervention: A systematic review and meta-analysis of randomized
controlled trials. BMC Cardiovasc Disord. 16:2492016. View Article : Google Scholar : PubMed/NCBI
|