Introduction
The rise of multidrug-resistant bacteria poses a
severe threat to human health and is a major cause of death in the
clinical setting (1,2). Among such bacteria, Pseudomonas
aeruginosa (PA) is an opportunistic pathogen and a leading
cause of nosocomial infections (3).
Numerous infections caused by PA occur in immunocompromised
patients and this species is the major cause of
ventilator-associated pneumonia (4).
Pneumonia caused by PA frequently leads to acute lung injury and
secondary sepsis, resulting in high infection-associated mortality.
Antibiotics are effective for the majority of the infections caused
by PA; however, PA has a natural resistance and the capacity for
exposure-induced resistance against antibiotics with various
mechanisms of action, leading to an increasing rate of resistance
(5). Therefore, developing
antibiotics with novel mechanisms of action has attracted the
interest of researchers.
Anti-microbial peptides (AMPs), which are encoded by
specific genes, are a class of low-molecular-weight polypeptides
with potent anti-microbial activity against a broad spectrum of
microorganisms (6). They are a
fundamental component of innate immunity and are nontoxic to
mammalian cells. AMPs initially bind to the negatively charged
bacterial cell membrane by electrostatic interactions and then
insert into the hydrophobic core and perturb its structure to
increase the bacterial membrane permeability, which induces the
leakage of cytoplasmic components and ultimately the death of the
microorganism (7). Due to the unique
bactericidal mechanism of AMPs, it is not easy for bacteria to
develop resistance through evolution, and the entire evolutionary
process is slow; therefore, AMPs are among the most promising
candidates for novel antibiotics for the treatment of
multidrug-resistant bacteria (8).
In a previous study, the AMP chensinin-1 was
isolated from the skin secretions of the Chinese brown frog,
Rana chensinensis, comprising 18 amino acid residues in the
sequence SAVGRHGRRFGLRKHRKH (9,10).
Chensinin-1 exhibited a moderate anti-microbial activity against
gram-positive bacteria, but no activity against gram-negative
bacteria, which may be due to its low hydrophobicity,
amphipathicity and random coil conformation in the membrane
environment. Chensinin-1 is able to form aggregates when it
attaches to the outer cell membrane, as indicated by the quenching
of the fluorescence intensity of rhodamine-labeled chensinin-1 in
the presence of lipopolysaccharides, which serve as the major
component of the outer membrane of gram-negative bacteria. Previous
research has indicated that in AMP sequences, bulky and hydrophobic
Trp residues are not present in a large proportion, but that such
residues may facilitate the anchoring and insertion of the peptide
into the bilayer surface of the cell membrane (11,12).
Therefore, to improve the broad-spectrum anti-microbial activity of
chensinin-1, a novel mutant analog of chensinin-1, MC1, was
designed by replacing three Gly residues with Trp residues. MC1 is
an 18-amino acid peptide with the sequence SAVWRHWRRFWLRKHRKH
(13). MC1 exhibits potent
anti-microbial activity against selected gram-positive bacteria and
gram-negative bacteria, including PA cells. Mechanistically, the
action of the AMP MC1 is initiated through electrostatic
interactions, causing the adsorption of AMPs onto the surface of
the negatively charged cell membrane. The majority of AMPs then
perform membrane permeabilization by inserting into the hydrophobic
core of the outer membrane and disrupting the bacterial membrane,
leading to cell death. Of note, MC1 has no hemolytic activity and
is therefore suitable as a novel antibiotic. However, it remains
elusive whether MC1 possesses bactericidal activity against
multidrug-resistant pathogens encountered in the clinic. In the
present study, the anti-bacterial activity of MC1 against
multi-drug resistant PA (MRPA), which was isolated from a clinical
setting, was investigated in vitro. In particular, the
ability of PA to grow in a biofilm may enhance its aversion of host
defenses and resistance to chemotherapy, and therefore, the ability
of MC1 to inhibit biofilm formation was also examined in the
present study. For this, the effect of MC1 on the relative
expression of specific biofilm-associated genes in multi-drug
resistant PA was investigated.
Materials and methods
Peptide synthesis
The AMP MC1 was synthesized by KareBay Biochem Inc.
(Ningbo, China) using a standard Fmoc solid-phase peptide synthesis
protocol. The peptides were purified to near homogeneity (95%) by
reverse-phase high-performance liquid chromatography using a Vydac
218TP1022 C-18 column (2.2 × 25 cm; Separations Group, Hesperis,
CA, USA) with a mobile phase of acetonitrile/water/trifluoroacetic
acid. The relative mass of the peptide was determined using
matrix-assisted laser desorption ionization-time of flight mass
spectrometry (Shimadzu, Kyoto, Japan).
Bacterial strains
PA was acquired from the China General
Microbiological Culture Collection Centre (Beijing, China). The
MRPA strain was obtained from the Department of Central Laboratory
of Hunan Cancer Hospital (Changsha, China) (14) and exhibited multidrug resistance to
amikacin, cefepime, aztreonam, ciprofloxacin and piperacillin.
Antibiotic susceptibility testing of the strains had been performed
by the Department of Central Laboratory of Hunan Cancer Hospital
(15), the precautions taken with
regard to biosafety when handling the pathogens were described
previously (16).
Anti-microbial assay
The minimum inhibitory concentration (MIC) of the
MC1 peptide for the multidrug-resistant strain MRPA and the
susceptible strain PA was determined using the two-fold dilution
method (17). The peptide was
two-fold serially diluted to achieve concentrations between 1.56
and 200 µM. Subsequently, 50 µl of the peptide solution mixed with
50 µl of a log-phase bacterial inoculum [2×105
colony-forming units (CFU)/ml] in PBS was added to the wells of a
96-well microtiter plate. The cultures were incubated for 24 h at
37°C in air. The absorbance at 600 nm for each sample was recorded
using a microtiter plate reader. The MIC was defined as the lowest
peptide concentration that inhibited 95% of bacterial growth.
Bactericidal kinetics assay
The bactericidal kinetics of the peptide against PA
and MRPA were assessed by generating time-kill curves according to
a previously described method (9).
Log-phase bacterial cultures were incubated with the peptide at its
MIC at 37°C for 0–180 min. After being washed twice with sterile
Meuller-Hinton broth (MHB; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and centrifuged at 4°C and 1,064 × g for 10 min, the
surviving bacteria were diluted 102- or
105-fold and then spread on agar plates (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Bacterial colonies were counted after the plates were incubated for
24 h at 37°C. Polymyxin B (PMB), the positive control drug, was
tested under the same conditions. Bactericidal kinetics were
determined by plotting the number of surviving bacteria against the
time.
Post-antibiotic effect (PAE)
PA and MRPA bacteria were grown until they reached
the log phase and then diluted to 2×106 CFU/ml. The
bacteria were incubated with MC1 at its MIC. After being washed
twice with sterile MHB, the cultures were centrifuged at 4,000 × g
for 10 min at 4°C, and the pellets were re-suspended and incubated
with shaking at 37°C for 8 h. The cell numbers were determined
using the spiral-plating method (18). PMB-treated bacteria were included as
the positive control. The time required for the colonies to reach
1.0 log10 CFU was recorded. The post-antibiotic effect
(PAE) was defined as the time difference between an experimental
culture and the control culture to achieve an increase of 1.0
log10 CFU/ml.
Membrane depolarization assay
Bacterial membrane depolarization was measured using
the previously reported method (19). Mid-log phase bacterial cells were
centrifuged, washed twice with 5 mM HEPES containing 20 mM glucose
and 100 mM KCl and then re-suspended in HEPES buffer at a final
concentration of 2×106 CFU/ml. After EDTA was added at a
final concentration of 0.5 mM, the bacterial suspensions were
incubated with 3,3′-dipropylthiadicarbocyanine iodide (DiSC3-5; 4
µM) to allow for the uptake of the DiSC3-5 probe in a 96-well
microtiter plate. Once DiSC3-5 was taken up by the bacteria, MC1
was added to the bacterial samples at a final concentration of 1-,
2- or 4-fold of its MIC, and the change in fluorescence intensity
was recorded.
Bacterial outer membrane
permeability
The outer membrane permeability was analyzed using
the 1-N-phenylnaphthylamine (NPN) dye (20). The bacterial cells were grown to
mid-log phase, harvested by centrifugation and then washed and
re-suspended in buffer (5 mM HEPES, 1 mM NaN3) at a
density of 2×106 CFU/ml. NPN was added to 500 µl of the
diluted bacterial cells at a final concentration of 10 µM, and the
peptide was then added at increasing concentrations. After 1 h, the
basal fluorescence intensity was recorded with an excitation
wavelength of 350 nm and an emission maximum of 420 nm using a
microplate reader. Gentamicin with MC1 (the two were incubated at
the following concentrations: 1.56, 3.13, 6.25, 12.5, 25 and 50µM)
served as positive controls.
Biofilm susceptibility assay
Biofilm formation was detected using a previously
published method (17). The
bacterial cells were grown to mid-log phase and diluted to
2×105 CFU/ml. The peptide was two-fold diluted from 50
to 1.56 µM and added to 50 µl of the bacterial suspension in a
96-well microtiter plate. The untreated control groups were setup
at the same time. The planktonic cells were removed after
incubation at 37°C for 24 h, and the biofilms in the wells were
washed two times with PBS. The adherent bacteria were fixed with
methanol at room temperature for 15 min, and each well was stained
with 0.1% (w/v) crystal violet (CV) dye at room temperature for 5
min and washed with water. Subsequently, 200 µl of 95% ethanol was
added to each CV-stained well. The absorbance of the biofilm
biomass was measured at 600 nm. PMB was used as a positive control.
The percentage of inhibition for each sample concentration was
calculated according to the following equation: Inhibition
(%)=1-(Absorbancesample/Absorbancecontrol)×100%
(21,22).
Activity against 1-day-old biofilms was determined
according to a previously described method (10). The bacterial cells were grown to
mid-log phase and diluted to 2×105 CFU/ml. Equal volumes
of water and bacteria (50 µl) were added to a 96-well microtiter
plate and then incubated at 37°C for 24 h. The biofilms were washed
with PBS and incubated with different concentrations of peptide for
24 h. Subsequently, the adherent bacteria were fixed with methanol
at room temperature for 15 min and each well was stained with 0.1%
(w/v) CV dye at room temperature for 5 min and washed with water
prior to addition of 200 µl of 95% ethanol to each CV-stained well.
After agitation for 30 min, the absorbance at 600 nm was measured
with a microtiter plate reader. The minimum biofilm reduction
concentration was defined as the minimum concentration of the
peptide required to reduce the biofilm by >50%. PMB was used as
a positive control.
Polysaccharide Psl assay
Psl is a crucial adhesive scaffolding component of
the biofilm matrix, promoting cell-cell interactions and surface
attachment (23,24), which can be examined by using the
liquid Congo red (CR) method according to a published protocol
(5). The mid-log phase bacterial
suspension [optical density at 600 nm (OD600), ~1.0] was
inoculated with the MC1 peptide at a final concentration of 1-, 4-,
8- or 16-fold of its MIC in unsalted Luria-Bertani medium (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China)
containing 40 µg/ml CR. Subsequently, the sample was incubated with
agitation overnight at 37°C. To determine the OD600, 1
ml of the culture liquid was removed. The remaining bacterial cells
were centrifuged for 10 min at 4°C and 4,000 × g, and the
supernatant was measured at 490 nm to determine the binding ability
of bacterial cells to unbound CR, a dye that detects neutral
polysaccharides or polysaccharides (25).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Mid-log phase bacterial cells were divided into two
groups: One group was treated with AMPs at a final concentration
equal to the MIC for 24 h and the other group was incubated without
the peptides. One milliliter of sample was removed and centrifuged
at 13,000 × g for 1 min at 4°C. The supernatant was removed, 10
mg/ml lysozyme (Takara Biotechnology Co., Ltd., Dalian, China) was
added to the sample and the sample was incubated at 4°C for 10 min.
Subsequently, 1 ml TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added, and the samples were
vortexed for 20 sec and incubated at 4°C for 5 min. The supernatant
was transferred into a fresh 1.5 ml Eppendorf tube, chloroform was
added, and the tube was vortexed for 1 min. The sample was
incubated for 15 min to develop a milky appearance and centrifuged
at 4°C. Subsequently, an equal volume of isopropanol was added to
the supernatant, and the sample was incubated for 4 min and
centrifuged at 12,000 × g for 15 min at 4°C. Anhydrous ethanol was
added for precipitation, the supernatant was removed by
centrifugation, and 50 µl DEPC-treated water was added. The RNA
bands were separated by agarose gel electrophoresis.
The extracted RNA was reverse-transcribed into
complementary DNA and qPCR was performed using a Super ScriptÔ III
One-Step RT-PCR System with Platinum™ Taq DNA Polymerase
(cat. no. 12574026; Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol and an ABI Prism 7000
sequence detection system (Thermo Fisher Scientific, Inc.). The
primers were designed and synthesized by Takara Biotechnology Co.,
Ltd. The following primers were used: PlsA (gene ID, 879717)
forward, 5′-AAACGCTACGGCTACAACAACC-3′ and reverse,
5′-TATTCGCTGACCGCCTCCT-3′; PelA (gene ID, 878833) forward,
5′-ACGCCCTTCGCCTATCTGT-3′ and reverse,
5′-GAGGTCCATTACCTGGCTGTTC-3′; alginate (Alg)D (gene ID, 879004)
forward, 5′-CTCATCACCAGCCACGACA-3′ and reverse,
5′-AGCACCAGCACATCGGAAC-3′; and GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
GAPDH expression was used as an internal control. The reaction
conditions were set as follows: 94°C for 30 sec, followed by 40
cycles at 94°C for 5 sec, 55°C for 15 sec and 72°C for 10 sec.
After normalization to the internal control GAPDH, fold changes
were calculated using the comparative cycle threshold method
(26).
Statistical analysis
All experiments were repeated three times,
independently. Values are expressed as the mean ± standard
deviation. Differences between groups were assessed using one-way
analysis of variance followed by the Student-Newman-Keuls post-hoc
test in SAS 9.2 software (SAS Institute Inc., Shanghai, China).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Anti-bacterial activity of MC1 against
multidrug-resistant bacteria
The AMP MC1 exhibited anti-bacterial activity
against the tested multidrug-resistant bacteria. In detail, MC1
exhibited a marked anti-bacterial activity against PA, with an MIC
of 6.25 µM, while an MIC of 25 µM was obtained for the MRPA strain,
which was significantly higher than that for the susceptible PA
strain.
Bactericidal kinetics and PAE of
MC1
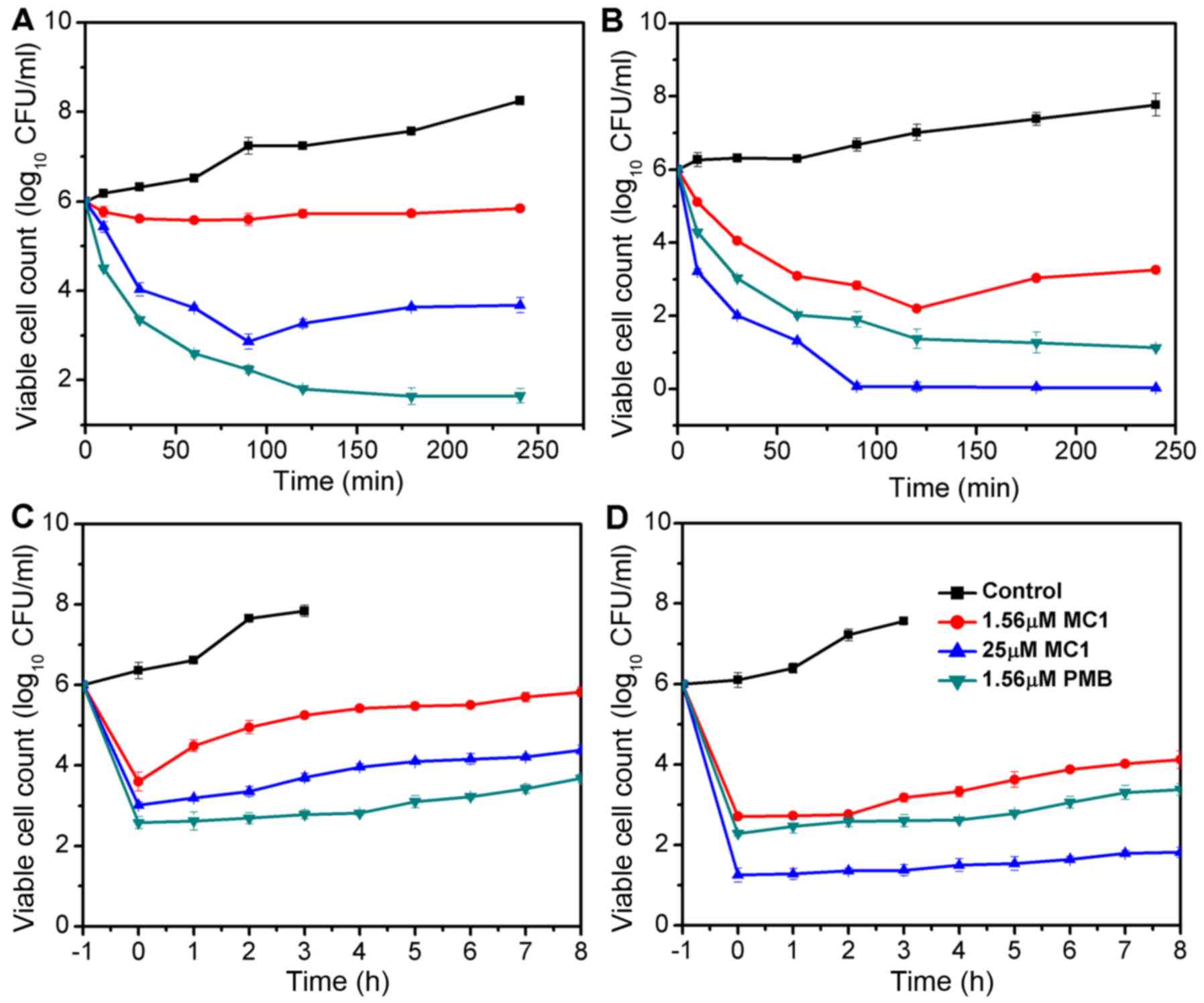
The bactericidal kinetics curves revealed that at
its MIC, MC1 killed the MRPA cells in a time-dependent manner; the
growth of MRPA cells was completely inhibited at 90 min when the
concentration of MC1 was 25 µM and a reduction of >4 log10
CFU/ml was observed. MC1 had no effect on the growth of MRPA cells
at a concentration of 1.56 µM (Fig.
1A), at which the growth of PA cells was inhibited, but the
exponentially growing PA cells were completely eliminated after
incubation with 25 µM MC1 for 90 min (Fig. 1B). PMB served as the positive control
and exhibited a similar anti-bacterial activity against PA and
MRPA. The PAE is defined as persistent suppression of bacterial
growth after a brief exposure (1–2 h) of bacteria to an antibiotic
(27). No PAE was observed when MRPA
cells were treated with 1.56 µM MC1, but when the concentration was
increased to 25 µM, an effect was detected at 3 h (Fig. 1C). A PAE for PA was observed at
peptide concentrations of 1.56 and 25 µM at 4–6 h (Fig. 1D).
MC1 affects the inner membrane
permeability of PA cells
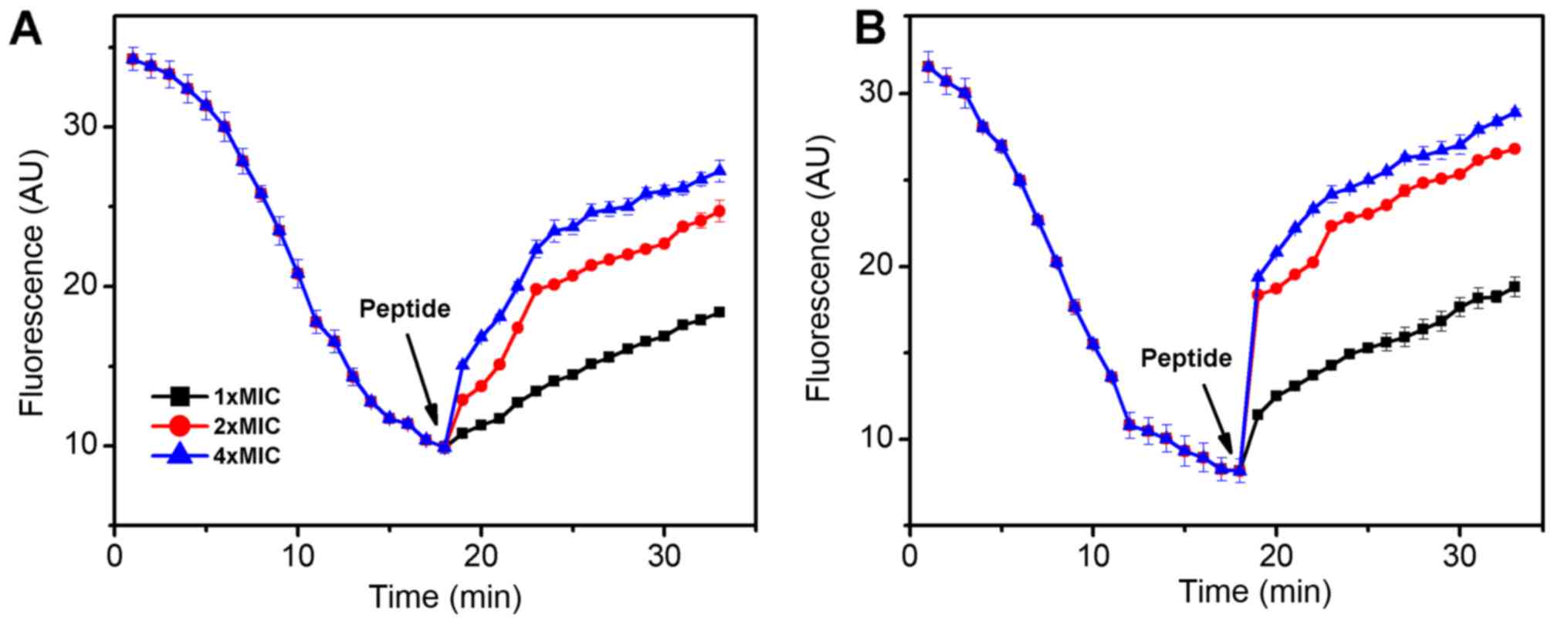
The fluorescent probe DiSC3-5 was used to determine
the effect of the AMP MC1 on the cytoplasmic membrane
depolarization of PA and MRPA. As presented in Fig. 2, when the dye was added to the
bacterial cells, its fluorescence decreased rapidly as the dye
self-quenched in the membranes. At 18 min, the fluorescence reached
a steady state. The peptide was then added and the fluorescence
increased rapidly. As depolarization was completed, the maximum
fluorescence intensity was 27 absorption units (AU) for MRPA and 29
AU for PA. The slope change of the susceptible PA strain was
clearly steeper than that of the MRPA strain, indicating that the
capacity of MC1 to change the inner membrane permeability of the
MRPA was relatively low. The slope changes were positively
associated with the concentration of the peptide.
Outer membrane permeability is
affected by MC1
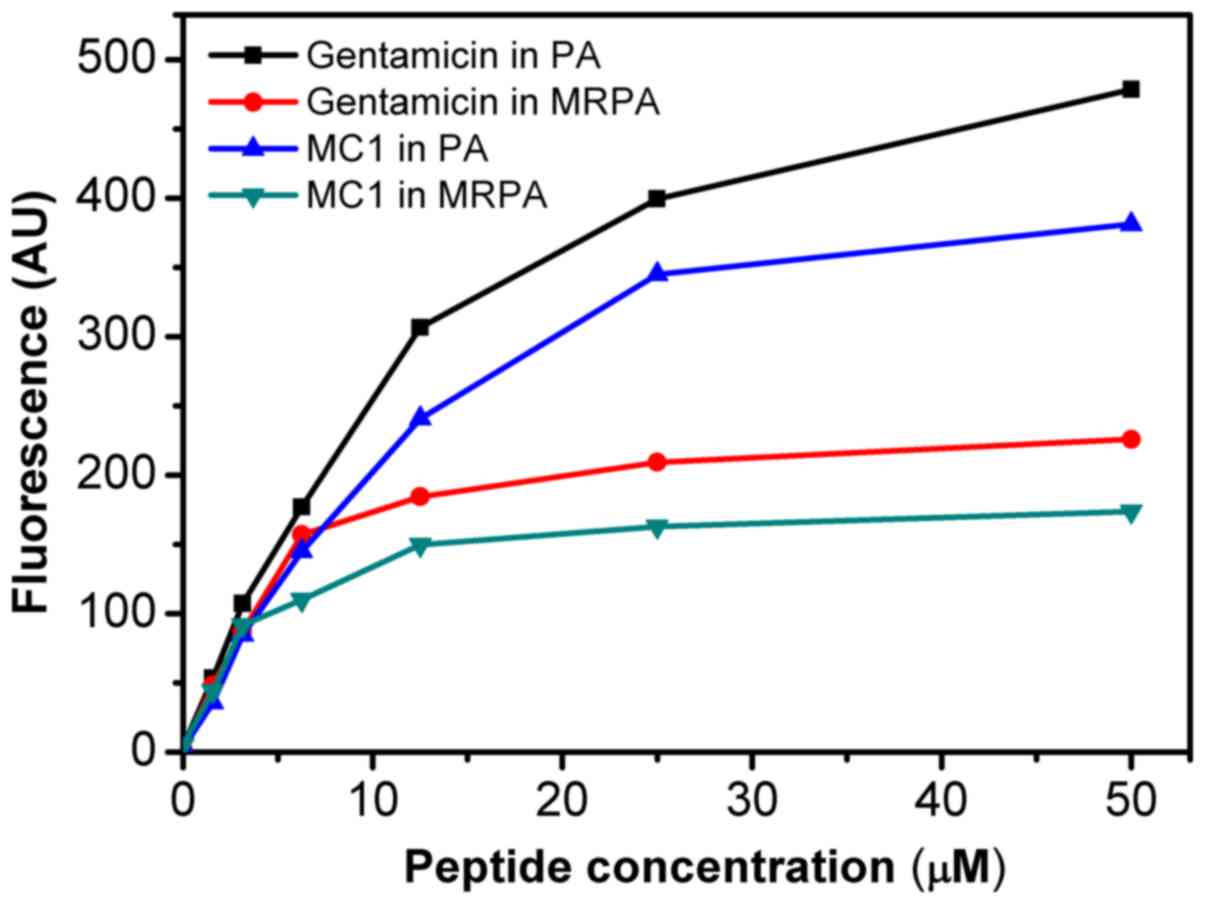
As presented in Fig.
3, the AMP MC1 dose-dependently permeabilized the outer
membrane of MRPA, reflected by the change in the fluorescence
intensity of the NPN dye. Initially, the fluorescence intensity
increased gradually. At the peptide concentration of 25 µM, a
fluorescence intensity of 165 AU was reached and the increase
stagnated at concentrations beyond this. However, at the same
concentration, the ability of the peptide to permeabilize the outer
membrane of the susceptible PA strain was relatively higher, as the
maximum fluorescence intensity reached 350 AU, suggesting that the
ability of the AMP MC1 to permeabilize the outer membrane of PA is
greater than that to permeabilize the outer membrane of the
drug-resistant strain. However, the permeability of the sensitive
and drug-resistant strains also depended on the concentration of
the peptide. The permeability of MC1 in the drug-resistant strain
was lower than that in the sensitive strain. Furthermore, the
ability of the AMP MC1 to permeabilize the outer membrane of the
bacteria was inferior to that of the positive control
gentamicin.
Anti-biofilm activity of the AMP
MC1
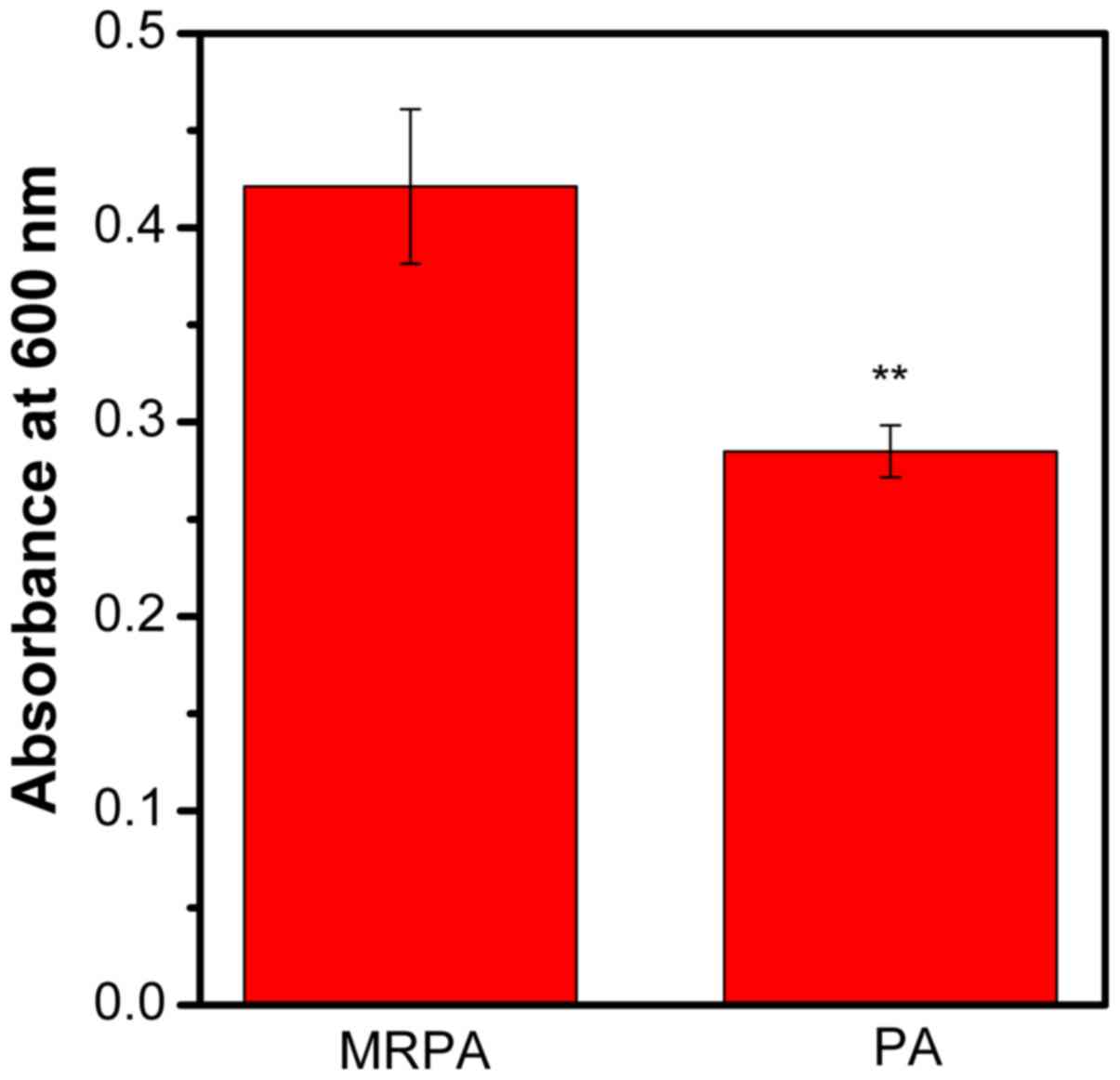
The biomass of PA and MRPA was quantified by CV. As
presented in Fig. 4, compared to the
sensitive strain, the multidrug-resistant strain had a greater
biofilm biomass after 24 h of incubation. It may be concluded that
the ability of MRPA to form a biofilm is stronger than that of the
susceptible strain under the same culture conditions.
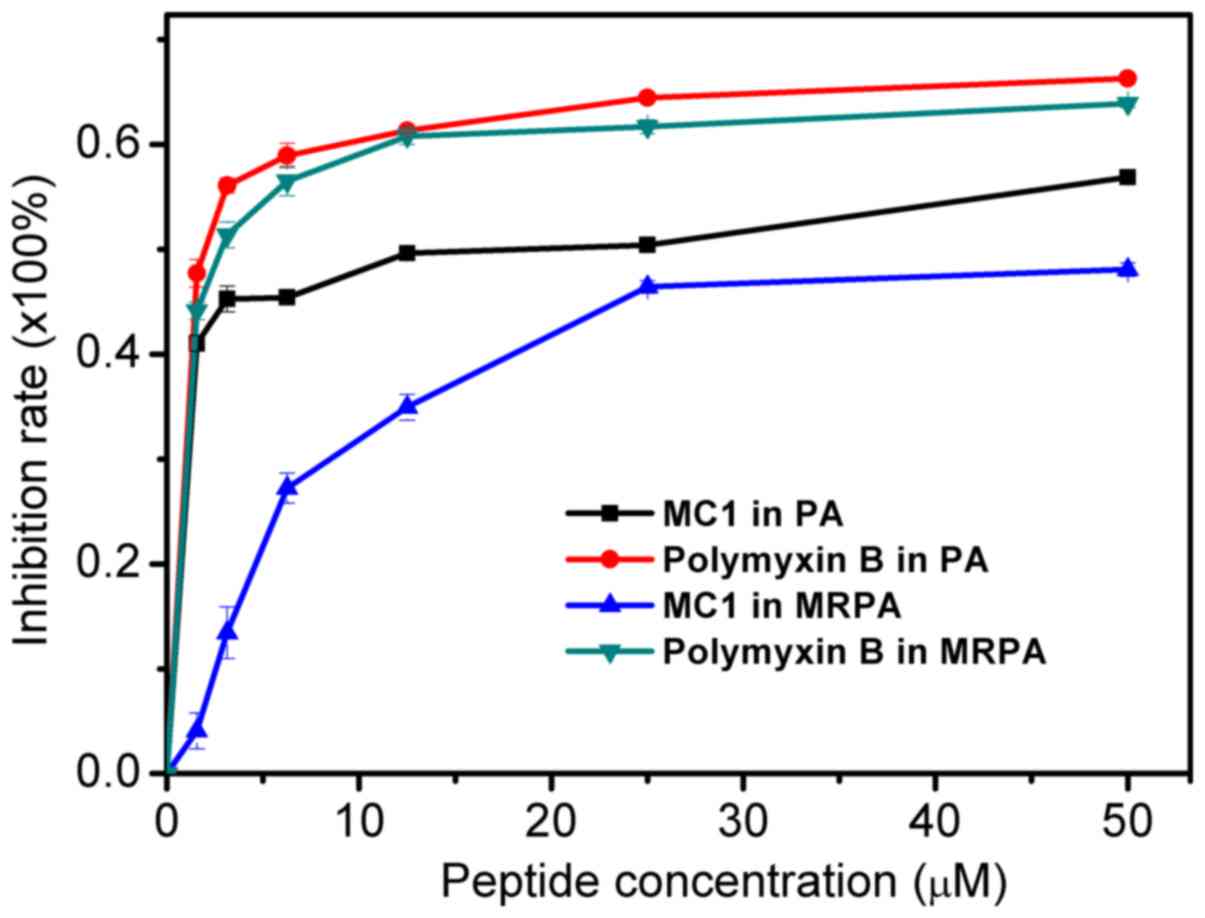
The inhibitory effect of the AMP on the MRPA biofilm
was determined by measuring the OD600. As presented in
Fig. 5, the inhibition of biofilm
formation by the peptide occurred in a dose-dependent manner. For
MRPA, with MC1 at a concentration of 1.56 µM, the inhibition rate
was only ~4%. However, for the susceptible strain, the inhibition
rate was ~41%. For MC1 at a concentration of 25 µM, the inhibition
rate of MRPA was ~46%, which was similar to that observed for
biofilms of the sensitive strain with 1.56 µM peptide. The results
suggested that MC1 inhibits biofilm formation, and as the
concentration of the AMP increases, the inhibition rate of the
biofilm also increases, but the inhibition was less distinct for
MRPA than for PA. Furthermore, the positive control PMB more
effectively inhibited the biofilm formation of PA and MRPA than
MC1, and its effects on these two strains were similar.
Effect of MC1 on the biofilm
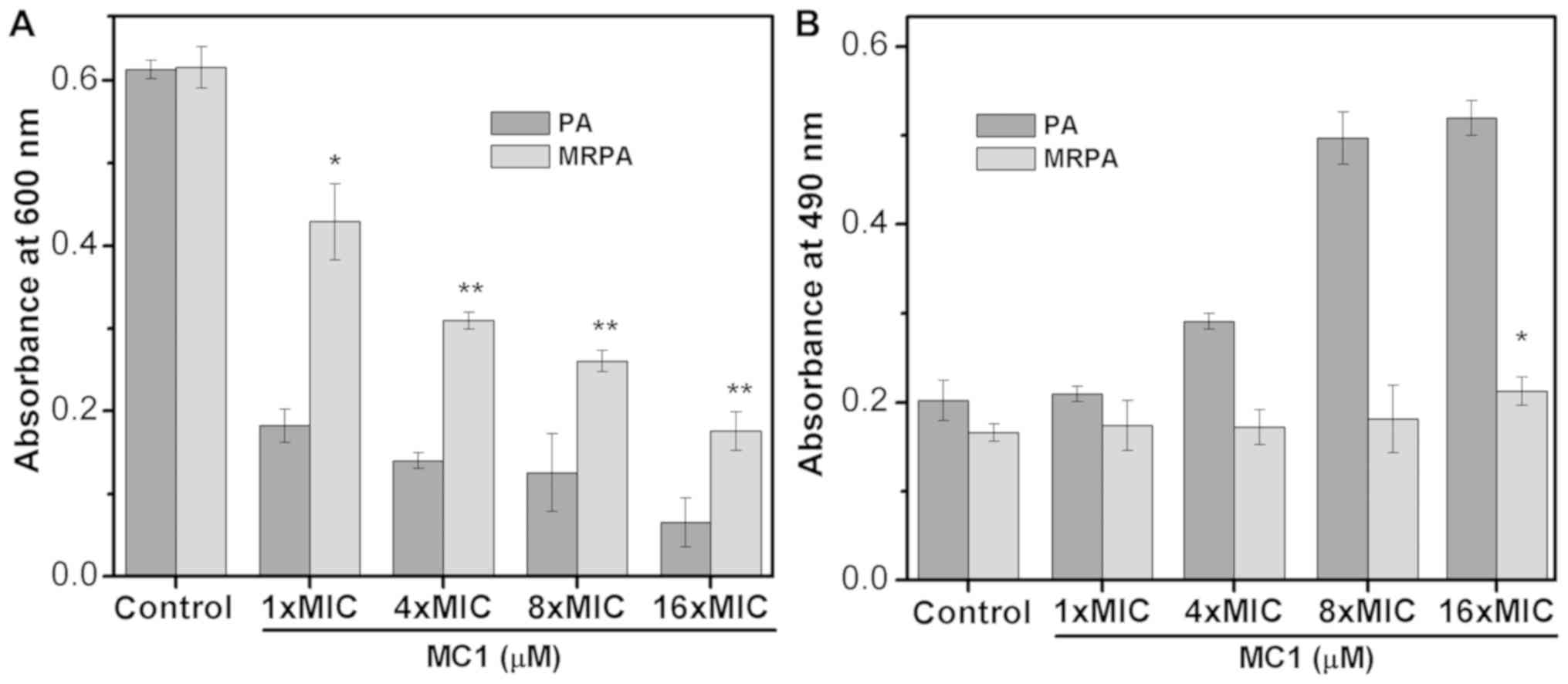
polysaccharide Psl
Previous studies have indicated that at least three
types of polysaccharide, namely Psl, Pel and AlgD, are produced by
PA to constitute the biofilm (28,29). The
biofilms of the sensitive strain and the multidrug-resistant strain
mainly contain two polysaccharides, Psl and Pel, which are
important factors in preventing antibiotics from entering
drug-resistant cells. Therefore, the effect of AMPs on the
synthesis of the biofilm polysaccharide Psl reflects the effect of
AMPs on biofilms (Fig. 6A and B). In
the present study, the OD600 represented suspended cells
and the OD490 indicated that the dye did not bind to the
bacteria. When Psl is overproduced, the binding of Psl to the dye
CR increases the OD600 value but decreases the
absorbance at 490 nm. As the number of bacteria decreases, the
synthesis of Psl is also inhibited, which decreases the binding
capacity of the dye, and the OD490 increases. The
experimental results indicate that the OD600 values of
the multidrug-resistant strain were larger than those of the
sensitive strain, indicating that the multidrug-resistant strain
exhibited less cell death compared with the PA strain. With the
addition of the AMP MC1, the OD600 value decreased and
the OD490 value increased, suggesting that MC1 inhibited
Psl synthesis. This inhibition was dependent on the peptide
concentration. In addition, at a peptide concentration of 1X MIC
(6.25 µM for PA and 25 µM for MRPA strain), the OD490
value of the multidrug-resistant bacteria was less than that of the
sensitive bacteria, indicating that the multidrug-resistant strain
produced more biofilm, which suggested that MRPA had a relatively
greater capacity to synthesize Psl.
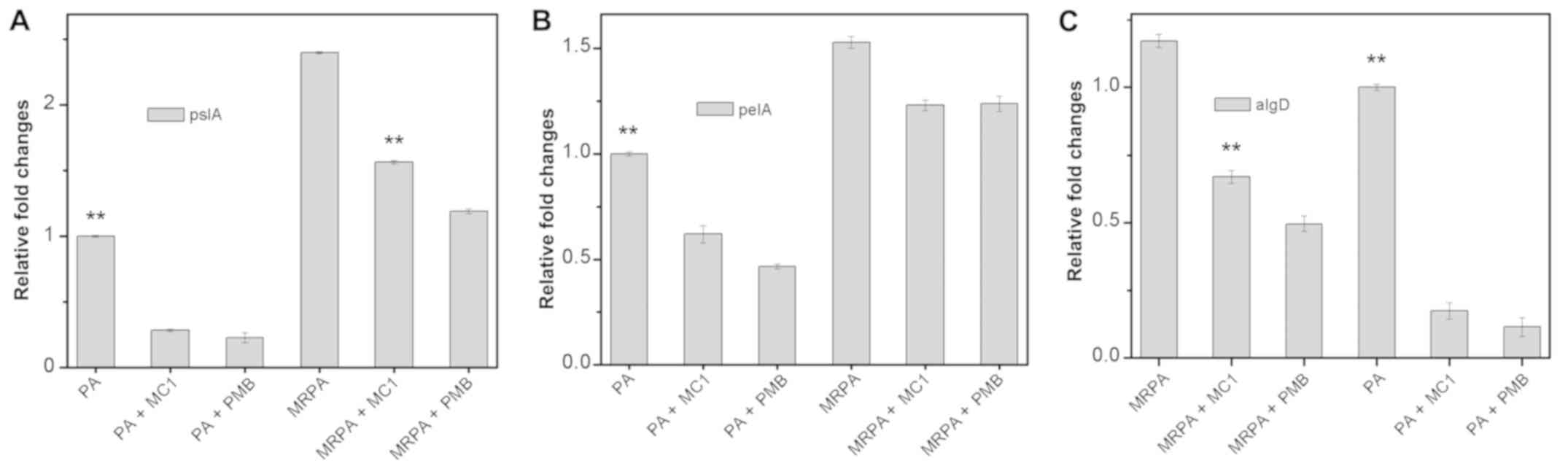
Gene expression of the biofilm
components PslA, PelA and AlgD is affected by MC1
To examine the relative expression of
polysaccharide-associated genes in the multidrug-resistant strain
during biofilm formation, RT-qPCR was used to determine the effect
of MC1 on the relative expression of the genes PelA, PslA and AlgD,
which encode for biofilm components. PMB was used as a positive
control drug. As presented in Fig.
7, the level of transcription of the PslA and PelA genes in the
MRPA cells was almost 2-fold higher than that observed in the PA
cells. In the absence of MC1 and PMB, the three genes were stably
expressed, and the gene expression levels of MRPA were higher than
those in the sensitive strain. When MC1 was added, all three genes
were inhibited, and the relative expression of the genes was
downregulated, particularly that of PslA.
Discussion
P. aeruginosa is resistant to most
antibiotics due to its low outer membrane permeability (30). The present study focused on the
anti-microbial activity of the AMP MC1 against MRPA and its effect
on biofilm formation. The membrane permeability of pathogenic
microorganisms affects whether AMP molecules enter pathogenic
microorganisms and kill them. Therefore, the effect of the MC1
peptide on the permeability of the internal and external membranes
of a multidrug-resistant strain and a sensitive strain was tested.
Depolarization of the bacterial plasma membrane provides a direct
assessment of effects on membrane permeability (31). A depolarization experiment
demonstrated that the degree of depolarization of the
multidrug-resistant strain and the sensitive strain by MC1 were
similar at 30 min, but the slope of the depolarization trend was
clearly different as the fluorescence intensity was increased from
9.9 AU to 15.06 for MRPA, while the fluorescence intensity was
sharply increased from 8.15 AU to 18.35 AU in 1 min for PA in the
presence of 4 × MIC at the interval between 17 and 18 min. However,
as the peptide concentration increased, there was no significant
increase in depolarization for PA and MRPA. An outer membrane
penetration test indicated that the permeability of the AMP MC1 in
the sensitive strain was significantly higher than that observed in
the multidrug-resistant strain, which was also dependent on the
peptide concentration. However, with increasing peptide
concentrations, the permeability of MRPA exhibited relatively
lesser increases and approached a maximum, which may be due to the
low permeability of the outer membrane.
The mechanism of drug resistance in MRPA is
associated with its biofilm formation ability. By quantifying the
biofilm formed by the bacteria within 24 h, it was determined that
the multidrug-resistant strain MRPA was able to produce more
biofilm under normal conditions. By comparing the inhibitory effect
of the AMP MC1 on the biofilm formation of the sensitive strain PA
and the multidrug-resistant strain MRPA, it was revealed that MC1
inhibited the biofilm formation of the sensitive strain PA at a low
concentration, while the inhibition rate in the MRPA groups was
relatively low, which indicated that MRPA produced more biofilm
biomass and was resistant to the inhibitory effects on biofilm
formation. In the biomass inhibition experiment using biofilms
produced by the sensitive strain PA and the multidrug-resistant
strain MRPA over 24 h, MC1 had a comparatively greater effect on
the decomposition of the biofilm from the sensitive strain. In
addition, as MRPA produced more biofilm than the sensitive strain,
the effect on biofilm decomposition was low at the same peptide
concentration.
With respect to biofilm components, the effect of
the AMP MC1 on the amount of the biofilm polysaccharides, Psl and
Pel, and the expression of the genes PslA, PelA and AlgD, which
encode for biofilm components, was assessed. It was revealed that,
compared to the susceptible strain PA, the multidrug-resistant
strain MRPA contained a larger amount of PslA, PelA and AlgD, and
the polysaccharide Psl in each strain was reduced with the addition
of AMP. The RT-qPCR results suggested that the AMP MC1 inhibited
the relative expression of polysaccharide-associated genes. In
general, the multidrug-resistant strain MRPA and the susceptible
strain PA produce biofilm; however, the biofilm formation by MRPA
is faster, and the structure is denser, so the biofilms are more
difficult to decompose (32,33). P. aeruginosa produces at least
three polysaccharides (alginate, Pel, and Psl) to stabilize the
biofilm structure (28,29). The data in the current study also
demonstrated that MC1 significantly inhibited Pel and Psl synthesis
in MRPA cells as the transcription levels of the algD and PslA
genes were significantly downregulated following the treatment of
MRPA 0108 cells with MC1. Therefore, a peptide-driven
downregulation of polysaccharide biosynthesis may occur as MC1
inhibited the expression of the algD and PslA genes to decrease the
structural stability of biofilms and interfere with the formation
of MRPA-containing biofilms.
For the development of novel antibiotics against
multi-drug-resistant pathogens, the bacterial cell membrane may
serve as the major target, as the evolution of membrane composition
changes may be a slow process. Therefore, membrane-targeted AMPs
are expected to be effective compared with traditional antibiotics
with a single target. MC1 exhibited a similar ability to permeate
the cell membrane of the susceptible and the MRPA strain,
suggesting that it has the potential to be developed as an
anti-microbial agent with membrane-perforating activity against
MRPA.
In summary, the present study demonstrates that the
AMP MC1 decreases the drug resistance of MRPA by reducing the outer
membrane permeability and the production of biofilm biomass. The
AMP MC1 inhibited biofilm formation and exhibited an anti-biofilm
effect. In addition, MC1 potently inhibited the expression of
biofilm-associated genes. These results indicated that MC1 may
serve as an effective antibiotic against multidrug-resistant
bacterial strains.
Acknowledgements
Not applicable.
Funding
The present study was supported by Hunan Cancer
Hospital (grant no. 303050).
Availability of data and materials
Data are available from the corresponding authors on
reasonable request.
Authors' contributions
ZY and JL conceived the study, designed the
experiments, analysed the data and wrote the manuscript. ZY, YK, ZL
and TL performed the experiments.
Ethical approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lyczak JB, Cannon CL and Pier GB:
Establishment of Pseudomonas aeruginosa infection: Lessons from a
versatile opportunist. Microbes Infect. 2:1051–1060. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rowe SM, Miller S and Sorscher EJ: Cystic
Fibrosis. N Engl J Med. 352:1992–2001. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicas TI and Hancock RE: Pseudomonas
aeruginosa outer membrane permeability: Isolation of a porin
protein F-deficient mutant. J Bacteriol. 153:281–285.
1983.PubMed/NCBI
|
|
4
|
Breidenstein EB, de la Fuente-Núñez C and
Hancock RE: Pseudomonas aeruginosa: All roads lead to resistance.
Trends Microbiol. 19:419–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pasupuleti M, Schmidtchen A and Malmsten
M: Antimicrobial peptides: Key component of the innate immune
system. Crit Rev Biotechnol. 32:143–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Xiang Q, Zhang Q, Huang Y and Su Z:
Overview on the recent study of antimicrobial peptides: Origins,
functions, relative mechanisms and application. Peptides.
37:207–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shang D, Sun Y, Wang C, Wei S, Ma L and
Sun L: Membrane interaction and antibacterial properties of
chensinin-1, an antimicrobial peptide with atypical structural
features from the skin of Rana chensinensis. Appl Microbiol
Biotechnol. 96:1551–1560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shang D, Yu F, Li J, Zheng J and Li Y:
Molecular cloning of cDNAs encoding antimicrobial peptide
precursors from the skin of the Chinese brown frog, Rana
chensinensis. Zoolog Sci. 26:220–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan DI, Prenner EJ and Vogel HJ:
Tryptophan- and arginine-rich antimicrobial peptides: Structures
and mechanisms of action. Biochim Biophys Acta. 1758:1184–1202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yau WM, Wimley WC, Gawrisch K and White
SH: The preference of tryptophan for membrane interfaces.
Biochemistry. 37:14713–14718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong W, Mao X, Guan Y, Kang Y and Shang D:
Antimicrobial and anti-inflammatory activities of three chensinin-1
peptides containing mutation of glycine and histidine residues. Sci
Rep. 7:402282017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pal T, Abraham B, Sonnevend A, Jumaa P and
Conlon JM: Brevinin-1BYa: A naturally occurring peptide from frog
skin with broad-spectrum antibacterial and antifungal properties.
Int J Antimicrob Agents. 27:525–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saiman L, Mehar F, Niu WW, Neu HC, Shaw
KJ, Miller G and Prince A: Antibiotic susceptibility of multiply
resistant Pseudomonas aeruginosa isolated from patients with cystic
fibrosis, including candidates for transplantation. Clin Infect
Dis. 23:532–537. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reller LB, Schoenknecht FD, Kenny MA and
Sherris JC: Antibiotic susceptibility testing of Pseudomonas
aeruginosa: Selection of a control strain and criteria for
magnesium and calcium content in media. J Infect Dis. 130:454–463.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Working party on antibiotic sensitivity
testing of the britishsociety for antimicrobial chemotherapy.
report of the working party on antibiotic sensitivity testing of
the British Society for Antimicrobial Chemotherapy. A guide to
sensitivity testing. J Antimicrob Chemother. 27:41–43. 1991.
|
|
17
|
Sun Y, Dong W, Sun L, Ma L and Shang D:
Insights into the membrane interaction mechanism and antibacterial
properties of chensinin-1b. Biomaterials. 37:299–311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilchrist JE, Campbell JE, Donnelly CB,
Peeler JT and Delaney JM: Spiral plate method for bacterial
determination. Appl Microbiol. 25:244–252. 1973.PubMed/NCBI
|
|
19
|
Saravanan R, Mohanram H, Joshi M, Domadia
PN, Torres J, Ruedl C and Bhattacharjya S: Structure, activity and
interactions of the cysteine deleted analog of tachyplesin-1 with
lipopolysaccharide micelle: Mechanistic insights into
outer-membrane permeabilization and endotoxin neutralization.
Biochim Biophys Acta. 1818:1613–1624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beckloff N, Laube D, Castro T, Furgang D,
Park S, Perlin D, Clements D, Tang H, Scott RW, Tew GN and Diamond
G: Activity of an antimicrobial peptide mimetic against planktonic
and biofilm cultures of oral pathogens. Antimicrob Agents
Chemother. 51:4125–4132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shang D, Zhang Q, Dong W, Liang H and Bi
X: The effects of LPS on the activity of Trp-containing
antimicrobial peptides against Gram-negative bacteria and endotoxin
neutralization. Acta Biomater. 33:153–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L, Jackson KD, Landry RM, Parsek MR and
Wozniak DJ: Analysis of Pseudomonas aeruginosa conditional psl
variants reveals roles for the psl polysaccharide in adhesion and
maintaining biofilm structure postattachment. J Bacteriol.
188:8213–8221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Friedman L and Kolter R: Two genetic loci
produce distinct carbohydrate-rich structural components of the
Pseudomonas aeruginosa biofilm matrix. J Bacteriol. 186:4457–4465.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsukawa M and Greenberg EP: Putative
exopolysaccharide synthesis genes influence Pseudomonas aeruginosa
biofilm development. J Bacteriol. 186:4449–4456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei GX, Campaqna AN and Bobek LA: Effect
of MUC7 peptides on the growth of bacteria and on Streptococcus
mutans biofilm. J Antimicrob Chemother. 57:1100–1009. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma KK, Sangraulah H and Mediratta PK:
Some new concepts in antibacterial drug therapy. Indian J Pharm.
34:390–396. 2002.
|
|
28
|
Ryder C, Byrd M and Wozniak DJ: Role of
polysaccharides in Pseudomonas aeruginosa biofilm development. Curr
Opin Microbiol. 10:644–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghafoor A, Hay ID and Rehm BH: Role of
exopolysaccharides in Pseudomonas aeruginosa biofilm formation and
architecture. Appl Environ Microbiol. 77:5238–5246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie J, Gou Y, Zhao Q, Wang K, Yang X, Yan
J, Zhang W, Zhang B, Ma C and Wang R: Antimicrobial activities and
membrane-active mechanism of CPF-C1 against multidrug-resistant
bacteria, a novel antimicrobial peptide derived from skin
secretions of the tetraploid frog Xenopus clivii. J Pept Sci.
20:876–884. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Torrent M, Navarro S, Moussaoui M, Nogués
MV and Boix E: Eosinophil cationic protein high-affinity binding to
bacteria wall lipopolysaccharides and peptidoglycans. Biochemistry.
47:3544–3555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XZ, Zhang L and Poole AK: Interplay
between the MexA-MexB-OprM multidrug efflux system and the outer
membrane barrier in the multiple antibiotic resistance of
Pseudomonas aeruginosa. J Antimicrob Chemother. 45:433–436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Høiby N, Bjarnsholt T, Givskov M, Molin S
and Ciofu O: Antibiotic resistance of bacterial biofilms. Int J
Antimicrob Agents. 35:322–332. 2010. View Article : Google Scholar : PubMed/NCBI
|