Introduction
The blood-brain barrier (BBB) is a specialized
tissue that restricts certain substances from entering the brain
and protects normal neuronal function (1). The BBB is composed of cerebral
endothelial cells, astrocytes and pericytes (2). Brain endothelial cells form the
structural basis of the BBB and are characterized by the presence
of tight junctions (TJs). TJs are composed of multiple protein
complexes, including occludin, claudin-5 and TJ protein-1 (ZO-1)
(3). Under normal conditions, TJs
seal the gaps between endothelial cells and prevent the
paracellular movement of molecules, wherein pathological insults
caused by neurological disorders may lead to TJ disruption and BBB
leakage, aggravating brain damage (4).
Ischemic stroke, which accounts for ~85% of all
cases of stroke, is one of the most common diseases worldwide with
a high disability and mortality rates (5). The obstruction of blood vessels reduces
oxygen and glucose supply to the cerebral tissue and results in an
energy failure, which triggers a cascade of events, including the
release of proinflammatory cytokines, elevated levels of reactive
oxygen species (ROS) and the activation of matrix
metalloproteinases (MMPs) (6). These
lead to TJ protein degradation, which can be exacerbated by
thrombolytic therapy (7). Enhanced
BBB permeability induces the occurrence of deleterious
complications, including brain edema and hemorrhage transformation
(8). Numerous compounds, including
α-lipoic acid, ruscogenin and calycosin-7-O-β-D-glucoside, were
reported to protect the ischemia-induced BBB injury through
decreasing the generation of ROS or inhibiting the activity of MMPs
(9–11). Therefore, targeting BBB integrity
suggests a promising therapeutic approach for ischemic stroke
treatment.
The epidermal growth factor (EGF) is a potent
mitogen for mediating neuronal proliferation and inducing central
nerve system (CNS) progenitor cells to produce astrocytes and
neurons (12). EFG bioavailability
in the brain is ensured through production in the CNS by glial
cells and neurons and through uptake from the peripheral
circulation (13). EGF has been
examined as a neurotrophic factor against stroke in animal models
in vivo (14). It also
reduces BBB permeability by activating the EGF receptor and the
downstream mitogen-activated protein kinase (MAPK) signaling
pathway (15,16). However, further investigation is
required as to whether EGF may preserve BBB integrity against
ischemic insult.
In the current study, an in vitro
oxygen-glucose deprivation (OGD) model was established based on
bEnd3 cells, to explore the benefit of EGF on BBB integrity and
protection against ischemic injury. TJ protein expression levels
were measured in addition to endothelial permeability and cell
viability, to demonstrate the protective effect of EGF against
ischemic injury by improving BBB integrity.
Materials and methods
Materials
bEnd3 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin
solution and Hank's balanced salt solution (HBSS) were purchased
from HyClone (GE Healthcare Life Sciences, Logan, UT, USA). Lucifer
yellow (LY) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Rabbit claudin-5 antibody (cat. no. ab15106)
was obtained from Abcam (Cambridge, UK). Rabbit ZO-1 antibody (cat.
no. 21773-1-AP) was purchased from ProteinTech Group, Inc.
(Chicago, IL, USA). Mouse GAPDH antibody (cat. no. C1312),
horseradish peroxide (HRP)-conjugated goat anti-rabbit (cat. no.
C1309) and anti-mouse (cat. no. C1308) IgG antibodies, the Cell
Counting kit-8 (CCK8) and the bicinchoninic acid (BCA) assay kit
were obtained from Applygen Technologies, Inc. (Beijing, China).
Polyvinylidene difluoride (PVDF) membranes and the chemiluminescent
HRP substrate (cat. no. WBKLS0100) were purchased from EMD
Millipore (Billerica, MA, USA). The 24-well polyester Transwell
inserts (pore size, 0.4 µM; diameter, 6.5 mm) were obtained from
Corning Inc. (Corning, NY, USA).
Cell culture and treatment
bEnd3 cells were cultured in DMEM supplemented with
10% FBS and 1% penicillin-streptomycin at 37°C with 5%
CO2 at a density of 3×105/well in six-well
plates. After 24 h, confluent bEnd3 cells were placed with
glucose-free and serum-free DMEM (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and incubated in an anaerobic
chamber (1% O2) flushed with 95% N2 and 5%
CO2 for 4, 6 and 12 h (10). For reoxygenation (RO), cells were
subsequently returned to normoxic conditions and incubated in
glucose-containing and serum-free standard DMEM for 24 h. For the
control group, the bEnd3 cells were incubated in glucose-containing
and serum-free standard DMEM medium for the same period of time
under normoxic conditions for the entire duration of the
experiment. EGF-administered groups were treated with mouse EGF
(250 ng/ml; ProteinTech Group, Inc.) diluted with standard DMEM
following a 4-h OGD period at the beginning of the RO process.
Cell viability
bEnd3 cells (5×103/well) were seeded in
96-well plates and incubated for 24 h prior to OGD treatment for 4,
6 and 12 h as detailed above. A total of 10 µl CCK8 solution was
subsequently added to each well and incubated for 1 h at 37°C.
Absorbance was measured at 450 nm using a plate reader. Changes in
cell viability were expressed compared with respective normoxic
groups.
Western blot analysis
Cells were harvested following RO and homogenized in
radioimmunoprecipitation assay buffer (Applygen Technologies, Inc.)
containing the protease inhibitor. The protein concentration was
determined by BCA assays. Proteins (40 µg) were separated on 12 and
8% SDS-PAGE gels for claudin-5 and ZO-1 analysis, respectively, and
transferred to PVDF membranes. Following blocking with 5% non-fat
milk for 1 h at room temperature, membranes were incubated with
primary antibodies of claudin-5 (dilution, 1:1,000), ZO-1
(dilution, 1:1,000) and GAPDH (dilution, 1:2,000) at 4°C overnight,
followed by incubation with secondary antibodies (dilution,
1:5,000) for 1 h at room temperature. Protein bands were visualized
by chemiluminescence detection using a FluorChem FC2 system (Cell
Biosciences, Inc., Santa Clara, CA, USA) and band densities were
quantified by Image J (version 1.43; National Institute of Health,
Bethesda, MD, USA). Bands were normalized to GAPDH and expressed in
comparison with respective normoxic controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from bEnd3 cells following
RO using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol
and quantified using a spectrophotometer. RNA (1 µg) was
reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit
with gDNA Eraser (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. qPCR was performed using the SYBR Premix
Ex Taq II kit (Takara Bio, Inc.) according to the manufacturer's
protocol using the following thermocycling conditions: 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 20 sec and
72°C for 30 sec. Primer sequences were as follows: Claudin-5,
forward, 5′-GTTAAGGCACGGGTAGCACT-3′ and reverse,
5′-TACTTCTGTGACACCGGCAC-3′; ZO-1, forward,
5′-AAGTTGGCAAGAGAGGAGCC-3′ and reverse, 5′-CAACCGCATTTGGCGTTACA-3′;
GAPDH, forward, 5′-ACGTCTGCCACGATAACACC-3′ and reverse,
5′-CTGCATGATTGGGTCACGTC-3. GAPDH was used as internal control and
mRNA expression levels were quantified using the 2−ΔΔCq
method (17).
Permeability assay
bEnd3 cells (3.3×104/well) were seeded in
Transwell inserts and incubated for 5 days at 37°C prior to
performing the OGD. Following 4 h of OGD and 24 h of RO treatment,
LY (50 µM) dissolved in HBSS was added to the upper compartment and
the lower compartment contained blank HBSS. At indicated time
points (15, 30, 45 and 60 min), samples were taken from the lower
compartment and analyzed using a plate reader (excitation, 430 nM;
emission, 540 nM). The permeability coefficient (Papp)
was measured by the following equation:
Papp=A/(SxC0), where A represents the rate of
drug accumulated at the lower compartment, S represents the
membrane area and C0 is the initial drug concentration
at the upper compartment.
Statistical analysis
Data are expressed as the mean ± standard deviation,
representative of three replicates. Differences between groups were
assessed by one-way analysis of variance followed by Tukey's post
hoc test using SPSS (version 19.0; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
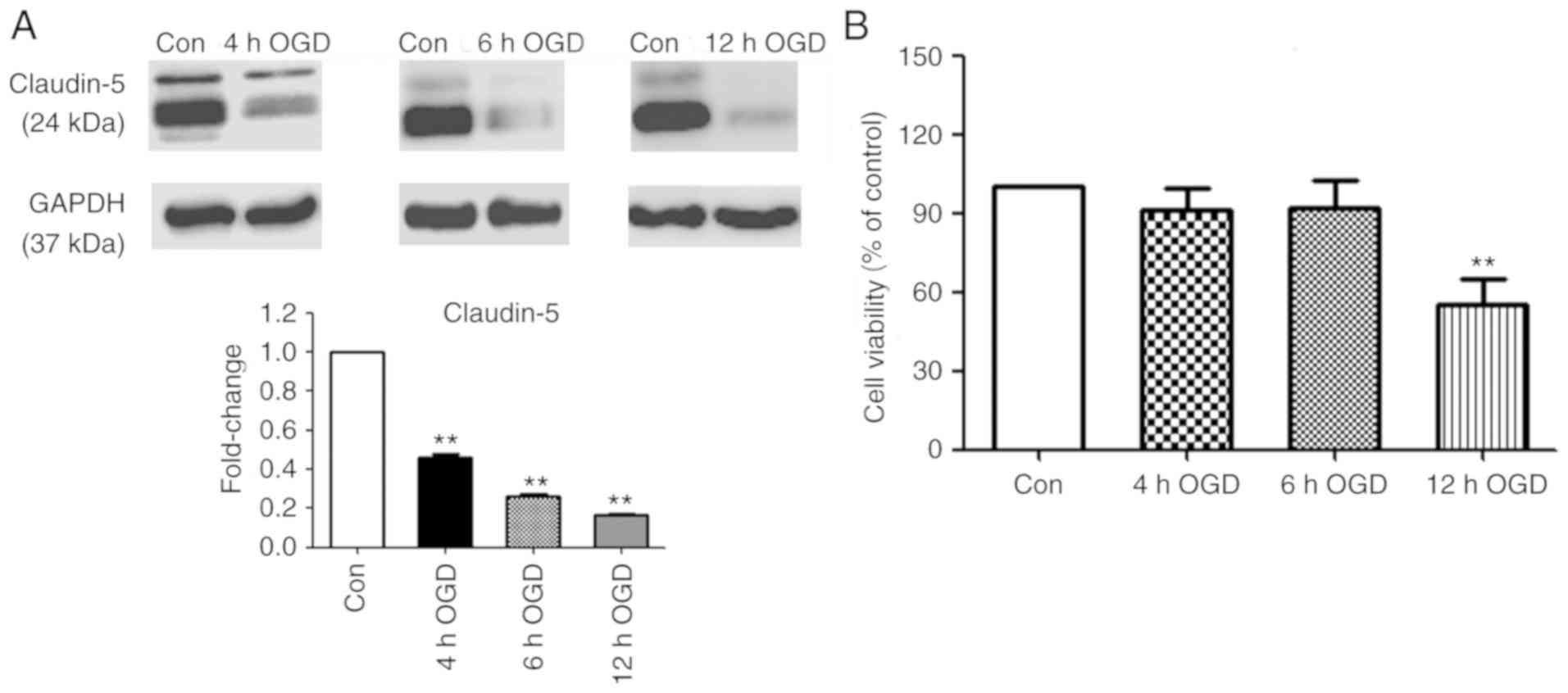
Effects of OGD on TJ protein
expression and cell viability
Claudin-5 is a key TJ protein that determines BBB
permeability and integrity (18). As
an indicator of ischemia-induced BBB disruption, claudin-5
expression was measured in bEnd3 cells, following exposure to OGD
for various periods and 24 h RO. As presented in Fig. 1A, 4, 6 and 12 h OGD exposure resulted
in a time-dependent decrease in claudin-5 protein expression
compared with the normoxic controls. Cell viability analysis
revealed no significant difference between the control and
treatment groups following 4 and 6 h OGD exposure. In contrast, 12
h OGD exposure revealed a significant reduction of 45% in cell
viability compared with the normoxic control (P<0.01; Fig. 1B).
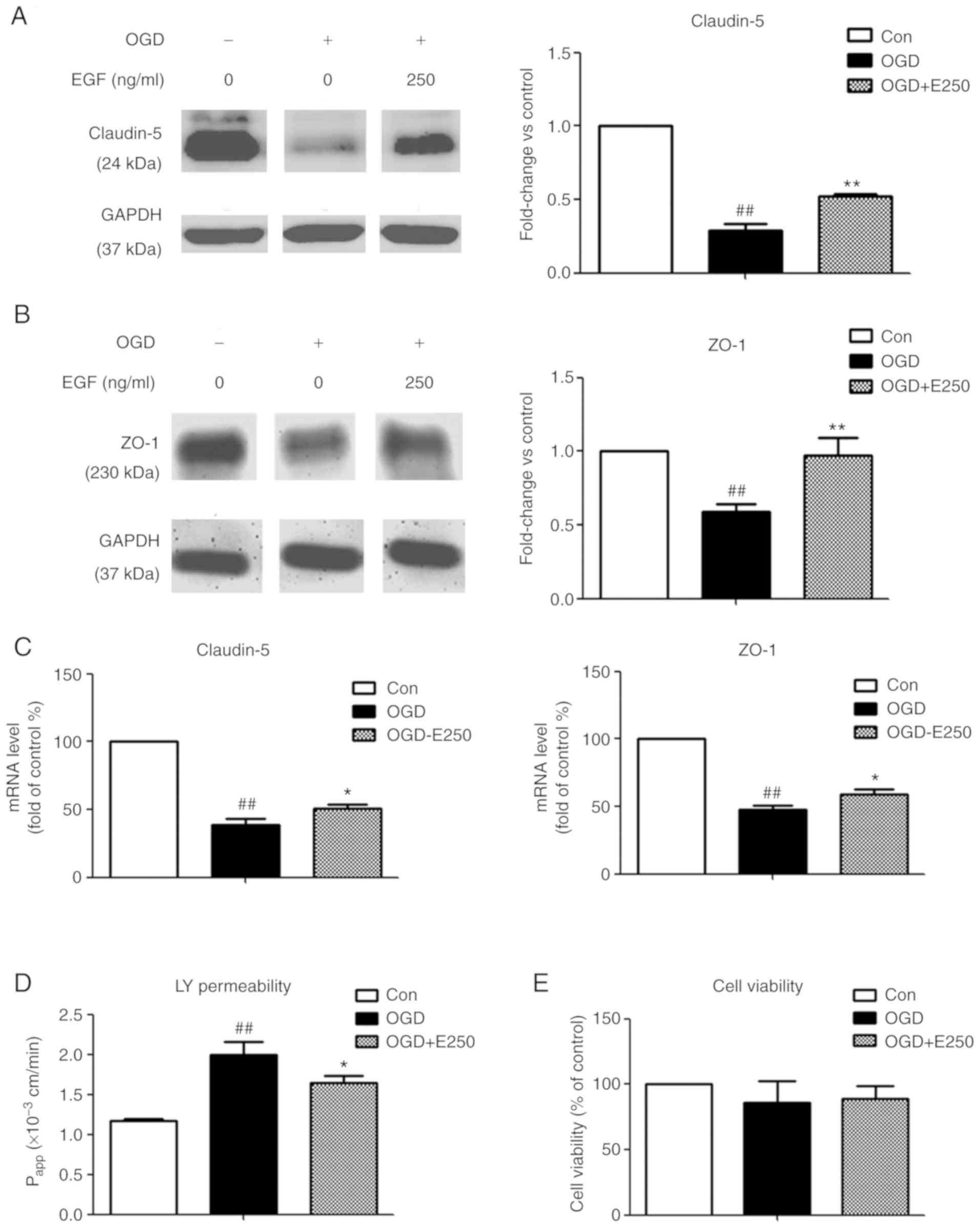
EGF attenuates OGD-induced BBB
disruption
To identify protective effects of EGF on
ischemia-induced BBB disruption, EGF was added to bEnd3 cells
immediately following 4 h OGD exposure prior to RO and subsequently
TJ protein expression levels were measured. In addition,
endothelial cell permeability of LY and cell viability were
determined. As presented in Fig. 2A and
B, 4 h OGD exposure caused a significant decrease in claudin-5
and ZO-1 protein expression compared with the control (71.3 and
41.2%, respectively; P<0.01), which was significantly
ameliorated by EGF treatment (P<0.01 vs. OGD). However, the
differences in the decrease of claudin-5 levels between the same
groups in Fig. 1 (54.2%) and
Fig. 2 (71.3%) may be attributed to
the differential cell growth status and density prior to performing
OGD in separate experiments. A significant decrease in mRNA
expression by 61.5 and 52.1% was observed for claudin-5 and ZO-1 in
the OGD group compared with the control group (P<0.01), while
EGF significantly reversed this decrease (P<0.05; Fig. 2C). In addition, EGF treatment
significantly attenuated an increase in endothelial permeability of
LY following 4 h OGD exposure (P<0.05 vs. OGD; Fig. 2D). No significant differences were
observed in cell viability following EGF treatment compared with
the OGD and normoxic control groups (P>0.05; Fig. 2E). Results indicated that the
protective effect of EGF on BBB integrity may be via enhancing TJ
protein expression and decreasing BBB permeability.
Discussion
Cerebral endothelial cells are a crucial component
of the neurovascular unit and are implicated in many biological
functions, including regulating vascular tone, providing trophic
support for neurons and mediating inflammatory responses (9). They also contribute to BBB integrity by
forming TJs, which block the paracellular flux (19). However, neurological disorders,
including ischemic stroke, damage the cerebral endothelium leading
to TJ dysfunction and BBB leakage, which are associated with
complications, including brain edema and hemorrhage transformation
(20). Therefore, there is an
increasing interest in the protection of BBB integrity in stroke
therapy (21).
The current study established an in vitro OGD
model using the mouse brain microvascular endothelial cell line
bEnd3 to mimic clinical ischemic stroke (22,23). An
increasing OGD exposure duration was observed to be associated with
a decrease in claudin-5 protein expression, indicating a potential
ischemia-induced BBB disruption. A 4 h OGD exposure was selected
for subsequent studies as it closely reflected the limited
therapeutic window of 4.5 h in ischemic stroke in a clinical
setting (6). However, in contrast to
previous studies, this duration of OGD exposure had no significant
effect on cell viability. This may be attributed to the difference
in oxygen levels during the OGD period, 1% here compared with 0.3%
previously reported (24), which
resulted in varying cell damage in the RO period.
The BBB phenotype of cerebral endothelial cells is
regulated by the release of cytokines in the microenvironment
(25). Glial-derived neurotrophic
factor and basic fibroblast growth factor enhance TJ function,
while proinflammatory cytokines, including interleukin-6 and tumor
necrosis factor-α may damage BBB integrity (26). EGF has been reported to preserve
endothelial integrity and protect TJ disruption against hydrogen
peroxide (27). In the present study
it was demonstrated that EGF upregulated the mRNA and protein
expression of claudin-5 and ZO-1 and reduced the endothelial cell
permeability of LY against ischemic insults in vitro. As OGD
and EGF exerted no significant influence on cell viability,
according to the conditions evaluated in the present study, the
protective effect of EGF on BBB integrity was mainly attributed to
the elevation in TJ protein expression levels. Furthermore, MAPK
and protein kinase B (Akt) signaling, downstream pathways of EGF,
serve important roles in cell proliferation, survival and apoptosis
(28). Their activation participates
in the regulation of TJ protein expression and TJ function
(29–31). Therefore, further investigation on
whether EGF preserves BBB integrity against ischemic stroke by
modulating the MAPK and Akt signaling pathways is required.
Additionally, electron microscopy is an effective approach for
observing BBB disruption at the ultrastructure level (32) and this method maybe explored in
future BBB studies.
In summary, EGF ameliorated TJ protein degradation
and inhibited increased BBB permeability induced by OGD treatment
in bEnd3 cells. The aforementioned findings indicated a beneficial
effect of EGF on BBB integrity against ischemic insult.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81703376) and the
Youth Foundation of Beijing Tiantan Hospital (grant no.
2016-YQN-11).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY and ZZ designed the study. SY performed all
experiments. SY and HJ interpreted the data and drafted the
manuscript. ZZ critically reviewed and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang S, Mei S, Jin H, Zhu B, Tian Y, Huo
J, Cui X, Guo A and Zhao Z: Identification of two immortalized cell
lines, ECV304 and bEnd3, for in vitro permeability studies of
blood-brain barrier. PLoS One. 12:e01870172017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cecchelli R, Berezowski V, Lundquist S,
Culot M, Renftel M, Dehouck MP and Fenart L: Modelling of the
blood-brain barrier in drug discovery and development. Nat Rev Drug
Discov. 6:650–661. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cardoso FL, Brites D and Brito MA: Looking
at the blood-brain barrier: Molecular anatomy and possible
investigation approaches. Brain Res Rev. 64:328–363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ronaldson PT and Davis TP: Blood-brain
barrier integrity and glial support: Mechanisms that can be
targeted for novel therapeutic approaches in stroke. Curr Pharm
Des. 18:3624–3644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Zhu X, Chen M, Ge Q, Shen Y and Pan
S: Resveratrol protects PC12 cells against OGD/R-induced apoptosis
via the mitochondrial-mediated signaling pathway. Acta Biochim
Biophys Sin (Shanghai). 48:342–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khatri R, McKinney AM, Swenson B and
Janardhan V: Blood-brain barrier, reperfusion injury, and
hemorrhagic transformation in acute ischemic stroke. Neurology. 79
(13 Suppl 1):S52–S57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warach S and Latour LL: Evidence of
reperfusion injury, exacerbated by thrombolytic therapy, in human
focal brain ischemia using a novel imaging marker of early
blood-brain barrier disruption. Stroke. 35 (11 Suppl
1):S2659–S2661. 2004. View Article : Google Scholar
|
|
8
|
Zhu H, Wang Z, Xing Y, Gao Y, Ma T, Lou L,
Lou J, Gao Y, Wang S and Wang Y: Baicalin reduces the permeability
of the blood-brain barrier during hypoxia in vitro by increasing
the expression of tight junction proteins in brain microvascular
endothelial cells. J Ethnopharmacol. 141:714–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie R, Li X, Ling Y, Shen C, Wu X, Xu W
and Gao X: Alpha-lipoic acid pre- and post-treatments provide
protection against in vitro ischemia-reperfusion injury in cerebral
endothelial cells via Akt/mTOR signaling. Brain Res. 1482:81–90.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin
M, Ma X, Zhou K, Qi J, Yu B and Kou J: Ruscogenin attenuates
cerebral ischemia-induced blood-brain barrier dysfunction by
suppressing TXNIP/NLRP3 inflammasome activation and the MAPK
pathway. Int J Mol Sci. 17(pii): E14182016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu S, Gu Y, Jiang JQ, Chen X, Xu M, Chen X
and Shen J: Calycosin-7-O-β-D-glucoside regulates nitric
oxide/caveolin-1/matrix metalloproteinases pathway and protects
blood-brain barrier integrity in experimental cerebral
ischemia-reperfusion injury. J Ethnopharmacol. 155:692–701. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pillai DR, Shanbhag NC, Dittmar MS,
Boqdahn U and Schlachetzki F: Neurovascular protection by targeting
early blood-brain barrier disruption with neurotrophic factors
after ischemia-reperfusion in rats*. J Cereb Blood Flow Metab.
33:557–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Plata-Salamán CR: Epidermal growth factor
and the nervous system. Peptides. 12:653–663. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang YF, Cooke MJ, Sachewsky N, Morshead
CM and Shoichet MS: Bioengineered sequential growth factor delivery
stimulates brain tissue regeneration after stroke. J Control
Release. 172:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu W, Wang P, Shang C, Chen L, Cai H, Ma
J, Yao Y, Shang X and Xue Y: Endophilin-1 regulates blood-brain
barrier permeability by controlling ZO-1 and occludin expression
via the EGFR-ERK1/2 pathway. Brain Res. 1573:17–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Liu W, Wang P, Xue Y, Su Q, Zeng C
and Shang X: Endophilin-1 regulates blood-brain barrier
permeability via EGFR-JNK signaling pathway. Brain Res. 1606:44–53.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ronaldson PT and Davis TP: Targeting
blood-brain barrier changes during inflammatory pain: An
opportunity for optimizing CNS drug delivery. Ther Deliv.
2:1015–1041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilhelm I and Krizbai IA: In vitro models
of the blood-brain barrier for the study of drug delivery to the
brain. Mol Pharm. 11:1949–1963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi KH, Kim HS, Park MS, Kim JT, Kim JH,
Cho KA, Lee MC, Lee HJ and Cho KH: Regulation of caveolin-1
expression determines early brain edema after experimental focal
cerebral ischemia. Stroke. 47:1336–1343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo J, Krause DN, Horne J, Weiss JH, Li X
and Duckles SP: Estrogen-receptor-mediated protection of cerebral
endothelial cell viability and mitochondrial function after
ischemic insult in vitro. J Cereb Blood Flow Metab. 30:545–554.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Jin X, Liu KJ and Liu W: Matrix
metalloproteinase-2-mediated occludin degradation and
caveolin-1-mediated claudin-5 redistribution contribute to
blood-brain barrier damage in early ischemic stroke stage. J
Neurosci. 32:3044–3057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman NA, Rasil ANHM, Meyding-Lamade U,
Craemer EM, Diah S, Tuah AA and Muharram SH: Immortalized
endothelial cell lines for in vitro blood-brain barrier models: A
systematic review. Brain Res. 1642:532–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ku JM, Taher M, Chin KY, Grace M, McIntyre
P and Miller AA: Characterization of a mouse cerebral microvascular
endothelial cell line (bEnd.3) after oxygen glucose deprivation and
reoxygenation. Clin Exp Pharmacol Physiol. 43:777–786. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Igarashi Y, Utsumi H, Chiba H,
Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y,
Nakagawa T, Mori M and Sawada N: Glial cell line-derived
neurotrophic factor induces barrier function of endothelial cells
forming the blood-brain barrier. Biochem Biophys Res Commun.
261:108–112. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abbott NJ, Rönnbäck L and Hansson E:
Astrocyte-endothelial interactions at the blood-brain barrier. Nat
Rev Neurosci. 7:41–53. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Basuroy S, Seth A, Elias B, Naren AP and
Rao R: MAPK interacts with occludin and mediates EGF-induced
prevention of tight junction disruption by hydrogen peroxide.
Biochem J. 393:69–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park JH and Han HJ: Caveolin-1 plays
important role in EGF-induced migration and proliferation of mouse
embryonic stem cells: Involvement of PI3K/Akt and ERK. Am J Physiol
Cell Physiol. 297:C935–C944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Campbell M, Collery R, McEvoy A, Gardiner
TA, Stitt AW and Brankin B: Involvement of MAPKs in
endostatin-mediated regulation of blood-retinal barrier function.
Curr Eye Res. 31:1033–1045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sumanasekera WK, Sumanasekera GU,
Mattingly KA, Dougherty SM, Keynton RS and Klinge CM: Estradiol and
dihydrotestosterone regulate endothelial cell barrier function
after hypergravity-induced alterations in MAPK activity. Am J
Physiol Cell Physiol. 293:C566–C573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo D, Zhao J and Rong J: Plant-derived
triterpene celastrol ameliorates oxygen glucose deprivation-induced
disruption of endothelial barrier assembly via inducing tight
junction proteins. Phytomedicine. 23:1621–1628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu D, Mai HC, Liang YB, Xu BD, Xu AD and
Zhang YS: Beneficial role of rosuvastatin in blood-brain barrier
damage following experimental ischemic stroke. Front Pharmacol.
9:9262018. View Article : Google Scholar : PubMed/NCBI
|