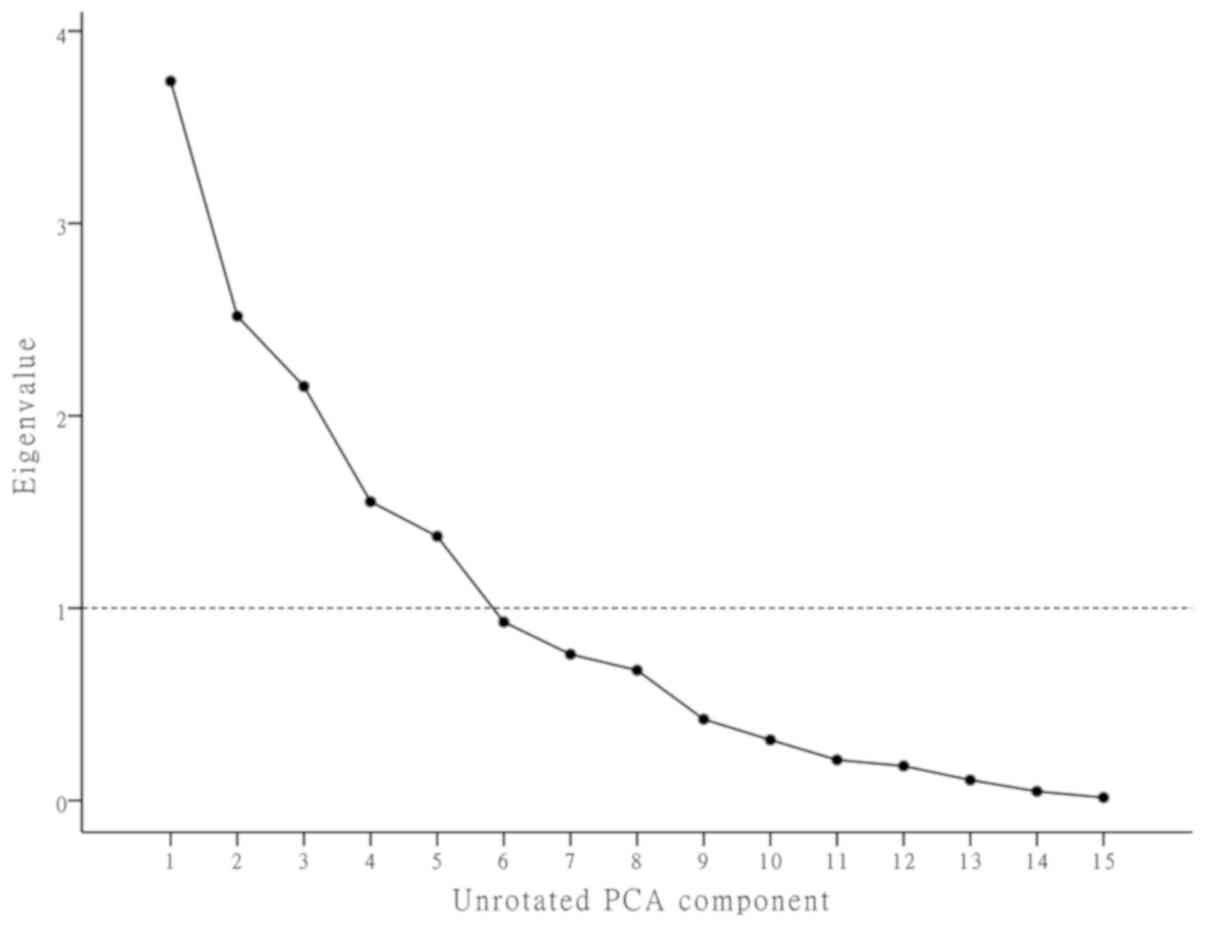
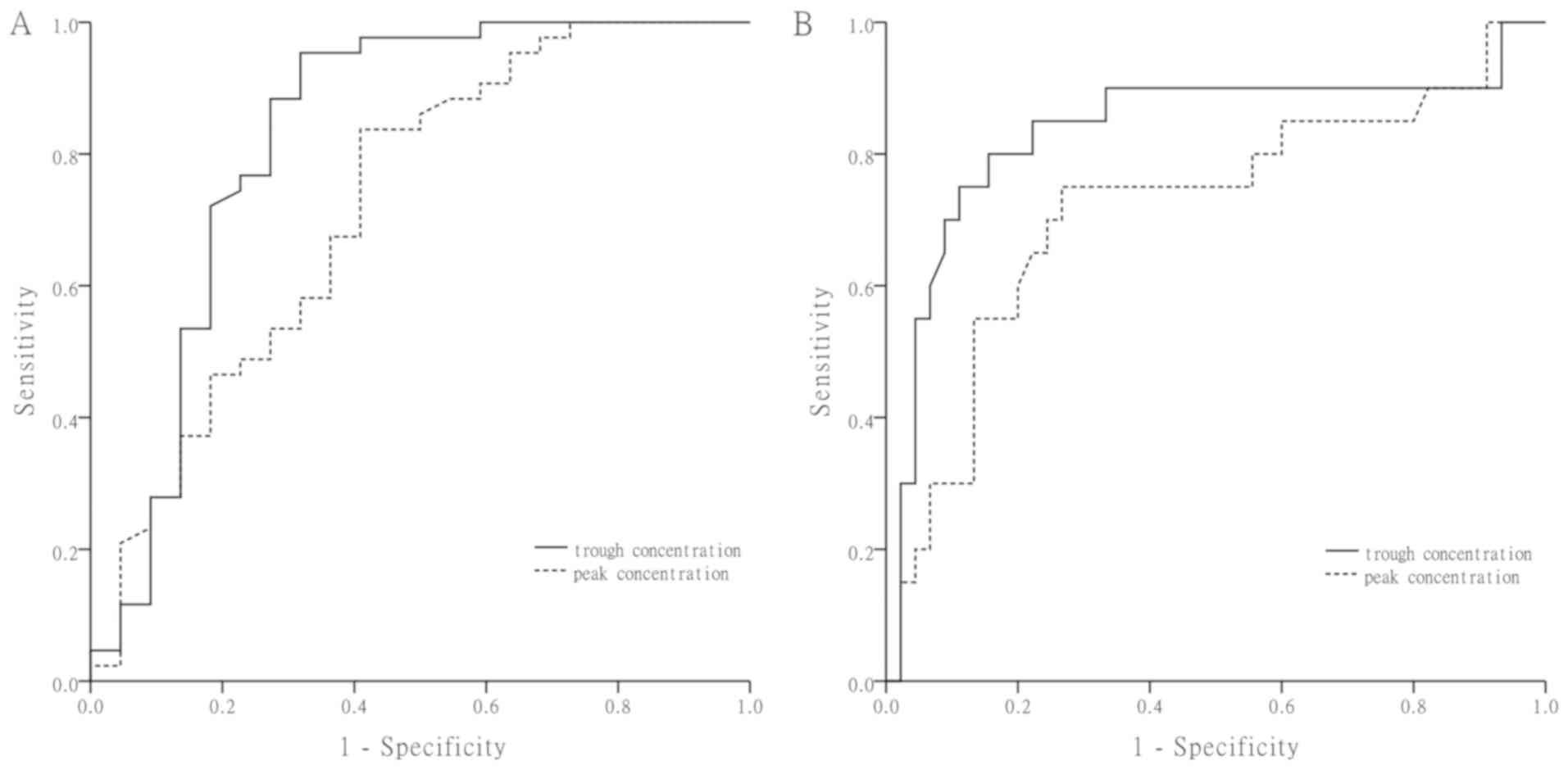
|
1
|
National Nosocomial Infections
Surveillance System: National Nosocomial Infections Surveillance
(NNIS) System report, data summary from January 1992 through June
2004, issued October 2004. Am J Infect Control. 32:470–485. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haque NZ, Zuniga LC, Peyrani P, Reyes K,
Lamerato L, Moore CL, Patel S, Allen M, Peterson E, Wiemken T, et
al: Relationship of vancomycin minimum inhibitory concentration to
mortality in patients with methicillin-resistant Staphylococcus
aureus hospital-acquired, ventilator-associated, or
health-care-associated pneumonia. Chest. 138:1356–1362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin JH, Norris R, Barras M, Roberts J,
Morris R, Doogue M and Jones RD G: Therapeutic monitoring of
vancomycinin adult patients: A consensus review of the American
society of health-system pharmacists, the infectious diseases
society of America, and the society of infectious diseases
pharmacists. Clin Biochem Rev. 31:21–24. 2010.PubMed/NCBI
|
|
4
|
Moellering RC Jr: Vancomycin: A 50-year
reassessment. Clin Infect Dis. 42 Suppl 1:S3–S4. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stevens DL: The role of vancomycin in the
treatment paradigm. Clin Infect Dis. 42 Suppl 1:S51–S57. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah-Khan F, Scheetz MH and Ghossein C:
Biopsy-proven acute tubular necrosis due to vancomycin toxicity.
Int J Nephrol. 2011:4368562001.
|
|
7
|
Htike NL, Santoro J, Gilbert B, Elfenbein
IB and Teehan G: Biopsy-proven vancomycin-associated interstitial
nephritis and acute tubular necrosis. Clin Exp Nephrol. 16:320–324.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumoto K, Takesue Y, Ohmagari N,
Mochizuki T, Mikamo H, Seki M, Takakura S, Tokimatsu I, Takahashi
Y, Kasahara K, et al: Practice guidelines for therapeutic drug
monitoring of vancomycin: A consensus review of the Japanese
society of chemotherapy and the Japanese society of therapeutic
drug monitoring. J Infect Chemother. 19:365–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rybak M, Lomaestro B, Rotschafer JC,
Moellering R Jr, Craig W, Billeter M, Dalovisio JR and Levine DP:
Therapeutic monitoring of vancomycin in adult patients: A consensus
review of the American society of health-system pharmacists, the
infectious diseases society of America, and the society of
infectious diseases pharmacists. Am J Health Syst Pharm. 66:82–98.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakoulas G, Moise-Broder PA, Schentag J,
Forrest A, Moellering RC Jr and Eliopoulos GM: Relationship of MIC
and bactericidal activity to efficacy of vancomycin for treatment
of methicillin-resistant Staphylococcus aureus bacteremia. J Clin
Microbiol. 42:2398–2402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xin HW, Tong HY, Dong QR, Li Q, Wu XC, Yu
AR, Xiong L and Li WL: Monitoring of blood concentration and
individualized administration of vancomycin and norvancomycin in
207 cases. Chin J Pharmacoepidemiol. 21:166–169. 2012.(In
Chinese).
|
|
12
|
Chen CY, Zhu SY, Zhou KT, Zhao YY and Xu
P: Retrospective analysis of nephrotoxicity and efficacy of
vancomycin trough concentrations in patients with severe pneumonia.
Chin J Mod Appl Pharm Pharm Mod Appl Pharm. 33:1188–1194. 2016.(In
Chinese).
|
|
13
|
Cockcroft DW and Gault MH: Prediction of
creatinine clearance from serum creatinine. Nephron. 16:31–41.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galbraith JI, Moustaki I, Bartholomew DJ
and Steele F: The analysis and interpretation of multivariate data
for social scientists. Chapman and Hall/CRC. 56:2802002.
|
|
15
|
Jolliffe IT: Principal Component Analysis.
Springer. (New York, NY). 1986. View Article : Google Scholar
|
|
16
|
Levine DP: Vancomycin: A history. Clin
Infect Dis. 42 Suppl 1:S5–S12. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matzke GR, Zhanel GG and Guay DR: Clinical
pharmacokinetics of vancomycin. Clin Pharmacokinet. 11:257–282.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura T, Takano M, Yasuhara M and Inui
K: In-vivo clearance study of vancomycin in rats. J Pharm
Pharmacol. 48:1197–1200. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dieterich C, Puey A, Lin S, Swezey R,
Furimsky A, Fairchild D, Mirsalis JC and Ng HH: Gene expression
analysis reveals new possible mechanisms of vancomycin-induced
nephrotoxicity and identifies gene markers candidates. Toxicol Sci.
107:258–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishino Y, Takemura S, Minamiyama Y,
Hirohashi K, Ogino T, Inoue M, Okada S and Kinoshita H: Targeting
superoxide dismutase to renal proximal tubule cells attenuates
vancomycin-induced nephrotoxicity in rats. Free Radic Res.
37:373–379. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oktem F, Arslan MK, Ozguner F, Candir O,
Yilmaz HR, Ciris M and Uz E: In vivo evidences suggesting the role
of oxidative stress in pathogenesis of vancomycin-induced
nephrotoxicity: Protection by erdosteine. Toxicology. 215:227–233.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong S, Valderrama E, Mattana J, Shah HH,
Wagner JD, Esposito M and Singhal PC: Vancomycin-induced acute
granulomatous interstitial nephritis: Therapeutic options. Am J Med
Sci. 334:296–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lodise TP, Patel N, Lomaestro BM, Rodvold
KA and Drusano GL: Relationship between initial vancomycin
concentration-time profile and nephrotoxicity among hospitalized
patients. Clin Infect Dis. 49:507–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong-Beringer A, Joo J, Tse E and Beringer
P: Vancomycin-associated nephrotoxicity: A critical appraisal of
risk with high-dose therapy. Int J Antimicrob Agents. 37:95–101.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki Y, Kawasaki K, Sato Y, Tokimatsu I,
Itoh H, Hiramatsu K, Takeyama M and Kadota J: Is peak concentration
needed in therapeutic drug monitoring of vancomycin? A
pharmacokinetic-pharmacodynamic analysis in patients with
methicillin-resistant staphylococcus aureus pneumonia.
Chemotherapy. 58:308–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwamoto T, Kagawa Y and Kojima M: Clinical
efficacy of therapeutic drug monitoring in patients receiving
vancomycin. Biol Pharm Bull. 26:876–879. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeffres MN, Isakow W, Doherty JA, Micek ST
and Kollef MH: A retrospective analysis of possible renal toxicity
associated with vancomycin in patients with health care-associated
methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther.
29:1107–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bosso JA, Nappi J, Rudisill C, Wellein M,
Bookstaver PB, Swindler J and Mauldin PD: Relationship between
vancomycin trough concentrations and nephrotoxicity: A prospective
multicenter trial. Antimicrob Agents Chemother. 55:5475–5479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Yin Y, Liu XZ, Yao HJ, Li LX, Chen
JH, Chen T, Lu XT, Bu SH and Zhang J: Retrospective analysis of
vancomycin nephrotoxicity in elderly chinese patients.
Pharmacology. 95:279–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han HK, An H, Shin KH, Shin D, Lee SH, Kim
JH, Cho SH, Kang HR, Jang IJ, Yu KS and Lim KS: Trough
concentration over 12.1 mg/l is a major risk factor of
vancomycin-related nephrotoxicity in patients with therapeutic drug
monitoring. Ther Drug Monit. 36:606–611. 2014. View Article : Google Scholar : PubMed/NCBI
|