Introduction
Globally, obesity is a serious health problem due to
its strong association with increased dyslipidemia, cardiovascular
disease (including hypertension, stroke, myocardial infarction),
insulin resistance, glucose metabolism disorders, osteoarthritis
and some cancers (1,2). According to a recent report, the
increase in the world obesity rate from 1975 to 2014 was 3.2–10.8%
in men, and 6.4–14.4% in women (3).
This trend will have a significant impact on world health and the
economy. The main causes of obesity are the delicious and energetic
diets rich in fat, consumption imbalances, low momentum, fat, sugar
and salt (1). The most common
methods for preventing and treating obesity are maintaining an
optimal weight by calibrating lifestyle habits by introducing
well-structured calorie restriction and exercise programs (4). Lifestyle corrections are very
challenging for obese patients, who subsequently resort to the use
of medications as an adjunct. However, factors such as the cost and
potential side effects are the main causes for failure of weight
and obesity management with medication. Recent reports indicate
that many herbal extracts, including Paullinia cupana, Plantago
psyllium, Garcinia cambogia, and Ilex paraguariensis,
are relatively safe for controlling weight, by promoting the lipid
and carbohydrate metabolism (5).
Thus, herbal extracts from natural sources are likely to be safe
and effective alternatives for treating obesity.
Commonly known as mulberry in Korea, Morus
alba (family: Mulberry), grows in northern China, and has been
cultivated from India to the Middle East, southern Europe, and
recently North America. Pharmacological studies have reported the
roots and bark to have antibacterial (6), antioxidant and hypoglycemic (7,8), and
neuroprotective, anti-ulcer, analgesic and anti-inflammatory
activities (9–12). Several studies have also presented
the beneficial effects of mulberry leaves on lipid metabolism.
Kobayashi et al (13)
reported that administration of mulberry leaves to rats for 7 weeks
induced the upregulation of fatty acid oxidation and downregulation
of lipogenesis-related genes. Other studies reported that the
mulberry leaf extract significantly reduced the lipid accumulation
and lipoprotein-related protein expression in 3T3-L1 adipocytes
(14). Additionally, the mulberry
leaf extract stimulates the glucose uptake in rat adipocytes, and
reduces the adipocyte development in white adipose tissue extracted
from db/db mice through inhibition of oxidative stress (15–17).
Cordyceps militaris (C. militaris) is an insect
pathogen belonging to Ascomycota, and is traditionally used in
Chinese and East Asian medicine (18,19).
Cordycepin isolated from C. militaris is effective in
anticancer (20), antitumor
(21), antimicrobial (22) and antioxidant (23) therapy. Recent reports indicate that
C. militaris-fermented products are beneficial in
attenuating liver fibrosis (24,25);
similar effects were observed with a mixture of C. militaris
and a secondary metabolite secreted from mushroom (26). In our previous study, we reported
that the extract of Morus alba leaves fermented with C.
militaris (EMfC) has a lipolytic effect on adipocytes isolated
from SD rats (27). However, no
detailed study was performed to evaluate the inhibitory effect of
EMfC on obesity.
Based on previous reports, we hypothesized that the
EMfC induces its anti-obesity effects through the regulation of
lipid metabolism. We therefore used mice with high-fat diet-induced
obesity to evaluate changes in fat accumulation and lipid profiles,
as well as examined the underlying mechanisms of adipogenesis,
lipogenesis and lipolysis.
Materials and methods
Preparation of EMfCs
One of the authors (Professor Young Whan Choi)
characterized the samples of mulberry leaves collected from
plantations in the Sangju district of Korea in October 2015.
Voucher specimens (accession no. Mul-PDRL-1) were deposited in the
herbarium of the Pusan National University (Miryang, Korea).
Professor Sang Mong Lee of the Department of Life Science and
Environmental Biochemistry, Pusan National University, kindly
provided the C. militaris used for fermentation. The
Jeongeup Agriculture Cooperative Federations for Silkworm Farming
(Jeongeup, Korea) supplied the silkworm pupae powder.
Briefly, the mulberry leaves were powder using an
electric blender, after drying in a hot-air drying machine (JSR,
Seoul, Korea) for 24 h at 60°C. The mulberry powder was sterilized
by autoclaving at 121°C for 60 min, mixed with 50% silkworm powder
(SWP), and the mixture inoculated with 10% C. military
(v/w). The samples were incubated in a shaking incubator (cat. no.
SI-600R; Lab Companion, Seoul, Korea) at 150 rpm and 25°C, and
fermented for 4 weeks. On the last day of fermentation, the mixture
was harvested from the flask and extracted with 95% EtOH. To
prepare the extracts of fermented mulberry powder, the extracted
powder was mixed with the solvent (95% EtOH) in a fixed liquid
ratio (mulberry powder:solvent, ratio 1:10) and sonicated for 1 h
using a JAC ultrasonic device (KODO, Hwangseong, Korea).
Centrifuging at 3,000 rpm for 10 min separated the supernatant from
the sonicated extract. The pellet was resuspended in 9 ml of the
solvent, and further sonicated using the same conditions. This
procedure was repeated once more, and the resultant supernatant was
collected, filtered through a 0.4 µm filter (cat. no. HAWP04700;
Millex-LH, MerckMillipore, Darmstadt, Germany), and evaporated
using a vacuum evaporator (cat. no. R-300, BUCHI Corporation, New
Castle, Delaware, USA). Finally, lyophilization of the EMfC was
achieved using the circulating extraction equipment (IKA
Labortechnik, Staufen, Germany). The extracts were dissolved in
DMSO (cat. no. D2660, Sigma-Aldrich Co., St Louis, MO, USA) at a
concentration of 50 mg/ml before use.
Design of animal experiment
Eight-week-old C57BL/6 male mice were purchased from
Samtako Bio-Korea Inc. (Osan, Korea), and provided ad
libitum access to water and a standard irradiated chow diet
(Samtako Bio-Korea Inc.). During the experiment, mice were
maintained in a pathogen-free state under a strict light cycle
(lights on at 08:00 h and off at 20:00 h) at 23±2°C and 50±10%
relative humidity. All C57BL/6 mice were handled at the Pusan
National University-Laboratory Animal Resources Center, accredited
by the Korea Food and Drug Administration (FDA; Accredited Unit no.
000231) and AAALAC International (Accredited Unit no. 001525).
The Pusan National University Animal Ethics
Committee (PNU-2017-1519) approved the protocol for animal study.
All animals were acclimatized on a normal diet (D12450K; Research
Diets, New Brunswick, NJ, USA) for 1 week. The C57BL/6 mice were
then divided into 4 study groups (7 mice/group): (1) Control group fed a normal diet (No
treatment group), (2) group fed HFD
(high fat diet) plus vehicle (olive oil+1% DMSO) (HFD+Vehicle
treated group), (3) group fed HFD
plus 10 mg/kg Orlistat (Sigma-Aldrich Co.) (HFD+OT treated group),
and (4) group fed HFD plus 50 mg/kg
EMfC (HFD+EMfC treated group). For 12 weeks, mice of all HFD
treatment groups consumed HFD containing 60% kcal fat purchased
from Research Diets (cat. no. D12492; Research Diets, Inc., New
Brunswick, USA). After 24 h of the final EMfC treatment, all mice
were euthanized using CO2 gas, after which the tissue
samples were acquired and stored in Eppendorf tubes at −70°C until
assay.
Measurement of body weight, dietary
intake and organ weight
Throughout the experimental period, the body weight
of mice treated with Vehicle, OT or EMfC were measured daily at
10:00 am using an electronic balance (cat. no. AD-2.5; Mettler
Toledo, Greifensee, Switzerland) according to the KFDA guidelines.
In addition, the weights of liver and abdominal organs collected
from the sacrificed C57BL/6 mice were determined using the same
method employed to measure the body weight. Feed intake was
measured at the same time once a week during the study period,
using a chemical balance (Mettler Toledo, Switzerland).
Serum biochemical analysis
After 12 weeks of feeding experimental diets, blood
was collected from the abdominal veins of all C57BL/6 mice after
fasting for 18 h. The blood samples were incubated for 30 min at
room temperature in serum separating tubes (BD Biosciences,
Franklin Lakes, NJ, USA). Serum was obtained by centrifugation at
1,500 × g for 15 min. Serum glucose, TC (total cholesterol), TG
(triglyceride), high-density lipoprotein (HDL)-cholesterol, and
low-density lipoprotein (LDL)-cholesterol levels were analyzed by
the automatic chemical analyzer (BS-120 Chemistry Analyzer;
Mindray, Shenzhen China). Additionally, serum was analyzed for
alkaline phosphatase (ALP), alanine aminotransferase (ALT),
aspartate aminotransferase (AST), blood urea nitrogen (BUN), and
creatinine (Crea) by using an automatic biochemical analyzer
(BS-120; Mindray). All assays were conducted in duplicate using
fresh serum.
Histopathological analysis
Liver and fat tissues dissected from mice of all
subset groups were fixed in 10% neutral buffered formaldehyde (pH
6.8) overnight. The dehydrated liver tissue was then embedded in
paraffin wax. Next, a series of liver and fat sections (4 µm) were
cut from the paraffin-embedded tissues using a Leica microtome
(cat. no. DM500; Leica Microsystems, Bannockburn, IL, USA). These
sections were then deparaffinized with xylene (cat. no. 8587-4410;
Daejung, Gyeonggi-do, Korea), rehydrated with graded ethanol
(decreasing concentrations of 100-70%), and finally washed with
distilled water. The slides with liver sections were stained with
hematoxylin (cat. no. MHS16) and eosin (cat. no. HT110332; both
Sigma-Aldrich Co.), washed with dH2O, and pathological
changes were assessed using the Leica Application Suite (Leica
Microsystems).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Frozen liver tissue was chopped with scissors and
homogenized in RNA Bee solution (cat. no. CS-105B; Tet-Test Inc.,
Friendswood, TX, USA). Total RNA molecules were isolated by
centrifugation at 15,000 rpm for 15 min, after which the
concentration was measured by UV spectroscopy. The complementary
DNA (cDNA) was synthesized by Invitrogen Superscript II reverse
transcriptase (cat. no. 4376600; Thermo Scientific, Wilmington,
Delaware, USA). Quantitative PCR was performed with the cDNA
template (2 µl) and 2X Power SYBR-Green (6 µl; Toyobo Life Science,
Osaka, Japan) containing specific primers. The primer sequences for
target gene expression identification used were as follows: PPARγ
sense, 5′-GAGTTCATGCTTGTGAAGGATGCAAGG-3′ and anti-sense,
5′-CATACTCTGTGATCTCTTGCACG-3′; C/EBPα sense,
5′-GTGGACAAGAACAGCAACGAGTACC-3′ and anti-sense,
5′-GGAATCTCCTAGTCCTGGCTTGC-3′; β-actin sense,
5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and anti-sense,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. qPCR was performed for 40 cycles
using the following parameters: Denaturation at 95°C for 15 sec,
followed by annealing and extension at 70°C for 60 sec.
Fluorescence intensity was measured at the end of the extension
phase of each cycle. The threshold value for the fluorescence
intensities of all samples was set manually. The reaction cycle at
which the PCR products exceeded this fluorescence intensity
threshold during the exponential phase of PCR amplification was
considered as be the threshold cycle (Cq). Expression of the target
gene was quantified relative to that of the housekeeping genes
β-actin, based on comparison of the Cqs at a constant fluorescence
intensity, as per Livak and Schmittgen's method (28).
Western blot analysis
Liver tissue (50 mg) collected from each group was
homogenized using PRO-PREPTM Solution (cat. no. 170841; iNtRON
Biotechnology Inc., Sungnam, Korea), and total protein extracts
were collected by centrifugation at 13,000 rpm for 5 min. The
prepared proteins were subsequently subjected to 10% SDS-PAGE for 2
h at 100 V, and transferred to a nitrocellulose membrane (GE
Healthcare, Little Chalfont, UK) for 2 h at 40 V in transfer buffer
(25 mM Trizma-base, 192 mM glycine, and 20% methanol). Membranes
were then exposed to appropriate dilutions of primary antibodies
and allowed to hybridize overnight at 4°C: anti-perilipin antibody
(cat. no. 9349S), anti-p-perilipin antibody (cat. no. 9621),
anti-HSL antibody (cat. no. 4107S), anti-p-HSL antibody (cat. no.
4139S), anti-ATGL antibody (cat. no. 2183S) and anti-β-actin
antibodies (cat. no. 4967S; all Cell Signaling Technology, Danvers,
MA, USA). After removal of the antibodies, the membranes were
washed three times in a 10 mM Trizma-base (150 mM NaCl and 0.05%
Tween-20) solution for 10 min. The membranes were subsequently
incubated with horseradish peroxidase-conjugated anti-secondary
antibody for 1 h at room temperature, after which they were washed
again as described above, and developed using the Enhanced
Chemiluminescence Reagent Plus kit (cat. no. DG-WF100; Dogen,
Seoul, Korea). Finally, the results were quantified using the Image
Analyzer System (Fluorchem FC2; Alpha Innotech, CA, USA) and
expressed as the fold-increase over control values.
Statistical significance analysis
One-way analysis of variance was performed using
SPSS 10.10 (SPSS, Inc., Chicago, IL, USA) in order to determine the
variance and identify significant differences between the treated
group and other groups, as well as between the vehicle group and
other HFD fed groups. All values are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of EMfC on body weight and
lipid profile of serum
To evaluate the anti-obesity effects of EMfC, we
analyzed the changes in body weight and serum lipid profile of
HFD-induced obesity mice treated with EMfC for 12 weeks (Fig. 1). Although no significant differences
were detected in the body weight of HFD+Vehicle treated group and
HFD+EMfC treated group, the serum lipid profile differed after EMfC
treatment. As shown in Fig. 1B, all
lipid profile factors were 40–180% higher in the HFD+Vehicle
treated group than in the No treatment group. However, the
concentration of LDL, TG, TC and glucose significantly decreased in
the HFD+EMfC treated group as compared to the HFD+Vehicle treated
group (P<0.005; Fig. 1B).
Additionally, EMfC treatment resulted in enhanced HDL levels
(Fig. 1B). These results suggest
that EMfC induces a recovery of the serum lipid profile in
HFD-induced obesity mice, although it is unable to inhibit weight
gain due to HFD.
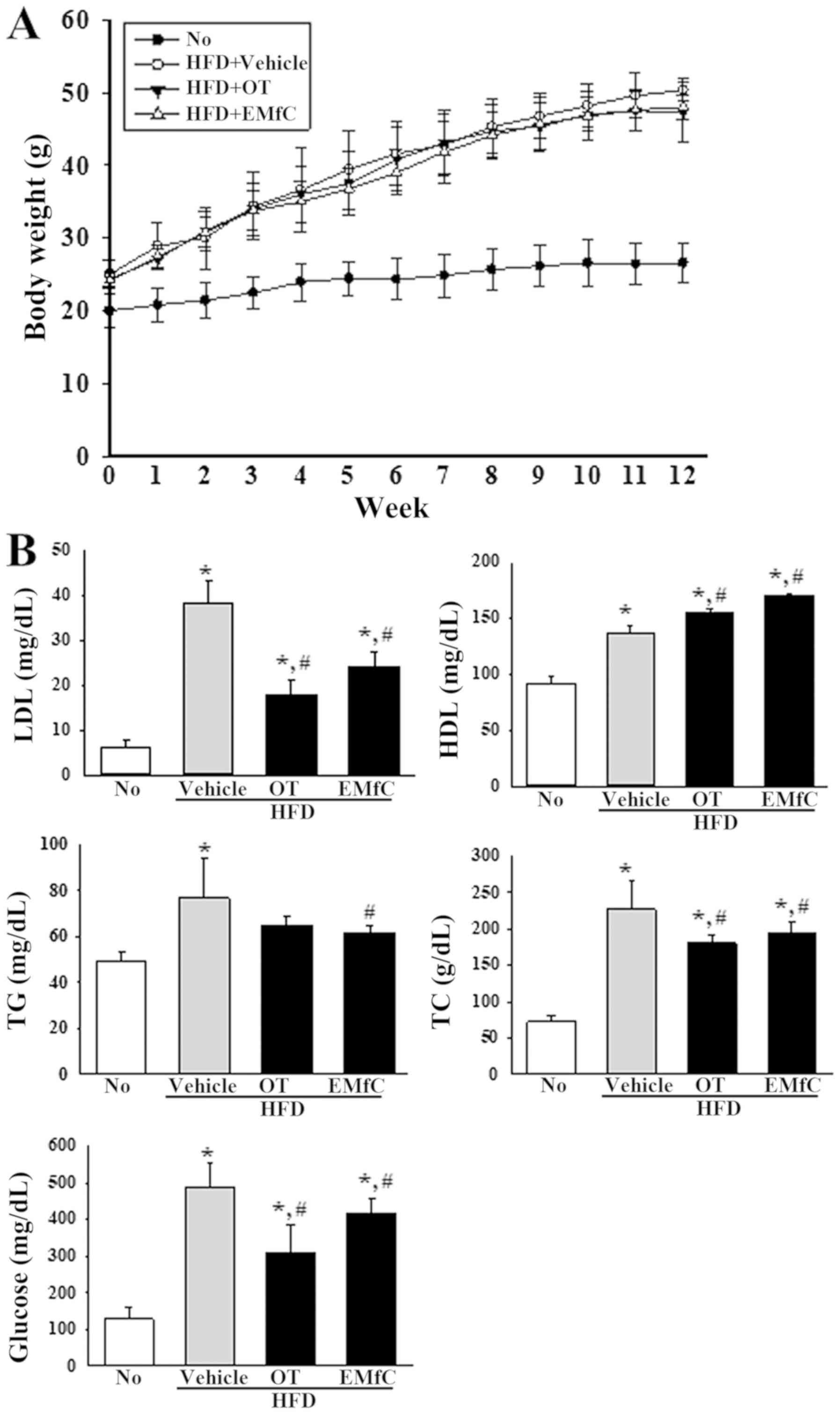 | Figure 1.Assessment of the body weight and
serum lipid profile. (A) Comparison of body weight following
treatment with Vehicle, OT or EMfC in HFD-induced obese C57BL/6
mice. Percentage body weight gain from 0 to 12 weeks. (B) LDL, HDL,
TG, TC and glucose content were measured in serum from all mice in
the experimental groups. Each group included seven animals. Data
are presented as mean percentage weight gain ± standard deviation.
*P<0.05 vs. the No treatment group; #P<0.05 vs.
the HFD+Vehicle treated group. LDL, low density lipid; HDL, high
density lipid; TG, triglyceride; TC, total cholesterol; OT,
Orlistat; HFD, high fat diet; EMfC, extract of mulberry leaves
fermented with Cordyceps militaris. |
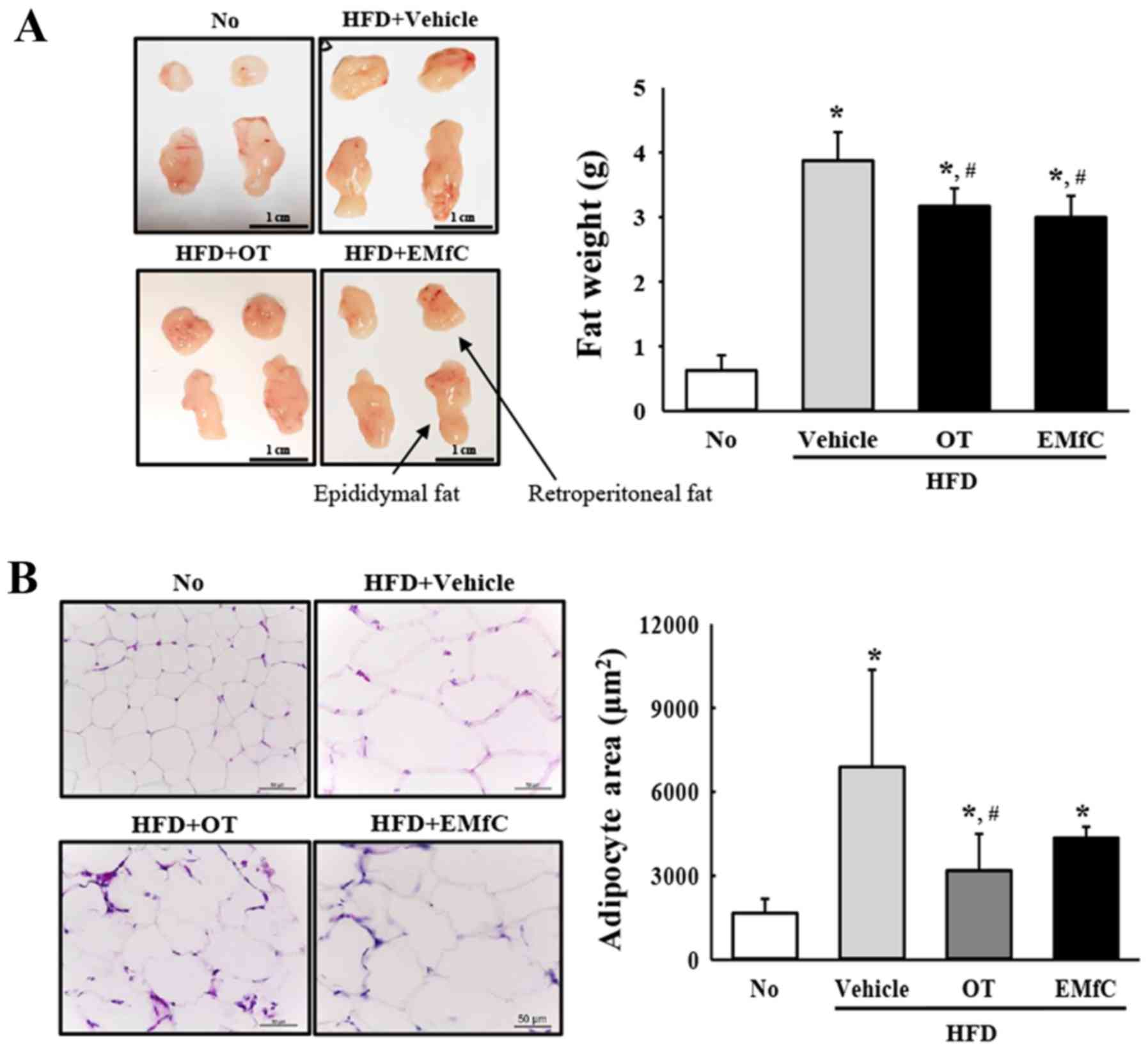
Effect of EMfC on abdominal fat
accumulation
In order to investigate the effect of EMfC on
abdominal fat accumulation, we assessed the amount of abdominal fat
and the size of adipocytes by H&E staining. The HFD+Vehicle
treated group had higher content of abdominal fat as compared to
the No treatment group (P<0.005). However, levels significantly
decreased in the HFD+EMfC and HFD+OT treated groups as compared to
the HFD+Vehicle treated group (P<0.005; Fig. 2A). A similar pattern was observed for
the average area of single adipocyte of fat tissue. HFD+EMfC and
HFD+OT treated groups showed significant decrease of the adipocyte
area as compared with the HFD+Vehicle treated group (P<0.005;
Fig. 2B). These results suggest that
EMfC prevents the accumulation of abdominal fat in HFD-induced
obesity mice.
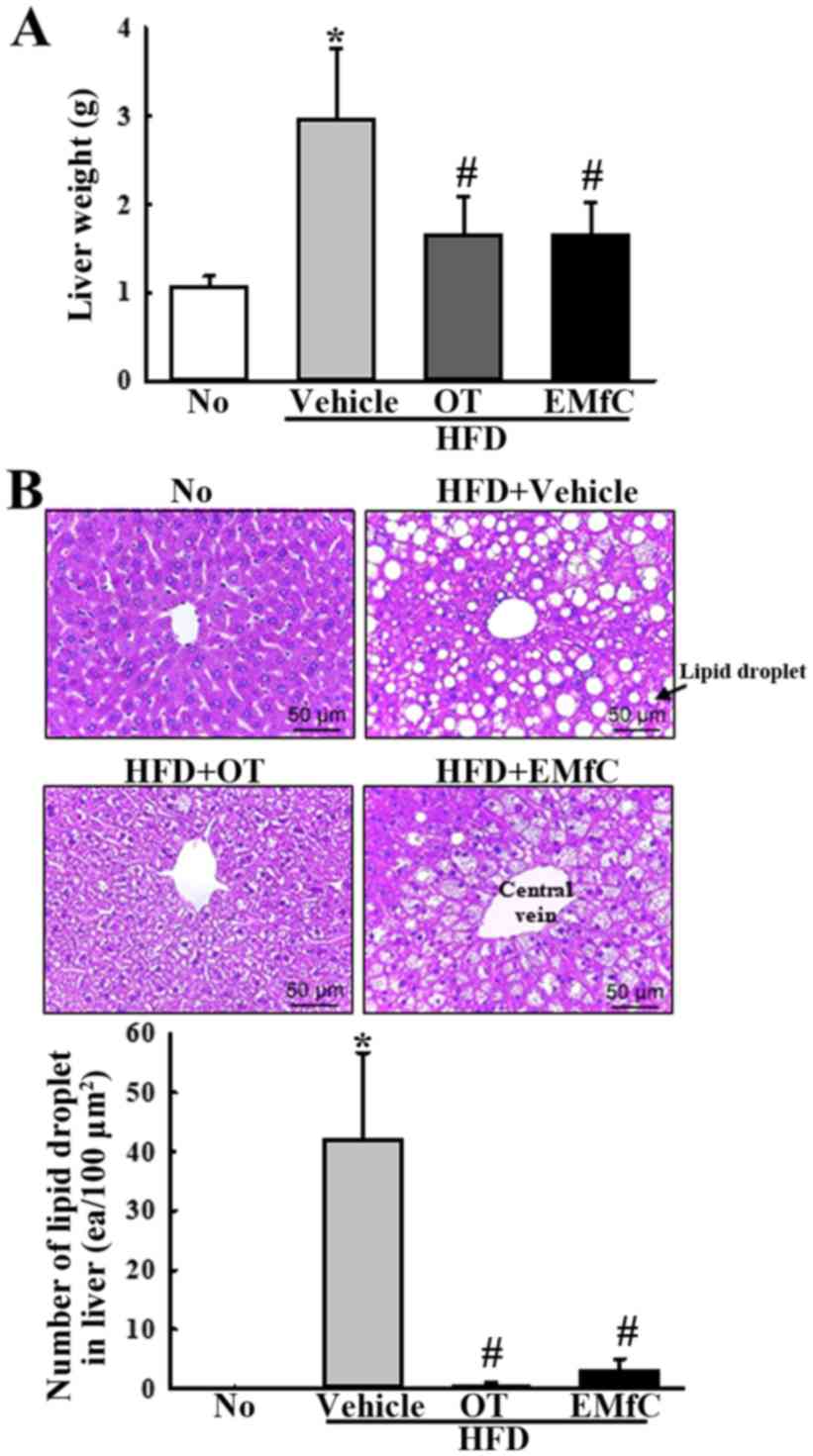
Effects of EMfC on hepatic steatosis
of HFD-induced obesity mice
Liver weight alterations and hepatic steatosis are
common obesity-related phenomena. Therefore, we measured the
changes in weight and fat accumulation of liver in HFD-induced
obesity mice treated with EMfC. The HFD+Vehicle treated group
showed increased liver weight compared to the No treatment group
(P<0.005). These changes recovered with OT or EMfC treatment
(Fig. 3A). Also, significant changes
were detected in the number of lipid droplet in the liver tissues
of all groups stained with H&E. The liver section of the
HFD+Vehicle treated group showed dramatic increase in the number of
lipid drops compared to the No treatment group. In comparison, only
a few lipid drops were seen in the HFD+OT and HFD+EMfC treated
groups (P<0.005; Fig. 3B). These
results indicate that EMfC significantly inhibits the liver
hypertrophy, fat accumulation and hepatic steatosis in HFD-induced
obesity mice.
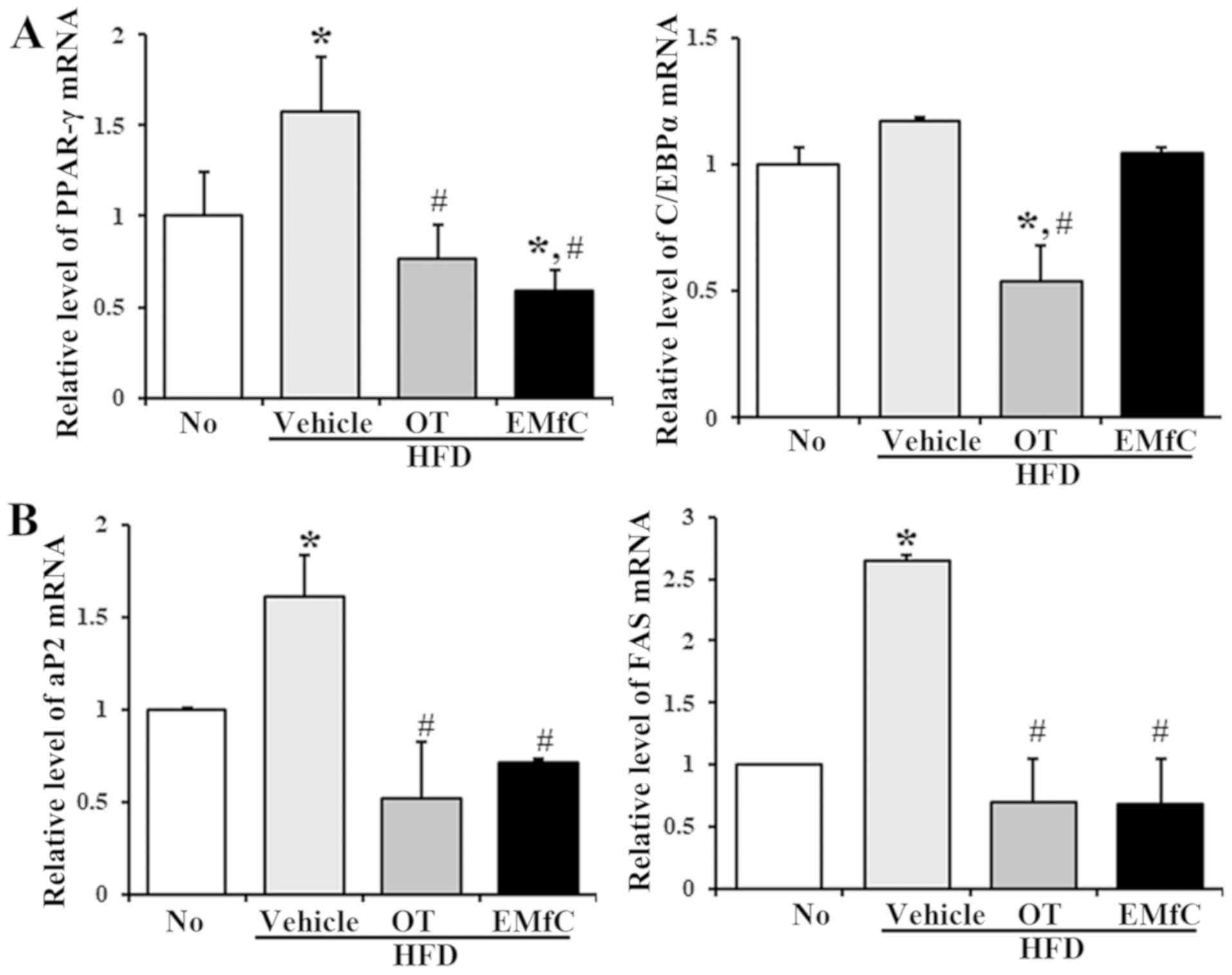
Effects of EMfC on adipogenesis and
lipogenesis of HFD-induced obese mice
To further elucidate the mechanisms by which EMfC
affects obesity, we measured the mRNA levels of several key genes
that affecting adipogenesis and lipogenesis in the liver tissue. As
shown in Fig. 4, there were
significant changes in the mRNA levels of adipogenesis and
lipogenesis related genes after EMfC treatment. The mRNA levels of
PPARγ and C/EBPα for adipogenesis increased after HFD feeding
(HFD+Vehicle treated group) compared to the No treatment group
(P<0.005). However, these levels were dramatically decreased
after OT or EMfC treatment (HFD+OT and HFD+EMfC treated groups,
respectively), although no changes were seen in the C/EBPα level
(Fig. 4A). The gene expression of
aP2 and FAS were showed dramatically increased in the HFD+Vehicle
treated group, but significantly decreased in the HFD+OT and
HFD+EMfC treated groups when compared to the No treatment group
(P<0.005; Fig. 4B). These results
indicate that EMfC treatment probably inhibits adipogenesis and
lipogenesis in the liver tissue through the regulation of PPARγ,
aP2 and FAS expression.
Effect of EMfC on lipolysis of
HFD-induced obese mice
We finally investigated the molecular mechanism for
lipolytic activity of EMfC by analyzing the expression and
activation of key enzymes involved in triglyceride metabolism. We
evaluated the phosphorylation or expression of three major enzymes,
including perilipin, HSL and ATGL. We observed increased protein
levels of ATGL in the liver tissues after treatment in the HFD+OT
and HFD+EMfC groups, as compare to No treatment and HFD+Vehicle
treated groups (P<0.005; Fig. 5).
The protein levels of perilipin and HSL significantly decreased
after OT and EMfC treatment, compared to the HFD+Vehicle treated
group. Interestingly, the phosphorylation of perilipin and HSL were
significantly enhanced in the HFD+EMfC treated group, while an
increase in phosphorylation of perilipin was observed in the HFD+OT
treated group (P<0.005; Fig. 5).
Our data indicates that increased lipolytic ability of EMfC is
mainly dependent on increased expression of ATGL and increased
activation of perilipin, as well as increased activation of HSL,
unlike OT. We therefore suggest that compared to OT, EMfC is likely
to be more efficient in contributing to lipolysis.
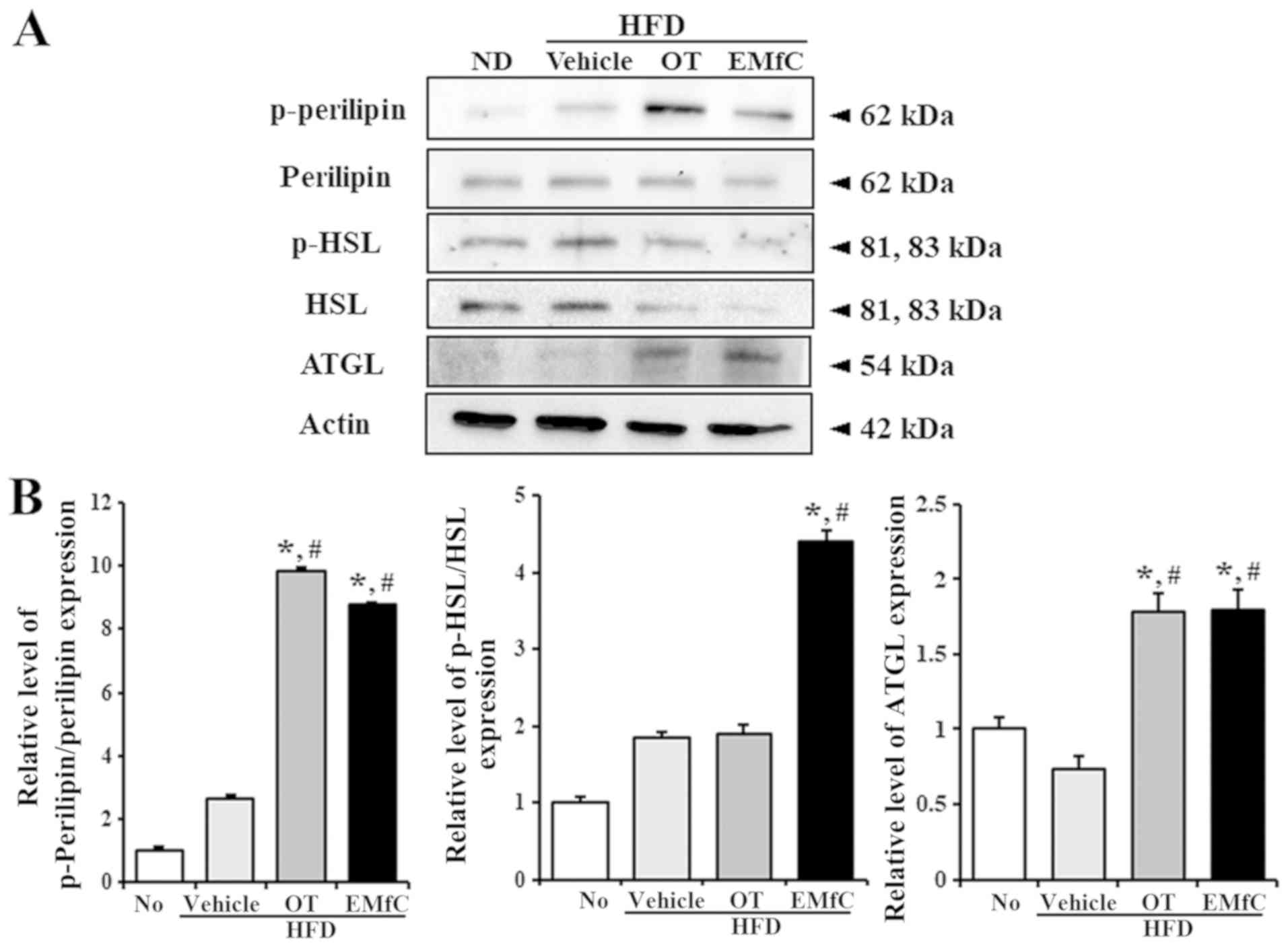 | Figure 5.Expression of lipolysis associated
proteins in liver tissues. (A) Western blot analysis measured the
phosphorylation or expression of several lipolysis associated
proteins, including (B) perilipin, p-perilipin, HSL, p-HSL and
ATGL. The intensity of each band was determined using an imaging
densitometer, and the relative levels of the four proteins were
calculated based on the intensity of actin. Five to six rats per
group were assayed in triplicate by western blotting. Data are
presented as the mean ± standard deviation of three replicates.
*P<0.05 vs. the No treatment group; #P<0.05 vs.
the HFD+Vehicle treated group. HFD, high fat diet; OT, Orlistat;
EMfC, extract of mulberry leaves fermented with Cordyceps
militaris; p-, phosphorylated; HSL, hormone-sensitive lipase;
ATGL, adipose triglyceride lipase. |
Discussion
Recent pharmacological approaches for treating and
controlling obesity have resorted to the use of drugs to induce
anorexia, inhibit nutritional absorption, and promote weight loss
(2). Clinically available
anti-obesity agents include pancreatic lipase inhibitors such as
orlistat, and appetite suppressants that act on the central nervous
system, such as lorcaserin (29,30).
Since body fat accumulation is a major risk factor for chronic
diseases including hyperlipidemia, diabetes, cardiovascular disease
and cancer, interest has increased for developing of new drugs
having lipolytic and lipogenic activities (31,32).
Numerous studies reported that several lipolytic agents, including
norepinephrine, isoproterenol, phoscholine, dibutyl-cAMP (DBcAMP)
and theophylline induce lipolysis in adipocytes (33). However, these anti-obesity drugs are
associated with serious side effects such as constipation,
insomnia, vomiting, headache, abdominal pain, and myocardial
infarction (34). Therefore, the
development of anti-obesity agents derived from natural products
that are effective in weight management in suppressing and
decomposing the fat accumulation, and having minimal side effects,
are required (35). In this study,
to analyze the anti-obesity effect of EMfC, we used orlistat as a
positive control for assessing the anti-obesity effect in
HFD-induced obese mice. At the end of the 12 weeks study period,
although the HFD treated mice had maximum weight gain, there was no
effective weight loss with orlistat and EMfC treatments also. This
could be because orlistat is an anti-obesity agent involved in fat
metabolism rather than weight loss effect. Secondly, the
concentration of orlistat used in the study is 10 mg/kg, which is a
low concentration as compared to the commonly used concentration of
10–50 mg/kg. The third reason is that although the final effect of
orlistat is reduction of body weight following the suppression of
fat absorption, 12 weeks is considered the period when no effective
weight loss has occurred. Subsequently, we expect similar effects
of orlistat and EMfC on fat metabolism, resulting in weight loss
during longer study protocols.
In addition, to study the changes in body weight as
a consequence of changes in dietary intake (data not shown), a
slight decrease was observed in dietary intake of the experimental
group (including the vehicle group), with no obvious difference
between. We believe this slightly reduction in consumption may be
due to the vehicle, and not olistat and EMfC.
Our study data reveals that EMfC treatment inhibits
the visceral (epididymal and abdominal) adipose tissue mass,
adipocyte cell size, serum LDL, serum TG, serum TC and serum
glucose in obese mice induced by HFD. In addition, decreased liver
weight and decreased lipid drop after EMfC exposure suggests that
EMfC has an anti-obesity effect by being involved in liver lipid
metabolism. Interestingly, the serum analysis of the experimental
group including EMfC treatment for 12 weeks showed no adverse side
effects such as hepatotoxicity and kidney toxicity (data not
shown). Taken together these results suggest that the reduction in
fat mass observed in HFD+EMfC treated mice is due not to the
toxicity of EMfC, but due to the direct pharmacological action on
adipose tissue.
EMfC treatment in HFD fed mice decreased the serum
TG, TC, LDL and glucose levels proportionate to the decrease in
adipose tissue mass. Conversely, the serum HDL concentration
dramatically increased after EMfC and OT treatment. triglycerides
(TGs) accumulate in adipose tissues by forming lipid drops of
adipocytes (36). Our data displayed
that the level of TG only marginally after EMfC treatment.
Microscopic and histologic examinations revealed reduction in the
mass of adipose tissue and adipocyte cell sizes of the HFD+EMfC
treated groups as compared to the HFD+Vehicle treated group. The
liver weight and number of lipid drops also decreased in the
HFD+EMfC treated organ. Although the effect on serum level
reduction of accumulated TGs is marginal, EMfC reduces adipose
tissue due to decreasing of adipocyte size and cell number, and it
is therefore possible to control cardiovascular diseases by
decreasing the concentration of LDL and TC in serum. Therefore, we
believe that the association between cytokine and hormones involved
in fat accumulation (such as adipokine and leptin), and the
relationship with cardiovascular diseases should be studied
further, with respect to the anti-obesity effects of EMfC.
Adipose tissue is responsible for systemic energy
homeostasis. PPARγ, a predominant transcription factor in adipose
tissue, plays an important role in adipocyte differentiation, lipid
storage, and glucose homeostasis (37,38).
PPARγ regulates the gene expression of enzymes involved in the
lipogenesis of adipose tissue, such as FAS, aP2, and lipoprotein
lipase (39,40). FAS is a major lipogenic enzyme that
catalyzes the biosynthesis of long chain fatty acids from
acetyl-CoA precursors (41), and aP2
is an intracellular fatty acid binding protein that binds
long-chain fatty acids (42). In
this study, EMfC significantly reduced the mRNA levels of PPARγ,
aP2, and FAS in the HFD-induced obesity mice. These results suggest
that EMfC treatment improves the intracellular fatty acid
metabolism by inhibiting the lipogenic gene expression.
Interestingly, there was no inhibition in the expression of C/EBPα,
a transcription factor similar to PPARγ and involved in
lipogenesis, after EMfC administration. Orlistat inhibits the
expression of two transcription factors (PPARγ and C/EBPα) involved
in lipid synthesis, suggesting that EMfC has a different mechanism
for inhibiting lipid synthesis.
In addition to the synthesis of TG stored in adipose
tissue, lipolysis (hydrolysis) is an important mechanism for fat
accumulation in adipose tissue. ATGL (PNPLA2, desnutrin) is an
important enzyme that initiates TG degradation and produces
diacylglycerol (DG). It serves to initiate the sequential process
of TG digestion, limiting the rate at the first step and
determining the maximum rate for fatty acid mobilization (43–45). We
observed that EMfC increases the amount of ATGL protein expression,
similar to orlistat treatment. Perilipin is activated by the
phosphorylation by phosphorylated PKA, which in turn activates the
lipase of ATGL (46). Perilipin
phosphorylation also increases after exposure to orlistat or EMfC,
suggesting that EMfC has a similar lipid-breakdown mechanism to the
orlistat induced mechanism. Interestingly, the phosphorylation of
HSL, where ATGL degrades the DG into monoglycerides, occurs more
strongly in EMfC than in orlistat (47), suggesting that EMfC has better
activity in lipolysis than orlistat.
In conclusion, our study demonstrates that EMfC
treatment successfully reduces the accumulation of adipose tissue
and adipocyte cell size, and decrease the serum LDL, TG, TC and
glucose levels in HFD-induced obese mice. In addition, there is a
decrease in the liver weight and lipid drops, suppression in the
expression of the lipogenic gene, and induction in the expression
and activation of lipolysis protein. The strong effects of EMfC on
fat loss and fatty liver loss without any accompanying adverse
health effects suggests EMfC to be a potential anti-obesity drug
that results in lipolysis.
Acknowledgements
The abstract was presented at the Korean Association
for Laboratory Animal Science Winter Symposium Jan 24-Jan 27 2018
in Wonju, Kanwon-do, Korea and published as abstract no. OR-S-06 in
Lab Anim Res 34 (Suppl. 1): 2018. The authors would like to thank
their animal technician, Miss. Jin Hyang Hwang, for directing the
animal care at the Laboratory Animal Resources Center in Pusan
National University.
Funding
The present study was supported by grants from the
Korea Institute of Planning & Evaluation for Technology of
Food, Agriculture and Forestry (grant no. 116027-032-HD030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MRL, JEK, JYC, JJP, HRK, BRS, YWC, KMK and DYH
participated in the design of the study, sample preparation, animal
experiments and data analyses. HS performed the data analysis and
assisted with manuscript preparation.
Ethics approval and consent to
participate
The Pusan National University Animal Ethics
Committee (no. PNU-2017-1519) approved the protocol for the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMfC
|
extract of mulberry leaves fermented
with Cordyceps militaris
|
|
HFD
|
high fat diet
|
|
TG
|
triglyceride
|
|
TC
|
total cholesterol
|
References
|
1
|
Haslam DW and James WP: Obesity. Lancet.
366:1197–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Després JP and Lemieux I: Abdominal
obesity and metabolic syndrome. Nature. 444:881–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
NCD Risk Factor Collaboration (NCD-RisC):
Worldwide trends in diabetes since 1980: A pooled analysis of 751
population-based studies with 4.4 million participants. Lancet.
387:1513–1530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maguire T and Haslam D: The Obesity
Epidemic and its Management. Matthew Wright: Pharmaceutical Press;
London: pp. 264–267. 2010
|
|
5
|
Pittler MH, Schmidt K and Ernst E: Adverse
events of herbal food supplements for body weight reduction:
Systematic review. Obes Rev. 6:93–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gunjal S, Ankola AV and Bhat K: In vitro
antibacterial activity of ethanolic extract of Morus alba
leaf against periodontal pathogens. Indian J Dent Res. 26:533–536.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raman ST, Ganeshan AK, Chen C, Jin C, Li
SH, Chen HJ and Gui Z: In vitro in vivo antioxidant activity
of flavonoid extracted from mulberry fruit (Morus alba L.).
Pharmacogn Mag. 12:128–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Xiang L, Wang C, Tang C and He X:
Antidiabetic and antioxidant effects and phytochemicals of mulberry
fruit (Morus alba L.) polyphenol enhanced extract. PLoS One.
8:e711442008. View Article : Google Scholar
|
|
9
|
Jo SP, Kim JK and Lim YH:
Antihyperlipidemic effects of stilbenoids isolated from Morus
alba in rats fed a high-cholesterol diet. Food Chem Toxicol.
65:213–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Beshbishy HA, Singab AN, Sinkkonen J
and Pihlaja K: Hypolipidemic and antioxidant effects of Morus
alba L. (Egyptian mulberry) root bark fractions supplementation
in cholesterol-fed rats. Life Sci. 78:2724–2733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eo HJ, Park JH, Park GH, Lee MH, Lee JR,
Koo JS and Jeong JB: Anti-inflammatory and anti-cancer activity of
mulberry (Morus alba L.) root bark. BMC Complement Altern
Med. 14:2002014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan EW, Lye PY and Wong SK:
Phytochemistry, pharmacology, and clinical trials of Morus
alba. Chin J Nat Med. 14:17–30. 2016.PubMed/NCBI
|
|
13
|
Kobayashi Y, Miyazawa M, Kamei A, Abe K
and Kojima T: Ameliorative effects of mulberry (Morus alba
L.) leaves on hyperlipidemia in rats fed a high-fat diet: Induction
of fatty acid oxidation, inhibition of lipogenesis, and suppression
of oxidative stress. Biosci Biotechnol Biochem. 74:2385–2395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang SJ, Park NY and Lim Y:
Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1
adipocytes. Nutr Res Pract. 8:613–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ann JY, Eo HY and Lim YS: Mulberry leaves
(Morus alba L.) ameliorate obesity-induced hepatic
lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed
mice. Genes Nutr. 10:462015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naowaboot J, Pannangpetch P,
Kukongviriyapan V, Prawan A, Kukongviriyapan U and Itharat A:
Mulberry leaf extract stimulates glucose uptake and GLUT4
translocation in rat adipocytes. Am J Chin Med. 40:163–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugimoto M, Arai H, Taura Y, Murayama T,
Khaengkhan P, Nishio T, Ono K, Ariyasu H, Akamizu T, Ueda Y, et al:
Mulberry leaf ameliorates the expression profile of adipocytokines
by inhibiting oxidative stress in white adipose tissue in db/db
mice. Atherosclerosis. 204:388–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sung GH, Hywel-Jones NL, Sung JM,
Luangsa-Ard JJ, Shrestha B and Spatafora JW: Phylogenetic
classification of Cordyceps and the clavicipitaceous fungi.
Stud Mycol. 57:5–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Silva DD, Rapior S, Sudarman E, Stadler
M, Xu J, Alias SA and Hyde KD: Bioactive metabolites from
macrofungi: Ethnopharmacology, biological activities and chemistry.
Fungal Divers. 62:1–40. 2013. View Article : Google Scholar
|
|
20
|
De Silva DD, Rapior S, Fons F, Bahkali AH
and Hyde KD: Medicinal mushrooms in supportive cancer therapies: An
approach to anti-cancer effects and putative mechanisms of action.
Fungal Divers. 55:1–35. 2012. View Article : Google Scholar
|
|
21
|
Pao HY, Pan BS, Leu SF and Huang BM:
Cordycepin stimulated steroidogenesis in MA-10 mouse Leydig tumor
cells through the protein kinase C Pathway. J Agric Food Chem.
60:4905–4913. 2102. View Article : Google Scholar
|
|
22
|
Kim JR, Yeon SH, Kim HS and Ahn YJ:
Larvicidal activity against Plutella xylostella of
cordycepin from the fruiting body of Cordyceps militaris.
Pest Manag Sci. 58:713–717. 2012. View
Article : Google Scholar
|
|
23
|
Ramesh T, Yoo SK, Kim SW, Hwang SY, Sohn
SH, Kim IW and Kim SK: Cordycepin (3′-deoxyadenosine) attenuates
age-related oxidative stress and ameliorates antioxidant capacity
in rats. Exp Gerontol. 47:979–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA
and Zhu HB: Cordycepin prevents hyperlipidemia in hamsters fed a
high-fat diet via activation of AMP-activated protein kinase. J
Pharm Sci. 113:395–403. 2010. View Article : Google Scholar
|
|
25
|
Kim SS, Lim KS, Kim HJ, Chong MS, Cho HE,
Choi YH, et al: Effects of extracts from mixed culture with
Tricholoma Matsutake mycelium and Cordyceps Militaris
mycelium on mlood glucose in streptozotocin-induced diabetic rats.
Korean J Orient Physiol Pathol. 22:365–370. 2008.
|
|
26
|
Hung YP and Lee CL: Higher anti-liver
fibrosis effect of cordyceps militaris-fermented product
cultured with deep ocean water via inhibiting proinflammatory
factors and fibrosis-related factors expressions. Mar Drugs.
15(pii): E1682017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee MR, Kim JE, Yun WB, Choi JY, Park JJ,
Kim HR, Song BR, Choi YW, Kim KM and Hwang DY: Lipolytic effect of
novel extracts from mulberry (Morus alba) leaves fermented
with Cordyceps militaris in the primary adipocytes derived
from SD rats. Lab Anim Res. 33:270–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hofbauer KG, Nicholson JR and Boss O: The
obesity epidemic: Current and future pharmacological treatments.
Annu Rev Pharmacol Toxicol. 47:565–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma B and Henderson DC: Sibutramine:
Current status as an anti-obesity drug and its future perspectives.
Expert Opin Pharmacother. 9:2161–2173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lean ME: Pathophysiology of obesity. Proc
Nutr Soc. 59:331–336. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Apostolopoulou M, Savopoulos C, Michalakis
K, Coppack S, Dardavessis T and Hatzitolios A: Age, weight and
obesity. Maturitas. 71:115–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morimoto C, Kameda K, Tsujita T and Okuda
H: Relationships between lipolysis induced by various lipolytic
agents and hormone-sensitive lipase in rat fat cells. J Lipid Res.
42:120–127. 2001.PubMed/NCBI
|
|
34
|
Bray GA: Drug treatment of obesity. Rev
Endocr Metab Disord. 2:403–418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mayer MA, Höcht C, Puyó A and Taira CA:
Recent advances in obesity pharmacotherapy. Curr Clin Pharmacol.
4:53–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vázquez-Vela ME, Torres N and Tovar AR:
White adipose tissue as endocrine organ and its role in obesity.
Arch Med Res. 39:715–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schoonjans K, Staels B and Auwerx J: The
peroxisome proliferator activated receptors (PPARS) and their
effects on lipid metabolism and adipocyte differentiation. Biochim
Biophys Acta. 1302:93–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rosen ED, Walkey CJ, Puigserver P and
Spiegelman BM: Transcriptional regulation of adipogenesis. Genes
Dev. 14:1293–1307. 2000.PubMed/NCBI
|
|
39
|
Kliewer SA and Willson TM: The nuclear
receptor PPARgamma-bigger than fat. Curr Opin Genet Dev. 8:576–581.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Spiegelman BM: PPAR-gamma: Adipogenic
regulator and thiazolidinedione receptor. Diabetes. 47:507–514.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boizard M, Le Liepvre X, Lemarchand P,
Foufelle F, Ferré P and Dugail I: Obesity-related overexpression of
fatty-acid synthase gene in adipose tissue involves sterol
regulatory element-binding protein transcription factors. J Biol
Chem. 273:29164–29171. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Coe NR and Bernlohr DA: Physiological
properties and functions of intracellular fatty acid-binding
proteins. Biochim Biophys Acta. 1391:287–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zimmermann R, Strauss JG, Haemmerle G,
Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger
G, Eisenhaber F, Hermetter A and Zechner R: Fat mobilization in
adipose tissue is promoted by adipose triglyceride lipase. Science.
306:1383–1386. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haemmerle G, Lass A, Zimmermann R,
Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C,
Eder S, et al: Defective lipolysis and altered energy metabolism in
mice lacking adipose triglyceride lipase. Science. 312:734–737.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ahmadian M, Duncan RE, Varady KA, Frasson
D, Hellerstein MK, Birkenfeld AL, Samuel VT, Shulman GI, Wang Y,
Kang C and Sul HS: Adipose overexpression of desnutrin promotes
fatty acid use and attenuates diet-induced obesity. Diabetes.
58:855–866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tansey JT, Sztalryd C, Hlavin EM, Kimmel
AR and Londos C: The central role of perilipin a in lipid
metabolism and adipocyte lipolysis. IUBMB Life. 56:379–385. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marcelin G and Chua S Jr: Contributions of
adipocyte lipid metabolism to body fat content and implications for
the treatment of obesity. Curr Opin Pharmacol. 10:588–593. 2010.
View Article : Google Scholar : PubMed/NCBI
|