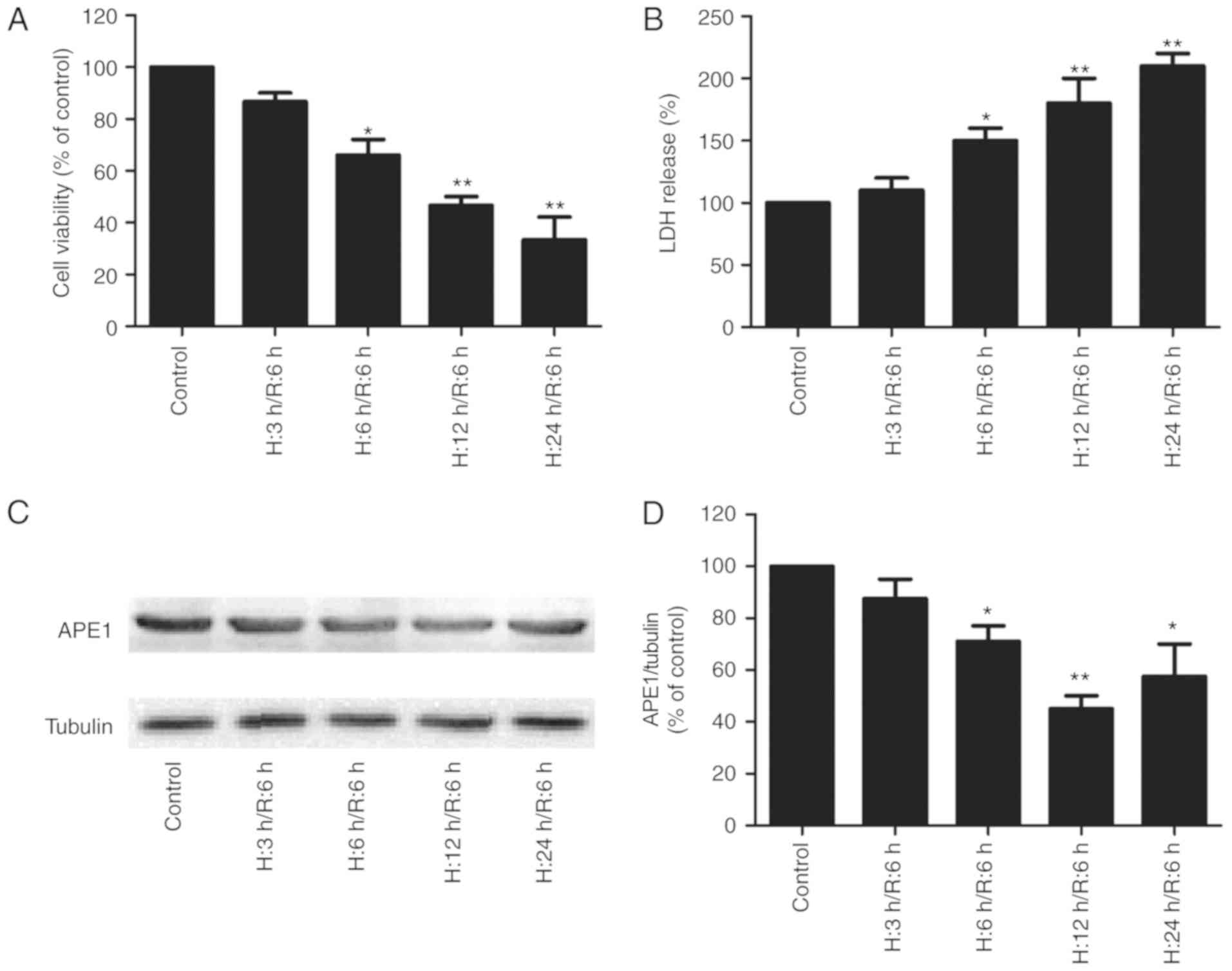
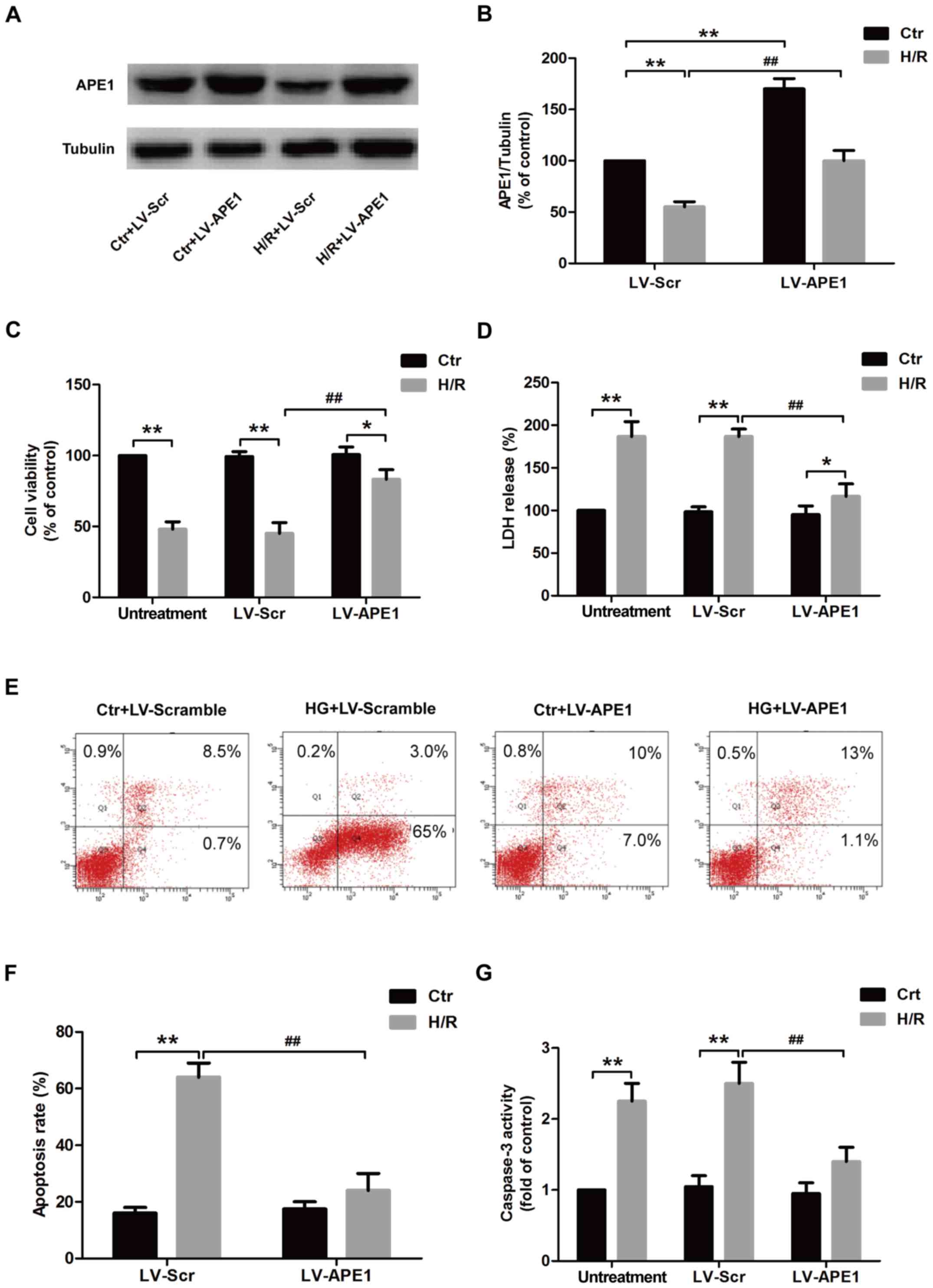
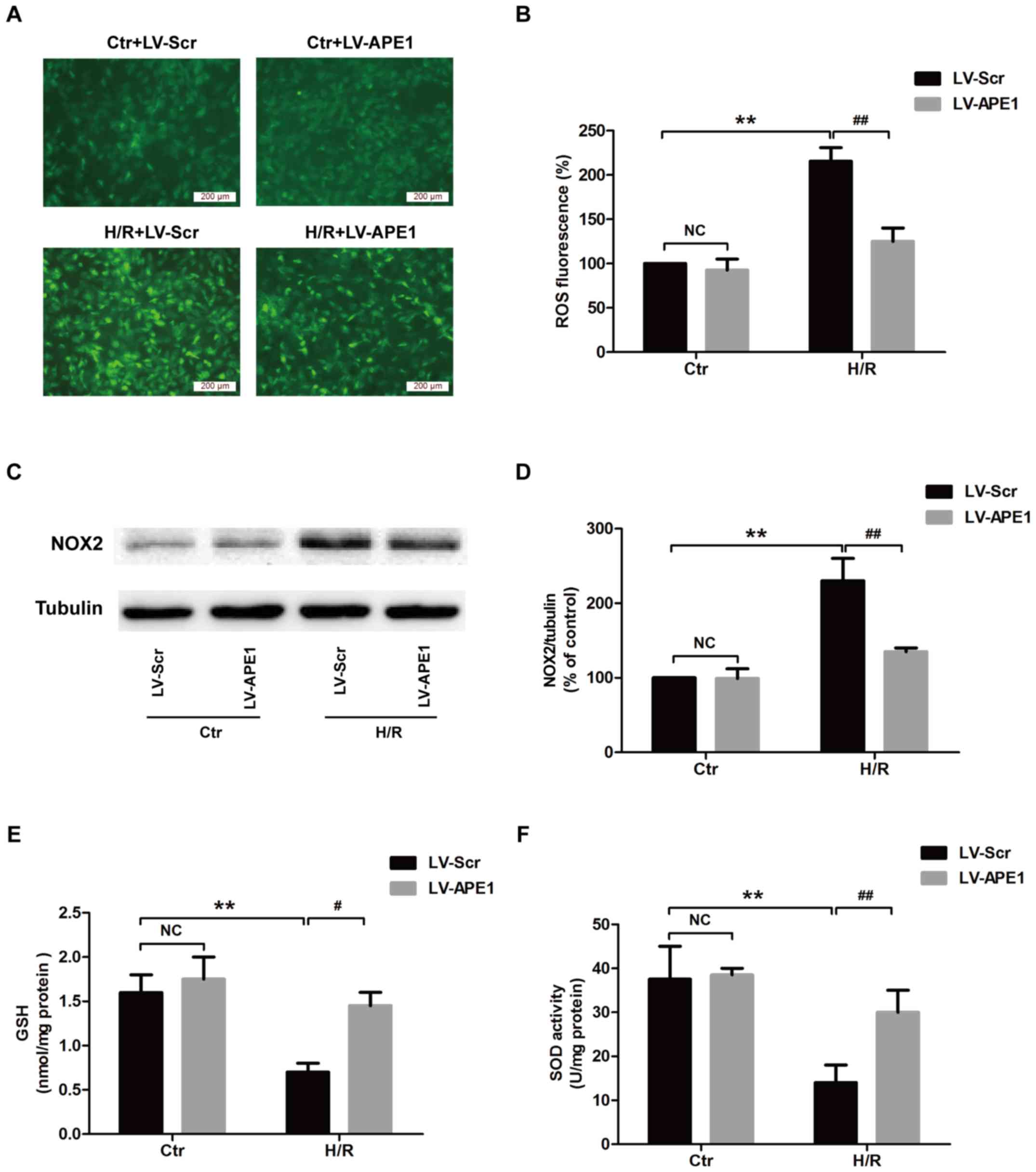
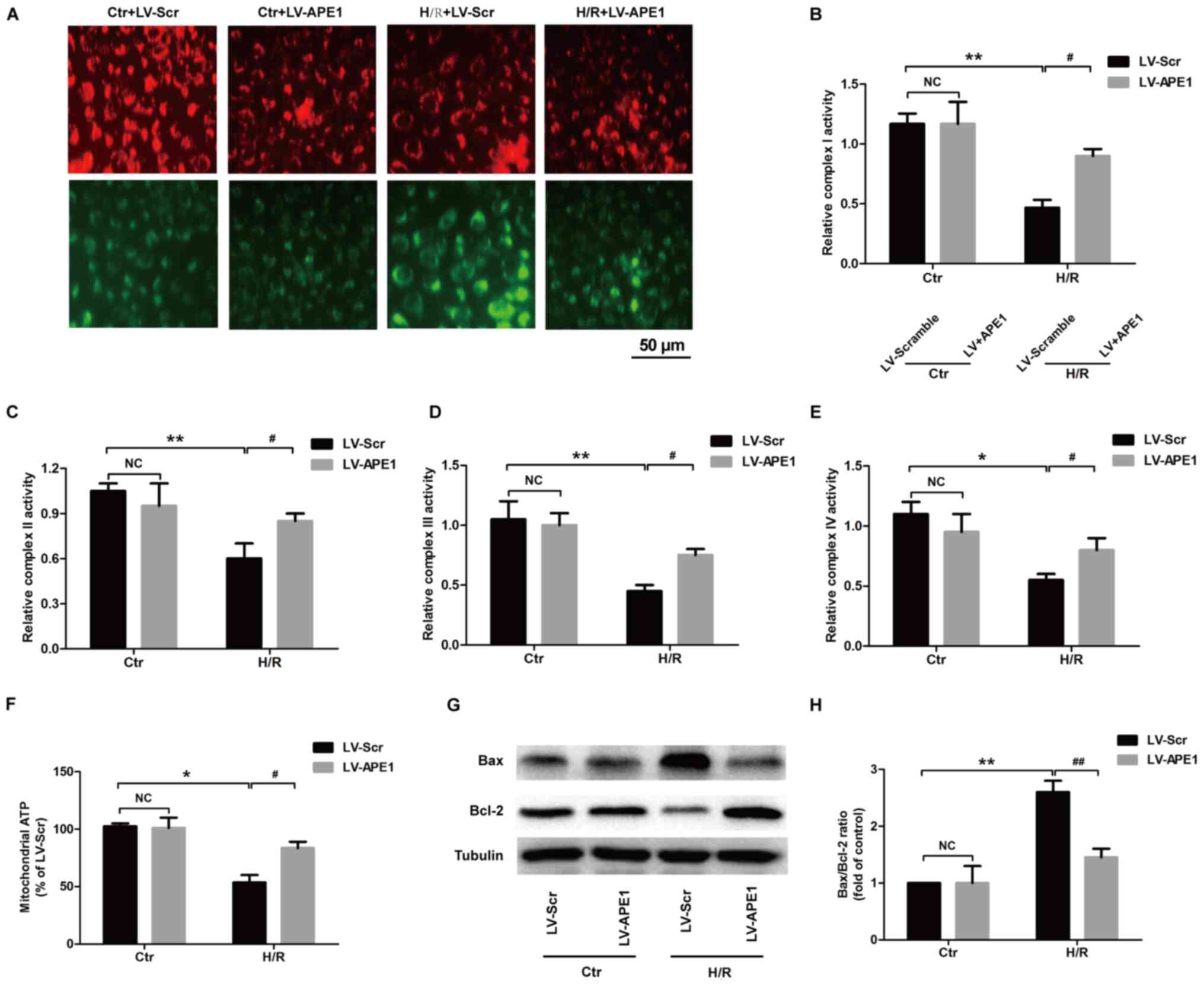
|
1
|
Ambrosio G and Tritto I: Reperfusion
injury: Experimental evidence and clinical implications. Am Heart
J. 138:S69–S75. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia Z, Chen Y, Fan Q and Xue M: Oxidative
stress-mediated reperfusion injury: Mechanism and therapies. Oxid
Med Cell Longev. 2014:3730812014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun L, Fan H, Yang L, Shi L and Liu Y:
Tyrosol prevents ischemia/reperfusion-induced cardiac injury in
H9c2 cells: Involvement of ROS, Hsp70, JNK and ERK, and apoptosis.
Molecules. 20:3758–3775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selvaraju V, Joshi M, Suresh S, Sanchez
JA, Maulik N and Maulik G: Diabetes, oxidative stress, molecular
mechanism, and cardiovascular disease-an overview. Toxicol Mech
Methods. 22:330–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Armstrong SC: Protein kinase activation
and myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:427–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao L, Bei Y, Lin S, Zhang H, Zhou Y,
Jiang J, Chen P, Shen S, Xiao J and Li X: Exercise training
protects against acute myocardial infarction via improving
myocardial energy metabolism and mitochondrial biogenesis. Cell
Physiol Biochem. 37:162–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ertracht O, Malka A, Atar S and Binah O:
The mitochondria as a target for cardioprotection in acute
myocardial ischemia. Pharmacol Ther. 142:33–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi S, Joo HK and Jeon BH: Dynamic
regulation of APE1/Ref-1 as a therapeutic target protein. Chonnam
Med J. 52:75–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thakur S, Sarkar B, Cholia RP, Gautam N,
Dhiman M and Mantha AK: APE1/Ref-1 as an emerging therapeutic
target for various human diseases: Phytochemical modulation of its
functions. Exp Mol Med. 46:e1062014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tell G, Quadrifoglio F, Tiribelli C and
Kelley MR: The many functions of APE1/Ref-1: Not only a DNA repair
enzyme. Antioxid Redox Signal. 11:601–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thakur S, Dhiman M, Tell G and Mantha AK:
A review on protein-protein interaction network of APE1/Ref-1 and
its associated biological functions. Cell Biochem Funct.
33:101–112. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leak RK, Li P, Zhang F, Sulaiman HH, Weng
Z, Wang G, Stetler RA, Shi Y, Cao G, Gao Y and Chen J:
Apurinic/apyrimidinic endonuclease 1 upregulation reduces oxidative
DNA damage and protects hippocampal neurons from ischemic injury.
Antioxid Redox Signal. 22:135–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aonuma T, Takehara N, Maruyama K, Kabara
M, Matsuki M, Yamauchi A, Kawabe J and Hasebe N:
Apoptosis-resistant cardiac progenitor cells modified with
apurinic/apyrimidinic endonuclease/redox factor 1 gene
overexpression regulate cardiac repair after myocardial infarction.
Stem Cells Transl Med. 5:1067–1078. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin SA, Seo HJ, Kim SK, Lee YR, Choi S,
Ahn KT, Kim JH, Park JH, Lee JH, Choi SW, et al: Elevation of the
serum apurinic/apyrimidinic endonuclease 1/redox factor-1 in
coronary artery disease. Korean Circ J. 45:364–371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tell G, Damante G, Caldwell D and Kelley
MR: The intracellular localization of APE1/Ref-1: More than a
passive phenomenon? Antioxid Redox Signal. 7:367–384. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tell G, Crivellato E, Pines A, Paron I,
Pucillo C, Manzini G, Bandiera A, Kelley MR, Di Loreto C and
Damante G: Mitochondrial localization of APE/Ref-1 in thyroid
cells. Mutat Res. 485:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barchiesi A, Wasilewski M, Chacinska A,
Tell G and Vascotto C: Mitochondrial translocation of APE1 relies
on the MIA pathway. Nucleic Acids Res. 43:5451–5464. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torres-Gonzalez M, Gawlowski T, Kocalis H,
Scott BT and Dillmann WH: Mitochondrial 8-oxoguanine glycosylase
decreases mitochondrial fragmentation and improves mitochondrial
function in H9C2 cells under oxidative stress conditions. Am J
Physiol Cell Physiol. 306:C221–C229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Qiu L, Liu X, Hou Z and Yu B: PINK1
alleviates myocardial hypoxia-reoxygenation injury by ameliorating
mitochondrial dysfunction. Biochem Biophys Res Commun. 484:118–124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen D, Jin Z, Zhang J, Jiang L, Chen K,
He X, Song Y, Ke J and Wang Y: HO-1 protects against
hypoxia/reoxygenation-induced mitochondrial dysfunction in H9c2
cardiomyocytes. PLoS One. 11:e01535872016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halestrap AP and Richardson AP: The
mitochondrial permeability transition: A current perspective on its
identity and role in ischaemia/reperfusion injury. J Mol Cell
Cardiol. 78:129–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Z, Lin S, Wu W, Tan H, Wang Z, Cheng C,
Lu L and Zhang X: Ghrelin prevents doxorubicin-induced
cardiotoxicity through TNF-alpha/NF-kappaB pathways and
mitochondrial protective mechanisms. Toxicology. 247:133–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Legrand C, Bour JM, Jacob C, Capiaumont J,
Martial A, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D, et
al: Lactate dehydrogenase (LDH) activity of the cultured eukaryotic
cells as marker of the number of dead cells in the medium
[corrected]. J Biotechnol. 25:231–243. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M and Wilson DM III: Human
apurinic/apyrimidinic endonuclease 1. Antioxid Redox Signal.
20:678–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ushio-Fukai M: Localizing NADPH
oxidase-derived ROS. Sci STKE. 2006:re82006.PubMed/NCBI
|
|
27
|
Capetanaki Y: Desmin cytoskeleton: A
potential regulator of muscle mitochondrial behavior and function.
Trends Cardiovasc Med. 12:339–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ibanez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Q, Shang M, Zhang M, Wang Y, Chen Y,
Wu Y, Liu M, Song J and Liu Y: Microvesicles derived from
hypoxia/reoxygenation-treated human umbilical vein endothelial
cells promote apoptosis and oxidative stress in H9c2
cardiomyocytes. BMC Cell Biol. 17:252016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuznetsov AV, Javadov S, Sickinger S,
Frotschnig S and Grimm M: H9c2 and HL-1 cells demonstrate distinct
features of energy metabolism, mitochondrial function and
sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta.
1853:276–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu
W, Bennett MVL and Chen J: Oxidative stress and DNA damage after
cerebral ischemia: Potential therapeutic targets to repair the
genome and improve stroke recovery. Neuropharmacology. 134:208–217.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pines A, Perrone L, Bivi N, Romanello M,
Damante G, Gulisano M, Kelley MR, Quadrifoglio F and Tell G:
Activation of APE1/Ref-1 is dependent on reactive oxygen species
generated after purinergic receptor stimulation by ATP. Nucleic
Acids Res. 33:4379–4394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin JH, Choi S, Lee YR, Park MS, Na YG,
Irani K, Lee SD, Park JB, Kim JM, Lim JS and Jeon BH: APE1/Ref-1 as
a serological biomarker for the detection of bladder cancer. Cancer
Res Treat. 47:823–833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeon BH, Gupta G, Park YC, Qi B, Haile A,
Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, et al:
Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO
production and vascular tone. Circ Res. 95:902–910. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hall JL, Wang X, Van Adamson, Zhao Y and
Gibbons GH: Overexpression of Ref-1 inhibits hypoxia and tumor
necrosis factor-induced endothelial cell apoptosis through nuclear
factor-kappab-independent and -dependent pathways. Circ Res.
88:1247–1253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Y, Chen J, Zhao T and Fan Z: Granzyme
K degrades the redox/DNA repair enzyme Ape1 to trigger oxidative
stress of target cells leading to cytotoxicity. Mol Immunol.
45:2225–2235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Angkeow P, Deshpande SS, Qi B, Liu YX,
Park YC, Jeon BH, Ozaki M and Irani K: Redox factor-1: An
extra-nuclear role in the regulation of endothelial oxidative
stress and apoptosis. Cell Death Differ. 9:717–725. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maslov LN, Naryzhnaia NV, Podoksenov IuK,
Prokudina ES, Gorbunov AS, Zhang I and Peĭ ZhM: Reactive oxygen
species are triggers and mediators of an increase in cardiac
tolerance to impact of ischemia-reperfusion. Ross Fiziol Zh Im I M
Sechenova. 101:3–24. 2015.(In Russian). PubMed/NCBI
|
|
39
|
Circu ML and Aw TY: Glutathione and
modulation of cell apoptosis. Biochim Biophys Acta. 1823:1767–1777.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zweier JL and Talukder MA: The role of
oxidants and free radicals in reperfusion injury. Cardiovasc Res.
70:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Frossi B, Tell G, Spessotto P, Colombatti
A, Vitale G and Pucillo C: H(2)O(2) induces translocation of
APE/Ref-1 to mitochondria in the Raji B-cell line. J Cell Physiol.
193:180–186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tomkinson AE, Bonk RT and Linn S:
Mitochondrial endonuclease activities specific for
apurinic/apyrimidinic sites in DNA from mouse cells. J Biol Chem.
263:12532–12537. 1988.PubMed/NCBI
|
|
44
|
Li MX, Wang D, Zhong ZY, Xiang DB, Li ZP,
Xie JY, Yang ZZ, Jin F and Qing Y: Targeting truncated APE1 in
mitochondria enhances cell survival after oxidative stress. Free
Radic Biol Med. 45:592–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Siddiqui A, Rivera-Sánchez S, Castro Mdel
R, Acevedo-Torres K, Rane A, Torres-Ramos CA, Nicholls DG, Andersen
JK and Ayala-Torres S: Mitochondrial DNA damage is associated with
reduced mitochondrial bioenergetics in Huntington's disease. Free
Radic Biol Med. 53:1478–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Saleh AM, Aljada A, El-Abadelah MM, Sabri
SS, Zahra JA, Nasr A and Aziz MA: The pyridone-annelated isoindigo
(5′-Cl) induces apoptosis, dysregulation of mitochondria and
formation of ROS in leukemic HL-60 cells. Cell Physiol Biochem.
35:1958–1974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abarikwu SO and Farombi EO: Atrazine
induces apoptosis of SH-SY5Y human neuroblastoma cells via the
regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway.
Pestic Biochem Physiol. 118:90–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar : PubMed/NCBI
|